Abstract
Background:
Asymptomatic bacteriuria frequently occurs among all ages with the possibility of developing into urinary tract infections, and the antimicrobial resistance patterns of the etiologic organisms are essential for appropriate therapy. Thus, we investigated the virulence and antimicrobial resistance patterns of common urinary bacteria in asymptomatic students of Niger Delta University, Amassoma, Bayelsa State, Nigeria in a cross-sectional study.
Materials and Methods:
Clean catch mid-stream early morning urine samples collected from 200 asymptomatic University students of aged ranges 15–30 years were cultured, screened and common bacteria were identified using standard microbiological procedures. The isolates were screened for hemolysin production and their susceptibility to antibiotics was determined using standard disc assay method.
Results:
A total prevalence rate of 52.0% significant bacteriuria was detected and it was significantly higher among the female with a weak association (χ2 = 6.01, phi = 0.173, P = 0.014). The Klebsiella pneumoniae and Staphylococcus aureus isolates were most frequently encountered among the isolated bacteria and 18 (12.7%) of all the bacterial isolates produced hemolysins. All the bacterial isolates exhibited 50–100% resistance to the tested beta-lactam antibiotics, tetracycline and co-trimoxazole. The isolated bacteria were 85-100% multi-drug resistant. However, most of the isolates were generally susceptible to gentamicin and ofloxacin. The phenotypic detection of extended-spectrum beta-lactamases was 9 (9.6%) among the tested Gram-negative bacterial isolates.
Conclusions:
The observed high proportions of multidrug resistant urinary bacteria among asymptomatic University students call for the need of greater control of antibiotic use in this study area.
KEY WORDS: Antimicrobial resistance, asymptomatic bacteriuria, Bayelsa State, Nigeria, students, urinary pathogens
Urinary tract infections (UTIs) are one of the most common bacterial infections which affect the human population of all ages regardless of their sex in both community and hospital settings.[1,2] It is the second most common type of infection that accounts for about 8.3 million doctor visit every year and it occurs far more frequently in women than in men because of the female's short urethra, which is adjacent to the genital and intestinal tracts openings.[3,4]
Asymptomatic bacteriuria is a condition that refers to the presence of bacteria in two consecutive clear-voided urine specimens with both yielding positive cultures of at least 105 cfu/ml of the same uropathogen in a patient without urinary symptoms.[4,5] Asymptomatic bacteriuria commonly occurs at varying prevalence by age, sex, sexual activity, and the presence of genitourinary abnormalities with the prevalence of bacteriuria usually increases with age especially in females living in the community.[5]
Asymptomatic bacteriuria is known to be highly prevalent among the elderly, pregnant women and people with diabetes and bladder catheters who are more likely to experience systematic UTI. Hence, the volume of reports on these groups of people than the general healthy individuals living in the community.[6] Notwithstanding, the screening of young sexually active individuals in a particular community for significant asymptomatic bacteriuria and the patterns of antibiotic resistance of the isolated uropathogens will be a very useful guide for the empirical treatment of UTIs, since UTIs affects humans of all ages and not a selected group.[7] The frequently encountered bacteria in patients with UTIs include Escherichia coli, Klebsiella species, Pseudomonas aeruginosa, Proteus spp., Streptococcus faecalis, Staphylococcus aureus, Staphylococcus saprophyticus and Enterobacter species, but Gram-negative bacteria especially E. coli and Klebsiella species have been reported by several researchers to be the most predominant organisms in UTIs cases.[8,9]
Antibiotic resistance has become a problem of global proportions affecting virtually all bacteria that commonly cause human illness.[10] The clinical management of UTIs has been hampered by the increasing speed at which some urinary bacteria develop resistance to frequently used antimicrobial agents.[11] The incidence of multidrug resistance among common uropathogens has been associated with unregulated use of antibiotics in most developing countries including Nigeria which favor the huge use and misuse of antibiotics within the hospital, agricultural and community settings.[12,13]
The study of asymptomatic bacteriuria among University students and other healthy individuals in the community have been very few in Nigeria, with reports from Ibadan (South West), Keffi (North Central) and Port Harcourt (South-South) while there is none from Bayelsa State.[14,15,16] Thus, this study of determining the bacterial profile and antibiotic resistance patterns of uropathogens among asymptomatic students of Niger Delta University in Amassoma, Bayelsa state, Nigeria will both contribute to the knowledge in this field and serve as a means of providing guideline for the empirical treatment of UTIs in this area of Nigeria.
Materials and Methods
Study design
A cross-sectional study was carried out among 200 healthy undergraduate students of Niger Delta University, Amassoma, Bayelsa State, as a representative of healthy young sexually active individuals in the community. The objective of the study was to investigate the level of carriage of common urinary pathogens by these healthy individuals, their virulence potentials, and antibiotic resistance rate. A study questionnaire was used to recruit those who claimed not to have symptoms associated with UTIs and to obtain their basic demographic data.
Setting
The asymptomatic students were drafted from various faculties of learning in the University from April to August 2012 (5 months) and only students who had not used antibiotics within the last 2 weeks prior to this survey were admitted into the study.
Ethical approval and consent
A written informed consent was signed by all the participants before enrollment, and the study was approved by the ethical committee of the institution before the commencement of the study.
Collection of samples and bacteriology
Each study participant collected the clean catch midstream urine into the sterile and wide mouthed glass bottles following the prescribed instructions for the urine collection. The urine samples were processed in the laboratory within 2 h of collection. Each of the urine samples was mixed and a calibrated sterile wire loop of 4.0 mm diameter designed to deliver 0.01 ml was used to inoculate Cysteine Lactose Electron Deficient agar and MacConkey agar (Oxoid, UK) plates and streaked for discrete colonies before being incubated at 37°C for 24 h for bacterial growth. The samples that gave bacterial count ≥105 colonies per ml (after multiplying the plate count by 100) were considered as significant bacteriuria while those that gave <105 colonies per ml were considered as insignificant bacteriuria.[17] The characteristic discrete colonies on the plates were identified using microbiological techniques which include colony morphology on selective media, hemolytic pattern on blood agar, Gram's stain reaction and biochemical characteristics for both Gram-positive and Gram-negative bacteria.
Antimicrobial susceptibility testing
Antimicrobial susceptibility tests of all the pure bacterial isolates were performed with 12 antibiotic discs from Oxoid, UK using the modified Kirby-Bauer disc diffusion technique as specified by Clinical Laboratory Standard Institute.[18] The standard suspension of each isolate was prepared using its overnight colony culture, which was adjusted to the turbidity of 0.5 McFarland standard before being used to swab the surface of a dried Mueller-Hinton (MH) agar (Oxoid, UK) plate. The following antimicrobial discs were placed on the MH agar (in duplicates) after 20 min of inoculation: Ampicillin (AMP 10 µg), tetracycline (TET 30 µg), co-trimoxazole (SXT 25 µg), ofloxacin (OFX 5 µg), ciprofloxacin (CIP 5 µg), chloramphenicol (C 30 µg), gentamicin (CN 10 µg), ceftazidime (CAZ 30 µg), cefotaxime (CTX 30 µg) and nitrofurantoin (F 30 µg) for Gram-negative bacteria. The third generation cephalosporins (ceftazidime and cefotaxime) were replaced with cefoxitin (FOX 30 µg) for Gram-positive bacteria and these isolates were subjected to vancomycin 2–16 µg/ml using agar dilution method. The plates containing enterobacteria were incubated at 37°C while those for Staphylococcus species were incubated at 35°C for 24 h. The zone diameter of inhibition around each antimicrobial disc was measured and interpreted using the Clinical and Laboratory Standards Institute chart while the Staphylococcus isolates that were resistant to vancomycin at 2 µg/ml were subjected to 4–16 µg/ml concentrations.
Detection of extended spectrum beta-lactamase producing organisms
The Gram-negative bacteria that were resistant to the third generation cephalosporins (cefotaxime and ceftazidime) were subjected to combination discs of cefotaxime/clavulanic acid, ceftazidime/clavulanic acid, single disc of cefotaxime and ceftazidime on the same MH agar plates and incubated at 37°C for 24 h for the phenotypic detection of extended spectrum beta-lactamase (ESBL) enzymes. The bacteria is said to be an ESBL producing organism, if the zone diameter difference between each combination disc and its single disc on the organism's plate is ≥ 5 mm.[18]
Statistical analysis
The groups differences were tested using the Chi-square test (or Fisher's exact test when expected frequencies were too low), with the assumed level of statistical significance at a P < 0.05 while the strength and direction association or relationship was measured using the phi coefficient/Cramer's V tests where applicable. Data was performed with SPSS for Windows, version 15.0 (SPSS Inc., Chicago, IL).
Results
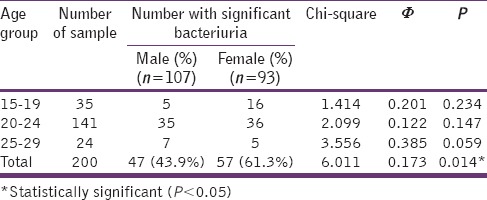
Of all the 200 University students which comprised 107 (53.3%) females and 93 (46.5%) males recruited for this study, 155 (77.5%) of their urine samples yielded positive bacterial growth with 104 (52.0%) of them having significant bacteriuria. The prevalence of significant bacteriuria among the students was significantly higher with a weak association among female than the male (χ2 = 6.01, phi = 0.173, P = 0.014) but the observed differences among the different age groups were not statistically significant [Table 1].
Table 1.
Age and gender distribution of students with significant bacteriuria
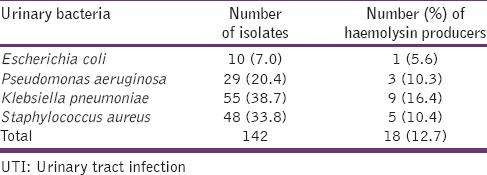
A total of 142 isolates of common UTI bacteria were identified from all the 155 samples with positive growths. Multiple bacteria of different species were recovered from 18 (11.6%) of the samples, and the number of isolates with hemolysin production was 18 (12.7%) as shown in [Table 2].
Table 2.
Common UTI bacteria and their virulence trait (haemolysin production)
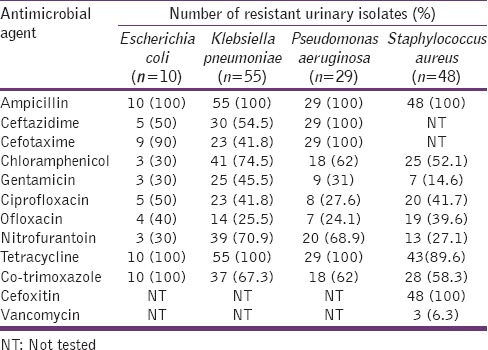
All the tested Gram-positive and Gram-negative bacteria exhibited 50–100% resistance to beta-lactam antibiotics, tetracycline, and co-trimoxazole. Klebsiella pneumoniae was found to be generally resistant to all the tested agents whilst P. aeruginosa was totally insensitive to the tested beta-lactam antibiotics and tetracycline. All the isolates of S. aureus were phenotypic methicillin-resistant strains. The most effective agents against all the isolated bacteria, although with moderate levels of resistance were gentamicin and ofloxacin [Table 3].
Table 3.
The antimicrobial resistance profile of common urinary isolates from asymptomatic subjects
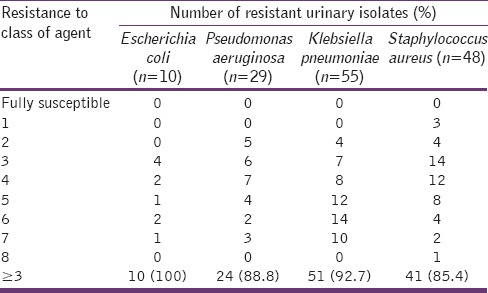
Multiple antibiotic resistance in this study was defined as the resistance of an isolate to at least one antimicrobial agent in at least three classes of antimicrobial agents used.[19] The isolates of the common urinary bacteria from asymptomatic University students exhibited a multiple antibiotic resistance of 85–100% as shown in Table 4.
Table 4.
Multi-drug resistance of the isolated bacteria
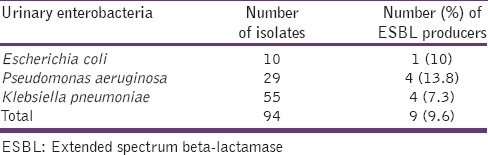
A total of 9 (9.6%) Gram-negative bacteria isolates showed a phenotypic production of ESBLs enzymes and the distribution of the producers among the bacteria is shown in Table 5.
Table 5.
The frequency of ESBL producers among the enterobacteria
Discussion
Our study found a total prevalence of 52.0% significant bacteriuria among the young asymptomatic University students with a significantly higher level among the females than the males (P = 0.014). This observed rate of significant bacteriuria is higher than the findings of Ngwai et al.[14] in Keffi (North Central) and Olowe et al.[15] in Oshogbo (South West) Nigeria, among similar individuals without overt clinical symptoms. The high prevalence among the subjects might be due to contaminants from procedural error or perennial skin. However, significantly higher level of bacteriuria observed among the females is in agreement with the findings of several earlier studies[6,14,15,20] and it might be related to the short course of the female urethra and its proximity to the anorectal region.[21,22]
The prevalence of common UTI bacteria among the samples from the asymptomatic young adults revealed K. pneumoniae and S. aureus as the most frequently recovered organisms whilst E. coli was the least. This observation is in contrast to several reports, which identified E. coli as the most common uropathogen causing asymptomatic bacteriuria.[15,23,24] However, this study aligns with the findings of Dada-Adegbola and Muili[25] who reported K. pneumoniae as the leading urinary pathogen. Furthermore, the increasing isolation of S. aureus as one of the major causes of UTI has also been reported, thereby suggesting the importance of these bacteria in UTIs. This observation might be due to these organisms increasing virulence and prevalence in the gastrointestinal tract.[16,26,27]
The presence of virulence markers in bacteria measures the organism's degree of pathogenicity, and hemolysin production is one of the common virulence traits in bacteria. Hemolysin is the bacterial toxin that liberates hemoglobin from red blood cells by destroying their structural integrity. Our study's prevalence of hemolysins production among all the isolates was low (12.7%), suggesting the reason why the volunteers were not having any overt clinical symptoms. However, the risk of tissue pathology of the renal system could be high among those harboring the hemolysin producing bacteria, and such injury could facilitate bacterial entry into the bloodstream.[28,29] The detection of 18 (12.7%) samples with different species of bacteria among the study asymptomatic subjects suggests the possibility of multiple infections among people with UTIs, and its consequences could be an increased cost of treatments and long hospital visits when the empirical treatments are targeted at a particular class of bacteria.
Antimicrobial resistance has been known to be a global health problem affecting the treatment of patients with UTIs and the situation is alarming in developing countries where there is little or no control of antimicrobial use. This study's results of antimicrobial resistance showed all the tested bacteria to be 60–100% resistant to ampicillin and co-trimoxazole. These agents were not only among the older antimicrobial agents but were also among the recommended first line drugs of choice for the treatment of UTIs.[30] This, therefore, suggests that their usefulness for the empiric therapy of UTIs is limited. The high resistance of these bacteria to ampicillin, co-trimoxazole and tetracycline has been attributed to their frequent uses in the hospital, community, and agricultural settings since they are older antibiotics with cheaper prices.[11] The observation of total resistance of P. aeruginosa isolates to the tested third generation cephalosporin suggests the ineffectiveness of these agents to this organism and thus, they might not be suitable for empiric treatment of UTIs especially with the emerging prevalence of multiple co-infections. Of all the tested agents, gentamicin and ofloxacin were observed to be the most effective agents against the isolated bacteria although with some moderate levels of resistance. Thus, these agents may be suitable for the empiric treatment of mixed infections. The observed moderate levels of resistance to these agents which are not frequently being used like the older drugs show the increasing rate at which the treatment of UTIs is becoming a difficult task.
All the isolates of S. aureus were observed to be phenotypic methicillin-resistant strains which had 40% resistance to the fluoroquinolones but were highly susceptible to vancomycin. These strains of methicillin-resistant S. aureus (MRSA) have been known to have very limited treatment options and their presence in healthy young adults suggests the possibility of increasing prevalence of UTIs with very limited treatment options because MRSA is a potential transmitter of its resistance genes to susceptible pathogens of the same or different species.
The common urinary bacteria isolated in this study were found to exhibit 85–100% multiple antibiotic resistance indicating that the isolates have been widely exposed to various classes of antimicrobial agents and thus they possess varying antibiotic resistance genes that could be transferred across species. ESBLs are enzymes produced by some Gram-negative bacteria that mediate resistance to extended spectrum third generation cephalosporins. Consequently, the ESBL producing bacteria possess resistance determinants to many other important groups of antibiotics.[31,32,33] This study's phenotypic detection of ESBLs among Gram-negative isolates revealed a prevalence rate of 9.6%, which suggests that the observed high multidrug resistance among these isolates might not be fully due to ESBLs enzymes but to other factors, which were not examined in this study. However, its detection among isolates of asymptomatic students even though low, is worrisome because it indicates the increasing rate at which these bacteria transmit resistance genes to same or different species. Thus, proper and regular hand washing techniques can help control its spread to the healthy population.
Conclusion
The results of this study revealed a high proportion of significant bacteriuria with high multi-drug resistant bacteria among the asymptomatic students of Niger Delta University, Bayelsa State. These findings, therefore, highlight the need for constant monitoring of antimicrobial susceptibility patterns of common pathogens among the healthy population as a necessary tool in adopting measures to control their spread to vulnerable individuals in the population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to the students of Niger Delta University, Amassoma, Bayelsa State for their voluntary participation and the staff of Pharmaceutical Microbiology and Biotechnology Laboratory for their technical assistance during the period of the study.
References
- 1.Schlager TA. Urinary tract infections in infants and children. Infect Dis Clin North Am. 2003;17:353–65. doi: 10.1016/s0891-5520(03)00009-6. ix. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B, Brown P. Epidemiology of urinary tract infections: Transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17:227–41. doi: 10.1016/s0891-5520(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 3.Haider G, Zehra N, Munir AA, Haider A. Risk factors of urinary tract infection in pregnancy. J Pak Med Assoc. 2010;60:213–6. [PubMed] [Google Scholar]
- 4.Demilie T, Beyene G, Melaku S, Tsegaye W. Urinary bacterial profile and antibiotic susceptibility pattern among pregnant women in North West Ethiopia. Ethiop J Health Sci. 2012;22:121–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Colgan R, Nicolle LE, McGlone A, Hooton TM. Asymptomatic bacteriuria in adults. Am Fam Physician. 2006;74:985–90. [PubMed] [Google Scholar]
- 6.Rodhe N, Mölstad S, Englund L, Svärdsudd K. Asymptomatic bacteriuria in a population of elderly residents living in a community setting: Prevalence, characteristics and associated factors. Fam Pract. 2006;23:303–7. doi: 10.1093/fampra/cml007. [DOI] [PubMed] [Google Scholar]
- 7.Foxman B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 8.Tambekar DH, Dhanorkar DV, Gulhane SR, Khandelwal VK, Dudhane MN. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotechnol. 2006;5:1562–5. [Google Scholar]
- 9.Loh K, Sivalingam N. Urinary tract infections in pregnancy. Malays Fam Physician. 2007;2:54–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Livermore DM. Bacterial resistance: Origins, epidemiology, and impact. Clin Infect Dis. 2003;36:S11–23. doi: 10.1086/344654. [DOI] [PubMed] [Google Scholar]
- 11.Gupta K, Sahm DF, Mayfield D, Stamm WE. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: A nationwide analysis. Clin Infect Dis. 2001;33:89–94. doi: 10.1086/320880. [DOI] [PubMed] [Google Scholar]
- 12.Matute AJ, Hak E, Schurink CA, McArthur A, Alonso E, Paniagua M, et al. Resistance of uropathogens in symptomatic urinary tract infections in León, Nicaragua. Int J Antimicrob Agents. 2004;23:506–9. doi: 10.1016/j.ijantimicag.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Bours PH, Polak R, Hoepelman AI, Delgado E, Jarquin A, Matute AJ. Increasing resistance in community-acquired urinary tract infections in Latin America, five years after the implementation of national therapeutic guidelines. Int J Infect Dis. 2010;14:e770–4. doi: 10.1016/j.ijid.2010.02.2264. [DOI] [PubMed] [Google Scholar]
- 14.Ngwai YB, Iliyasu H, Young E, Owuna G. Bacteriuria and antimicrobial susceptibility of Escherichia coli isolated from urine of Asymptomatic University Students in Keffi, Nigeria. Jundishapur J Microbiol. 2012;5:323–7. [Google Scholar]
- 15.Olowe O, Makanjuola O, Olabiyi K, Akinwusi P, Alebiosu C, Isawumi M, et al. Asymptomatic bacteriuria among elderly and middle-aged rural community-dwellers in South-Western Nigeria. Infect Drug Resist. 2013;6:55–8. doi: 10.2147/IDR.S44724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank-Peterside N, Oguike N. Asymptomatic significant bacteriuria among students of the University of Port-Harcourt, Nigeria. Niger J Microbiol. 2006;20:1252–7. [Google Scholar]
- 17.Cheesbrough M. Cambridge, UK: Cambridge University Press; 2002. District Laboratory Practice in Tropical Countries. Part 2. [Google Scholar]
- 18.Wayne, Pennsylvania 19087 USA: Clinical and Laboratory Standards Institute; 2012. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Eighteenth informational supplement, M100-S18. [Google Scholar]
- 19.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 20.Varli M, Guruzb H, Arasa S, Yalcina A, Atlia T, Turgaya M. Asymptomatic bacteriuria among the elderly living in the community: Prevalence, risk factors and characteristics. Eur Geriatr Med. 2012;3:87–91. [Google Scholar]
- 21.Harrington RD, Hooton TM. Urinary tract infection risk factors and gender. J Gend Specif Med. 2000;3:27–34. [PubMed] [Google Scholar]
- 22.Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177–82. doi: 10.1086/315827. [DOI] [PubMed] [Google Scholar]
- 23.Roos V, Ulett GC, Schembri MA, Klemm P. The asymptomatic bacteriuria Escherichia coli strain 83,972 outcompetes uropathogenic E. coli strains in human urine. Infect Immun. 2006;74:615–24. doi: 10.1128/IAI.74.1.615-624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watts RE, Hancock V, Ong CL, Vejborg RM, Mabbett AN, Totsika M, et al. Escherichia coli isolates causing asymptomatic bacteriuria in catheterized and noncatheterized individuals possess similar virulence properties. J Clin Microbiol. 2010;48:2449–58. doi: 10.1128/JCM.01611-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dada-Adegbola HO, Muili KA. Antibiotic susceptibility pattern of urinary tract pathogens in Ibadan, Nigeria. Afr J Med Med Sci. 2010;39:173–9. [PubMed] [Google Scholar]
- 26.Ngwai YB, Bakare OR. Asymptomatic bacteriuria and antimicrobial susceptibility of Staphylococcus aureus from University students in Keffi, Nigeria. Int J Microbiol Bioinform. 2012;2:5–8. [Google Scholar]
- 27.Onanuga A, Awhowho GO. Antimicrobial resistance of Staphylococcus aureus strains from patients with urinary tract infections in Yenagoa, Nigeria. J Pharm Bioallied Sci. 2012;4:226–30. doi: 10.4103/0975-7406.99058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brauner A, Katouli M, Ostenson CG. P-fimbriation and haemolysin production are the most important virulence factors in diabetic patients with Escherichia coli bacteraemia: A multivariate statistical analysis of seven bacterial virulence factors. J Infect. 1995;31:27–31. doi: 10.1016/s0163-4453(95)91271-1. [DOI] [PubMed] [Google Scholar]
- 29.Ovalle A, Levancini M. Urinary tract infections in pregnancy. Curr Opin Urol. 2001;11:55–9. doi: 10.1097/00042307-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Wolff O, Maclennan C. Evidence behind the WHO guidelines: Hospital care for children: What is the appropriate empiric antibiotic therapy in uncomplicated urinary tract infections in children in developing countries? J Trop Pediatr. 2007;53:150–2. doi: 10.1093/tropej/fmm030. [DOI] [PubMed] [Google Scholar]
- 31.Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Fishman NO, Bilker WB, et al. Risk factors for increasing multidrug resistance among extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species. Clin Infect Dis. 2005;40:1317–24. doi: 10.1086/429239. [DOI] [PubMed] [Google Scholar]
- 32.Melzer M, Petersen I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J Infect. 2007;55:254–9. doi: 10.1016/j.jinf.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Akinjogunla OJ, Odeyemi AT, Olasehinde GI. Epidemiological studies of urinary tract infection among postmenopausal women in Uyo Metropolis, South-South, Nigeria. J Am Sci. 2010;6:1674–81. [Google Scholar]


