Abstract
Background:
Recurrent or residual pituitary adenomas previously treated by transsphenoidal surgery are not uncommon. There are no strongly established guidelines to perform treatment of such cases. The objective of this study is to elucidate the effect of transsphenoidal reoperation in residual or recurrent pituitary adenomas.
Methods:
We made a systematic review of the literature to elucidate this effect through electronic search in MEDLINE/PubMed and Cochrane Central database. PRISMA statement was used as a basis for this systematic review and analysis of the risk of bias was made according to the Grading of Recommendations, Assessment, Development and Evaluation recommendations.
Results:
In this review, fifteen studies were finally pooled analyzed. Although remission rates (RRs) and follow-up periods varied widely, from 149 patients with growth hormone-secreting tumors the mean RR was 44.5%, from 273 patients with adrenocorticotropic hormone-secreting tumors the mean RR was 55.5% and among 173 patients with nonsecreting tumors, RR was 76.1%. There was significant higher RR in nonsecreting tumors. Mean follow-up was 32.1 months. No difference was found between microscopic and endoscopic techniques.
Conclusions:
A second transsphenoidal surgery is accompanied by a chance of remission in approximately half of cases with secreting tumors. In nonsecreting ones, success is higher.
Key Words: Endoscopy, microsurgery, pituitary adenoma, transsphenoidal, treatment
INTRODUCTION
Treatment of pituitary adenomas is often surgical, especially in hormone-secreting tumors or those with a mass effect on surrounding structures, such as the optic chiasm.[1,2,3,4,5] The presence of residual tumor and tumor recurrence in the postoperative period are not uncommon. Treatment of both conditions remains subject to individual selection since no established guidelines on the type of treatment option for these cases is currently available.[1,6,7,8,9]
In addition, it is well known that risks are higher in patients subjected to repeated transsphenoidal surgery than in patients without prior therapy.[4,5,6,7,8] Thus, it is important to conduct a critical analysis of the effect of repeated surgery.
The objective of this study is to elucidate the effects of transsphenoidal reoperation for pituitary adenomas.
METHODS
A systematic review of the literature on the effects of repeated transsphenoidal surgery was performed using the MEDLINE (via PubMed) database and the Cochrane Central Register of Controlled Trials. The PRISMA statement and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) recommendations were followed.
Structure of the search strategy
The literature search was based on structured questions, which defined the criteria for inclusion of papers, based on types of participants (patients), intervention and control, follow-up, and types of studies, following the PICOT strategy.
Types of participants: Adult patients, of both genders, with pituitary adenoma, who experienced tumor recurrence or residual tumor after primary surgery and underwent revision surgery. Prolactinomas were excluded from evaluation due to the established concept of its treatment with dopaminergic agonists.
We excluded patients who either underwent radiotherapy (RT)/radiosurgery or who were receiving medical therapy to attain remission previously to the second transsphenoidal surgery.
Types of intervention/exposure: Transsphenoidal reoperation of pituitary adenomas.
Outcomes: The outcome selected for the study was the remission rate (RR) after second operation (reoperation).
Follow-up time or extraction of outcomes: We included all studies that reported follow-up time.
Types of study: We aimed to include clinical trials; if not possible, we accepted all cohorts. Case reports and studies evaluating <10 cases were excluded.
Language: Articles published in English, Portuguese, French, and Spanish from 1964 to 2014 were considered for analysis.
Analysis of risk of bias: The risk of bias was analyzed in accordance with the GRADE criteria.
Search strategies
The search strategy used is detailed below:
“Pituitary neoplasms” [MeSH Terms] OR “pituitary” [All Fields] AND “recurrence” [MeSH Terms] AND Transsphenoidal [All Fields] AND “surgery” [Subheading] OR “surgery” [All Fields] OR “surgical procedures, operative” [MeSH Terms] OR “surgical” [All Fields] AND “procedures” [All Fields] AND “operative” [All Fields] OR “operative surgical procedures” [All Fields] OR “surgery” [All Fields].
Searches were performed by two authors independently, and disagreements were resolved by discussion.
Statistics
RR was described as a percentage, mean, standard deviation, range, and confidence intervals. Follow-up time was described as mean, standard deviation, and range.
A meta-analysis of pooled data was made according to the type of tumor. The RRs of the surgical operated pituitary adenomas were compared across pituitary tumors types according to the method proposed by Neyeloff et al.[20] for observational data.
A comparison between secreting and nonsecreting tumors was performed with Chi-square test.
RESULTS
Search
The search strategy retrieved 410 articles [Figure 1]. Twenty-two papers were initially selected by their titles alone. Eight articles were initially selected by the electronic search. One other study known to the authors was added, and six additional studies were included after cross-referencing of selected papers. The final sample included 15 publications for analysis.
Figure 1.
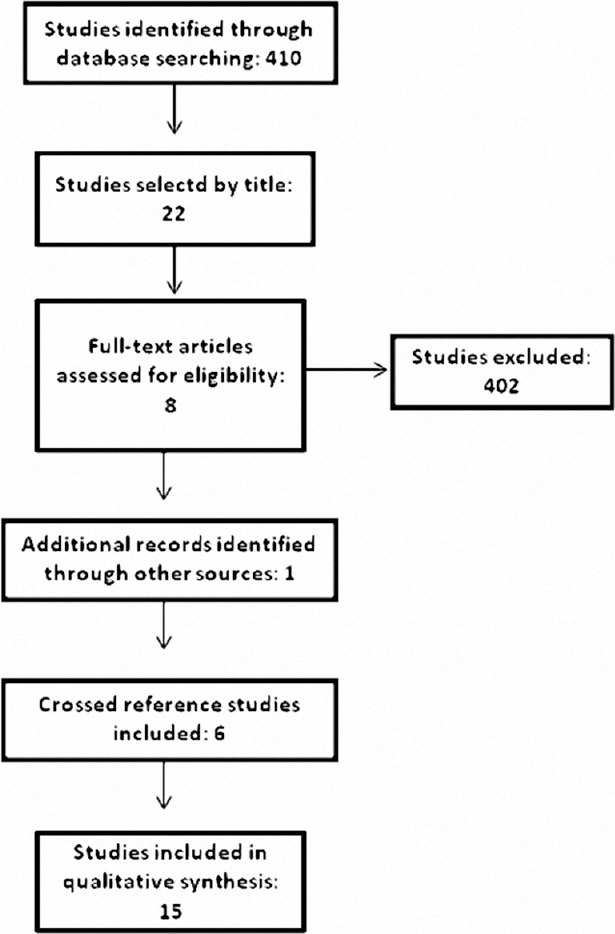
Search strategy and results
Data extraction [Table 1]
Table 1.
Data extraction of 15 studies enrolled
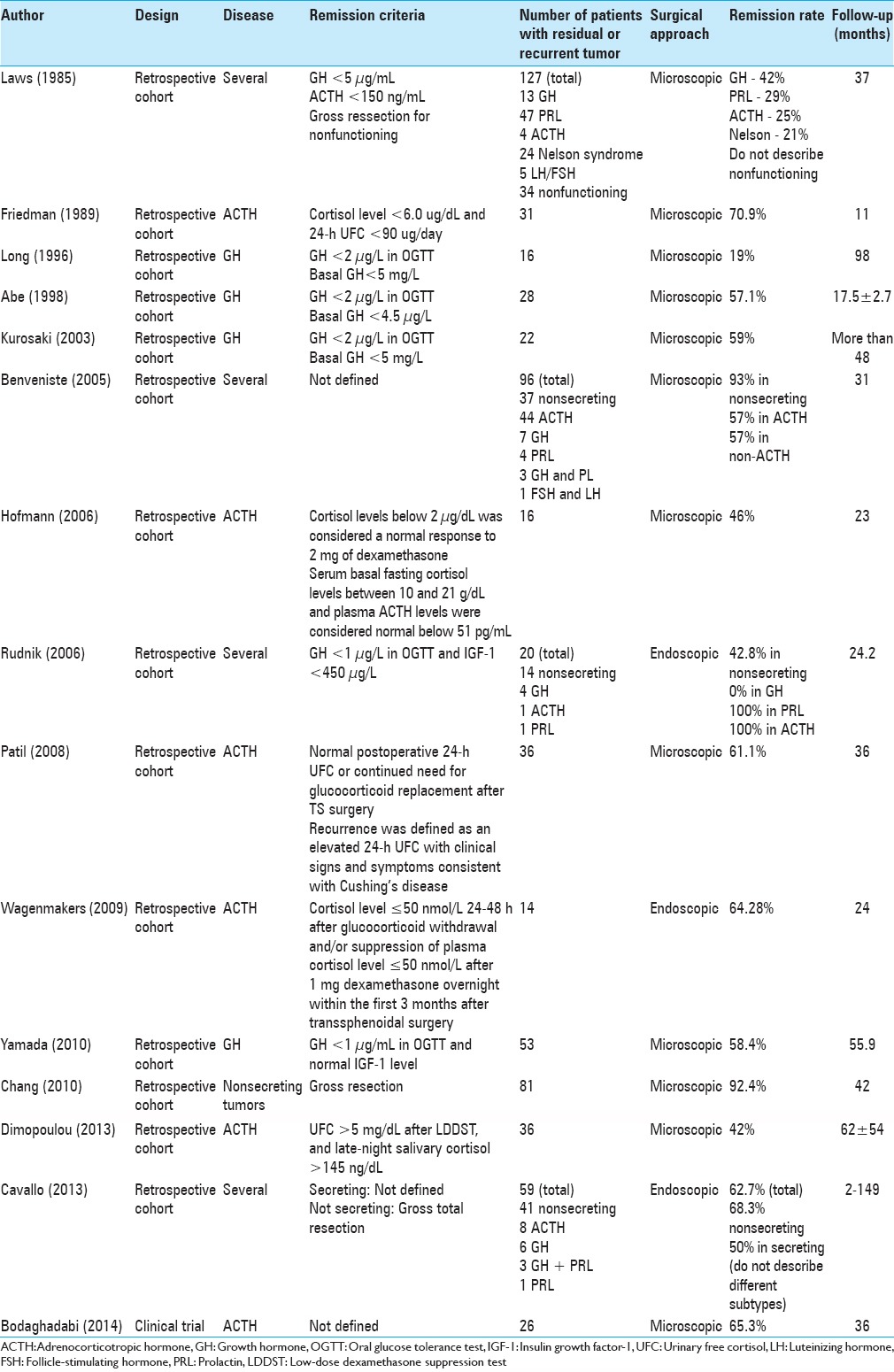
Laws et al.[16] analyzed 158 patients with pituitary tumors who underwent secondary transsphenoidal surgery, of whom 127 had adenomas. Thirteen were growth hormone (GH)-secreting, 47 were prolactin (PRL)-secreting, 4 were adrenocorticotropic hormone (ACTH)-secreting, and 34 were nonsecreting. Mean follow-up time was 37 months. RRs were 42% for GH tumors, 29% for PRL tumors, and 25% for ACTH tumors; no data were available for remission in nonsecreting tumors.[16]
Friedman et al.[13] reported the outcomes of 31 patients with Cushing's disease after second transsphenoidal surgery. Over 11 months of follow-up, 22 patients (70.96%) achieved remission.[13]
Long et al.[17] described the results of secondary transsphenoidal surgery in 16 patients with GH-secreting adenomas. After an average follow-up of 8.2 years, the authors reported normalization of GH levels in 19% of patients and a >50% reduction in GH levels in 69% of patients. For pooled analysis, we considered 19% as the RR.[13]
Abe and Lüdecke[1] analyzed 270 patients with GH-secreting adenomas, of whom 28 experienced recurrence and underwent secondary surgery. Of the 28 recurrent tumors, 18 could be resected and 10 could not. Among the 18 patients operated, 16 achieved remission (57.14%). The follow-up time was 17.5 ± 2.7 months.[1]
Kurosaki et al.[15] analyzed 22 patients with GH-secreting tumors. The RR was 59% with a follow-up of more than 4 years.[15]
Benveniste et al.[4] studied 96 patients undergoing reoperation for residual tumor or recurrence of pituitary adenomas without RT. Mean follow-up was 31 months. Overall, 37 patients had nonsecreting tumors, whereas 7 had GH-secreting and 44 had ACTH-secreting tumors. Surgery for recurrent or residual mass was associated with gross resection rates of 93% in nonsecreting tumors and remission in 57% in secreting tumors.[4]
Hofmann et al.[14] studied 16 patients with a previously operated pituitary tumor who presented with recurrent Cushing's disease. Of these 16 patients, 13 underwent selective adenomectomy and 3 underwent hemihypophysectomy. Six patients had remission of Cushing's disease while seven remained affected (three of whom had tumors with cavernous sinus invasion). One of these underwent bilateral adrenalectomy and improved, and another underwent RT and adrenalectomy. The follow-up period was 23 months.[14]
Rudnik et al.[22] evaluated 20 patients who underwent endoscopic surgery of recurrent adenomas or residual tumor not completely removed during the first surgical procedure. The analyzed group had 14 nonsecreting adenomas, 4 GH-secreting adenomas, 1 PRL-secreting adenoma, and 1 ACTH-secreting adenoma. Of these, 19 were macroadenomas, while 1 was a microadenoma. The follow-up period after surgery was between 12 and 42 months (mean, 24.2 months). The gross resection rate was 42.8% in nonsecreting tumors, and RR was 33.3% in secreting tumors. While ACTH and PRL-secreting tumors were associated with 100% remission, GH tumors had 0%.[22]
Patil et al.[21] reviewed the medical records of 455 patients who underwent surgery for Cushing's disease between 1992 and 2006. Mean follow-up was 36 months. Of these patients, 40 underwent repeat surgery, and 36 patients with clinical and biochemical evidence of recurrent Cushing's disease after the first surgery who had endocrine follow-up were included. Overall, 22 patients (61.1%) achieved remission after the second surgery.[21]
Wagenmakers et al.[24] described 24 patients treated for persistent Cushing's disease over 24 months of follow-up. Remission after the second surgery was achieved in 10 of 14 patients who underwent repeated surgery.[24]
Yamada et al.[25] studied 53 cases of the recurrent GH-secreting tumor with a follow-up period of 55.9 months. Patients were divided into groups (A, B, C) according to their endocrine profile in the early postoperative period: Group A, success, in 26 of 53 (49%); Group B, insufficient, in 9 of 53 (16.98%); and Group C, unsuccessful, in 18 of 53 (33.9%).[25]
Chang et al.[8] studied 81 patients in two groups: Gross resection versus subtotal resection and RT. The mean follow-up was 3.6 years. Gross resection could be reached in 92.4%.[8]
Dimopoulou et al.[11] evaluated 120 patients with Cushing's disease, of whom 36 underwent a second surgery. The RR was 42%. Follow-up time was 62 ± 54 months.[11]
Cavallo et al.[7] performed transsphenoidal endoscopic surgery for residual or recurrent pituitary adenomas in 59 patients. Of these, 31 had previously undergone surgery via a microsurgical transsphenoidal approach, 22 by means of an endoscopic transsphenoidal route, and 6 through a transcranial route. Forty-one patients presented with nonsecreting tumors, while 18 were hormone-secreting (8 ACTH, 6 GH, 3 GH + PRL, and 1 PRL). Follow-up ranged from 2 to 149 months.[7]
Gross total resection was achieved in 37 cases (62.7%). On average, 38.4% of gross total resections left residual lesions which regrew (60.8% in nonsecreting adenomas, 15 of 24; 14.3% insecreting adenomas, 1 of 7); the recurrence rate was 74.6% (76.5% in nonsecreting adenomas, 13 of 17; 72.7% in secreting adenomas, 8 of 11).[7]
Bodaghabadi et al.[5] evaluated 52 patients with relapse of Cushing's disease after initial surgical therapy who were treated with further surgery versus stereotactic radiosurgery (gamma knife). Patients were divided into two balanced groups (n = 26 each). The mean follow-up was 3 years, and the outcome measure was a disease-free interval. The groups were homogeneous before treatment of relapse, with no differences in age, gender, type of adenoma (micro or macro), and hormone levels. The time to recurrence was 38 months among patients undergoing radiosurgery and 30 months in the surgery group (P = 0.36). Nine patients in the surgery group experienced recurrence, versus 10 patients in the gamma knife group.[5]
Analysis of data pooled by tumor type
Growth hormone-secreting
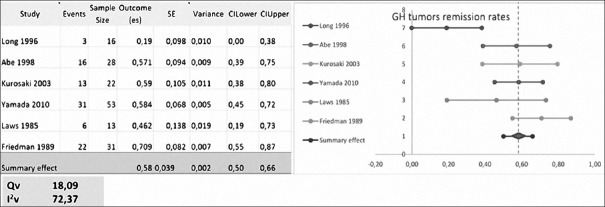
Yamada, Kurosaki, Rudnik, Cavallo, Benveniste, Abe, Laws, and Long included a total of 149 patients with residual or recurrent GH-secreting tumors. Data from Benveniste and Cavallo were not pooled due to a low number of studied patients.
Laws used basal GH levels as remission criteria. Long, Abe and Kurosaki used basal GH and GH levels after glucose tolerance test (GTT). Rudnik and Yamada used GH levels after GTT and insulin growth factor-1 levels [Table 1].
The mean RR was 44.5 ± 23.85% (range, 0–72.8%) with repeated transsphenoidal surgery.[1,4,7,15,16,17,22,25] Meta-analysis excluded Rudnik and Cavallo, each with <10 patients evaluated [Figure 2].
Figure 2.
Meta-analysis of growth hormone-secreting tumors remission rates
Adrenocorticotropic hormone-secreting
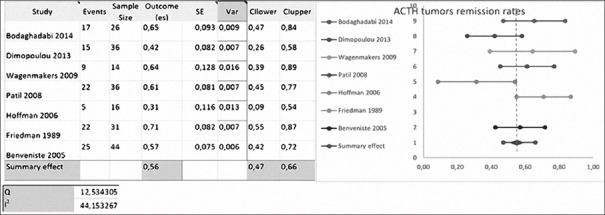
Hofmann, Patil, Bodaghabadi, Wagenmakers, Dimopoulou, Benveniste, Cavallo, Rudnik, Laws, and Friedman included 213 cases of recurrent ACTH-secreting tumors.
Laws used ACTH as remission criteria. Friedman, Hofmann, and Wagenmakers used basal cortisol levels and urinary cortisol levels as remissions criteria. Patil used urinary cortisol levels, and Dimopoulou used urinary and salivary cortisol levels [Table 1]. Cavallo, Bodaghabadi, Benveniste, and Rudnik did not define clear remission criteria for ACTH-secreting tumors.
The mean RR was 55.5 ± 15.89% (range, 25–72.7%) with repeated transsphenoidal surgery.[4,5,7,11,13,14,21,22,24] Meta-analysis excluded Laws, Rudnik and Cavallo, each with <10 patients evaluated [Figure 3].
Figure 3.
Meta-analysis of adrenocorticotropic hormone-secreting tumors remission rates
Nonsecreting tumors
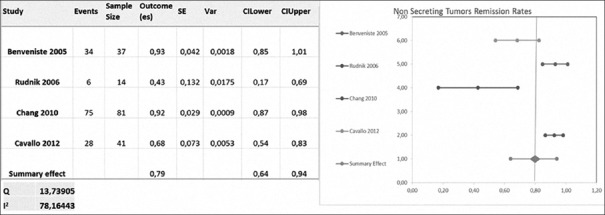
Benveniste, Chang, Rudnik, and Cavallo included 173 nonsecreting tumors. Remission was defined as an image criteria revealing gross resection [Table 1].
The gross resection rate was 76.05 ± 23.52% (ranging from 42.8% to 93%) with repeated transsphenoidal surgery [Figure 4].[4,7,8,22]
Figure 4.
Meta-analysis of nonsecreting tumors remission rates
Endoscopic versus microscopic surgery
Yamada, Kurosaki, Benveniste, Abe, Laws, Long, Hofmann, Patil, Bodaghabadi, Dimopoulos, Friedman and Chang reported transsphenoidal surgery using microscopic techniques. The mean RR reported was 56.5 ± 19.7%.[1,4,5,8,11,13,14,15,21,25]
Rudnik, Cavallo, and Wagenmakers published surgical series of repeated transsphenoidal surgery using the endoscopic technique.[7,22,24] The mean RR was 54.9 ± 14.7%. There was no statistical difference in RRs between the two groups.[7,22,24]
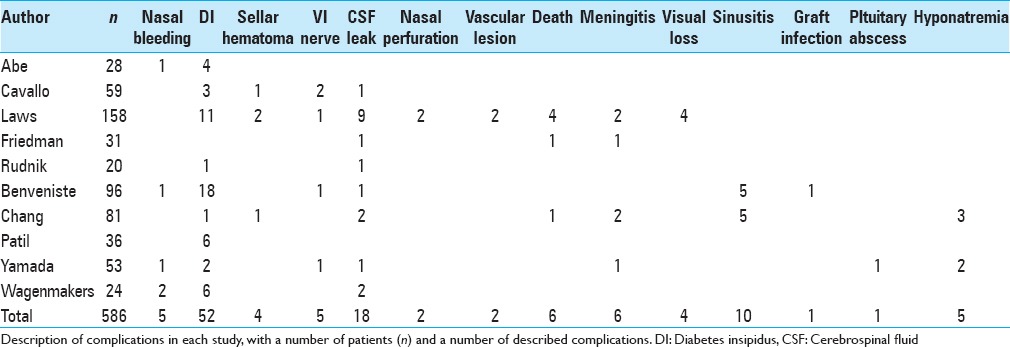
Complications in repeated transsphenoidal surgery
Among 15 studies evaluated, 3 did not even describe complications (Dimopoulou et al.; Long et al.; Bodaghabadi et al.) and 2 described no complications among their patients (Hofmann et al.; Kurosaki et al.). Finally, 10 studies described complications [Table 2]. There were 121 complications among 586 patients (20.6%). Most frequent complications were diabetes insipidus in 52 patients (8.8%), a cerebrospinal fluid leak in 18 patients (3.0%) and sinusitis in 10 patients (1.7%). There were 6 cases of death, 4 of them being from one single study. Other complications are described in Table 2.
Table 2.
Complications after repeated transsphenoidal surgery
Synthesis of results
The rate of gross resection after repeated transsphenoidal surgery was approximately 76.05% for nonsecreting tumors. In secreting lesions, the average RR was 55.5% in ACTH-secreting and 44.5% in GH-secreting tumors. In short, RRs were higher in nonsecreting tumors than in secreting tumors (P = 0.001, Chi-square test).
Bias
Only one of the publications included was a randomized trial (Bodaghabadi et al.),[5] which compared the results of surgery and radiosurgery. Since the goal of this review was to disclose the effect of surgery, we only used the results of the surgical arm for analysis. All other studies evaluated were observational studies of homogeneous cohorts of patients with recurrent and residual pituitary tumors.[5]
Several studies evaluated homogeneous samples of recurrent tumors. However, Benveniste, Rudnik, Cavallo, and Laws evaluated a range of tumors but reported remission as an outcome in different subtypes.[4,7,16,22] Laws et al. did not report the RR for nonsecreting tumors.[16]
All studies described the type of intervention and the outcome measure evaluated in this study, i.e., the average rate of tumor remission.
The length of follow-up varied widely, from 2 to 149 months (mean, 32.1 months), and was significantly different across studies (Chi-square, P < 0.002). None of the studies reported the significant loss to follow-up. However, Chang et al. found that postoperative images were available for only 74.07% of patients.[8]
DISCUSSION
The second surgery is a common situation in the treatment of pituitary adenomas, which are lesions especially prone to recurrence or regrowth.[2,6,9,10,12,18,19,23] Previous studies have already stressed the importance of the first surgery in avoiding reoperation, as it is believed that residual tumor lying within the pituitary gland, dura mater of the sella turcica, or cavernous sinus (by extension from the dura) may be the cause of regrowth.[10] At repeat surgery, the residual tumor may be found at or immediately contiguous to the site where the lesion was found originally.[10]
Remission is defined differently in many of the quoted papers. Nevertheless, recurrence of pituitary tumors is a fact and must be addressed.
Currently, there is no guideline for the management of such cases, and treatment protocols are largely based on individual experience rather than on evidence-based literature. RT and radiosurgery, which are almost always reserved for cases of failed surgical treatment, are being increasingly applied to recurrent tumors, with encouraging results.[2] However, no studies have adequately addressed the ideal protocol for treatment of recurrent or residual pituitary adenomas. In fact, some tumors, such as acromegaly, would be amenable to perform adjuvant therapy prior to reoperation, and it would be very difficult to evaluate these treatments in a pooled analysis of data. Future trials are encouraged to answer these topics.
In our literature review, we were able to compare the results of reoperation and radiosurgery in only one randomized trial, which included 26 patients in each arm.[5] All other pooled cohort studies evaluated a total of 509 patients who underwent reoperation without a control group. Thus, we were not able to compare the effects of surgery versus those of other treatment modalities, but we were able to estimate the effect of reoperation.
Stratification of tumors by hormone type revealed striking differences in RRs and prognosis. The average RR was 44.5%in GH-secreting tumors; in ACTH-secreting tumors, 55.5%; and in nonsecreting tumors, 76.05% (P = 0.001).
Although the main interest of any intervention with systematic review always will be randomized trials, still now several questions cannot be answered with this type of publications due to the lack of papers until this moment. Then, nonrandomized trials represent the best available evidence. The cohort studies have many potential biases that the data interpretation must be done with caution. As no comparative intervention was selected to be the scope of this review, the main concern in performing a retrospective meta-analysis, i.e. differences in compared populations may be initially disregarded. The data evaluation was done in this study still did not take into consideration that follow-up time could be an independent factor for lower RRs. This must be considered as a limitation when analyzing the results of this study.
In addition, remission criteria used in literature are distinct and varied among published papers describing tumoral recurrence [Table 1]. The reader must bear in mind that there is not any paper describing long-term follow-up. Some recurrences may be delayed particularly with Cushing's disease.
In summary, the pooled RR in the published literature after transsphenoidal reoperation for residual or recurrent pituitary adenomas was 45.5% in GH-secreting tumors, 55.5% in ACTH-secreting tumors and 76.05% in nonsecreting tumors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Lindolfo Carlos Heringer, Email: heringerlindolfo@terra.com.br.
Matheus Fernandes de Oliveira, Email: mafernoliv@yahoo.com.br.
José Marcus Rotta, Email: josemarcusrotta@gmail.com.
Ricardo Vieira Botelho, Email: bitbot@uol.com.br.
REFERENCES
- 1.Abe T, Lüdecke DK. Recent results of secondary transnasal surgery for residual or recurring acromegaly. Neurosurgery. 1998;42:1013–21. doi: 10.1097/00006123-199805000-00036. [DOI] [PubMed] [Google Scholar]
- 2.Aghi MK. Management of recurrent and refractory Cushing disease. Nat Clin Pract Endocrinol Metab. 2008;4:560–8. doi: 10.1038/ncpendmet0947. [DOI] [PubMed] [Google Scholar]
- 3.Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84:843–9. doi: 10.1136/jnnp-2012-303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benveniste RJ, King WA, Walsh J, Lee JS, Delman BN, Post KD. Repeated transsphenoidal surgery to treat recurrent or residual pituitary adenoma. J Neurosurg. 2005;102:1004–12. doi: 10.3171/jns.2005.102.6.1004. [DOI] [PubMed] [Google Scholar]
- 5.Bodaghabadi M, Riazi H, Aran S, Bitaraf MA, Alikhani M, Alahverdi M, et al. Repeated transsphenoidal surgery or gamma knife radiosurgery in recurrent cushing disease after transsphenoidal surgery. J Neurol Surg A Cent Eur Neurosurg. 2014;75:91–7. doi: 10.1055/s-0033-1345688. [DOI] [PubMed] [Google Scholar]
- 6.Cappabianca P, Alfieri A, Colao A, Cavallo LM, Fusco M, Peca C, et al. Endoscopic endonasal transsphenoidal surgery in recurrent and residual pituitary adenomas: Technical note. Minim Invasive Neurosurg. 2000;43:38–43. doi: 10.1055/s-2000-8814. [DOI] [PubMed] [Google Scholar]
- 7.Cavallo LM, Solari D, Tasiou A, Esposito F, de Angelis M, D’Enza AI, et al. Endoscopic endonasal transsphenoidal removal of recurrent and regrowing pituitary adenomas: Experience on a 59-patient series. World Neurosurg. 2013;80:342–50. doi: 10.1016/j.wneu.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Chang EF, Sughrue ME, Zada G, Wilson CB, Blevins LS, Jr, Kunwar S. Long term outcome following repeat transsphenoidal surgery for recurrent endocrine-inactive pituitary adenomas. Pituitary. 2010;13:223–9. doi: 10.1007/s11102-010-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark K. Surgery for the recurrent pituitary adenoma. Clin Neurosurg. 1980;27:309–14. doi: 10.1093/neurosurgery/27.cn_suppl_1.309. [DOI] [PubMed] [Google Scholar]
- 10.Dickerman RD, Oldfield EH. Basis of persistent and recurrent Cushing disease: An analysis of findings at repeated pituitary surgery. J Neurosurg. 2002;97:1343–9. doi: 10.3171/jns.2002.97.6.1343. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulou C, Schopohl J, Rachinger W, Buchfelder M, Honegger J, Reincke M, et al. Long-term remission and recurrence rates after first and second transsphenoidal surgery for Cushing's disease: Care reality in the Munich Metropolitan Region. Eur J Endocrinol. 2013;170:283–92. doi: 10.1530/EJE-13-0634. [DOI] [PubMed] [Google Scholar]
- 12.Ding D, Starke RM, Sheehan JP. Treatment paradigms for pituitary adenomas: Defining the roles of radiosurgery and radiation therapy. J Neurooncol. 2014;117:445–57. doi: 10.1007/s11060-013-1262-8. [DOI] [PubMed] [Google Scholar]
- 13.Friedman RB, Oldfield EH, Nieman LK, Chrousos GP, Doppman JL, Cutler GB, Jr, et al. Repeat transsphenoidal surgery for Cushing's disease. J Neurosurg. 1989;71:520–7. doi: 10.3171/jns.1989.71.4.0520. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann BM, Hlavac M, Kreutzer J, Grabenbauer G, Fahlbusch R. Surgical treatment of recurrent Cushing's disease. Neurosurgery. 2006;58:1108–18. doi: 10.1227/01.NEU.0000215945.26764.92. [DOI] [PubMed] [Google Scholar]
- 15.Kurosaki M, Luedecke DK, Abe T. Effectiveness of secondary transnasal surgery in GH-secreting pituitary macroadenomas. Endocr J. 2003;50:635–42. doi: 10.1507/endocrj.50.635. [DOI] [PubMed] [Google Scholar]
- 16.Laws ER, Jr, Fode NC, Redmond MJ. Transsphenoidal surgery following unsuccessful prior therapy. An assessment of benefits and risks in 158 patients. J Neurosurg. 1985;63:823. doi: 10.3171/jns.1985.63.6.0823. [DOI] [PubMed] [Google Scholar]
- 17.Long H, Beauregard H, Somma M, Comtois R, Serri O, Hardy J. Surgical outcome after repeated transsphenoidal surgery in acromegaly. J Neurosurg. 1996;85:239–47. doi: 10.3171/jns.1996.85.2.0239. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin N, Eisenberg AA, Cohan P, Chaloner CB, Kelly DF. Value of endoscopy for maximizing tumor removal in endonasal transsphenoidal pituitary adenoma surgery. J Neurosurg. 2013;118:613–20. doi: 10.3171/2012.11.JNS112020. [DOI] [PubMed] [Google Scholar]
- 19.Meij BP, Lopes MB, Ellegala DB, Alden TD, Laws ER., Jr The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg. 2002;96:195–208. doi: 10.3171/jns.2002.96.2.0195. [DOI] [PubMed] [Google Scholar]
- 20.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. doi: 10.1186/1756-0500-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patil CG, Veeravagu A, Prevedello DM, Katznelson L, Vance ML, Laws ER., Jr Outcomes after repeat transsphenoidal surgery for recurrent Cushing's disease. Neurosurgery. 2008;63:266–70. doi: 10.1227/01.NEU.0000313117.35824.9F. [DOI] [PubMed] [Google Scholar]
- 22.Rudnik A, Zawadzki T, Galuszka-Ignasiak B, Bazowski P, Duda I, Wojtacha M, et al. Endoscopic transsphenoidal treatment in recurrent and residual pituitary adenomas - First experience. Minim Invasive Neurosurg. 2006;49:10–4. doi: 10.1055/s-2006-932126. [DOI] [PubMed] [Google Scholar]
- 23.Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA., Jr Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: Outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab. 2013;98:3190–8. doi: 10.1210/jc.2013-1036. [DOI] [PubMed] [Google Scholar]
- 24.Wagenmakers MA, Netea-Maier RT, van Lindert EJ, Timmers HJ, Grotenhuis JA, Hermus AR. Repeated transsphenoidal pituitary surgery (TS) via the endoscopic technique: A good therapeutic option for recurrent or persistent Cushing's disease (CD) Clin Endocrinol (Oxf) 2009;70:274–80. doi: 10.1111/j.1365-2265.2008.03334.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamada S, Fukuhara N, Oyama K, Takeshita A, Takeuchi Y. Repeat transsphenoidal surgery for the treatment of remaining or recurring pituitary tumors in acromegaly. Neurosurgery. 2010;67:949–56. doi: 10.1227/NEU.0b013e3181ec4379. [DOI] [PubMed] [Google Scholar]