Abstract
Background:
Hyperglycemia is frequently encountered in critically ill patients and has been shown to contribute to both morbidity and mortality. We aimed to study the predictive role of blood glucose level in clinical outcomes of mechanically ventilated patients with traumatic brain injury during intensive care unit (ICU) stay and to explore its relationship with Glasgow coma scale (GCS) and acute physiology and chronic health examination (APACHE) II scores that are used in the evaluation of ICU patients as predictor.
Materials and Methods:
A total of 185 patients with craniocerebral trauma who were hospitalized in the ICU were included in the study. Comparisons of mean glucose values (MGVs) and APACHE II scores between survivors and nonsurvivors were made with Student's t-test and chi-square test. Survival analysis was performed with log rank (Mantel-Cox) test and Cox regression was used for mortality risk factors analysis.
Results:
MGVs at the initial, last, and all measurements were significantly higher for nonsurvivors than for survivors. Hazard rate at any given time point for patients with mean glucose value (MGV) between 150 and 179 was found to be 3.691 times that of patients with MGV values between 110 and 149. The hazard rate at any given time point for patients with MGV values ≥180 was found to be 6.571 times that of patients with MGV values between 110 and 149.
Conclusion:
High glucose level is an independent risk factor for mortality in mechanically ventilated ICU patients with traumatic brain injury.
Keywords: Acute physiology and chronic health examination (APACHE) II, Glasgow coma scale (GCS), glucose, hyperglycemia, intensive care unit (ICU), mortality, traumatic brain injury
INTRODUCTION
In the management of critically ill, intensive care patients with mechanical ventilation, various assessment parameters are being used in order to predict patient outcome and mortality with efficient treatment. Multiple scoring systems including Glasgow coma scale (GCS) and acute physiology and chronic health examination (APACHE) II scores, and several biochemical parameters have been developed for the early prediction of severity of patients with traumatic injury to facilitate early treatment in an intensive care unit (ICU).
Blood glucose level, especially hyperglycemia had been commonly studied to predict the patient outcome in several critical situations such as trauma, traumatic brain injury, and burns in intensive care patients.[1,2,3,4] Hyperglycemia is frequently encountered in critically ill patients even without the presence of diabetes and has been shown to contribute to both morbidity- and mortality-worsening outcomes.[1,5]
The occurrence of hyperglycemia in critically ill patients is multifactorial. In critically ill patients, increased levels of circulating catecholamines and glucocorticoids resulting from sympathetic-adrenomedullary response lead to hyperglycemia. It is also a result of hypermetabolic response to stress. It was also stated that insulin resistance with insulin deficiency, impaired glucose metabolism, and use of medications such as corticosteroids, caloric enteral, and parenteral nutritional supplements are the other factors leading to hyperglycemia.[4,6,7,8,9]
Both GCS and APACHE II scoring systems are widely used in the evaluation of intensive care patients — GCS for getting information about the level of consciousness and APACHE II for systemic evaluation based on physiological variables.
In this study, we delineated the predictive role of blood glucose level in the clinical outcomes of mechanically ventilated patients with traumatic brain injury during ICU stay and to explore its relationship with GCS and APACHE II scores that are used in the evaluation of ICU patients as predictor.
MATERIALS AND METHODS
This study is an analysis of data collected from a prospective observational study. A total of 185 patients who were admitted to the ICU of Haydarpaşa Numune Research and Training Hospital between January 2011 and December 2012 were included in the study. The inclusion criteria were adult patients with craniocerebral trauma, hospitalized in the ICU with mechanical ventilation (Maquet Servo i: Model no: 06449701, Sweden) for at least 72 h, and nondiabetic patients. Data of 112 patients were included in the analysis because 73 patients were excluded due to the following criteria: 38 of them died and 23 were extubated before 72 h, 7 were subsequently found to be diabetic and 5 were transferred to another health center.
The patients’ medical records, fasting plasma glucose values, GCS (at admission and daily) and APACHE II scores within the first 24 h, and their medications were noted throughout their ICU stay. The mortality rate and length of hospital stay in critically ill patients were evaluated by their range of glucose, GCS, and APACHE II scores.
Serum glucose values were recorded daily starting at 7 AM and repeated every 1 h, 2h, or 4 h while the patients had insulin infusion according to algorithm. Daily mean glucose levels as well as the daily high and low values in a 24-h period were also recorded. Insulin infusion (Alaris Plus, UK) was adjusted to maintain blood glucose level between 110 mg/dL and 149 mg/dL. For this study, hyperglycemia was described as serum blood glucose >180 mg/dL and hypoglycemia was defined as serum blood glucose ≤70 mg/dL. The glucose levels were checked hourly to see if the serum levels fell below 70.
All the patients were maintained with hemodynamic parameters with mean arterial pressure of 70 mmHg (by providing adequate intravascular volume replacement with saline or blood if required and using ionotrophic agents if needed) and adequate O2 supply; hyperthermia was avoided by using antipyretic medications and cooling blankets.
Statistical analyses
All analyses were performed using Number Cruncher Statistical System (NCSS) 2007 (Kaysville, UT, USA) and Stata 12 (College Station, TX, USA: StataCorp LP, TX, USA).
Comparison of mean glucose values (MGVs) obtained during ICU stay, at admission, and the last measurements between survivors and nonsurvivors were made with Student's t-test. Survival analysis according to MGVs and GCS were performed with log rank (Mantel-Cox) test. Proportional hazards assumption, the effects of the predictor variables upon survival being constant over time, was tested before Cox regression analyses were applied. The effects of APACHE II, MGV, and GCS upon survival time were performed with Cox regression analysis. Correlation between APACHE II scores and MGVs was determined by Pearson correlation coefficient. P value <0.05 was considered to be statistically significant.
RESULTS
Study population
The majority of the patients were males (female:male = 38:74) and the mean age of the entire population was 52.1 ± 23.5 years. The mean age of the patients who survived was 50.3 ± 23.3 years and mean age of the nonsurvivors was 53.6 ± 23.7 years. There was no difference between the age of the patients in the survivor group and the nonsurvivor group (P = 0.460).
The mean length of ICU stay was 24.4 ± 25.5 days in survivors and 14.8 ± 12.8 days in nonsurvivors. Survivors had a longer length of stay in the ICU than did nonsurvivors (P = 0.009).
Glucose values and hospital mortality
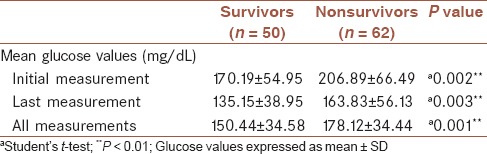
The MGVs obtained during ICU stay for each patient was calculated. Initial (at admission), last (before discharge), and overall glucose values of the survivors and nonsurvivors were compared. MGVs of the initial measurement, last measurement, and all measurements were significantly higher for nonsurvivors than for survivors [Table 1].
Table 1.
Comparison of the initial, last, and all glucose values in survivors and nonsurvivors
Gender, acute physiology and chronic health examination II, and hospital mortality
APACHE II scores were obtained during the first 24 h after admission. Survival analysis for gender (log rank (Mantel-Cox) χ2 =0.06; P = 0.803) and APACHE II score (log rank (Mantel-Cox) χ2 =1.75; P = 0.417) were not statistically significant. There was no difference between female and male survival analysis.
Survival analysis according to mean glucose values
The survival length was significantly higher for patients with MGVs between 110 mg/dL and 149 mg/dL than for MGVs between 150-179 mg/dL and ≥180 mg/dL (log rank (Mantel-Cox) χ2 = 24.66; P = 0.001). The highest survival rate, 69.2%, occurred among patients with MGVs between 110 mg/dL and 149 mg/dL. Patients with MGVs of 150-179 mg/dL had lesser, 30.0% survival rate compared with those with MGVs of 110-149 mg/dL. Survival rate decreased with further increases in MGVs, with the lowest survival rate (26.7%) seen among patients with MGVs exceeding 180 mg/dL [Figure 1].
Figure 1.
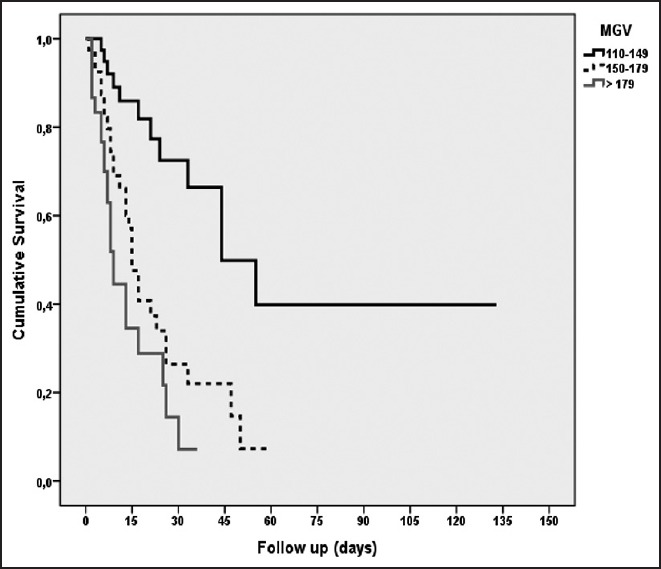
Survival analysis according to mean glucose values (MGVs); [log rank (Mantel-Cox) = 24.66; P = 0.001]
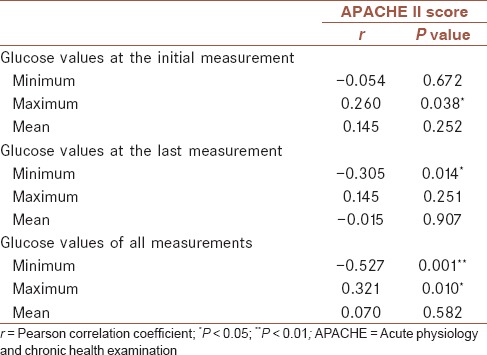
Correlation between glucose values and acute physiology and chronic health examination II scores
Correlation of APACHE II scores with MGVs obtained initially and at the last measurements and all glucose measurements was analyzed with Spearman correlation test [Table 2]. There was a correlation between maximum glucose values obtained at admission and APACHE II scores (r = 0.260; P = 0.038). Minimum glucose value obtained at the last measurement was negatively correlated with APACHE II scores (r = -0.305; P = 0.014). Among all measurements, minimum glucose values were found to be negatively correlated with APACHE II scores (r = -0.527; P = 0.001).
Table 2.
Correlation between glucose values and APACHE II scores
Overall survival analysis
Of the total 112 patients, 44.6% survived while 62 of them did not survive. The median survival duration was 21.00 ± 3.95 days. The last died case was seen on the 55th day; 1 month cumulative survival rate was 17.7% with standard deviation of 6.1%.
Survival analysis according to Glasgow coma and acute physiology and chronic health examination II scores
In the survival analysis according to GCS scores for the entire ICU stay, there was a statistically significant difference between the groups (log rank (Mantel-Cox) χ2 =7.69; P = 0.021). The survival length was significantly higher for patients with a GCS score of 9 and higher than for other groups [Figure 2]. There was no significant difference in survival analysis between groups according to APACHE II scores [Figure 3].
Figure 2.
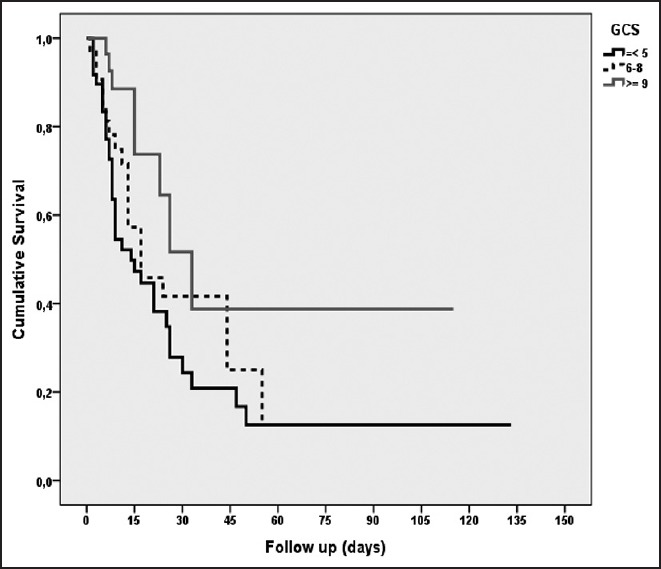
Survival analysis according to GCS; [log rank (Mantel-Cox) = 7.69; P = 0.021]
Figure 3.

Survival analysis according to APACHE II; [log rank (Mantel-Cox) = 1.75; P = 0.417]
Cox regression analysis of factors relating to time to mortality
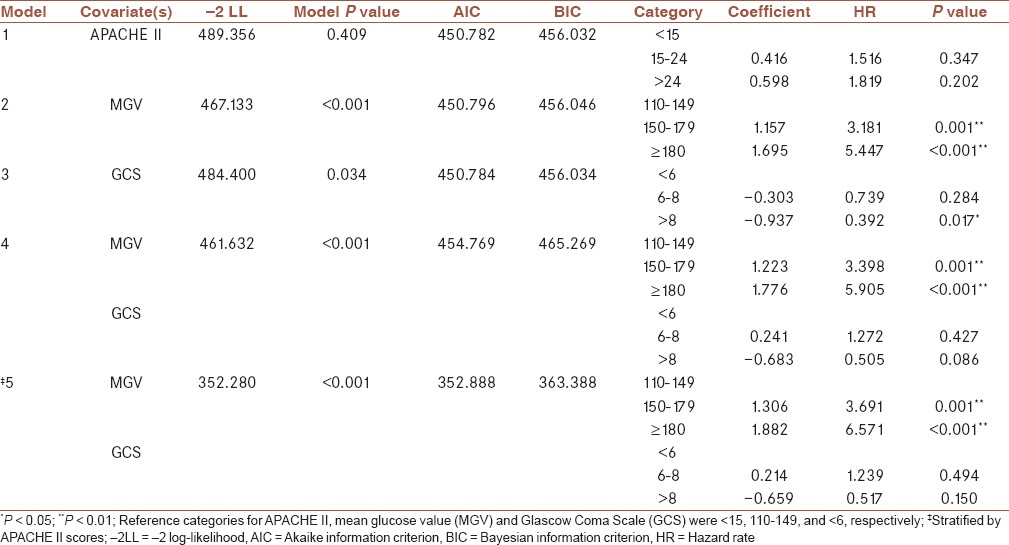
The results of proportional hazards assumption are presented in Table 3. APACHE II score violated the proportional hazards assumption (global P value: 0.033). MGV and GCS were found to be not violating proportional hazards assumption (global P values: 0.734 and 0.559, respectively). The results of Cox regression analyses are presented in Table 4. Five different modelings with different covariates were applied. Model 1 included only APACHE II scores, whereas model 2 included MGV only, model 3 included GCS only, and model 4 included both MGV and GCS as covariates. Since APACHE II violated the proportional hazards assumption, model 5 included MGV and GCS values and was stratified by APACHE II scores. Covariates in the model, -2 log-likelihood (-2LL), model P values, akaike information criterion (AIC) and Bayesian information criterion (BIC) values and coefficients, hazard ratios, and P values of related categories and variables are reported in Table 4. According to -2LL, AIC, and BIC, the most appropriate model seems to be model 5 since it had the lowest values for -2LL, AIC, and BIC. The hazard rate at any given time point for patients with MGV values between 150 and 179 was found to be 3.691 times that of patients with MGV values between 110 and 149. The hazard rate at any given time point for patients with MGV values ≥180 was found to be 6.571 times that of patients with MGV values between 110 and 149. GCS was not an independent risk factor of time to mortality.
Table 3.
Proportional hazards assumption analysis
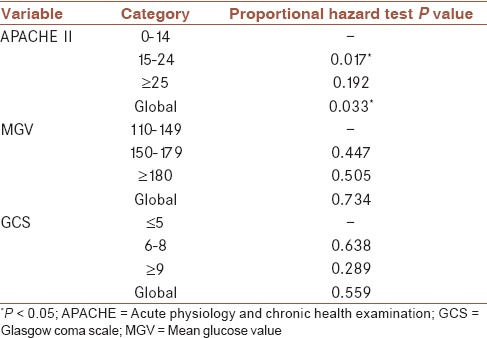
Table 4.
Cox regression analysis of factors affecting mortality
DISCUSSION
In this study, we found that admission glucose values, maximum glucose values, and calculated MGVs were in correlation with the mortality rate in mechanically ventilated intensive care patients with traumatic brain injury. Our study also demonstrated that GCS and APACHE II score as well as high glucose values were associated with poor clinical outcome.
Our results were consistent with previous studies that showed a correlation between high glucose values and increased mortality in patients with traumatic brain injury.[10,11] In a retrospective study, Griesdale et al. demonstrated that any episode of hyperglycemia defined as glucose value higher than 200 mg/dL was associated with 3.6-fold increased risk of mortality in patients with traumatic brain injury.[10] Krinsley et al. studied a consecutive series of 1,826 critically ill patients and reported the association between hyperglycemia and hospital mortality, apart from mean and maximum glucose values being higher for nonsurvivors than for survivors and also for grouping for APACHE II scores.[5]
However, such studies did not show the effects of GCS score and APACHE II score with hyperglycemia on mortality. In contrast, our data showed that APACHE II score was higher in nonsurvivors and there was a correlation between maximum glucose values obtained at admission and APACHE II scores.
We used GCS and APACHE II score in addition to glucose values to predict patient outcome. The neurological condition of the patients was assessed by GCS and acute physiology and chronic diseases of patients were evaluated by using APACHE II.
The survival rate was significantly higher for patients with GCS score of 9 and higher than for other groups. However, APACHE II score and GCS were not independent risk factors for affecting mortality and for the prediction of mortality of traumatized patients in ICU. Zali et al. compared the sensitivity, specificity, and outcome prediction by GCS and APACHE II in neurosurgical ICU patients and they concluded that APACHE II was more valuable for mortality prediction in patients with multitrauma, and they also stated that especially in emergencies GCS score was simple, less-time consuming, and gave effective information on head injuries.[12]
Stress-induced catecholamine release resulting in hyperglycemia can be used to assess the severity of the disease. Thus, the mean, initial, and maximum glucose values during ICU stay of nonsurvivors were higher in our study. The highest survival rate, 69.2%, occurred among patients with MGVs between 110 mg/dL and 149 mg/dL. Among patients with MGVs between 150 mg/dL and 179 mg/dL, the survival rate decreased to 30.0%. Further increases in MGVs had progressive associations with mortality rates.
Also, in the study of James S. Krinsley and Jean-Charles Preiser, targeted blood glucose range between 70 mg/dL and 140 mg/dL >80% was demonstrated to be strongly associated with survival in critically ill patients without diabetes independent of ICU length of stay (LOS) and severity of illness.[13]
Within APACHE II scoring, with groupings of 0-14, 15-24, and 25 and higher, glucose values were higher among nonsurvivors than among survivors. Finally, Cox regression analysis confirmed that increasing MGVs was the only independent predictor of mortality.
There was no significant difference in age between nonsurvivors and survivors. However, previous studies also found that older patients with traumatic brain injury had higher long-term but not short-term mortality rates. The majority of patients in our study population were males and this finding is consistent with the literature survey regarding traumatic brain injury that has a male predominance.[4,10,12]
The initial glucose values in our study were associated with worse survival. Our results were consistent with previous studies that showed a correlation between high glucose values and increased severity and outcomes in patients with traumatic brain injury. In a prospective study conducted by Bian et al., it was demonstrated that hyperglycemia on the day of admission is a predictor for a 1-year mortality in patients with subarachnoid hemorrhage.[14]
The survival length was significantly higher for patients with MGVs between 110-149 mg/dL than for MGVs between 150-179 mg/dL and ≥180 mg/dL. Patients with MGVs between 110 mg/dL and 149 mg/dL had the highest survival rate (69.2%).
Young et al. studied the relationship between hyperglycemia at admission and neurological outcome in 59 patients with traumatic brain injury and they found that patients with admission glucose value greater than 200 mg/dL had worse GCS scores than did patients with glucose values lower than 200 mg/dL.[4]
In another study, Weir et al. studied 645 patients with ischemic stroke and 105 patients with hemorrhagic stroke. They found hyperglycemia in patients with ischemic stroke and hemorrhagic stroke, and predicted increased mortality and morbidity.[15]
Hyperglycemia at the time of admission in patients with chest pain was investigated and it was found to be an independent risk factor for major adverse cardiac event in patients with acute coronary syndrome.[16]
In the study of Liu-DeRyke, glucose level higher than 160 mg/dL within 24 h of admission after traumatic brain injury was demonstrated to be associated with poor outcomes irrespective of injury severity. They reported that day 1 peak glucose was the best predictor of mortality when compared to hospital days 2-5 glucose levels. Thus, the initial glucose levels were also higher in nonsurvivors in our study.[17]
Martin et al. investigated the relationship between blood glucose level at the time of admission and outcomes of patients who were admitted to the emergency department and they found that admission glucose levels were independently associated with increased mortality in patients who had no diabetes. In patients with type 2 diabetes, glycemic variability was found to be associated with increased length of hospital stay.[18]
However, such studies did not show the predictive roles of APACHE II score and GCS score assessed together with hyperglycemia in the survival rate.
CONCLUSION
In conclusion, this study suggested that high glucose value (≥180 mg/dL) is found to be an independent risk factor for mortality in mechanically ventilated intensive care patients with traumatic brain injury.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
BT contributed in the conception and design of the work, conducting the study, acquisition and interpretation of data, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. OE contributed in the conception and design of the work, conducting the study, acquisition and interpretation of data, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. ZB contributed in the conception and design of the work, conducting the study, acquisition and interpretation of data, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
REFERENCES
- 1.Bochicchio GV, Joshi M, Bochicchio KM, Pyle A, Johnson SB, Meyer W, et al. Early hyperglycemic control is important in critically injured trauma patients. J Trauma. 2007;63:1353–9. doi: 10.1097/TA.0b013e31815b83c4. [DOI] [PubMed] [Google Scholar]
- 2.Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–4. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Sung J, Bochicchio GV, Joshi M, Bochicchio K, Tracy K, Scalea TM. Admission hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;59:80–3. doi: 10.1097/01.ta.0000171452.96585.84. [DOI] [PubMed] [Google Scholar]
- 4.Young B, Ott L, Dempsey R, Haack D, Tibbs P. Relationship between admission hyperglycemia and neurologic outcome of severely brain-injured patients. Ann Surg. 1989;210:466–73. doi: 10.1097/00000658-198910000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–8. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 6.Bochicchio GV, Scalea TM. Glycemic control in the ICU. Adv Surg. 2008;42:261–75. doi: 10.1016/j.yasu.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Laird AM, Miller PR, Kilgo PD, Meredith JW, Chang MC. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma. 2004;56:1058–62. doi: 10.1097/01.ta.0000123267.39011.9f. [DOI] [PubMed] [Google Scholar]
- 8.Oddo M, Schmidt JM, Mayer SA, Chioléro RL. Glucose control after severe brain injury. Curr Opin Clin Nutr Metab Care. 2008;11:134–9. doi: 10.1097/MCO.0b013e3282f37b43. [DOI] [PubMed] [Google Scholar]
- 9.Rosner MJ, Newsome HH, Becker DP. Mechanical brain injury: The sympathoadrenal response. J Neurosurg. 1984;61:76–86. doi: 10.3171/jns.1984.61.1.0076. [DOI] [PubMed] [Google Scholar]
- 10.Griesdale DE, Tremblay MH, McEwen J, Chittock DR. Glucose Control and mortality in patients with severe traumatic brain injury. Neurocrit Care. 2009;11:311–6. doi: 10.1007/s12028-009-9249-1. [DOI] [PubMed] [Google Scholar]
- 11.Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma. 2005;58:47–50. doi: 10.1097/01.ta.0000135158.42242.b1. [DOI] [PubMed] [Google Scholar]
- 12.Zali AR, Seddighi AS, Seddighi A, Ashrafi F. Comparison of the acute physiology and chronic health evaluation score (APACHE) II with GCS in predicting hospital mortality of neurosurgical intensive care unit patients. Glob J Health Sci. 2012;4:179–84. doi: 10.5539/gjhs.v4n3p179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krinsley JS, Preiser JC. Time in blood glucose range 70 to 140 mg/dl >80% is strongly associated with increased survival in non-diabetic critically ill adults. Crit Care. 2015;19:179. doi: 10.1186/s13054-015-0908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian L, Liu L, Wang C, Hussain M, Yuan Y, Liu G, et al. Hyperglycemia within day 14 of aneurysmal subarachnoid hemorrhage predicts 1-year mortality. Clin Neurol Neurosurg. 2013;115:959–64. doi: 10.1016/j.clineuro.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ. 1997;314:1303–6. doi: 10.1136/bmj.314.7090.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner LS, Nguyen-Pham S, Greenslade JH, Parsonage W, D’Emden M, Than M, et al. Admission glycaemia and its association with acute coronary syndrome in Emergency Department patients with chest pain. Emerg Med J. 2015;32:608–12. doi: 10.1136/emermed-2014-204046. [DOI] [PubMed] [Google Scholar]
- 17.Liu-DeRyke X, Collingridge DS, Orme J, Roller D, Zurasky J, Rhoney DH. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit Care. 2009;11:151–7. doi: 10.1007/s12028-009-9228-6. [DOI] [PubMed] [Google Scholar]
- 18.Martin WG, Galligan J, Simpson S, Jr, Greenaway T, Burgess J. Admission blood glucose predicts mortality and length of stay in patients admitted through the emergency department. Intern Med J. 2015;45:916–24. doi: 10.1111/imj.12841. [DOI] [PubMed] [Google Scholar]


