Abstract
Aim:
Stress relaxant and antioxidant activities of ethanolic extract of fruit Spondias mangifera (EEFSM) and its isolated compound (Sm-01) were evaluated. The structure of Sm-01 was also elucidated.
Materials and Methods:
EEFSM at two different doses of 100 and 200 mg/kg (bw)/day and Sm-01 at dose of 10 mg/kg (bw)/day were screened for in vivo stress relaxant activity using anoxia stress tolerance, swimming endurance and cyclophosphamide-induced immune suppression model and in vitro antioxidant activity using 1,1-diphenyl-2-picrylhydrazyl (DPPH) model. The levels of Hb, red blood cell (RBC) and white blood cell (WBC) along with organ and body weights suppressed by cyclophosphamide were estimated. The structure of Sm-01 was elucidated by spectroscopy (ultraviolet, infrared, 1H-nuclear magnetic resonance [NMR],13 C-NMR and mass spectrometry) and chemical analyses.
Results:
Sm-01 was structurally elucidated as propan-1,2-dioic acid-3-carboxyl-β-D-glucopyranosyl-(6’→1”)-β-D-glucofuranoside. It was found that EEFSM and Sm-01 significantly increased the anoxia stress tolerance, swimming endurance and duration of stay on rotarod and normalized the levels of Hb, RBC, and WBC along with altered organ and body weights suppressed by cyclophosphamide. EEFSM and Sm-01 also exhibited significant antioxidant activity against DPPH free radical at the concentrations of 0.05, 0.5, and 1.0 mg/mL with obtained IC50 of 0.32 and 0.15 mg/mL, respectively.
Conclusions:
These findings demonstrated that extract and Sm-01 both possess significant stress relaxant and antioxidant activities favoring its use as adaptogens. The activities of the extract may be due to the Sm-01.
KEY WORDS: Propanetricarboxylic diglucoside, Spondias mangifera, stress relaxant
At present stressful environment, it is very difficult to look smart, maintain body charming, and powerful youth life for a longer time. Modern lifestyle has enhanced the exposure of human beings to stressful conditions resulting in the physical and psychological abnormalities. In a stressful environment, the energy requirement of organisms is increased resulting in the enhanced generation of free radicals causing oxidation of lipid, proteins, and nucleic acids.[1] During this process, the ability of the body's defense system to combat the oxidative stress may diminish.[2] Therefore, it is required to enhance the adaptability of human beings to stressful conditions. Natural product resources provide excellent raw materials for the discovery and development of novel oxidative stress defense and anti-ageing compounds.[3] Terpenoid, steroid, phenolics, flavonoids and acid glycosides obtained from natural products have been reported to exhibit a wide range of biological activities like antioxidant, mast cell stabilizing and adaptogenic properties.[4] Spondias mangifera Willd (S. mangifera) belonging to family Anacardiaceae is a glabrous tree with a characteristic pleasant smell of fruits, commonly known as Hog plum or Bile tree and Amrata in Ayurveda which is widely distributed on tropical and subtropical beaches and abundantly in the eastern and in North-East regions of India.[5,6] The juice of the ripe fruit is used in the preparation of jams and the unripe fruit is used in the preparation of condiments, pickles and also added to curry in the eastern region of India. The ripe fruit is unique fleshy drupe with sweet turpentine aromatic odor, used traditionally as an astringent, refrigerant, tonic aphrodisiac, and to cure “vata,” biliousness, ulcer, burning sensation, phthisis and blood complaints, dysentery, diarrhea, rheumatic articular, and muscular pain.[7] In Ayurveda, the fruit powder of S. mangifera with combination of Phyllanthus emblica, Terminalia citrina along with barks of Terminalia arjuna is used orally with drinking water for treatment of premature whitening of hair and fruit of S. mangifera is considered to promote hair growth.[8] The major chemical constituents of the S. mangifera fruit are ellagitannins, galloylgeranin, lignoceric acid, β-sitosterol and Vitamin A, thiamine, riboflavin and ascorbic acid.[9] The juice of the ripe fruit is a rich source of vitamins, highly acidic and has great nutraceutical potential. That's why; it should have adaptogenic and antioxidant activities and could be used as stress relaxant. Hence, an attempt has been made to evaluate the stress relaxant and antioxidant activities of the ethanolic extract of fruit Spondias mangifera (EEFSM) and the isolated acid glycoside “propan-1,2-dioic acid-3-carboxyl-β-D-glucopyranosyl-(6’′1”)-β-D-glucofuranoside” (isolated compound [Sm-01]).
Materials and Methods
Collection and authentication of plant specimen
The fruits of the plant were collected in the month of November from Dibrugarh University campus, Assam and authenticated by Prof. (Dr.) Muhibul Islam from the Department of Life Sciences, Dibrugarh University, Assam (Voucher specimen No.: DULS-32/09).
Extraction and isolation
The air-dried fruits of S. mangifera were made into a coarse powder. The dried fruit powder (1 kg) was defatted with petroleum ether and then extracted with 95% ethanol by using Soxhlet apparatus. The extract was then concentrated and dried on a rotavapor under reduced pressure to get reddish-brown solid mass (170 g). It was then subjected to column chromatography on silica gel (60–120) using solvents of varying polarities starting from petroleum ether, chloroform and methanol in different combinations. Elution of the column with chloroform:methanol (60:40) mixture yielded chocolate colored compound (Sm-01) (2.42 g).
Instrumentation and chemicals for structural elucidation of isolated compound
Melting point apparatus (Perfit), Silica gel (Qualigens, 60–120 mesh, Mumbai, India), Silica gel G (Qualigens, Mumbai, India), 1,1-diphenyl-2-picrylhydrazyl (DPPH, Sigma-Aldrich Co MO, USA), geriforte tablets (Himalaya drugs, India), cyclophosphamide (CYP, Khandelwal laboratories, Mumbai, India), rotarod (Medicraft, India) ultraviolet (UV) spectra scanned in methanol (Lambda Bio 20 Spectrophotometer Shimadzu-U Singapore), infrared (IR) spectra (Win IR FTS 135 instrument, Biorad, USA), 1H-nuclear magnetic resonance (NMR) 300 MHz and13 C-NMR 75 MHz spectra in CD3 OD (Brucker Spectrometer, Brucker, USA), MS (DART dry Helium, JEOL-AccuTOF JMS-T100LC). Spots were visualized by exposure to iodine vapors, UV Lamp 254 nm and by spraying with anisaldehyde sulphuric acid reagents. The solvents for isolation were obtained from Merck Mumbai, India.
Animals
Healthy adult Swiss albino mice of either sex weighing between 20 and 25 g were procured from Central Drug Research Institute Lucknow, India. They were maintained in clean, sterile, polypropylene cages at room temperature (21 ± 2°C) in 12 h dark/light control and fed with commercial pellet and water ad libitum. After randomization into various groups, the mice were quarantined for a period of a week for environmental and trainer handling acclimatization before initiation of experiments such as anoxia stress tolerance test, swimming endurance test and CYP-induced immune suppression activity. The experimental protocols were approved by Institutional Ethical Committee following the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) which complies with international norms of INSA, Indian National Science Academy (Approval No.: 1213/ac/08/CPCSEA/IU).
Administration of drugs
Ethanolic extract of fruit S. mangifera and the Sm-01 were used in all the experimental models. Geriforte tablets (50 mg/kg) was used as standard anti-stress drug which is a multi-constituent Ayurvedic drug with 35 herbal and natural constituents like Withania somnifera, Asparagus racemosus, Glycyrrhiza glabra, Centella asiatica, Terminalia chebula, Piper longum, Shilajit etc.[10] All the drugs were formulated into an emulsion using 2% gum acacia to obtain the desired dose on body weight basis (mg/kg) of the animal and administered orally using a ball ended feeding needle.
Safety profile study
An acute toxicity study of the EEFSM was carried out for the determination of LD50 by adopting the fixed dose method (Annexure 2d) of CPCSEA, OECD guideline No. 420.[11] The number of dead or surviving mice was recorded after 24 h.
Anoxia stress tolerance
Thirty Swiss albino mice were divided into five groups of six mice each (n = 6). The mice of the Group-I served as control and received vehicle alone (2% gum acacia). Group-II and III were treated with 100 and 200 mg/kg/day of EEFSM respectively. Group-IV was treated with 10 mg/kg/day of Sm-01 and Group-V was treated with 50 mg/kg/day of the standard drug, geriforte. All the groups were treated for 3 weeks. Every week after 1 h of drug administration, each animal was placed in an airtight glass container 250 mL and observed the time taken for appearance of the generalized clonic seizures (alternate limbs flexion and extension connected to loss of posture).[12] Thereafter, the mice were removed for recovery. The time duration from the entry of the animal into the hermetic vessel and the appearance of first convulsion was taken as time of anoxia tolerance.[13]
Swimming endurance and post swimming motor function test
Mice were divided into five groups of six mice each (n = 6). Mice of the Group-I served as control and received vehicle alone (2% gum acacia). Group-II and III were treated with 100 and 200 mg/kg/day of EEFSM respectively. Group-IV was treated with 10 mg/kg/day of Sm-01 and Group-V was treated with 50 mg/kg/day of the standard drug, geriforte. All the drugs were given orally once a day for seven days. On seventh day, 1 h after drug administration, all the mice were made to swim in a water tank (140 cm × 60 cm × 45 cm) maintained at room temperature until they sank. This was recorded as the swimming time.[14] Mice were removed and allowed to recover for about 5 min. They were subsequently tested for muscle coordination on a rotarod rotating at the speed of 15 rpm and the duration of stay on the rotarod was recorded.[15]
Cyclophosphamide induced immune suppression
Mice were divided into four groups of six mice each (n = 6).[16] Mice of the Group-I served as control and received vehicle alone (2% gum acacia). Group-II received 25 mg/kg/day of CYP alone. Group-III received 25 mg/kg/day of CYP along with 100 mg/kg/day of EEFSM. Group-IV received 25 mg/kg/day of CYP along with 10 mg/kg/day of Sm-01. All the groups were treated for 16 days. At the end of the treatment schedule, mice were anesthetized, and a blood sample was collected by cardiac puncture into tubes containing ethylenediaminetetraacetic acid as an anti-coagulant.[17] The vital organs (liver, kidney, spleen and heart) were carefully dissected out, cleaned of the adhering connective tissues, blotted, accurately weighed and relative organ weight was calculated. The white blood cell (WBC) count was done by Turke's method, red blood cell (RBC) by Hayem's method and hemoglobin by Sahli's method.[18,19,20] The body weights of the animal were also recorded prior to CYP treatment and every fourth day up to 16 days period.
Antioxidant activity
The free radical scavenging activity of EEFSM and Sm-01 against DPPH was determined by UV spectrophotometer at 517 nm. An aliquot of 0.05, 0.5 and 1.0 mg/mL of EEFSM and Sm-01 each were mixed in a test tube containing 3 mL of methanol and 0.5 mL of 1 mM DPPH. Ascorbic acid was used as a standard at the same concentrations. A blank solution was prepared containing the same amount of methanol and DPPH. The reaction mixture was incubated at 37°C for 30 min.[21] The radical scavenging activity was calculated using the following equation:
% Scavenging = ([Ac – As]/Ac) × 100
Where, Ac is absorbance of control, and As is absorbance of the sample.
Statistical analysis
A one-way analysis of variance followed by Dunnett's post-hoc test was performed by using Graph Pad Prism V2.01 (GraphPad Software, Inc., San Diego, California, USA). The data were expressed as the mean ± standard error of the mean and P < 0.05 was considered as statistically significant.
Results
Structural elucidation of isolated compound
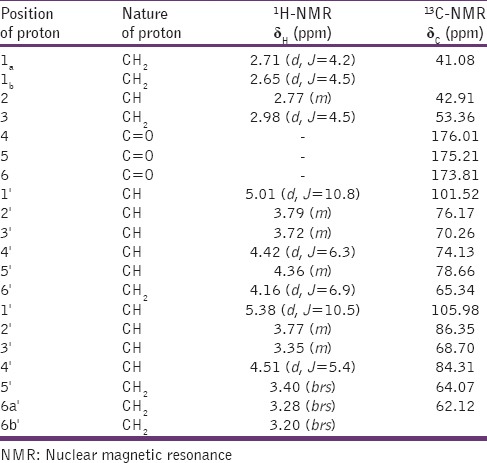
Chocolate colored compound; yield 2.42% (w/w); mp 136–138°C; Rf: 0.62 (MeOH: CHCl3, 6:1:2); UV-visible λmax (MeOH): 227 nm; IR νmax (KBr): 3426, 2925, 1485, 1752, 1220, 772/cm; 1H-NMR (300 Hz, MeOD) and13 C-NMR (75 Hz, MeOD) [spectral data: Table 1]; MS DART m/z (rel. int.): 500 (M)+ (C18H28O16), 500.4, 394.14, 362.13, 348.10, 331.14, 191.10, 162.
Table 1.
1H-NMR and 13C-NMR spectral data for compound Sm-01 (at 300 MHz in CD3OD, δ in ppm, J in Hz)
Safety profile study
Acute toxicity study of the EEFSM did not exhibit any signs of toxicity up to 2 g/kg body weight. Since, there was no mortality of the animals found at the highest dose.
Anoxia stress tolerance
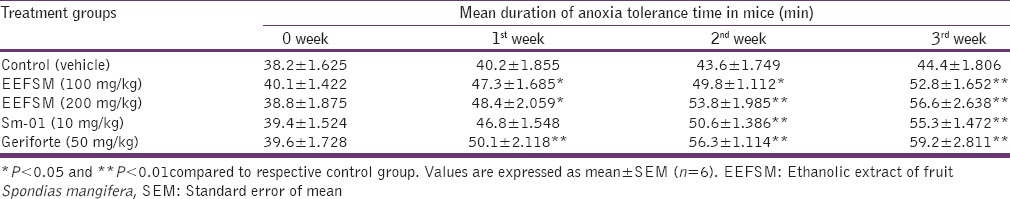
It was observed that EEFSM, Sm-01 and a standard drug (gerifort) enhanced the anoxia tolerance time significantly (P < 0.05 and P < 0.01). The anoxia tolerance effect was increased with increasing dose and duration of treatment [Table 2] indicating the significant stress relaxant activity.
Table 2.
Effect of EEFSM, Sm-01 and standard drug gerifort on anoxia stress tolerance test in mice
Effect on swimming endurance test and post swimming motor function test
The control group of mice swam for 131.2 ± 4.934 min, EEFSM treated mice at a dose 100 and 200 mg/kg/day swam for 152.7 ± 3.692 and 158.6 ± 4.735 min whereas Sm-01 treated mice swam for 155.4 ± 4.828 min at the dose of 10 mg/kg/day [Figure 1]. The duration of stay on rotarod were significantly increased from 8.12 ± 1.34 s in control group to 12.9 ± 1.31 s and 19.16 ± 1.08 s in the groups treated with EEFSM at the doses of 100 and 200 mg/kg/day respectively, whereas 16.11 ± 1.2 s in the groups treated with Sm-01 at the dose of 10 mg/kg/day [Figure 2].
Figure 1.
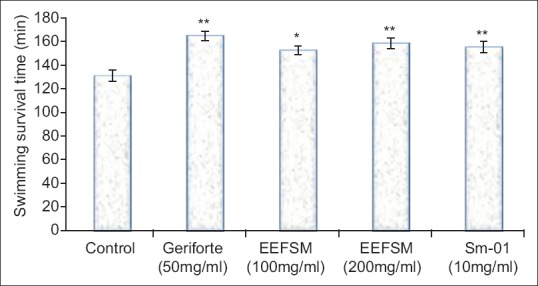
Effect of ethanolic extract of fruit Spondias mangifera, Sm-01 and gerifort on swimming endurance test in mice (values were represented as mean ± standard error of mean [n = 6]. *P < 0.05 and **P < 0.01 compared with respective control group)
Figure 2.
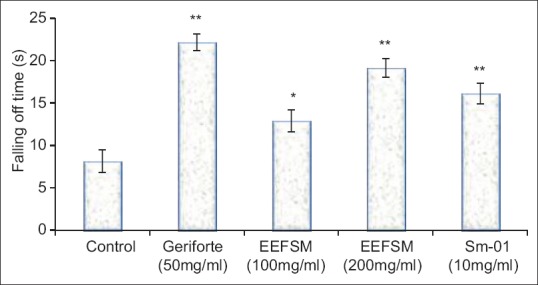
Effect of ethanolic extract of fruit Spondias mangifera, Sm-01 and gerifort on postswimming motor function in mice (values were represented as mean ± standard error of mean [n = 6]. *P < 0.05 and **P < 0.01 compared with respective control group)
Cyclophosphamide induced immune suppression
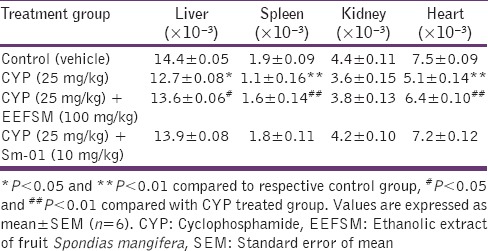
Administration of CYP (25 mg/kg/day) alone produced a significant decrease in the total RBC count, leukocyte count, hemoglobin and body weight. CYP given alongwith EEFSM (100 mg/kg/day) and Sm-01 (10 mg/kg) indicated good protection by increasing all the hematological parameters and maintaining the body weight [Table 3]. A decrease in the liver, spleen and heart weight were observed in mice treated with CYP alone. The weight of liver, spleen and heart were also normalized significantly (P < 0.05 and P < 0.01) by their treatment with CYP along with EEFSM and Sm-01 as compared to CYP alone treated group [Table 4].
Table 3.
Effect of EEFSM and Sm-01 on CYP induced changes in Hb, RBC and WBC counts
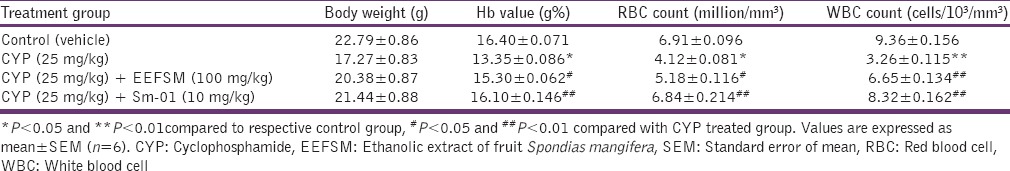
Table 4.
Effect of EEFSM and Sm-01 on CYP induced changes on the organ weight/body weight ratio of mice
Antioxidant activity
DPPH radical scavenging activity of the EEFSM and Sm-01 was compared with standard drug ascorbic acid. The calculated IC50 values of EEFSM and Sm-01 were found to be 0.32 and 0.15 mg/ml respectively; and that for the standard drug ascorbic acid was found to be 0.015 mg/mL [Figure 3].
Figure 3.
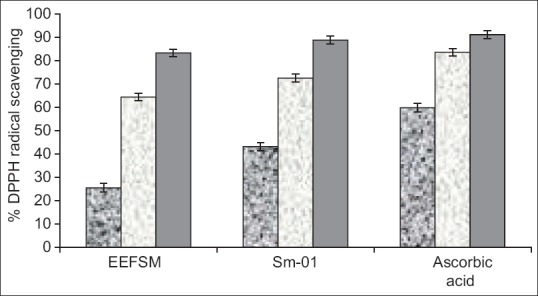
Dose dependent scavenging of 1,1-diphenyl-2-picrylhydrazyl radical by the ethanolic extract of fruit Spondias mangifera, Sm-01 and ascorbic acid at different doses of 0.05, 0.5 and 1.0 mg/mL (values were represented as mean ± standard error of mean [n = 4])
Discussion and Conclusion
The Sm-01 propanetricarboxylic diglucoside (Sm-01) gave positive Fehling's test for glycosides and showed IR absorption band for hydroxyl groups (3426, 3255/cm), ester group (1752/cm), pyranosyl (1485/cm), glycosidic linkage (1086/cm) and aliphatic chain (772/cm). Absorption band 3255 and 3426/cm arises from polymeric structures of hydrogen in hydroxyl groups and another strong band appears around 2925/cm assigned to the linear vibration of hydrogen in C—H groups of the compound. The carbonyl absorption band in the compound for ester occurs at 1752 and for acid at 1628/cm. Vibration of a C—O linkage has been shown to produce an absorption band at 1485/cm supporting the presence of pyranosyl group in the compound.[22] On the basis of MS and13 C NMR spectra, the molecular ion peak of Sm-01 was determined at m/z 500 (M)+ consistent to the molecular formula of propanetricarboxylic diglucoside, C18H28O16 [Figure 4]. The MS of Sm-01 exhibited prominent ion fragments generated at m/z 500 (C18H28O16), 338 (C12H18O11 glucose esterified with aglycon), 191 (C7H10O6 [2-methoxy-2-oxoethyl succinic acid] aglycon), 162.13 (C6H11O5 pyranosyl). The compound shows a characteristic fragmentation pattern with only a small molecular ion and a few abundant fragment ions. The 1H NMR spectrum of Sm-01 displayed two single proton doublet at δH 5.01 (J = 10.8 Hz) and 5.38 (J = 10.5 Hz) assigned to anomeric H – 1’ and H – 1” respectively and others sugar protons from δH3.79 to 3.20. Other methylene protons as a two proton multiplate at δH4.35 and as a broad singlet at δH3.28 and δH 3.20. The13 C NMR spectrum exhibited two acids carbon at δC176.01 (C-4) and 175.21 (C-5), two anomeric sugar carbon at δC 101.52 (C-1’) and 105.98 (C-1”), aglycone ester carbons at δC173.81(C-6) and other sugar carbons between δC 86.35 and 64.07. On the basis of these evidences, the structure of Sm-01 has been established as propan-1,2-dioic acid-3-carboxyl-β-D-glucopyranosyl-(6 → 1”)-β-D-glucofuranoside.[23,24]
Figure 4.
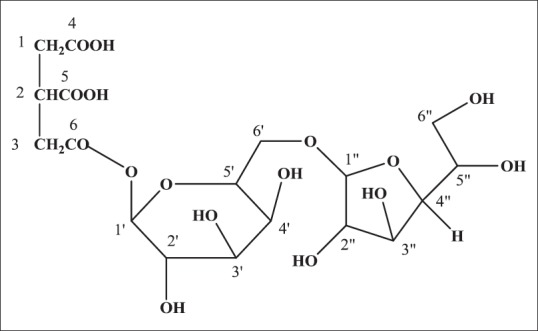
Structure of the isolated compound “propan-1,2-dioic acid-3-carboxyl-β-D-glucopyranosyl-(6’→1”)-β-D-glucofuranoside” (Sm-01)
Dose-related increase in anoxia stress tolerance time was observed during pretreatment with EEFSM and Sm-01 indicating their significant stress relaxant activity. It is also suggested that adaptogenic agents facilitate the conversion of energy in a cellular system of the organism and helps in adaptation.[12] Hence, it is found that EEFSM and Sm-01 facilitated the conversion of energy in a cellular system of organisms which could help adaptive process during anoxia stress tolerance.
The results of swimming endurance test and post swimming motor function test indicated clearly that the pretreatment with EEFSM and Sm-01 have the properties whereby they significantly increase the physical swimming endurance time, as well as stay on rotarod in mice. The enhanced swimming endurance in mice compared to the normal animals may be attributed to the flavonoids, acids glycosides and triterpenoids.[25]
The alterations in hematological parameters, organ and body weight, are usually observed in mice indicative of CYP toxicity.[26] The results demonstrated that treatment with EEFSM and Sm-01 was able to normalize the Hb, RBC, WBC and organ and body weights significantly (P < 0.05 and P < 0.01) and they were also able to reduce leukopenia and anemia induced by CYP.
EEFSM contained a considerable amount of phenolics, flavonoids and acid glycoside responsible for the antioxidant activity.[4] Thus, extract EEFSM and acid glycoside (Sm-01) showed a potent scavenging of DPPH free radicals. There was no scientific report before on the presence of this propanetricarboxylic diglucoside from EEFSM through several flavonoidal and acids glycosides.
The present study suggested that EEFSMand Sm-01 have significant stress relaxant and antioxidant activities. The study also affirms that the effectiveness of EESMF may be due to the presence of acid glycoside (Sm-01). Thus, EEFSM and Sm-01 is of highest therapeutic or nutritional importance and may be used in the treatment of patients with severely impaired or suppressed the immune system due to chemotherapy. The acid glycoside (Sm-01) may be used as a marker molecule for S. mangifera.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
One of the authors is thankful to Faculty of Pharmacy, Integral University, Lucknow, Uttar Pradesh for providing necessary facilities for the research. The authors are thankful to CDRI, Lucknow and Prof. (Dr.) Mohammad Ali, Jamia Hamdard for characterization and interpretation of isolated compound.
References
- 1.Bhaumik G, Srivastava KK, Selvamurthy W, Purkayastha SS. The role of free radicals in cold injuries. Int J Biometeorol. 1995;38:171–5. doi: 10.1007/BF01245384. [DOI] [PubMed] [Google Scholar]
- 2.Kondo H, Miura M, Itokawa Y. Oxidative stress in skeletal muscle atrophied by immobilization. Acta Physiol Scand. 1991;142:527–8. doi: 10.1111/j.1748-1716.1991.tb09191.x. [DOI] [PubMed] [Google Scholar]
- 3.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375–83. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 4.Yildirim A, Mavi A, Oktay M, Kara AA, Algur OF, Bilaloglu V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea Desf ex DC), sage (Salvia triloba L.), and black tea (Camellia sinensis) extracts. J Agric Food Chem. 2000;48:5030–4. doi: 10.1021/jf000590k. [DOI] [PubMed] [Google Scholar]
- 5.New Delhi; India: Publication and Information Directorate, CSIR; 1992. Anonymous. The Wealth of India, A Dictionary of Indian Raw Materials. Vol. 10; pp. 19–20. [Google Scholar]
- 6.1st ed. III. New Delhi: The Controller of Publications, Dept. of ISM and H; 2001. Anonymous. The Ayurvedic Pharmacopoeia of India, Govt. of India, Ministry of Health and Family Welfare, Part-I; p. 11. [Google Scholar]
- 7.Kritikar KR, Basu BD. Indian Medicinal Plants. Vol. 1. Dehradune, India: M/S Bishen Singh, Mahendra Pal Singh. International Book Distributors. 1975:672. [Google Scholar]
- 8.Sadia MM, Mahal MJ, Bhuiyan P, Zakaria AS, Datta B, Rana M, et al. Medicinal plants and formulations of the Goala tribe of Moulvibazar. Bangladesh Am Eurasian J Sustain Agric. 2012;6:254–60. [Google Scholar]
- 9.Tandon S, Rastogi RP. Studies on the chemical constituents of Spondias pinnata. Planta Med. 1976;29:190–2. doi: 10.1055/s-0028-1097651. [DOI] [PubMed] [Google Scholar]
- 10.Kannur DM, Hukkeri VI, Akki KS. Adaptogenic activity of Caesalpinia bonduc seed extracts in rats. J Ethnopharmacol. 2006;108:327–31. doi: 10.1016/j.jep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2:74–9. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivakumar H, Javed T, Prakash T, Nagendra Rao R, Jayakumar Swamy BH, Veerana Goud A. Adaptogenic activity of ethanolic extract of Tribulus terrestris L. J Nat Remedies. 2006;6:87–95. [Google Scholar]
- 13.Singh B, Gupta DK, Chandan BK. Adaptogenic activity of glycopeptido-lipid fraction from the alcoholic extract of Trichopus zeylanicus gaertn. Phytomedicine. 2001;8:283–91. doi: 10.1078/0944-7113-00038. [DOI] [PubMed] [Google Scholar]
- 14.Nimbakar SR, Patki VP, Patki MP. Pharmacological evaluation of anti-stress and androgenic activity of polyherbal formulation “A.P-3000”-containing Panax ginseng. Indian Drugs. 2001;38:27–39. [Google Scholar]
- 15.Gufran A, Yusuf KM, Naeem A, Khan T. The anti-stress activity of a gem-containing Unani formulation against diverse stressors. J Ethnopharmacol. 1998;59:187–93. doi: 10.1016/s0378-8741(97)00125-6. [DOI] [PubMed] [Google Scholar]
- 16.Davis S, Kuttan G. Suppressive effect of Cyp induced toxicity by Withania somnifera extract in mice. J Ethnopharmacol. 1998;62:209. doi: 10.1016/s0378-8741(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 17.Hoff J. Methods of blood collection in the mouse. Lab Anim (NY) 2000;29:47–53. [Google Scholar]
- 18.Dacie JV, Lewis SM. 11th ed. Hong Kong: Longman Group Ltd; 2001. Practical Haematology; pp. 11–7. [Google Scholar]
- 19.Easthan RD, Slade RR. 7th ed. Tokyo: Butter Work-Heinemann Ltd; 1993. Clinical Haematology; pp. 83–105. [Google Scholar]
- 20.Cavill IJ, Fisher JA, Souza P. Sequential blood counts and their variation in normal subjects. Clin Lab Haematol. 1993;3:91–3. [PubMed] [Google Scholar]
- 21.Schimada K, Fujikawa K, Yahara K, Nakamura T. Anti-oxidative properties of xanthan on the auto-oxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–8. [Google Scholar]
- 22.Silverstein RM, Webster FX. 6th ed. Singapore: John Wiley and Sons Inc; 2005. Spectrometric Identifications of Organic Compounds; pp. 81–99. [Google Scholar]
- 23.Hamid H, Kaur G, Abdullah T, Ali M, Athar M, Alam SM. Two new compounds from the Galls of Qiiercus infectoria with nitric oxide and superoxide inhibiting ability. Pharm Biol. 2005;43:317–23. doi: 10.1080/13880200590951711. [DOI] [PubMed] [Google Scholar]
- 24.Markham KR, Ternai B, Stanley R, Geiger H, Mabry TJ. Carbon-13 NMR studies of flavonoids, naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron. 1978;34:1389–97. [Google Scholar]
- 25.Klein R. Phytoecdysteroids. J Am Herbalists Guild. 2004;5:18–28. [Google Scholar]
- 26.Chahoud A, Ligensa L, Dietzel K, Faqui AS. Correlation between maternal toxicity and embryo/fetal effects. Reprod Toxicol. 1999;13:375–81. doi: 10.1016/s0890-6238(99)00035-0. [DOI] [PubMed] [Google Scholar]


