Abstract
Background:
Awake craniotomy is currently the gold standard for aggressive tumor resections in eloquent cortex. However, a significant subset of patients is unable to tolerate this procedure, particularly the very young or old or those with psychiatric comorbidities, cardiopulmonary comorbidities, or obesity, among other conditions. In these cases, typical alternative procedures include biopsy alone or subtotal resection, both of which are associated with diminished surgical outcomes.
Case Description:
Here, we report the successful use of a preoperatively obtained resting state functional connectivity magnetic resonance imaging (MRI) integrated with intraoperative neuronavigation software in order to perform functional cortical mapping in the setting of an aborted awake craniotomy due to loss of airway.
Conclusion:
Resting state functional connectivity MRI integrated with intraoperative neuronavigation software can provide an alternative option for functional cortical mapping in the setting of an aborted awake craniotomy.
Key Words: Awake craniotomy, functional mapping, intraoperative neuronavigation, resting state functional connectivity magnetic resonance imaging
BACKGROUND
The goal of an awake craniotomy for tumor resection is to remove as much pathologic tissue as possible while preserving cortical structures subserving functions such as speech and motor control.[1,7,16,17,21,38] In some cases, however, patients may not be able to tolerate an awake craniotomy, leading to biopsy alone or subtotal resection. Imaging advances including task-based functional magnetic resonance imaging (fMRI)[8,15,24] and more recently, resting state functional connectivity MRI[19,22,44] have provided useful information regarding the anatomic relationships between eloquent cortex and an underlying pathologic process. Typically, these advanced imaging modalities are utilized in a presurgical planning capacity with the gold standard for intraoperative functional cortical mapping remaining awake surgical technique with electrocortical stimulation (ECS).[39] Here, we report the successful use of a preoperatively obtained, resting-state functional connectivity MRI integrated into our intraoperative navigation software to guide aggressive resection after an aborted awake craniotomy due to the loss of the patient's airway making formal stimulation mapping impossible. Though preoperative imaging has been previously used in conjunction with ECS during awake neurosurgical procedures,[23,26,35,37] this account represents the first reported successful use of resting-state fMRI obtained preoperatively for functional cortical mapping as a secondary salvage maneuver when stimulation is not possible.
CASE DESCRIPTION
Clinical history and physical examination
Our patient was a 57-year-old right-handed gentleman with a remote history of colon cancer who presented with increasing headache frequency and severity with new onset complex partial seizures. MRI identified a left posterior frontotemporal tumor suggestive of a high-grade glioma [Figure 1]. Given the lesion's proximity to both speech and motor cortex the patient was offered an awake craniotomy integrated with intraoperative stereotactic navigation including tractography as well as electrophysiology and evoked potentials for speech and motor mapping to facilitate maximal safe surgical resection which he elected to proceed with.
Figure 1.
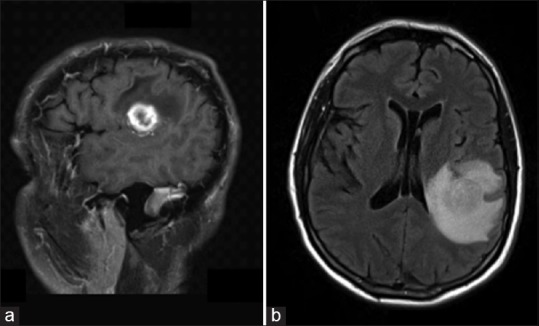
Preoperative anatomic magnetic resonance imaging. (a) Sagittal T1-weighted contrasted magnetic resonance imaging demonstrating a rim enhancing peri-sylvian mass consistent with primary glial neoplasm. (b) Axial fluid attenuated inversion recovery magnetic resonance imaging sequence demonstrating the same peri-sylvian mass with significant surrounding edema
Surgical procedure
The patient was placed in the three-quarter lateral position exposing the left side for craniotomy. He was mildly sedated with remifentanil and midazolam by the anesthesia team for surgical exposure. Supraorbital and posterior occipital nerve blocks were performed prior to fixing his head in an MRI compatible Mayfield cranial fixation device (Integra Life Sciences, Plainsboro NJ, USA). A circumferential field block was then performed, and the patient's head was coregistered with the intraoperative stereotactic navigation system (StealthStation, Medtronic, Inc., Minneapolis, MN, USA). Following coregistration, the surgical team was notified by anesthesia that the patient was vomiting and had aspirated, and they would need to place an endotracheal tube to protect the patient's airway moving forward. At this juncture, the following options were considered: Aborting the procedure, proceeding with biopsy alone or proceeding with surgical resection based on previously integrated preoperative resting state functional connectivity MRI.
The patient's preoperative resting state functional connectivity MRI suggested that there was a corridor through the parietal lobe that would allow for surgical resection without damage to adjacent eloquent cortex [Figures 2 and 3]. This option would provide the survival benefit of maximal surgical resection[4,5,28,29] in addition to obtain tissue for a pathologic diagnosis. The decision was made to proceed with surgical resection based on preoperative resting state functional imaging, the methods, and validity of which have been previously published.[22] Following surgical exposure, stereotactic navigation was again used to confirm the optimal gyrus from which to access the tumor [Figure 4]. A corticectomy was made in the selected gyrus, and the tumor margin was encountered deeply using a combination of sharp and blunt dissection. Upon arrival at the presumed tumor, stereotactic navigation was again used for confirmation. A peripheral margin was defined circumferentially around the tumor until the deep aspect of the tumor was reached. This portion appeared to involve the posterior aspect of the insular pia. Tumor resection proceeded uneventfully with periodic use of stereotactic confirmation of our resection margins as they related to eloquent pathways including cortical activations by resting state fMRI and en passage white matter fiber tracts by tractography. An intraoperative MRI was obtained demonstrating a small area of residual enhancement within the anterior resection cavity. Following the merger of this intraoperatively obtained imaging, it was recognized that this represented the portion of the tumor immediately abutting language cortex as identified on preoperative functional imaging [Figure 5]. Given this proximity to the eloquent cortex and our inability to obtain interactive language mapping with our patient, the risk of further resection was believed to outweigh its benefits and surgery concluded. The patient awoke without difficulty and demonstrated no language or motor deficits postoperatively.
Figure 2.
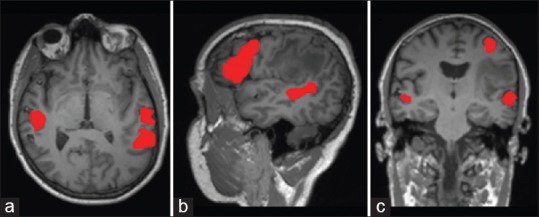
Preoperative resting state functional connectivity T1-weighted magnetic resonance imaging in the (a) axial, (b) sagittal and (c) coronal planes demonstrating language activations in red related to the patient's peri-sylvian mass
Figure 3.
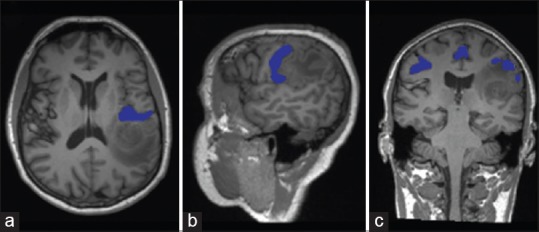
Preoperative resting state functional connectivity T1-weighted magnetic resonance imaging in the (a) axial, (b) sagittal and (c) coronal planes demonstrating motor activations in blue related to the patient's peri-sylvian mass
Figure 4.
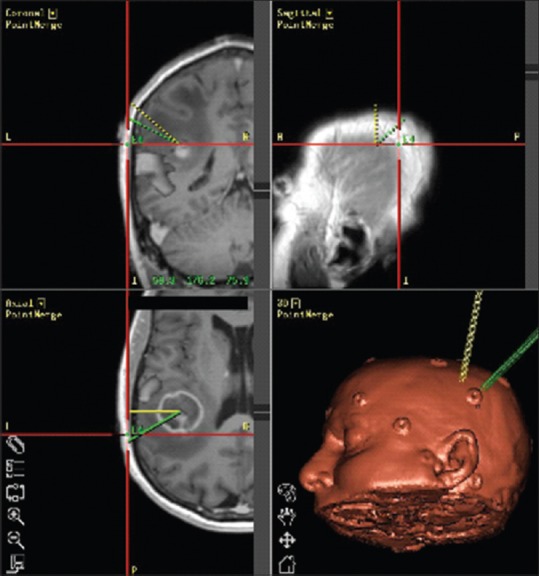
Intraoperative navigation system (StealthSystem, Medtronics, Inc., Minneapolis, MN, USA) demonstrating integrated resting state functional connectivity magnetic resonance imaging with preoperative anatomic T1-weighted sequence images
Figure 5.
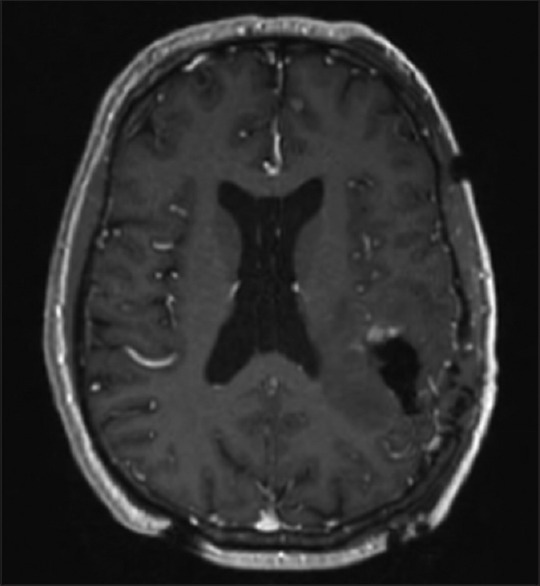
Postoperative anatomic magnetic resonance imaging demonstrating a small amount of residual enhancement within the anterior resection cavity, abutting motor activation as identified on preoperative resting state functional connectivity magnetic resonance imaging
CONCLUSION
Since its introduction in the 1990s, task-based fMRI has shown tremendous potential as an adjunctive tool to delineate areas of eloquent cortex for preoperative planning.[13] The benefits of task-based fMRI include its noninvasive technique and its ability to demonstrate task-related brain activity in individuals[27,40,41] but it also has certain limitations such as potentially long data acquisition times and the requirement that the patient be awake, interactive and cooperative during task performance.[25,34] These requirements have limited its application to patients with altered mental status, children, and the elderly in particular. More recently, resting state functional connectivity MRI has addressed some of these limitations by making use of spontaneous BOLD fluctuations that are present independent of tasks.[11] This allows more broad application to both functional mapping and preoperative planning.[34,44] Using previously described postprocessing imaging techniques, network connectivity can be readily defined using this resting state imaging modality.[12,22]
Currently, the gold standard for mapping eloquent cortex for neurosurgical procedures involves ECS testing during an awake craniotomy.[27] This technique requires patients to remain cooperative and interactive through the entirety of both the mapping and resection portions of a surgical procedure. These requirements as well as the possibility of intraoperative seizures or respiratory complications can limit this technique's efficacy overall. Intraoperative seizures can occur in 3–18% of awake craniotomies and respiratory complications may occur in up to 15–24% of awake craniotomies.[9,20,31,32,33,36] These and other complications can require the cessation of the awake portion of the procedure, limiting the extent of resection of a patient's tumor as it relates to functional cortex. In addition, to its limited success rate, only a subset of patients with pathologies that would benefit from an awake neurosurgical procedure are deemed to be candidates for such a technique. Baseline characteristics that would limit one's suitability for an awake procedure would include very young or old age, psychiatric comorbidities, cardiopulmonary comorbidities or obesity, among others.[2,6,10,14,30,31] Given these limitations, alternative mapping techniques could increase the number of patients who would be candidates for aggressive tumor resections despite tumor location within or adjacent to eloquent cortex.
Preoperative fMRI has been extensively compared to intraoperative ECS, generally with favorable results.[3,18,22] Lehéricy et al. report successful task-based fMRI mapping of 92% of sites that were positive under intraoperative ECS,[18] and Bizzi et al. report high sensitivity and specificity of task-based fMRI in mapping motor and language functions.[3] However, caution should be exercised interpreting findings from these advanced imaging techniques in the setting of an infiltrative tumor as neurovascular uncoupling, and other tumor-induced effects can impact the measured BOLD signal used for both task-based and resting-state fMRI.[34,40,42,43] Nevertheless, as evidenced by this case, advanced imaging techniques including integrated resting state functional connectivity MRI may be a viable alternative for patients who are not deemed candidates for an awake procedure or who are poorly tolerant of an awake procedure as in the case of our patient. Awake resection will remain the gold standard for management of eloquently located pathologies but for those patients who cannot undergo an awake procedure for any reason; we report a suitable alternative option to maximize tumor resection.
In conclusion, preoperative resting state functional connectivity MRI is a viable alternative for the management of eloquently located brain tumors when an awake craniotomy isn’t feasible or is poorly tolerated. Since resting state functional connectivity, MRI is both noninvasive and nonparticipatory, it is more broadly applicable than both task-based fMRI and awake ECS for mapping of the functional cortex. Its use for this purpose would allow tumor resections within eloquent cortex to be performed under a wider variety of circumstances. This, in turn, could lead to improved neurosurgical outcomes. The successful resection of our patient's left temporoparietal high-grade glioma using integrated preoperative fMRI instead of awake cortical stimulation in the setting of an aborted awake craniotomy supports the use of preoperatively acquired resting state functional connectivity MRI as an effective alternative to an awake cortical mapping.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Prag Batra, Email: prag@wustl.edu.
S. Kathleen Bandt, Email: skbandt@gmail.com.
Eric C. Leuthardt, Email: leuthardte@wudosis.wustl.edu.
REFERENCES
- 1.Ammirati M, Vick N, Liao YL, Ciric I, Mikhael M. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987;21:201–6. doi: 10.1227/00006123-198708000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Berger MS. The impact of technical adjuncts in the surgical management of cerebral hemispheric low-grade gliomas of childhood. J Neurooncol. 1996;28:129–55. doi: 10.1007/BF00250195. [DOI] [PubMed] [Google Scholar]
- 3.Bizzi A, Blasi V, Falini A, Ferroli P, Cadioli M, Danesi U, et al. Presurgical functional MR imaging of language and motor functions: Validation with intraoperative electrocortical mapping. Radiology. 2008;248:579–89. doi: 10.1148/radiol.2482071214. [DOI] [PubMed] [Google Scholar]
- 4.Brown PD, Maurer MJ, Rummans TA, Pollock BE, Ballman KV, Sloan JA, et al. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: The impact of the extent of resection on quality of life and survival. Neurosurgery. 2005;57:495–504. doi: 10.1227/01.neu.0000170562.25335.c7. [DOI] [PubMed] [Google Scholar]
- 5.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30(6 Suppl 19):10–4. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Carrabba G, Venkatraghavan L, Bernstein M. Day surgery awake craniotomy for removing brain tumours: Technical note describing a simple protocol. Minim Invasive Neurosurg. 2008;51:208–10. doi: 10.1055/s-2008-1073132. [DOI] [PubMed] [Google Scholar]
- 7.Ciric I, Ammirati M, Vick N, Mikhael M. Supratentorial gliomas: Surgical considerations and immediate postoperative results.Gross total resection versus partial resection. Neurosurgery. 1987;21:21–6. doi: 10.1227/00006123-198707000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. J Neurophysiol. 2000;83:3133–9. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- 9.Fadel N, Eldahab H, Wageh O, Wafik H. Awake craniotomy versus conventional general anaesthesia in surgical removal of low grade glioma primary experience of Kasr El-Aini Hospital. [Last accessed on 2013 Jun 18];Egypt J Anaesth. 2008 24:275–84. Available from: http://www.onlinelibrary.wiley.com/o/cochrane/clcentral/articles/935/CN-00789935/frame.html . [Google Scholar]
- 10.Fadul C, Wood J, Thaler H, Galicich J, Patterson RH, Jr , Posner JB.Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology. 1988;38:1374–9. doi: 10.1212/wnl.38.9.1374. [DOI] [PubMed] [Google Scholar]
- 11.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacker CD, Laumann TO, Szrama NP, Baldassarre A, Snyder AZ, Leuthardt EC, et al. Resting state network estimation in individual subjects. Neuroimage. 2013;82:616–33. doi: 10.1016/j.neuroimage.2013.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch J, Ruge MI, Kim KH, Correa DD, Victor JD, Relkin NR, et al. An integrated functional magnetic resonance imaging procedure for preoperative mapping of cortical areas associated with tactile, motor, language, and visual functions. Neurosurgery. 2000;47:711–21. doi: 10.1097/00006123-200009000-00037. [DOI] [PubMed] [Google Scholar]
- 14.Jääskeläinen J, Randell T. Awake Craniotomy in Glioma Surgery. In: Westphal M, Tonn JC, Ram Z, editors. Local Therapies for Glioma Present Status and Future Developments [Internet] Vienna: Springer Vienna; 2003. [Last accessed on 2013 Jun 20]. pp. 31–5. Available from: http://www.springerlink.com/index/10.1007/978-3-7091-6090-9_6 . [Google Scholar]
- 15.Jack CR, Jr, Thompson RM, Butts RK, Sharbrough FW, Kelly PJ, Hanson DP, et al. Sensory motor cortex: Correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology. 1994;190:85–92. doi: 10.1148/radiology.190.1.8259434. [DOI] [PubMed] [Google Scholar]
- 16.Kim SS, McCutcheon IE, Suki D, Weinberg JS, Sawaya R, Lang FF, et al. Awake craniotomy for brain tumors near eloquent cortex: Correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery. 2009;64:836–45. doi: 10.1227/01.NEU.0000342405.80881.81. [DOI] [PubMed] [Google Scholar]
- 17.Lanier WL. Brain tumor resection in the awake patient. Mayo Clin Proc. 2001;76:670–2. doi: 10.4065/76.7.670. [DOI] [PubMed] [Google Scholar]
- 18.Lehéricy S, Duffau H, Cornu P, Capelle L, Pidoux B, Carpentier A, et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: Comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg. 2000;92:589–98. doi: 10.3171/jns.2000.92.4.0589. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Buckner RL, Talukdar T, Tanaka N, Madsen JR, Stufflebeam SM. Task-free presurgical mapping using functional magnetic resonance imaging intrinsic activity. J Neurosurg. 2009;111:746–54. doi: 10.3171/2008.10.JNS08846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manninen PH, Balki M, Lukitto K, Bernstein M. Patient satisfaction with awake craniotomy for tumor surgery: A comparison of remifentanil and fentanyl in conjunction with propofol. Anesth Analg. 2006;102:237–42. doi: 10.1213/01.ANE.0000181287.86811.5C. [DOI] [PubMed] [Google Scholar]
- 21.Meyer FB, Bates LM, Goerss SJ, Friedman JA, Windschitl WL, Duffy JR, et al. Awake craniotomy for aggressive resection of primary gliomas located in eloquent brain. Mayo Clin Proc. 2001;76:677–87. doi: 10.4065/76.7.677. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell TJ, Hacker CD, Breshears JD, Szrama NP, Sharma M, Bundy DT, et al. A novel data-driven approach to preoperative mapping of functional cortex using resting-state functional magnetic resonance imaging. Neurosurgery. 2013;73:969–82. doi: 10.1227/NEU.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mogilner AY, Rezai AR. Epidural motor cortex stimulation with functional imaging guidance. Neurosurg Focus. 2001;11:E4. doi: 10.3171/foc.2001.11.3.5. [DOI] [PubMed] [Google Scholar]
- 24.Mueller WM, Yetkin FZ, Hammeke TA, Morris GL, 3rd, Swanson SJ, Reichert K, et al. Functional magnetic resonance imaging mapping of the motor cortex in patients with cerebral tumors. Neurosurgery. 1996;39:515–20. doi: 10.1097/00006123-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Murphy K, Bodurka J, Bandettini PA. How long to scan.The relationship between fMRI temporal signal to noise ratio and necessary scan duration? Neuroimage. 2007;34:565–74. doi: 10.1016/j.neuroimage.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'shea JP, Whalen S, Branco DM, Petrovich NM, Knierim KE, Golby AJ. Integrated image- and function-guided surgery in eloquent cortex: A technique report. Int J Med Robot. 2006;2:75–83. doi: 10.1002/rcs.82. [DOI] [PubMed] [Google Scholar]
- 27.Pereira LC, Oliveira KM, L’Abbate GL, Sugai R, Ferreira JA, da Motta LA. Outcome of fully awake craniotomy for lesions near the eloquent cortex: Analysis of a prospective surgical series of 79 supratentorial primary brain tumors with long follow-up. Acta Neurochir (Wien) 2009;151:1215–30. doi: 10.1007/s00701-009-0363-9. [DOI] [PubMed] [Google Scholar]
- 28.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–64. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 29.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas: Clinical article. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 30.Santini B, Talacchi A, Casagrande F, Casartelli M, Savazzi S, Procaccio F, et al. Eligibility criteria and psychological profiles in patient candidates for awake craniotomy: A pilot study. J Neurosurg Anesthesiol. 2012;24:209–16. doi: 10.1097/ANA.0b013e3182464aec. [DOI] [PubMed] [Google Scholar]
- 31.Sarang A, Dinsmore J. Anaesthesia for awake craniotomy – Evolution of a technique that facilitates awake neurological testing. Br J Anaesth. 2003;90:161–5. doi: 10.1093/bja/aeg037. [DOI] [PubMed] [Google Scholar]
- 32.See JJ, Lew TW, Kwek TK, Chin KJ, Wong MF, Liew QY, et al. Anaesthetic management of awake craniotomy for tumour resection. Ann Acad Med Singapore. 2007;36:319–25. [PubMed] [Google Scholar]
- 33.Serletis D, Bernstein M. Prospective study of awake craniotomy used routinely and nonselectively for supratentorial tumors. J Neurosurg. 2007;107:1–6. doi: 10.3171/JNS-07/07/0001. [DOI] [PubMed] [Google Scholar]
- 34.Shimony JS, Zhang D, Johnston JM, Fox MD, Roy A, Leuthardt EC. Resting-state spontaneous fluctuations in brain activity: A new paradigm for presurgical planning using fMRI. Acad Radiol. 2009;16:578–83. doi: 10.1016/j.acra.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinoura N, Yamada R, Kodama T, Suzuki Y, Takahashi M, Yagi K. Preoperative fMRI, tractography and continuous task during awake surgery for maintenance of motor function following surgical resection of metastatic tumor spread to the primary motor area. Minim Invasive Neurosurg. 2005;48:85–90. doi: 10.1055/s-2004-830227. [DOI] [PubMed] [Google Scholar]
- 36.Skucas AP, Artru AA. Anesthetic complications of awake craniotomies for epilepsy surgery. Anesth Analg. 2006;102:882–7. doi: 10.1213/01.ane.0000196721.49780.85. [DOI] [PubMed] [Google Scholar]
- 37.Stapleton SR, Kiriakopoulos E, Mikulis D, Drake JM, Hoffman HJ, Humphreys R, et al. Combined utility of functional MRI, cortical mapping, and frameless stereotaxy in the resection of lesions in eloquent areas of brain in children. Pediatr Neurosurg. 1997;26:68–82. doi: 10.1159/000121167. [DOI] [PubMed] [Google Scholar]
- 38.Szelényi A, Bello L, Duffau H, Fava E, Feigl GC, Galanda M, et al. Intraoperative electrical stimulation in awake craniotomy: Methodological aspects of current practice. Neurosurg Focus. 2010;28:E7. doi: 10.3171/2009.12.FOCUS09237. [DOI] [PubMed] [Google Scholar]
- 39.Taylor MD, Bernstein M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: A prospective trial of 200 cases. J Neurosurg. 1999;90:35–41. doi: 10.3171/jns.1999.90.1.0035. [DOI] [PubMed] [Google Scholar]
- 40.Tieleman A, Deblaere K, Van Roost D, Van Damme O, Achten E. Preoperative fMRI in tumour surgery. Eur Radiol. 2009;19:2523–34. doi: 10.1007/s00330-009-1429-z. [DOI] [PubMed] [Google Scholar]
- 41.Tomczak RJ, Wunderlich AP, Wang Y, Braun V, Antoniadis G, Görich J, et al. fMRI for preoperative neurosurgical mapping of motor cortex and language in a clinical setting. J Comput Assist Tomogr. 2000;24:927–34. doi: 10.1097/00004728-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Ulmer JL, Hacein-Bey L, Mathews VP, Mueller WM, DeYoe EA, Prost RW, et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: Implications for preoperative assessments. Neurosurgery. 2004;55:569–79. doi: 10.1227/01.neu.0000134384.94749.b2. [DOI] [PubMed] [Google Scholar]
- 43.Ulmer JL, Krouwer HG, Mueller WM, Ugurel MS, Kocak M, Mark LP. Pseudo-reorganization of language cortical function at fMR imaging: A consequence of tumor-induced neurovascular uncoupling. AJNR Am J Neuroradiol. 2003;24:213–7. [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang D, Johnston JM, Fox MD, Leuthardt EC, Grubb RL, Chicoine MR, et al. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: Initial experience. Neurosurgery. 2009;65(6 Suppl):226–36. doi: 10.1227/01.NEU.0000350868.95634.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]


