Abstract
Background:
Spontaneous complete thrombosis of a giant aneurysm and its parent artery is a rare event. Their spontaneous recanalization is even rarer, with few reports.
Case Description:
A 17-year-old male patient presenting blurred vision and headache, with a history of seizures, was referred to our service. After further investigation with cranial computed tomography, magnetic resonance imaging (MRI), and cerebral angiography (CAG), it was diagnosed a thrombosed aneurysm of the posterior cerebral artery (PCA) and also complete thrombosis of the PCA. Three years later, he experienced visual worsening. A new MRI scan indicated flow both through the aneurysm and the left PCA, which was further confirmed by CAG. We decided for a noninterventional treatment combined with strict clinical follow-up. The patient continues to present with the previous neurological deficit, without recurrence of headaches.
Conclusions:
Thrombosis is not the final event in the natural history of giant aneurysms, and partial thrombosis does not preclude the risk of rupture. Thrombosed aneurysms may display additional growth brought about by wall dissections or intramural hemorrhages. Their treatment may be either surgical or involve endovascular procedures such as embolization. Thrombosed giant aneurysms are dynamic and unstable lesions. A noninterventional treatment is feasible, but aneurysmal growth or recanalization may suggest the need for a more active intervention.
Key Words: Giant intracranial aneurysm, intracranial aneurysm, intracranial thrombosis, posterior cerebral artery
INTRODUCTION
Although thrombosis in giant aneurysms is a relatively common phenomenon, complete thrombosis is uncommon, and usually spares the parent vessel.[17] When such an event occurs nonetheless, it may either remain silent or cause compressive and ischemic symptoms. Its radiological characteristics may suggest the existence of a neoplastic mass lesion.[17]
Recanalization of thrombosed aneurysms, although recorded in the literature, is a rare event.[10,17] The aim of this paper is to report on the case of a patient diagnosed with a giant aneurysm of the posterior cerebral artery (PCA) that progressed to spontaneous thrombosis of the aneurysm and its parent artery, both of which later exhibited spontaneous recanalization. A brief literature review on the subject related to the case is presented.
CASE REPORT
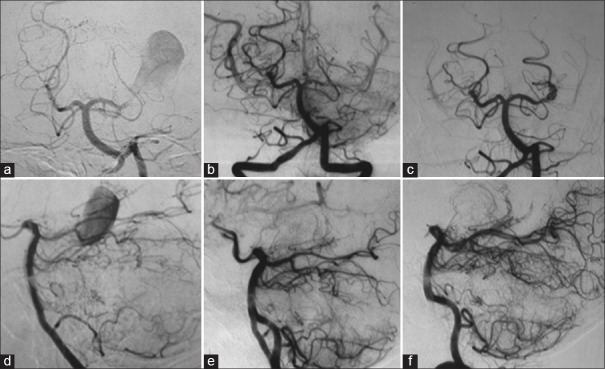
A 17-year-old male patient was admitted to a different institution than ours complaining of blurred vision, headache, and three episodes of generalized tonic-clonic seizures, which had occurred 3 months before his admission. Upon neurological examination, the single alteration found was a visual deficit, observed with a direct confrontation visual field examination. A campimetry examination showed right homonymous hemianopsia. A cranial computed tomography (CT) scan showed a hyperdense expansive lesion adjacent to the left PCA [Figure 1a] with partial and homogeneous opacification following contrast injection [Figure 1b]. The main diagnostic hypothesis was a partially thrombosed giant aneurysm of the PCA. Subsequently, a cerebral angiography (CAG) was conducted, which demonstrated a giant saccular aneurysm of the P2 segment of the left PCA [Figure 2a and d].
Figure 1.
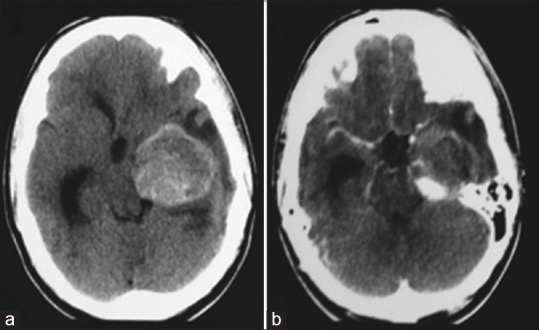
(a and b) Cranial computed tomography in axial acquisitions showing an expansive lesion in the topography of the left posterior cerebral artery, with an important mass effect. In b, one can observe the partial opacification by means of a venous contrast
Figure 2.
Left vertebral angiography showing a giant aneurysm of the left posterior cerebral artery (a and d); left vertebral angiography after 1 month demonstrating the occlusion of the left posterior cerebral artery at its P2 segment, without opacification of the aneurysm (b and e); (c and f) left vertebral angiography performed 3 years after the first one, showing recanalization of the left posterior cerebral artery and aneurysm (a, b, and c: Towne's view; d, e, and f: Lateral view)
One month later, the patient was sent for endovascular treatment in our service. A cranial magnetic resonance imaging (MRI) scan was performed and showed a lesion with the heterogeneous signal, suggesting a totally thrombosed giant aneurysm of the P2 segment of the left PCA, with occlusion of the artery at its P2 segment [Figure 3a and b]. Given this result, we performed another CAG, which confirmed the total occlusion of the aneurysm and the left PCA [Figure 2b and e]. The patient was discharged with the prescription of an anticonvulsant, which was gradually discontinued after 6 months.
Figure 3.
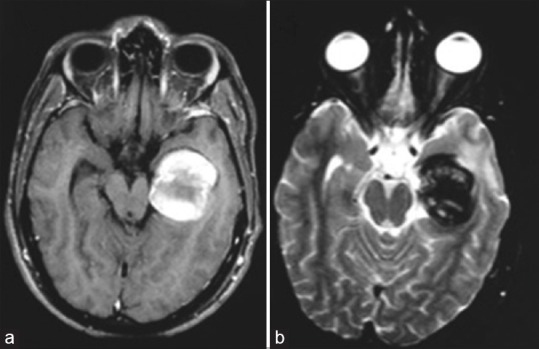
Cranial magnetic resonance imaging axial acquisition (a: T1-weighted; b: T2-weighted) showing an expansive lesion, with heterogeneous signal, suggesting a totally thrombosed giant aneurysm with occlusion of the posterior cerebral artery
About 3 years after, the patient experienced visual worsening, having come back to our service. A cranial MRI scan was then performed, which showed flow through both the left PCA and its aneurysm, with no signs of bleeding. A CAG was also conducted, which confirmed the spontaneous recanalization of the aneurysm and the PCA [Figure 2c and f].
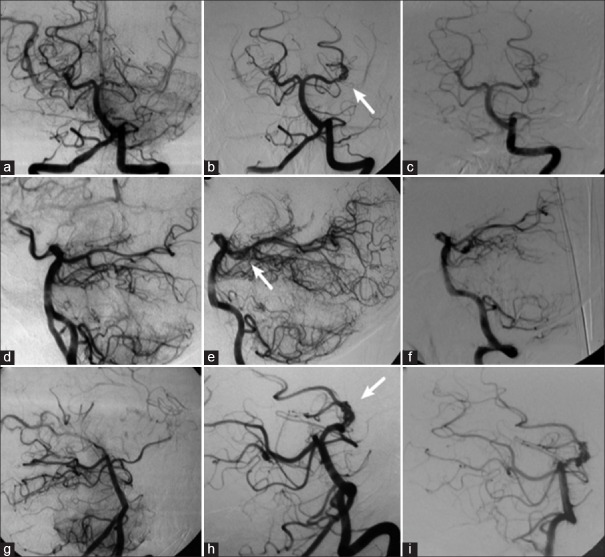
We chose a noninterventional treatment aimed at managing the condition, with strict clinical follow-up. A CAG performed 1 year later revealed the patency of both the aneurysm and the left PCA, with no major differences in comparison with the previous examination [Figure 4]. The patient continues to present with the previous neurological deficit due to the aneurysmal mass effect, yet with no additional headache episodes. He is still being given a noninterventional treatment combined with clinical follow-up.
Figure 4.
Left vertebral angiography demonstrating occlusion of the left posterior cerebral artery at its P2 segment, without opacification of the aneurysm (a, d, and g); in (b, e, and h), the left vertebral angiography performed 3 years after the occlusion shows recanalization of the left posterior cerebral artery and aneurysm (arrow); in (c, f, and i), the new left vertebral angiography shows unchanged aspect of the aneurysm 1 year after recanalization (a, b, and c: Towne's view; d, e, and f: Lateral view; g, h, and i: Right anterior oblique view)
DISCUSSION
Aneurysms with a diameter as large as 25 mm or more are classified as giant aneurysms.[19] They make up between 3% and 13% of all intracranial aneurysms.[19] The PCA is the location of only 1.4% of intracranial aneurysms,[17,19] but those located in this artery are, more often than not, large or gigantic in size.[17]
Spontaneous partial thrombosis of giant aneurysms is a fairly common finding, occurring in up to 60% of such lesions.[10,17] Complete thrombosis, however, is a rare event and may occur in association with parent vessel occlusion.[17] Spontaneous recanalization following complete thrombosis, as observed in this case, is a rare phenomenon, with few previous reports found in the searched literature.[10,17]
The additional growth of thrombosed giant aneurysms is a well-described phenomenon. The suggested pathophysiological mechanisms for this growth include successive aneurysmal wall dissections;[8] recurrent hemorrhages from proliferated intramural capillaries;[15] and the presence of intrathrombotic endothelized vascular channels.[9] It is proposed that thrombosed giant aneurysms constitute a singular clinicopathological entity, different from the nonthrombosed ones.[9] Such hypothesis is supported by histopathological evidence of inflammatory macrophages in the wall of both thrombosed and giant aneurysms,[1] which are typically absent in nonthrombosed.
Some authors[17] observed that the clinical presentation of giant aneurysms with spontaneous thrombosis, in the absence of subarachnoid hemorrhage (SAH), frequently includes paroxysmal neurological signs, such as seizures and ischemic events. Stroke and transient ischemic attack due to thromboembolism from aneurysmal thrombus are rare events, despite being consistently described in association with partially thrombosed aneurysms.[4,6,22] Additional mechanisms for cerebral ischemic events associated with unruptured aneurysms are the occlusion of the parent artery provoked by the extension of the aneurysmal thrombus,[3,4,22] and the external compression of the artery due to the aneurysmal mass effect.[2,13]
The close relationship among the PCA, mesencephalon, and cranial nerves renders the giant aneurysms of the PCA particularly prone to cause a mass effect.[19] In the case here discussed, the patient experienced visual deficit probably due to compression of the temporal portion (Meyer's loop) of the optic radiation, apart from presenting with seizures at the beginning of the clinical presentation.
Despite the fact that spontaneous thrombosis is more frequently associated with giant aneurysms than with nongiant ones,[3] the latter comprise most of the cases of cerebral ischemia related to unruptured aneurysms.[4,6] It is also known that giant aneurysms pose a higher risk of rupture, with a 50% 5-year cumulative risk for those affecting the posterior circulation.[23] There is no evidence that partial thrombosis may preclude the possibility of aneurysmal bleeding.[5]
CAG is the gold standard for diagnosing intracranial aneurysms. Its major advantage is the possibility of a detailed assessment of the vascular anatomy, including the study of aneurysmal orientation through multiple planes.[19] However, as the angiographic examinations rely on the detection of contrast flow in the vascular lumen, they are likely to underestimate the size of partially thrombosed aneurysms, or even miss those totally thrombosed.[12]
In such context, CT angiography and MR angiography both seem to be more suitable for the measuring the volume of partially thrombosed aneurysms.[11] Those techniques have had an increasing role even in the assessment of patients with a suspected aneurysmal SAH, besides being useful for showing the relationship between the vascular lesion and bony structures.[21] On CT images, giant aneurysms appear as expansive lesions with well-defined margins,[12] such as those found in the presented case. In postcontrast acquisitions, they may show a peripheral ring enhancement in the aneurysmal wall, attributed to proliferated intramural capillaries or recent hemorrhages from these vessels.[14,19,20] the presence of peripheral calcifications and the “target sign” also suggests intraluminal thrombosis,[14,19,20] even though the latter is absent in totally thrombosed lesions.
In precontrast MRI scans, an “onion-skin” lesion, characterized by layers with different signal intensities, points to the presence of an aneurysmal thrombus.[18] Such a hypothesis should also be raised in the face of a nonenhancing mass lesion inside a dilated arterial segment.[11,18] In postcontrast, T1-weighted MRI images, the presence of either the flow void sign or vascular lumen enhancement is highly sensitive and specific for the diagnosis of an aneurysmal thrombus,[18] whereas T2-weighted MRI images may show perianeurysmal edema, frequently associated with thrombosed giant aneurysms, probably secondary to the inflammatory process in the wall of this type of lesion.[7]
Given the rarity of giant aneurysms of the P2-segment of the PCA, there are no major clinical trials assessing the specific therapeutic modalities for treating such lesions. The potential complications of thrombosed giant aneurysms lead to judicious appraisal on the need for intervention. The treatment goals are to prevent bleedings, relief the mass effect, and maintain an appropriate brain perfusion.[19] Factors that must be considered in the therapeutic choice include location and size of the lesion, presence of intraluminal thrombus, neck dimension, and the patient's clinical conditions, particularly their age and neurological status.[5,8,12,16,19]
The endovascular therapeutic modalities include aneurysm embolization using platinum coils or, more recently, flow-diverting stents. These techniques aim at the occlusion of the aneurysm while sparing the flow in the parent vessel. Another strategy is the therapeutic occlusion of the parent vessel (OPV) and the aneurysm.[8] Compared to embolization, OPV is associated with lower rates of aneurysm growth and the need for retreatment, besides higher rates of aneurysm volume reduction and neurological improvement.[8] Yet, its feasibility depends upon the existence of vascular anastomoses capable of supplying an adequate blood flow to the sacrificed arterial territory.[5,8]
The surgical options include aneurysmal clipping followed by thrombectomy and aneurysmal trapping either with or without revascularization. Given the possibility of thrombus dislodgment and distal embolism, surgical manipulation must be careful.[5,10] Wide neck and intraluminal thrombus make clipping a difficult proposition, with the possibility of clip migration and occlusion of the parent artery.[5,10] Revascularization procedures are especially useful when there are unfavorable conditions for embolization and clinical intolerance or unsatisfactory collateral circulation in the parent artery occlusion test.[5]
In this case, recanalization occurred in the artery and in the aneurysm neck only. Thus, only the most proximal portion of the aneurysm was recanalized, and this small region has not increased in size during follow-up. Hence, with no increase in a lesion with mass effect, and not occurring worsening of patient's symptoms, it was opted for a conservative treatment.
CONCLUSION
Thrombosed giant aneurysms are dynamic and unstable lesions, being prone to spontaneous recanalization and additional growth. There is no consensus about the ideal management of such lesions. A noninterventionist management approach is possible but requires strict follow-up, including serial imaging examinations. Any worsening in the neurological status or evidence of either recanalization or growth may be suggestive of the need for a direct approach.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Guilherme Brasileiro de Aguiar, Email: guilhermebraguiar@yahoo.com.br.
Mário Vítor Caldeira Pagotto, Email: mario_vitor_1@hotmail.com.
Mario Luiz Marques Conti, Email: mlconti@uol.com.br.
José Carlos Esteves Veiga, Email: jcemveiga@uol.com.br.
REFERENCES
- 1.Atlas SW, Grossman RI, Goldberg HI, Hackney DB, Bilaniuk LT, Zimmerman RA. Partially thrombosed giant intracranial aneurysms: Correlation of MR and pathologic findings. Radiology. 1987;162(1 Pt 1):111–4. doi: 10.1148/radiology.162.1.3786749. [DOI] [PubMed] [Google Scholar]
- 2.Batjer HH, Purdy PD. Enlarging thrombosed aneurysm of the distal basilar artery. Neurosurgery. 1990;26:695–9. doi: 10.1097/00006123-199004000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee RD, Tranmer BI, Sevick RJ, Karmy G, Curry BJ. Spontaneous thrombosis of an unruptured anterior communicating artery aneurysm.An unusual cause of ischemic stroke. Stroke. 1995;26:1945–9. doi: 10.1161/01.str.26.10.1945. [DOI] [PubMed] [Google Scholar]
- 4.Calviere L, Viguier A, Da Silva NA, Jr, Cognard C, Larrue V. Unruptured intracranial aneurysm as a cause of cerebral ischemia. Clin Neurol Neurosurg. 2011;113:28–33. doi: 10.1016/j.clineuro.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Choi IS, David C. Giant intracranial aneurysms: Development, clinical presentation and treatment. Eur J Radiol. 2003;46:178–94. doi: 10.1016/s0720-048x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JE, Itshayek E, Gomori JM, Grigoriadis S, Raphaeli G, Spektor S, et al. Spontaneous thrombosis of cerebral aneurysms presenting with ischemic stroke. J Neurol Sci. 2007;254:95–8. doi: 10.1016/j.jns.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Dengler J, Maldaner N, Bijlenga P, Burkhardt JK, Graewe A, Guhl S, et al. Perianeurysmal edema in giant intracranial aneurysms in relation to aneurysm location, size, and partial thrombosis. J Neurosurg. 2015;123:446–52. doi: 10.3171/2014.10.JNS141560. [DOI] [PubMed] [Google Scholar]
- 8.Ferns SP, van Rooij WJ, Sluzewski M, van den Berg R, Majoie CB. Partially thrombosed intracranial aneurysms presenting with mass effect: Long-term clinical and imaging follow-up after endovascular treatment. AJNR Am J Neuroradiol. 2010;31:1197–205. doi: 10.3174/ajnr.A2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krings T, Alvarez H, Reinacher P, Ozanne A, Baccin CE, Gandolfo C, et al. Growth and rupture mechanism of partially thrombosed aneurysms. Interv Neuroradiol. 2007;13:117–26. doi: 10.1177/159101990701300201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KC, Joo JY, Lee KS, Shin YS. Recanalization of completely thrombosed giant aneurysm: Case report. Surg Neurol. 1999;51:94–8. doi: 10.1016/s0090-3019(97)00346-7. [DOI] [PubMed] [Google Scholar]
- 11.Martin AJ, Hetts SW, Dillon WP, Higashida RT, Halbach V, Dowd CF, et al. MR imaging of partially thrombosed cerebral aneurysms: Characteristics and evolution. AJNR Am J Neuroradiol. 2011;32:346–51. doi: 10.3174/ajnr.A2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta RI, Salamon N, Zipser BD, Mehta RI. Best cases from the AFIP: Giant intracranial aneurysm. Radiographics. 2010;30:1133–8. doi: 10.1148/rg.304095199. [DOI] [PubMed] [Google Scholar]
- 13.Schaller B, Lyrer P. Focal neurological deficits following spontaneous thrombosis of unruptured giant aneurysms. Eur Neurol. 2002;47:175–82. doi: 10.1159/000047978. [DOI] [PubMed] [Google Scholar]
- 14.Schubiger O, Valavanis A, Hayek J. Computed tomography in cerebral aneurysms with special emphasis on giant intracranial aneurysms. J Comput Assist Tomogr. 1980;4:24–32. doi: 10.1097/00004728-198002000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Schubiger O, Valavanis A, Wichmann W. Growth-mechanism of giant intracranial aneurysms; demonstration by CT and MR imaging. Neuroradiology. 1987;29:266–71. doi: 10.1007/BF00451765. [DOI] [PubMed] [Google Scholar]
- 16.Sharma S. Evolution of giant P2-posterior cerebral artery aneurysm over 16 years: Saccular to serpentine.A case report. Neuroradiol J. 2009;22:605–11. doi: 10.1177/197140090902200514. [DOI] [PubMed] [Google Scholar]
- 17.Silva JM, Aguiar GB, Conti ML, Veiga JC. Spontaneous thrombosis of aneurysm and posterior cerebral artery. Rev Chil Neurocir. 2013;39:172–5. [Google Scholar]
- 18.Teng MM, Nasir Qadri SM, Luo CB, Lirng JF, Chen SS, Chang CY. MR imaging of giant intracranial aneurysm. J Clin Neurosci. 2003;10:460–4. doi: 10.1016/s0967-5868(03)00092-4. [DOI] [PubMed] [Google Scholar]
- 19.Türe U, Elmaci I, Ekinci G, Pamir MN. Totally thrombosed giant P2 aneurysm: A case report and review of literature. J Clin Neurosci. 2003;10:115–20. doi: 10.1016/s0967-5868(02)00276-x. [DOI] [PubMed] [Google Scholar]
- 20.Vorkapic P, Czech T, Pendl G, Oztürk E, Horaczek A. Clinico-radiological spectrum of giant intracranial aneurysms. Neurosurg Rev. 1991;14:271–4. doi: 10.1007/BF00383260. [DOI] [PubMed] [Google Scholar]
- 21.Westerlaan HE, van Dijk JM, Jansen-van der Weide MC, de Groot JC, Groen RJ, Mooij JJ, et al. Intracranial aneurysms in patients with subarachnoid hemorrhage: CT angiography as a primary examination tool for diagnosis - Systematic review and meta-analysis. Radiology. 2011;258:134–45. doi: 10.1148/radiol.10092373. [DOI] [PubMed] [Google Scholar]
- 22.Whittle IR, Dorsch NW, Besser M. Spontaneous thrombosis in giant intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1982;45:1040–7. doi: 10.1136/jnnp.45.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–10. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]