Abstract
Gastric varices (GV) are responsible for 10-30% of all variceal hemorrhage. However, they tend to bleed more severely with higher mortality. Around 35-90% rebleed after spontaneous hemostasis. Approximately 50% of patients with cirrhosis of liver harbor gastroesophageal varices. In this review, new treatment modalities in the form of endoscopic treatment options and interventional radiological procedures have been discussed besides discussion on classification and pathophysiology of GV.
Keywords: Endoscopic treatment, gastroesophageal varices, sclerotherapy
INTRODUCTION
Gastroesophageal varices have been seen in approximately 50% of patients with cirrhosis of the liver. Their presence correlates with the severity of liver disease. While only 40% of Child A patients has varices, they are present in 85% of Child C patients.[1,2] Variceal hemorrhage occurs at a yearly rate of 5-15%, and 6-week mortality after variceal hemorrhage is about 20%.[3,4] In general, variceal bleeding ceases spontaneously in 40-50% of patients, but incidence of early rebleeding ranges between 30% and 40% within first 6 weeks, and about 40% of all rebleeding episodes occur within the first 5 days.[5,6]
Gastric varices (GV) bleed less frequently than esophageal varices and are responsible for 10-30% of all variceal hemorrhages.[7] However, gastric variceal bleeding tends to be more severe with higher mortality. In addition, a high proportion of patients, around 35-90%, rebleed after spontaneous hemostasis.
New endoscopic treatment options and interventional radiological procedures have broadened the therapeutic armamentarium for GV. This review provides an overview of the classification and pathophysiology of GV, which have direct consequences for management; an introduction to current endoscopic and interventional radiological management options for GV.
CLASSIFICATION OF GASTRIC VARICES
There are three types of classification commonly used for GV.
Sarin's classification
Hashizome classification
Arakawa's classification.
Most commonly used classification is Sarin's classification of GV.
SARIN'S CLASSIFICATION
Gastric varices are categorized into four types based on the relationship with esophageal varices, as well as by their location in the stomach [Figure 1].[7]
Figure 1.
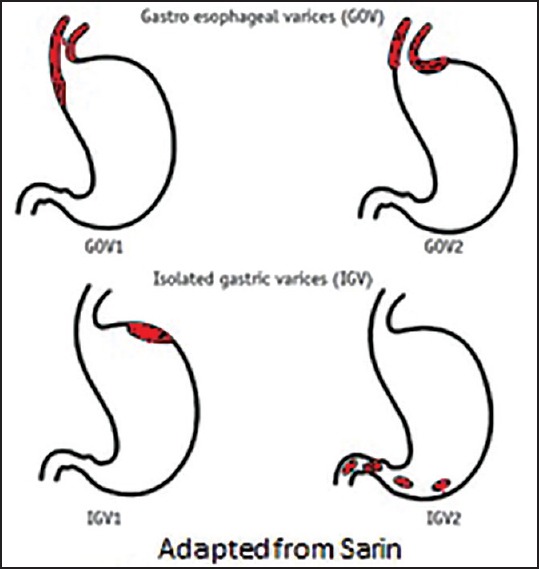
Sarin's classification for gastric varices
Gastroesophageal varix (GOV) type 1: Extension of esophageal varices along lesser
Gastroesophageal varix type 2: Extension of esophageal varices along great curve
Isolated gastric varix (IGV) type 1 and
Isolated gastric varix type 2: Varices in stomach or duodenum as shown in figure.
Gastroesophageal varix type 1 is the most common type, accounting for 74% of all GV. However, the incidence of bleeding is highest with IGV type 1, followed by GOV type 2. Overall, the most important predictor of hemorrhage is the size of varices, with the highest risk of first hemorrhage (15%/year) occurring in patients with large varices.[8] Other predictors of hemorrhage are decompensated cirrhosis (Child B or C) and the endoscopic presence of red wale marks.[8]
HASHIZOME CLASSIFICATION
It is based on clinically significant endoscopic findings, and particularly from the viewpoint of findings associated with the lightly risk of rupture, as in the classification of esophageal varices [Figure 2]. Thus, endoscopic findings of GV were classified according to their form, location, and color.[9] The form was classified into three types:
Figure 2.
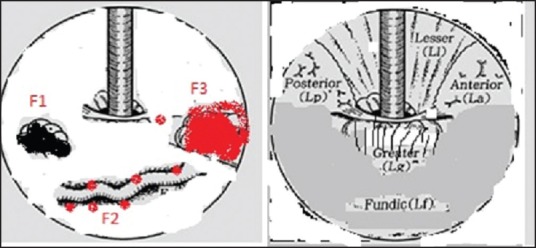
Hashizome classification of gastric varices
Tortuous (F1).
Nodular (F2).
Tumorous (F3).
The location was classified into five types and depends on hemodynamic factors;
Anterior (La).
Posterior (Lp).
Lesser curvature (Ll).
Greater curvature (Lg) of the cardia and.
Fundic area (Lf).
The colors can be classified in (a) white (Cw) or (b) red (Cr). The glossy, thin-walled focal redness on the varix was defined as red color spot (RC spot). The Hashizume group reported that the RC spot and larger forms were related to a significantly higher risk of gastric variceal bleeding. The form and location of Hashizome classification are shown in Figure 2.
Arakawa's classification[10] type I: Branches within the stomach wall are very few, and the supplying vessel, varix and draining vessel form a single continuous vein of a nearly unchanged caliber.
Ia: A single supplying vessel forms a fundic varix.
Ib: Plural supplying vessels join and form a varix that drains into a single draining vessel.
Type II: Beside the main supplying and the draining vessels, there are many branching vessels that exist within the stomach wall, namely varix has communications with vessels within the stomach wall.
Vascular anatomy
To achieve best results of treatment and at the same time minimizing the complications it is very important to understand relevant vascular anatomy. This holds true for all interventional modalities of treatment likely endoscopic, endoscopic ultrasound (EUS) and radiological management of GV. GV drain into the systemic vein via the esophageal-paraesophageal varices (gastroesophageal venous system), the inferior phrenic vein (IPV) (gastrophrenic venous system), or both.[11] These drainage types generally correspond to the classification system of Sarin et al.[7] GOV1 drains via esophageal and paraesophageal varices, IGV1 drains via the left IPV, and GOV2 drains via both esophageal varices and the IPV. GV form at the hepatopetal collateral pathway that develops secondary to localized portal hypertension and drain via the gastric veins, thereby corresponding with IGV2 [Figure 3].
Figure 3.
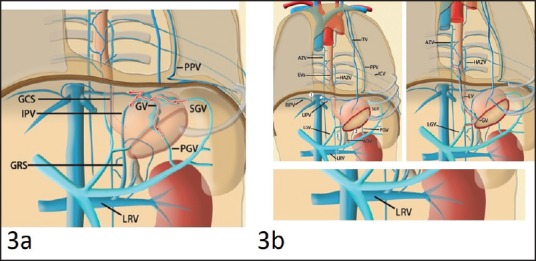
Drawings illustrate Isolated gastric varixs draining via the gastrophrenic venous system and also portal and systemic venous pathways that are potentially involved in gastric varices
Gastroesophageal venous system
Esophageal venous plexus which normally lies at the lower end of esophagus anastomoses with tributaries of the left gastric vein within and around the gastric wall. The esophageal vein drains via the gastroesophageal venous system into the superior vena cava (SVC). GOVs develop at this anastomosis between the left gastric vein and the azygos vein due to portal hypertension.
Gastrophrenic venous system
The gastric veins lie in the posterosuperior part of the gastric wall which have the potential to anastomose with the IPV at the bare area of the stomach (gastrophrenic ligament). The majority of IGVs form in a large portosystemic venous shunt that develops based on this potential anastomosis between the gastric vein and the left IPV due to portal hypertension.[12] The left IPV terminates either (a) inferiorly into the left renal vein (forming a gastrorenal shunt), often together with the left adrenal vein, or (b) transversely into the inferior vena cava (IVC) (forming a gastrocaval shunt. The proximal portion of the left IPV runs inferiorly to the diaphragm. Some of the peripheral branches run superiorly to the diaphragm and supply the superior surface of the muscular diaphragm; one of these peripheral branches anastomoses with the left pericardio phrenic vein The left IPV can also communicate with other peridiophragmatic and retroperitoneal veins, including the subcostal and intercostal veins, small anastomotic veins to the right IPV, and adrenal vein or with the azygos venous system.[13] Therefore, GV can also drain through these communications. Potential communication between these venous systems and the pulmonary vein has been demonstrated in anatomic studies and a small number of cases as one of the collateral pathways that develops as a result of obstruction of the SVC and portal hypertension.[14,15]
Modern imaging by computed tomography scan
The following venous systems are important to understand the venous anatomy of GV on multi-detector computed tomography (CT) scan [Figure 4].
Figure 4.
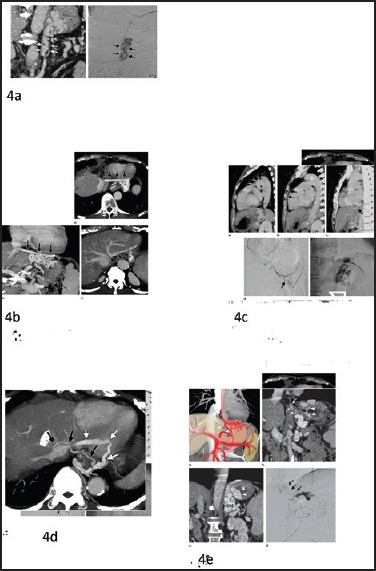
Multidetector compute pictures depicting venous anatomy of gastric varices
Gastroesophageal venous system
On oblique coronal reformatted multidetector CT images one can see that GOVs usually receive blood from the left gastric vein, which runs in the submucosal layer of the stomach along the lesser curvature and continues directly to esophageal varices beyond the esophagocardiac junction, finally joining the azygos and hemiazygos veins.
Gastrophrenic venous system
Gastrorenal shunt
A gastrorenal shunt is the most common drainage route of IGVs (80-85% of cases), running inferiorly and terminating in the left renal vein, often together with the left adrenal vein. The shunt frequently communicates with other phrenic or retroperitoneal drainage veins. Duplication or fenestration of gastrorenal shunts is rare.
Gastrocaval shunt
In 10-15% of cases gastro caval shunt forms main drainage route for IGVS. It consists of the left IPV passing transversely in front of the esophageal hiatus below the diaphragm and terminating in the IVC (60% of cases) or the left hepatic vein (40%). The proximal portion of the shunt runs inferiorly to the diaphragm, whereas the peripheral branches run superiorly to the diaphragm. The peripheral branch of the left IPV communicates with other peridiaphragmatic veins; thus, gastrocaval shunts are always associated with multiple accessory drainage veins around the diaphragm.
Pericardiophrenic vein
The left pericardiophrenic vein is often seen with a gastrorenal or gastrocaval shunt at multidetector CT as an accessory drainage vein from GV. In a few cases (5%), it can serve as a main drainage route of GV.[11]
The left pericardiophrenic vein anastomoses with the left IPV at the cardiac apex. It runs superiorly along the pericardium and in the left superior mediastinum then terminates into the left brachiocephalic vein.[16] It may join the left ITV or left superior costal vein.
Other drainage routes
These small veins are often difficult to identify at multidetector CT due to their small Caliber. These veins are as follows;
The veins running in the thoracic wall, diaphragm, and retroperitoneum are often observed as accessory drainage routes of GV. The subcostal-intercostal veins communicate anteriorly with the ITV and posteriorly with the azygos vein.[17]
There are small retroperitoneal and peridiaphragmatic veins, including an anastomotic vein between the bilateral IPVs, renal capsular vein, and unnamed retroperitoneal veins, that communicate with the gastrorenal shunt and the hemiazygos vein.
Combined drainage via gastroesophageal and gastrophrenic venous systems
In Sarin's classification of GV, GOV 2 drains via both gastroesophageal and gastrophrenic venous systems and account for 15% of GV[7] in this type of gastric varix, the anteromedial (cardiac) part of the varix is supplied by the left gastric vein and drains via the esophageal varices into the gastroesophageal venous system. The posterior (fundal) part of the varix is often supplied by the posterior gastric vein or short gastric vein and drains via the gastrophrenic venous system. In the balloon-occluded retrograde transvenous obliteration (BRTO) technique, sclerosant is injected via the gastrophrenic venous drainage system into the GV. Therefore, small parts of GV draining via the esophageal varices may remain after BRTO when the sclerosant does not fill or stagnate in them. This is called incomplete eradication and in such a scenario the high-pressure anteriomedial part needs transhepatic embolization approach or endoscopic band ligation.
Management
-
Endoscopic treatment modalities for gastric variceal bleeding.
- Gastric variceal sclerotherapy (GVS).
- Gastric variceal obturation (GVO) with glue.
- Gastric variceal band ligation (GVL) with or without detachable snares.
- Thrombin injection (bovine or human).
- Combined endoscopic therapy.
Endoscopic ultrasound-guided therapy.
Radiologic intervention - transjugular intrahepatic portosystemic shunt (TIPS) and BRTO.
Gastric variceal sclerotherapy
The endoscopic sclerotherapy has been less effective in the treatment of gastric variceal bleeding and eradication of GV as against esophageal varices where endoscopic sclerotherapy is one of the effective modes of treatment.[12,18] Because of the high volume of blood flow through GV compared with EV, resulting in rapid flushing away of the sclerosant in the bloodstream. GVS typically requires larger volumes of sclerosant than for EV[7,19] and fundal varices (GOV2 and IGV1) require significantly more sclerosant than GOV1.[20] This results in more side effects after GVS, such as fever, retrosternal and abdominal pain, and large ulcerations.[20] Perforations and mediastinitis are complications that are more serious, and the latter results in mortality in excess of 50%.
In acute GV bleeding, GVS has been reported to control bleeding in 60-100% of cases[20,21,22] but with unacceptably high rebleeding rates of up to 90%. Mucosal ulcers are also commonly seen, and cause rebleeding. Approximately, 50% of rebleeding is caused by sclerotherapy induced ulcers and is difficult to control, with a success rate between 9% and 44%.GVS appears to be least successful in controlling acute fundal variceal bleeding.[23,24]
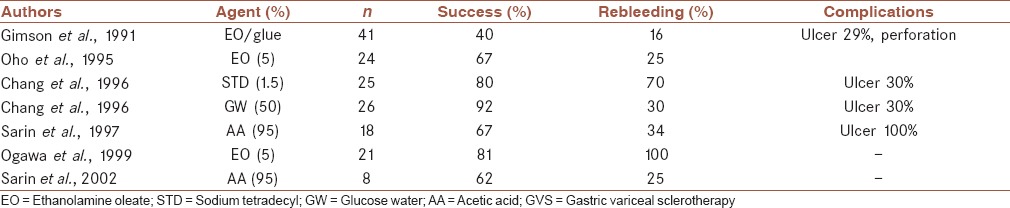
Gastric variceal sclerotherapy is an effective and appropriate treatment for treatment of acute GOV1 hemorrhage and for attempting secondary prophylactic GOV1 obliteration. It is not appropriate for patients with fundal varices (GOV2 or IGV1) because of the low rate of primary hemostasis, the low success rate for secondary variceal eradication, and the high rate of rebleeding and complications. Results of certain important trials on GVS are shown in Table 1.
Table 1.
GVS in the management of gastric variceal bleeding: Comparison from different studies
Gastric variceal obturation
Tissue adhesive such as N-butyl-2-cyanoacrylate, which is a monomer that rapidly undergoes exothermic polymerization on contact with the hydroxyl ions present in water, has been used for Gastric variceal obturation.
METHODS
After puncturing the varix lumen with the needle, cyanoacrylate is injected in 1-1.5 mL aliquots by using normal saline or sterile water (about 0.8-1.0 mL, equal to the dead space) to flush the glue into the varix. As the needle is withdrawn from varix, a steady stream of the flush solution is aimed at the puncture site. Additional glue is injected until the varix is ‘’hard” to palpation.[25]
Weeks to months after the injection, the mucosa overlying the glue cast sloughs off and the plug is extruded into the stomach. Initial hemostasis rates of over 90% can be achieved.
Complications
Complications are mainly thrombotic in nature and include cerebral embolization and stroke, portal vein embolization, splenic infarction, coronary emboli, with a series demonstrating fatal and nonfatal pulmonary emboli in up to 5% of cases. Use of undiluted cyanoacrylate was shown to be safe and effective and also to be associated with fewer complications, in contrast to the diluted form.[26,27]
Several recent long-term studies also reported hemostasis rates of 90% and low rebleeding rates of 15% to 30% with cyanoacrylate injection, with 1-3 injections needed to achieve obliteration, with higher eradication rates for GOV1 and GOV 2 than IGV1.[28,29] Even in young infants, the use of cyanoacrylate glue is safe and effective for the treatment of gastric variceal bleed.[30] At present, it is clear that GVO using tissue adhesives has high efficacy and safety for the control of acute GV bleed and for the prevention of GV rebleeding and is the treatment of choice.[31,32]
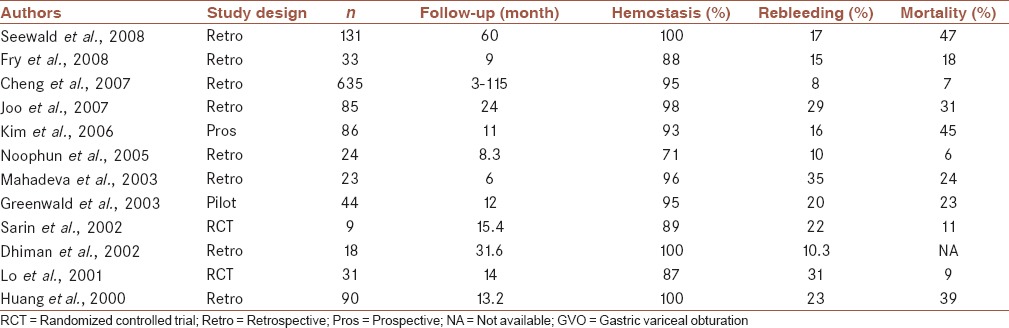
Results of certain trials on GOV are shown in Table 2.
Table 2.
GVO in the management of gastric variceal bleeding: Comparison from different studies
Gastric variceal band ligation
Variceal band ligation (VBL) is an established treatment modality for the prevention of esophageal variceal bleeding control of active bleeding and rebleeding. However for GV GVL is not the 1st choice of treatment and evidence for the use of GVL for acute gastric variceal bleeding is mixed, and at best GVL is a second alternative therapy to tissue adhesives. Although initial hemostasis may be achieved with GVL, the main disadvantage has been a high rate of rebleeding, probably from feeding vessels. Repeat endoscopy and VBL is thus necessary at 1- to 2-weekly intervals until eradication of varices or until only small residual varices remain.
In five uncontrolled studies[33,34,35] active GV bleed was controlled with 100% success with a very low rebleeding rate of 10-20%, and also the GV was obliterated in all cases.
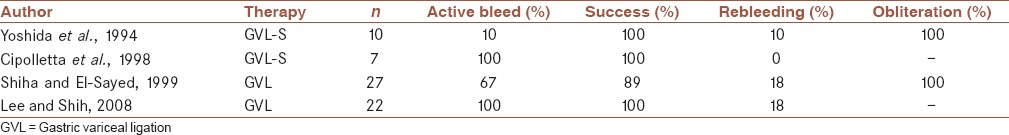
Results of some trials of GVL are shown in Table 3.
Table 3.
GVL in the management of gastric variceal bleeding: comparison from different studies
Thrombin
Thrombin which is commercially available as a sterile lypophilized powder principally affects hemostasis by converting fibrinogen to a fibrin clot. A 5-mL solution of thrombin containing 1000 units/mL of thrombin will clot a liter of blood in <60 s. In a patient with bleeding GV, thrombin is reconstituted, and 1 mL aliquot is injected into the bleeding varix.[36,37]
Yang et al.[38] retrospectively evaluated the use of human thrombin in 12 patients with GV bleeding. Immediate hemostasis was achieved in all patients in whom there was active bleeding from GVs at the time of endoscopy (n = 6). There were no immediate allergic reactions, thromboembolic complications or rebleeding. The results appeared promising, with immediate hemostasis achieved in 70% patients and with no patient having rebleed from GV over a follow-up period of 8 months.
Ramesh et al.[39] showed that endoscopic treatment of bovine thrombin is effective in 92% of patients with bleeding GVs, without any rebleeding in a follow-up period of almost 2 years.
Thrombin seems to be a promising therapy, and has the benefits of achieving excellent initial hemostasis and being easy to use with a good safety profile. Thrombin appears to be successful even in patients with GOV2, and may have a role in this difficult group.
Combined endoscopic therapy (endoscopic variceal ligation-injection sclerotherapy)
Endoscopic variceal ligation-injection sclerotherapy (EVLIS) is safe and effective in achieving hemostasis and obliteration in all patients as shown by Chun and Hyun.[40] The combination of variceal ligation and cyanoacrylate injection in GV effectively controlled acute bleed in 89% of patients; however 33% rebleed on follow-up.[41] Combination with sclerotherapy is unlikely to be accepted for the management of acute bleeding in view of the increased risk of iatrogenic complications, and the need for greater technical skill and procedure time. For secondary prophylaxis, combined therapy should be compared with standard established treatments.
Endoscopic ultrasound guided treatment
This is a new modality for treatment of GV and this has emerged as a valuable tool for diagnosis, treatment planning, evaluation of treatment efficacy, estimation of recurrent bleeding potential and also helps visualize varices, perforating veins, collateral veins and allows predict varices at high risk.
Romero-Castro et al. in their small case series injected cyanoacrylate-lipiodol into GV at the level of perforating veins, under EUS guidance.[29,42] All the procedures were successful, without recurrent bleeding or other complications during followup. They postulated that targeting perforating veins would produce the maximal blood-flow blockage, with the lower amounts of cyanoacrylate needed, therefore reducing the rate of potential local and systemic complications.
Romero-Castro et al. reported in other small case series of patients with GV treated with EUS guided coil embolization. They inserted coils into the perforating veins in order to block the blood flow. The varices were eradicated in three out of four patients, and no complications occurred in the successfully treated patients during five months of follow-up.[34,43]
Deployment of coil (ECA) as shown in Romero et al., use of 19 gauge needle and standard steel synthetic fibered 0.035 inches in diameter with straight length of 50-150 mm, coiled diameter of 8-20 mm, 3.2-5.6 configurator loops. The size of the coil was calculated according to the diameter of the vessel measured on EUS and was chosen to be approximately 20% larger, the needle was forwarded into target vessel under EUS guidance and the stylet withdrawn. The larger coil (20 mm) where deployed by using, the stiffer part of a 0.035-inch guide wire and a pusher. Smaller coils <15 mm were deployed by using floppy part of respective guide wire. Placing the needle tip opposite wall of vessel was avoided to allow enough space for coil to curl. If EUS vision becomes blurred contrast injection under fluoroscopy was used. Thrombosis of vessel was confirmed by injection of contrast.
Binmoeller et al. in their study on thirty patients combined EUS-guided coiling and cyanoacrylate glue injection by transesophageal approach to the gastric fundal varices. They hypothesized that coils with attached synthetic fibers (“wool coils”) inserted in to the varices prior to cyanoacrylate injection would function as a “barrier” for cyanoacrylate to outflow into the larger veins causing embolic events. In their procedure, after positioning and endosonographic visualization of GV from the esophagus (through the diaphragmatic crus muscle), a 19-gauge fine needle aspiration (FNA) needle was inserted through the esophageal wall and diaphragm muscle directly to the gastric varix, following coil delivery and 1 mL of cyanoacrylate with immediate repetition of the procedure as needed until varix obliteration.[44]
Steps of endoscopic ultrasound guided treatment
Active flow within gastroesophageal varices was confirmed by color Doppler before therapy.
Intraluminal water filling of the gastric fundus to improve acoustic coupling and visualization of GFV.
Echoendoscope positioned in the distal esophagus to sonographically visualize the gastric fundus in an anterograde fashion (the diaphragmatic crus muscle was identified between the esophageal wall and the GFV).
Endoscopic ultrasound-FNA needle (19 g) puncture into the GFV by using a transesophageal-transcrural approach.
Embolization coil (12-20-mm diameter) delivered into varix through the FNA needle by using the stylet as a pusher.
Immediate injection of 1 mL of 2-octyl-CYA after coil deployment through the same needle over 30 s by using normal saline solution to flush the glue through the catheter.
Color Doppler confirmed the absence of flow in varix after treatment. If the persistent flow was identified, an additional 1.0 mL of CYA glue was delivered. If varix had persistent flow and appeared large enough to accommodate another coil, the varix was repunctured with a new FNA needle, and the technique described was repeated.[44]
Other methods used for gastric varices treatment
Radiologic intervention
Transjugular intrahepatic portosystemic shunt
When patients with GV bleeding are unresponsive to initial endoscopic treatment, a second endoscopic therapy should be attempted if possible. If a second attempt fails or the severity of bleeding precludes further endoscopic therapy, salvage therapy using surgical shunts or TIPSs should be considered for refractory GV bleeding. Most current studies of TIPS focus on treatment for refractory GV bleeding and prevention of GV rebleeding. A recent study showed that the primary hemostasis rate of TIPS for acute GV bleeding is 92.3%. Other studies showed initial hemostasis of TIPS for acute refractory GV bleeding is between 87% and 100%, 58-62 with an approximate rebleeding rate of 10-30%.
Frequent complications of TIPS are encephalopathy and shunt stenosis/occlusion, with post-TIPS encephalopathy occurring in 4-16% of patients. The shunt dysfunction could be reduced by using polytetrafluoroethylene (PTFE)-covered stent. Considering the currently available evidence, TIPS with PTFE-covered stent is the treatment of choice for patients who failed first-line medical and endoscopic therapy.
Balloon-occluded retrograde transvenous obliteration
Kanagawa et al. first introduced the BRTO procedure in 1996. GVs are associated with a gastro-renal shunt (GRS) in 60-85%. The GRS drains blood flow into systemic circulation and provides a pathway for radiologists to treat GV. The outflow of GRS was blocked by inflating the balloon, and 5% ethanolamine oleateiopamidol was injected in a retrograde manner [Figure 5].
Figure 5.
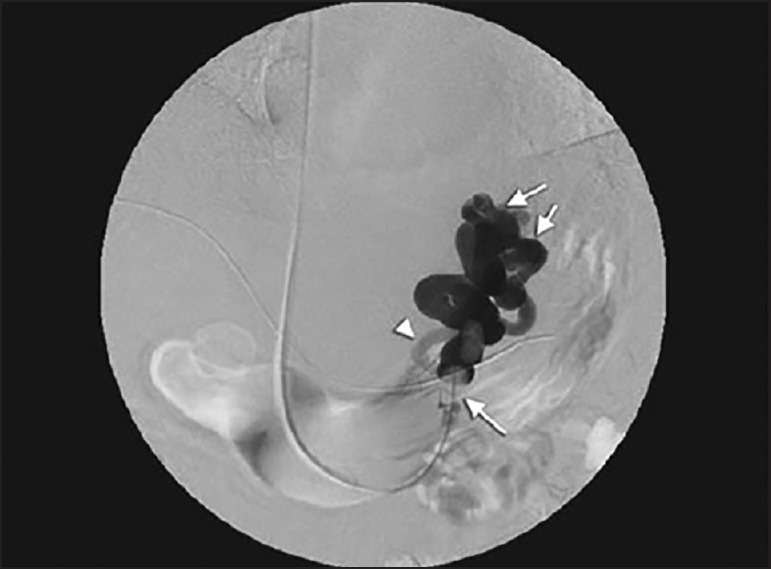
Ballon occluded retrograde venogram (thick arrow - Gastric varices; thin arrow - balloon catheter; arrow heads- inflow route)
The BRTO was used as either primary or secondary prophylaxis of GV in most series. Initial hemostasis of BRTO for acute GV bleeding ranges between 76.9% and 100%. It was noted that the re-bleeding rate was from 0% to 15.4%.BRTO had a similar initial hemostasis and lower rebleeding rate, compared with GVO or band ligation for acute GV bleeding. The BRTO had been shown to be as effective as TIPS for acute GV bleeding, without increasing hepatic encephalopathy. Portal pressure also increased significantly after BRTO, which caused worsening of esophageal variceal pressure. Other common complications of BRTO are hemoglobinuria, abdominal pain, pyrexia, and pleural effusion. Major complications are shock and atrial fibrillation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
This work was carried out in collaboration between all authors. Author ZAW designed the study, wrote the protocol, and wrote the first draft of the manuscript. Author RAB managed the literature search, edited the draft and analyzed accuracy. Authors ASB and RM performed the statistical and spectroscopy analysis. Author AC managed the experimental and review process. All authors read and approved the final manuscript.
Acknowledgments
Thanks to the research department of ILBS for support. There is no grant for this work.
REFERENCES
- 1.Navasa M, Parés A, Bruguera M, Caballería J, Bosch J, Rodés J. Portal hypertension in primary biliary cirrhosis. Relationship with histological features. J Hepatol. 1987;5:292–8. doi: 10.1016/s0168-8278(87)80035-1. [DOI] [PubMed] [Google Scholar]
- 2.Sanyal AJ, Fontana RJ, Di Bisceglie AM, Everhart JE, Doherty MC, Everson GT, et al. The prevalence and risk factors associated with esophageal varices in subjects with hepatitis C and advanced fibrosis. Gastrointest Endosc. 2006;64:855–64. doi: 10.1016/j.gie.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Carbonell N, Pauwels A, Serfaty L, Fourdan O, Leby VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652–9. doi: 10.1002/hep.20339. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Everhart JE. Improved survival after variceal hemorrhage over an 11-year period in the Department of Veterans Affairs. Am J Gastroenterol. 2000;95:3566–73. doi: 10.1111/j.1572-0241.2000.03376.x. [DOI] [PubMed] [Google Scholar]
- 5.D’Amico G, De Franchis R Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 6.Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80:800–9. [PubMed] [Google Scholar]
- 7.Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343–9. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- 8.North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983–9. doi: 10.1056/NEJM198810133191505. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume M, Kitano S, Yamaga H, Koyanagi N, Sugimachi K. Endoscopic classification of gastric varices. Gastrointest Endosc. 1990;36:276–80. doi: 10.1016/s0016-5107(90)71023-1. [DOI] [PubMed] [Google Scholar]
- 10.Arakawa M, Masuzaki T, Okuda K. Pathomorphology of esophageal and gastric varices. Semin Liver Dis. 2002;22:73–82. doi: 10.1055/s-2002-23208. [DOI] [PubMed] [Google Scholar]
- 11.Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Correlation between endoscopic and angiographic findings in patients with esophageal and isolated gastric varices. Dig Surg. 2001;18:176–81. doi: 10.1159/000050126. [DOI] [PubMed] [Google Scholar]
- 12.Johns TN, Evans BB. Collateral pathways in portal hypertension. Ann Surg. 1962;155:838–45. doi: 10.1097/00000658-196206000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibukuro K, Tsukiyama T, Mori K, Inoue Y. Precaval draining vein from paraesophageal varices: Radiologic-anatomic correlation. AJR Am J Roentgenol. 1999;172:651–4. doi: 10.2214/ajr.172.3.10063853. [DOI] [PubMed] [Google Scholar]
- 14.Kapur S, Paik E, Rezaei A, Vu DN. Where there is blood, there is a way: Unusual collateral vessels in superior and inferior vena cava obstruction. Radiographics. 2010;30:67–78. doi: 10.1148/rg.301095724. [DOI] [PubMed] [Google Scholar]
- 15.Widrich WC, Srinivasan M, Semine MC, Robbins AH. Collateral pathways of the left gastric vein in portal hypertension. AJR Am J Roentgenol. 1984;142:375–82. doi: 10.2214/ajr.142.2.375. [DOI] [PubMed] [Google Scholar]
- 16.Lawler LP, Corl FM, Fishman EK. Multi-detector row and volume-rendered CT of the normal and accessory flow pathways of the thoracic systemic and pulmonary veins. Radiographics. 2002;22:S45–60. doi: 10.1148/radiographics.22.suppl_1.g02oc05s45. [DOI] [PubMed] [Google Scholar]
- 17.Paquet KJ, Feussner H. Endoscopic sclerosis and esophageal balloon tamponade in acute hemorrhage from esophagogastric varices: A prospective controlled randomized trial. Hepatology. 1985;5:580–3. doi: 10.1002/hep.1840050409. [DOI] [PubMed] [Google Scholar]
- 18.Jalan R, Hayes PC. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut. 2000;46(Suppl 3-4):III1–III15. doi: 10.1136/gut.46.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarin SK, Lahoti D. Management of gastric varices. Baillieres Clin Gastroenterol. 1992;6:527–48. doi: 10.1016/0950-3528(92)90037-f. [DOI] [PubMed] [Google Scholar]
- 20.Sarin SK. Long-term follow-up of gastric variceal sclerotherapy: An eleven-year experience. Gastrointest Endosc. 1997;46:8–14. doi: 10.1016/s0016-5107(97)70202-5. [DOI] [PubMed] [Google Scholar]
- 21.Stray N, Jacobsen CD, Rosseland A. Injection sclerotherapy of bleeding oesophageal and gastric varices using a flexible endoscope. Acta Med Scand. 1982;211:125–9. doi: 10.1111/j.0954-6820.1982.tb01912.x. [DOI] [PubMed] [Google Scholar]
- 22.Trudeau W, Prindiville T. Endoscopic injection sclerosis in bleeding gastric varices. Gastrointest Endosc. 1986;32:264–8. doi: 10.1016/s0016-5107(86)71843-9. [DOI] [PubMed] [Google Scholar]
- 23.Korula J, Chin K, Ko Y, Yamada S. Demonstration of two distinct subsets of gastric varices. Observations during a seven-year study of endoscopic sclerotherapy. Dig Dis Sci. 1991;36:303–9. doi: 10.1007/BF01318201. [DOI] [PubMed] [Google Scholar]
- 24.Millar AJ, Brown RA, Hill ID, Rode H, Cywes S. The fundal pile: Bleeding gastric varices. J Pediatr Surg. 1991;26:707–9. doi: 10.1016/0022-3468(91)90015-l. [DOI] [PubMed] [Google Scholar]
- 25.Rengstorff DS, Binmoeller KF. A pilot study of 2-octyl cyanoacrylate injection for treatment of gastric fundal varices in humans. Gastrointest Endosc. 2004;59:553–8. doi: 10.1016/s0016-5107(03)02865-7. [DOI] [PubMed] [Google Scholar]
- 26.Dhiman RK, Chawla Y, Taneja S, Biswas R, Sharma TR, Dilawari JB. Endoscopic sclerotherapy of gastric variceal bleeding with N-butyl-2-cyanoacrylate. J Clin Gastroenterol. 2002;35:222–7. doi: 10.1097/00004836-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 27.D’Imperio N, Piemontese A, Baroncini D, Billi P, Borioni D, Dal Monte PP, et al. Evaluation of undiluted n-butyl-2-cyanoacrylate in the endoscopic treatment of upper gastrointestinal tract varices. Endoscopy. 1996;28:239–43. doi: 10.1055/s-2007-1005435. [DOI] [PubMed] [Google Scholar]
- 28.Belletrutti PJ, Romagnuolo J, Hilsden RJ, Chen F, Kaplan B, Love J, et al. Endoscopic management of gastric varices: Efficacy and outcomes of gluing with N-butyl-2-cyanoacrylate in a North American patient population. Can J Gastroenterol. 2008;22:931–6. doi: 10.1155/2008/389517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akahoshi T, Hashizume M, Shimabukuro R, Tanoue K, Tomikawa M, Okita K, et al. Long-term results of endoscopic Histoacryl injection sclerotherapy for gastric variceal bleeding: A 10-year experience. Surgery. 2002;131(1 Suppl):S176–81. doi: 10.1067/msy.2002.119501. [DOI] [PubMed] [Google Scholar]
- 30.Rivet C, Robles-Medranda C, Dumortier J, Le Gall C, Ponchon T, Lachaux A. Endoscopic treatment of gastroesophageal varices in young infants with cyanoacrylate glue: A pilot study. Gastrointest Endosc. 2009;69:1034–8. doi: 10.1016/j.gie.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 31.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–76. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Consolo P, Luigiano C, Giacobbe G, Scaffidi MG, Pellicano R, Familiari L. Cyanoacrylate glue in the management of gastric varices. Minerva Med. 2009;100:115–21. [PubMed] [Google Scholar]
- 33.Shiha G, El-Sayed SS. Gastric variceal ligation: A new technique. Gastrointest Endosc. 1999;49(4 Pt 1):437–41. doi: 10.1016/s0016-5107(99)70039-8. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T, Harada T, Shigemitsu T, Takeo Y, Miyazaki S, Okita K. Endoscopic management of gastric varices using a detachable snare and simultaneous endoscopic sclerotherapy and O-ring ligation. J Gastroenterol Hepatol. 1999;14:730–5. doi: 10.1046/j.1440-1746.1999.01941.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee TH, Shih LN. Clinical experience of endoscopic banding ligation for bleeding gastric varices. Hepatogastroenterology. 2008;55:766–9. [PubMed] [Google Scholar]
- 36.Yang WL, Tripathi D, Therapondos G, Todd A, Hayes PC. Endoscopic use of human thrombin in bleeding gastric varices. Am J Gastroenterol. 2002;97:1381–5. doi: 10.1111/j.1572-0241.2002.05776.x. [DOI] [PubMed] [Google Scholar]
- 37.Williams SG, Peters RA, Westaby D. Thrombin — An effective treatment for gastric variceal haemorrhage. Gut. 1994;35:1287–9. doi: 10.1136/gut.35.9.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Przemioslo RT, McNair A, Williams R. Thrombin is effective in arresting bleeding from gastric variceal hemorrhage. Dig Dis Sci. 1999;44:778–81. doi: 10.1023/a:1026626212129. [DOI] [PubMed] [Google Scholar]
- 39.Ramesh J, Limdi JK, Sharma V, Makin AJ. The use of thrombin injections in the management of bleeding gastric varices: A single-center experience. Gastrointest Endosc. 2008;68:877–82. doi: 10.1016/j.gie.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 40.Chun HJ, Hyun JH. A new method of endoscopic variceal ligation-injection sclerotherapy (EVLIS) for gastric varices. Korean J Intern Med. 1995;10:108–19. doi: 10.3904/kjim.1995.10.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugimoto N, Watanabe K, Watanabe K, Ogata S, Shimoda R, Sakata H, et al. Endoscopic hemostasis for bleeding gastric varices treated by combination of variceal ligation and sclerotherapy with N-butyl-2-cyanoacrylate. J Gastroenterol. 2007;42:528–32. doi: 10.1007/s00535-007-2041-0. [DOI] [PubMed] [Google Scholar]
- 42.Romero-Castro R, Pellicer-Bautista FJ, Jimenez-Saenz M, Marcos-Sanchez F, Caunedo-Alvarez A, Ortiz-Moyano C, et al. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: Results in 5 cases. Gastrointest Endosc. 2007;66:402–7. doi: 10.1016/j.gie.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Romero-Castro R, Pellicer-Bautista F, Giovannini M, Marcos-Sánchez F, Caparros-Escudero C, Jiménez-Sáenz M, et al. Endoscopic ultrasound (EUS)-guided coil embolization therapy in gastric varices. Endoscopy. 2010;42(Suppl 2):E35–6. doi: 10.1055/s-0029-1215261. [DOI] [PubMed] [Google Scholar]
- 44.Binmoeller KF, Weilert F, Shah JN, Kim J. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos) Gastrointest Endosc. 2011;74:1019–25. doi: 10.1016/j.gie.2011.06.030. [DOI] [PubMed] [Google Scholar]


