Abstract
The body responds to environmental stressors by triggering autonomic reflexes in the pulmonary receptors, baroreceptors, and chemoreceptors to maintain homeostasis. Numerous studies have shown that exposure to various gases and airborne particles can alter the functional outcome of these reflexes, particularly with respect to the cardiovascular system. Modulation of autonomic neural input to the heart and vasculature following direct activation of sensory nerves in the respiratory system, elicitation of oxidative stress and inflammation, or through other mechanisms is one of the primary ways that exposure to air pollution affects normal cardiovascular function. Any homeostatic process that utilizes the autonomic nervous system to regulate organ function might be affected. Thus, air pollution and other inhaled environmental irritants have the potential to alter both local airway function and baro-and chemoreflex responses, which modulate autonomic control of blood pressure and detect concentrations of key gases in the body. While each of these reflex pathways causes distinct responses, the systems are heavily integrated and communicate through overlapping regions of the brainstem to cause global effects. This short review summarizes the function of major pulmonary sensory receptors, baroreceptors, and carotid body chemoreceptors and discusses the impacts of air pollution exposure on these systems.
Keywords: C fibers, RARs, SARs, Baroreceptors, Carotid body, Air pollution
Introduction
Epidemiological studies have shown that air pollution exposure increases cardiovascular morbidity and mortality [1], especially in individuals made more susceptible by advanced age or chronic cardiovascular and lung disease. In fact, air pollution exposure has been shown to exacerbate cardiac and vascular pathophysiology attendant to hypertension, ischemia, heart failure, diabetes, coronary artery disease, and other pathophysiological conditions of the cardiovascular system [1]. Key components of air pollution associated with adverse health effects include fine and ultra-fine particulate matter (PM2.5 and UFP, with diameters less than 2.5 and 0.1 μm, respectively), and gases including ozone (O3), nitrogen dioxide (NO2), sulfur dioxide (SO2), and carbon monoxide (CO) [2]. For example, exposure to PM2.5 concentrations of as little as 10–20 μg/m3 caused significant increases in the risk of myocardial infarction 24 h after exposure [3]. In addition, individuals who had been hospitalized for coronary artery disease showed increases in T wave area (suggesting impairment in myocardial repolarization) after exposure to ambient levels of black carbon [4]. PM inhalation has also been associated with myocardial ischemia [5–7] and arrhythmias [7–9], especially in susceptible populations such as aged adults, the medically fragile, and those with underlying cardiovascular disease.
Our understanding of the pathophysiological mechanisms underlying air pollution-induced adverse clinical cardiovascular events has increased substantially since the first epidemiological studies discovered associations between PM exposure and increased cardiovascular morbidity and mortality. Currently, there are three mechanisms of action hypothesized that could explain the health effects of gaseous and particulate pollutants [1]. One theory posits that health effects result from the translocation of PM and/ or its components into the systemic circulation resulting from the direct interaction of the pollutant with blood vessel walls and/or the myocardium and causing attendant changes in vascular, mechanical, and electrical function. The second mechanism suggests that air pollution exposure elicits its effects by triggering pulmonary oxidative injury and systemic inflammation with an increase in pro-inflammatory biomarkers [1]. This local response can lead to systemic oxidative stress and inflammation characterized by an increase in activated white blood cells, platelets, and cytokine expression. Perhaps the major consequence of air pollutant-induced systemic inflammation is injury of the vascular endothelium, the inner lining of the blood vessel wall, thus promoting vasoconstriction, thrombosis, and inflammation [10]. The third mechanism for air pollution-induced cardiovascular dysfunction posits that pollutant inhalation activates airway sensory nerves, which due to neural plasticity, even in the short-term, can modify autonomic nervous system (ANS) control of cardiovascular function. Importantly, these three mechanisms can overlap, as seen in studies showing that oxidative stress can lead to changes in autonomic function [11, 12]. While evidence exists for all three mechanisms, changes in cardiac autonomic tone are often the most immediate consequences of air pollution exposure [1] and involve several different reflex arcs.
The ANS controls many of the visceral functions and is composed of two branches, the sympathetic branch that is responsible for the ‘‘fight or flight’’ response and the parasympathetic branch that is responsible for maintaining a homeostatic baseline. The body has a complex and multifaceted system that responds to environmental stressors or anything that causes fluctuation in normal function on a moment-to-moment basis, by triggering autonomic reflexes. This includes pulmonary pathways that respond to sensory activation/irritation, baroreceptor reflexes that respond to changes in blood pressure (BP), and chemoreceptor-initiated reflex responses to changes in pCO2, pO2, pH, and temperature. Given the growing interest in mechanisms that mediate short-term cardiovascular effects of air pollution, this review will discuss (1) the impacts of autonomic modulation on cardiovascular function, (2) evidence linking these three prominent autonomic reflex arcs to the cardiovascular effects associated with exposure to air pollution, and (3) how these responses are integrated.
Changes in Heart Rate Variability: Evidence for Altered Autonomic Tone
Much of the evidence linking changes in cardiac autonomic tone with exposure to air pollution comes from studies of heart rate variability (HRV). HRV is the degree of difference in the inter-beat intervals of successive heartbeats and is an indicator of the balance between the sympathetic and parasympathetic branches of the ANS [13]. High HRV is traditionally considered positive because the heart has the ability to respond to rapidly changing environments. Low HRV, reflecting increased sympathetic tone [13], is associated with an increased risk of cardiac arrhythmia [14] and an increased risk of mortality in people with heart disease [15, 16]. While low HRV has been reported with exposure to PM [17–21] and ozone [4, 22, 23], other studies have demonstrated associations between PM exposure and increased HRV [24–26]. Importantly, increased HRV may also have links to adverse health outcomes. Increased parasympathetic tone is a precursor to drug-induced torsade de pointes [27] (a precursor arrhythmia to ventricular fibrillation [28]) and is associated with increased apnea severity in obese patients [29], adverse cardiovascular events in type II diabetics [30], and increased mortality in heart failure [31]. While the mechanisms triggering changes in HRV, and thus autonomic tone, have not been fully delineated and are likely numerous and diverse in nature, the best studied mechanism with respect to acute air pollution-induced effects is the activation of pulmonary neural reflexes.
Airway Receptors
The respiratory system is innervated with multiple vagal sensory nerve types to ‘‘sense’’ the presence of various environmental irritants as well as stretch receptors that respond to changes in lung inflation (Table 1). The cell bodies of the sensory nerve fibers are located in the jugular and nodose ganglia; upon activation, these fibers send afferent signals to the nucleus tractus solitarius (NTS) in the brainstem, which initiates both higher central nervous system signals and an efferent flow of information via the autonomic nerves (Fig. 1) [32]. There are three major types of receptors by which the sensory nerve fibers are characterized in the airways: C-nerve fibers, rapidly adapting pulmonary receptors (RARs or irritant receptors), and slowly adapting pulmonary receptors (SARs or stretch receptors) [33]. The receptor types have overlapping locations in the airways and are designed to respond to different stimuli (Table 1).
Table 1.
Summary of locations, effects of activation, and activating air pollutants in the body’s reflex responses
| Receptor | Location | Irritant/pollutant | Effect |
|---|---|---|---|
|
C-nerve fibers TRPA, TRPV, J receptors |
Nose, larynx, trachea/ bronchi alveoli [34] | Cigarette smoke [40, 41], SO2 [41], acrolein [39], PM [41] | Apnea, dyspnea, cough, bronchoconstriction, lung inflation, injury, acute pulmonary vascular congestion [35–38] |
| Rapidly adapting pulmonary receptors (RARs) | Larynx, trachea/ bronchi [47] | Cigarette smoke, acrolein, ozone [42–44] | Cough, bronchoconstriction, mucus secretion, lung inflation, deflation, congestion of pulmonary vascular bed, prolonged inspiration [34, 37, 38, 48, 50] |
| Slowly adapting pulmonary receptors (SARs) | Trachea/ bronchi, visceral pleurae [34] | Lung inflation [34] | Changes heart rate and cardiac output [55–58] |
| Baroreceptors | Carotid sinus, aortic arch [59] | PM [67–69, 73], cigarette smoke [76], concentrated ambient particles [78] | Increased diastolic blood pressure [74], reduced HRV [74], increased sympathetic nerve activity [81], decreased baroreflex sensitivity [72, 75] |
| Chemoreceptors– carotid body | Bifurcation of carotid artery [87] | PM [91–94], acrolein [99] | Increased likelihood of arrhythmias [98], increased systolic, diastolic, and mean arterial blood pressure [99] |
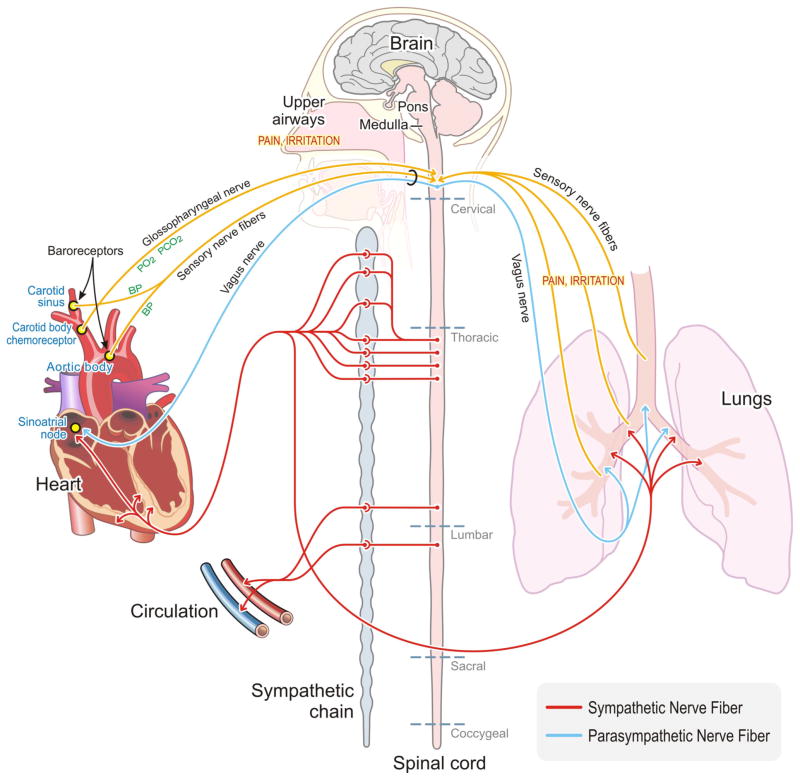
Fig. 1.
Airway, baroreceptor, and chemoreceptor responses in the body. Multiple autonomic and neural pathways control the body’s response to stimuli, including air pollution. The ANS is composed of the sympathetic and parasympathetic branches that innervate the major organs of the body including the heart and lungs. The sympathetic nervous system communicates through sympathetic nerve fibers (shown in red) and is responsible for the ‘‘fight or flight’’ response while the parasympathetic nervous system communicates through the vagus nerve (shown in blue) and is crucial in homeostatic control and regulation. The ANS communicates with the lungs through multiple efferent pathways while signals from the lung are then transmitted back to the brain through sensory nerve fibers (shown in yellow). The ANS also innervates the heart, and signals from the heart are transmitted back to the brain through sensory organs including the baroreceptors and carotid body chemoreceptors via sensory nerve fibers, specifically the afferent fibers of the glossopharyngeal nerve in the case of the carotid body (shown in yellow). The ANS is a complex system with multi-organ control, and exposure to air pollution can trigger responses through multiple levels of neural communication including pulmonary nerve fibers, baroreceptors, and the carotid body (Color figure online)
C-nerve fibers exist throughout the respiratory tract including the nose, larynx, trachea/bronchi, and alveoli [34]. These unmyelinated afferent fibers are activated by environmental pollutants and initiate chemoreflex responses that result in cough, bronchoconstriction, and dyspnea through both local and central pathways [35]. C-nerve fiber activation causes local responses with the release of Substance P as well as reflex bronchospasm and mucus secretion, and centrally mediated responses that trigger apnea followed by rapid shallow breathing [36]. A type of C fiber receptor known as juxtapulmonary capillary receptors (J receptors) have also been shown to be sensitive to lung inflation and will cause apnea if severely stimulated [37, 38]. Acrolein [39], cigarette smoke [40], and SO2 [41] potentiate C-nerve fiber airway chemoreflex responsiveness and result in prolonged apnea and increased bronchoconstriction. Some of these responses may be further augmented due to increased neuropeptide release and initiation of neuroinflammatory mechanisms as in the case of cigarette smoke exposure [41].
In recent years, increased attention has been paid to the direct targets of air pollutants, particularly the gaseous irritants. Bautista et al. [42] initially showed that the transient receptor potential ankyrin 1 (TRPA1) cation channel mediated the activation of C-nerve fiber by pungent substances like garlic and mustard oil, but also ubiquitous air pollutants like acrolein. Ozone was also found to stimulate C-nerve fibers through TRPA1 [43]. It is now quite clear that nasal, bronchial, and pulmonary C-nerve fiber subtypes play a role in the response to certain air pollution components through the activation of not only TRPA1 [43], but also transient receptor potential vanilloid 1 (TRPV1) [44], and purinergic P2X channels [45]; these receptors act like innate environmental sensors and initiate sensory nerve excitation.
C-nerve fiber activation often coincides with RAR activation [46], as the two sensory receptors have been shown to respond to similar irritants. RARs are myelinated fibers located in the larynx, trachea, and bronchi [47]. Similar to C-nerve fibers, activation of RARs leads to bronchoconstriction, cough, and increased mucus secretion [48]. However, RARs respond to both mechanical and chemical irritation depending on the location of the receptor and have an additive effect on the pulmonary reflex response when stimulated simultaneously with C-nerve fibers [49]. Although RAR and C-nerve fiber activation are similar in many respects, including their responses to air pollution, C-nerve fiber activation causes apnea and then recovery through rapid shallow breathing, while RAR activation more commonly causes cough and augmented breathing [34]. In addition to the varied changes in tidal volume and breathing frequency, stimulation of C-nerve fibers and RARs alters cardiovascular function due to global (i.e., not restricted to the respiratory system) autonomic modulation resulting from activation of the pulmonary chemoreflex. Inhalation of irritants in the nose, nasopharynx, and pharynx stimulates reflex bradycardia coupled with subsequent increases or decreases in HR depending on the location of activation in the airways [34]. In the lower airways, C-nerve fibers and RARs will respond strongly to inhaled irritants and lead to pronounced bradycardia and hypotension [50]. On the other hand, airway reflexes originating in the larynx have been shown to cause cardiac arrhythmia and ST depression, a non-specific electrocardiographic change often associated with myocardial ischemia [51]. Similarly, we demonstrated that the TRPA1 channels expressed on these fibers mediate increased cardiac arrhythmogenesis one day after diesel exhaust exposure [44]. In addition, RARs respond to changes in the fluid volume of the airways and play a role in respiratory reflexes during heart failure and may contribute to increased heart failure hospitalization and mortality associated with acute exposure to air pollution [52, 53].
Only the lower airways contain stretch receptors called SARs that, unlike C-nerve fibers and RARs, are likely not as sensitive to irritants. While clear evidence links air pollution exposure to C-nerve fiber and RAR activation, SARs do not, for the most part, respond to chemical stimuli and are not major drivers of pulmonary reflex responses to air pollutants. They are, however, extremely important regulators of breathing, as they are sensitive to lung inflation [34] and have an important role in controlling changes in HR and cardiac output as well as stimulating the cough reflex [54]. SARs have been shown to be critical mediators of respiratory sinus arrhythmia (RSA) [55], a naturally occurring variation in HR where HR increases during inspiration and decreases during expiration [56]. SARs control of RSA is critical in maintaining normal functioning of the cardiovascular system since it has been shown that disordered breathing can cause life threatening ventricular arrhythmias [57], while paced breathing can actually reduce BP in hypertensive individuals through improvement of another homeostatic reflex called the baroreflex [58]. The baroreceptor reflex is another important component of autonomic regulation and overlaps with airway reflexes to induce cardiovascular responses after air pollution exposure.
Baroreceptors
The baroreceptor reflex controls BP and is critical to maintaining normal cardiovascular function and perfusion throughout the body. Baroreceptors are stretch-sensitive mechanoreceptors located in the carotid sinus and aortic arch (Fig. 1) that are innervated by the glossopharyngeal and vagus nerve, respectively; these nerves transmit afferent signals to the nucleus of the solitary tract (NTS) in the brainstem [59] (Table 1). Baroreceptor reflex function is regulated through a negative feedback mechanism characterized by opposing activation/inhibition of the parasympathetic and sympathetic branches. An increase in BP causes the baroreceptors to fire more rapidly, which results in the inhibition of the sympathetic branch and contemporaneous activation of the parasympathetic branch causing a reflex decrease in HR with an attendant decrease in systemic BP. On the other hand, decreases in systemic BP decrease baroreceptor firing, which reduces the inhibitory effect on the sympathetic branch; this results in an increase in HR and subsequently BP. Studies using isolated carotid sinuses found that a reflex increase in systemic arterial BP and HR occurred when pressure was lowered in the sinus [60]. Upon receiving input from the baroreceptors, the NTS communicates with regions of the brain controlling the ANS and leads to an inhibition of the sympathetic nervous system and an activation of the parasympathetic nervous system [61]. Because the baroreceptor reflex is crucial in day-to-day, and indeed moment-to-moment, homeostatic control [62], abnormal functioning of this reflex may significantly increase the risk of adverse cardiovascular events [63, 64]. Studies have shown that desensitization of the baroreceptor reflex contributes to the development and progression of cardiovascular diseases [65] and is believed to contribute to cardiovascular disease in rats with hypertension [66].
Multiple studies have reported significant changes in BP following air pollution exposure. In a cohort of German citizens, individuals living in close proximity to high traffic had higher arterial BP and greater prevalence of hypertension than individuals living in low traffic areas [67]. Long-term exposure to PM, SO2, O3, and black carbon causes increases in arterial BP and incidence of hypertension [68–71], potentially suggesting increased sympathetic tone and baroreceptor desensitization [72]. Exposure of research volunteers to concentrated ambient fine PM caused significant increases in diastolic BP in healthy adults, and this increase was coupled with decreases in HRV (increased sympathetic tone) [73]. Similarly, a controlled human exposure by Huang et al. [74] found that exposure to PM2.5, black carbon, and NO2 significantly decreases HRV and increases BP in patients with underlying cardiovascular disease. Air pollution-induced increases in BP have also been coupled with baroreflex desensitization [72], showing that the baroreceptor reflex responses are likely involved during air pollution exposure. Although this study was performed in rats, the results provide key insight into the effect of air pollution on baroreflex responses.
Most studies that directly examined baroreflex sensitivity (BRS) activity following air pollution exposure point to a reduction in BRS. In humans, for example, exposure to 200 ppb SO2 was associated with decreased BRS [75]. The national standard for SO2 is 75 ppb for 1 h, and decreases in BRS at realistic exposure concentrations reveal the importance of measuring baroreceptor response when studying the effects of air pollutants. We previously demonstrated that whole-body exposure to acrolein causes baroreflex desensitization in both normotensive and hypertensive rats [72]. In addition, exposure to cigarette smoke in rats decreased BRS [76]. Rats instilled with carbon nanotubes, engineered nanomaterials that share many properties with the ultrafine component of air pollution, decreased arterial baroreceptor function after exposure [77]. By contrast, exposure to concentrated ambient particles (CAPs) in dogs increased arterial BP and BRS, which were reversed with alpha-adrenergic blockade [78]. These opposing findings by Bartoli et al. [78] may be due to the fact that the dogs were exposed via the trachea, bypassing irritant mechanisms of the upper airways and potentially modifying BRS responses. An additional possibility is that sympathetic activation resulted in increased HR and peripheral vascular resistance which caused increases in BP, while concurrent increases in parasympathetic tone blunted the HR response and resulted in only small HR changes [78]. These results highlight the complex nature of the autonomic response with both the sympathetic and parasympathetic branches working to regulate BP through opposing mechanisms. Low BRS has been associated with increased sympathetic modulation, including responses during intermittent hypoxia [79]. While low HRV (i.e., increased sympathetic tone) has been linked to increased cardiac morbidity and mortality, studies have shown that low BRS is an even stronger predictor [80] and may represent a shift toward sympathetic dominance [81]. Thus, cardiovascular effects of air pollution may in part be mediated by BRS-driven changes in autonomic tone. The precise cause of BRS desensitization related to air pollution exposure is uncertain although evidence suggests that factors associated with endothelium and endothelial dysfunction play a role. For instance, air pollution exposure alters prostacyclins, nitric oxide (NO), and factors released from aggregating platelets. Each of these factors is known to modulate baroreceptor nerve activity [82–84]. Even reactive oxygen species (ROS), which are increased in macrophages after acrolein exposure [85], can blunt baroreflex responses [86].
Chemoreceptors
There are two categories of chemoreceptors: (1) central chemoreceptors located in the brainstem and (2) peripheral chemoreceptors located in the aortic and carotid bodies [87] (Table 1). In mammals, the response to changes in oxygen, carbon dioxide, pH, and temperature is controlled by the carotid body, a major sensory organ located at the bifurcation of the carotid artery (Fig. 1). Activation of the carotid body initiates reflex cardiopulmonary changes that serve to maintain homeostasis. In response to hypoxia, for example, the carotid body triggers increases in sympathetic tone that result in elevations in BP and HR, as well as increased ventilation [88–90]. Such shifts in autonomic tone may be detrimental if they persist given that increased sympathetic tone is associated with elevated cardiovascular risk, which includes increased mortality rate in people with heart disease [16]. However, increased sympathetic modulation has been reported with PM and ozone exposure [1]; whether it occurs due to chemoreflex activation remains to be determined. Parallels between carotid body-mediated effects and cardiovascular responses associated with air pollution exposure suggest a linkage is plausible.
Although it is known that the carotid body is a key component of cardiovascular reflex responses, there is relatively little evidence linking carotid body activation with air pollution exposure. While the effects were small, several studies have reported changes in oxygen saturation, an important parameter of carotid body sensing, with exposure to air pollution including older individuals with cardiopulmonary disease [91], 80-year-old males [92], healthy adults with long-term traffic exposure [93], and healthy elderly volunteers [94, 95]. Furthermore, exposures to environmental tobacco smoke [96], SO2, and NO2 [97] were linked to abnormal cardiopulmonary sensitivity responses to hypoxia, indicating modified activity of the carotid body. In heart failure mice, Wang et al. [98] recently found that cardiac arrhythmias associated with exposure to particulate matter were in part due to altered sensitivity of the carotid body. In addition, our laboratory found that pretreatment with an inhibitor of carotid body signal transduction in rats prevented several adverse cardiovascular responses associated with acrolein exposure (i.e., increased systolic, diastolic, and mean arterial BP during exposure, and decreased cardiac contractility 1 day after exposure). In the same study, we found that acrolein exposure caused significant decreases in pO2 and significant increases in pCO2 during exposure, suggesting that carotid body activation may have been triggered by hypercapnia and/or hypoxia [99]. While we did not perform direct measurements of carotid body activation during acrolein exposure, changes in the concentrations of pO2 and pCO2 are known to activate carotid body signaling. Importantly these responses appeared to be mediated by carotid body-triggered changes in autonomic tone, suggesting that this may be a potential mechanism for the cardiovascular effects of air pollution.
Integration of Reflex Responses and Conclusions
While evidence exists that exposure to air pollution is associated with the activation of each of these autonomic reflex arcs in cardiovascular effects, in reality, these responses do not function independently and are highly integrated (Fig. 1). A striking example of the degree of interconnectedness between the reflex responses can be seen in the hypoxia ventilatory response (HVR) in obstructive sleep apnea. In OSA, obstruction of the upper airway during sleep causes both hypoxia and hypercapnia, which stimulate the carotid body chemoreceptors. This causes a reflex increase in ventilation, sympathetic tone, and arterial BP. The stimulation of these pathways is accompanied by both chemoreceptor and pulmonary mechanoreceptor activation, restoring normal ventilation, often causing individuals to awake from sleep [100]. In addition to immediate reflex responses, approximately half of all people who suffer from OSA will develop systemic hypertension, while others develop pulmonary hypertension affecting cardiac output [101], with potential alteration of BRS. The HVR shows that the reflex pathways are not only involved in exposure situations but also influence responses in multiple target locations.
Moreover, activation of the chemoreflex response has been shown to cause a resetting of the baseline function of the baroreflex [102], further displaying the interaction between reflex responses after stimulation. Exposure to hypoxia causes increases in HR and sympathetic nerve activity [103], and these changes are accompanied by baroreflex resetting to higher pressures and higher HRs [104]. These effects were largely independent of breathing changes and tidal volume and were instead attributed to peripheral chemoreceptor activation [102]. While breathing changes were not responsible for baroreflex resetting, exposure to hypoxia caused a rapid increase in ventilation [89], further displaying the interaction between the various reflexes responses. Moreover, pulmonary, baro-, and chemoreflexes have extensive influence over the ANS as well as overlapping pathways in the brainstem, making interaction and integration between the system not only plausible but highly likely [102]. Thus, as future research better defines the roles of unique reflex responses in modulating autonomic control of the heart, the constant interplay among pulmonary, baroreceptor, and chemoreceptor responses together likely determines the overall physiological response to an inhaled air pollutant.
Acknowledgments
The authors would like to thank John Havel of SRA International, Inc. for his graphics support as well as Drs. Wayne Cascio, Daniel L. Costa, M. Ian Gilmour, and Lindsay Stanek of the United States Environmental Protection Agency, and Dr. Samantha Snow of the University of North Carolina, Chapel Hill, for their thorough review of this manuscript.
Abbreviations
- ANS
Autonomic nervous system
- BRS
Baroreflex sensitivity
- COPD
Chronic obstructive pulmonary disease
- HRV
Heart rate variability
- HVR
Hypoxia ventilatory response
- NTS
Nucleus tractus solitarius
- OSA
Obstructive sleep apnea
- PM
Particulate matter
- RARs
Rapidly adapting pulmonary receptors
- SARs
Slowly adapting pulmonary receptors
- TRPA1
Transient receptor potential ankyrin 1
- TRPV1
Transient receptor potential vanilloid 1
Footnotes
Disclaimer: This paper has been reviewed and approved for release by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency. Approval does not signify that the contents necessarily reflect the views and policies of the U.S. EPA, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Contributor Information
Christina M. Perez, Email: clamb@email.unc.edu, Curriculum in Toxicology, University of North Carolina, Chapel Hill, NC 27599, USA
Mehdi S. Hazari, Environmental Public Health Division, NHEERL, U.S. Environmental Protection Agency, Mail Code: B143-01, Research Triangle Park, NC 27711, USA
Aimen K. Farraj, Environmental Public Health Division, NHEERL, U.S. Environmental Protection Agency, Mail Code: B143-01, Research Triangle Park, NC 27711, USA
References
- 1.Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: A statement for healthcare professionals form the expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109:2566–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 3.Peters A, von Klot S, Heier M, Trentinaglia I, Hörmann A, Wichmann HE, et al. Exposure to traffic and the onset of myocardial infarction. New England Journal of Medicine. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 4.Zanobetti A, Gold DR, Stone PH, Suh HH, Schwartz J, Coull BA, et al. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environmental Health Perspectives. 2010;118:324–330. doi: 10.1289/ehp.0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold DR, Litonjua AA, Zanobetti A, Coull BA, Schwartz J, MacCallum G, et al. Air pollution and ST-segment depression in elderly subjects. Environmental Health Perspectives. 2005;113:883–887. doi: 10.1289/ehp.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellenius GA, Coull BA, Godleski JJ, Koutrakis P, Okabe K, Savage ST, et al. Inhalation of concentrated ambient air particles exacerbates myocardial ischemia in conscious dogs. Environmental Health Perspectives. 2003;111:402–408. doi: 10.1289/ehp.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartell SM, Longhurst J, Tjoa T, Sioutas C, Delfino RJ. Particulate air pollution, ambulatory heart rate variability, and cardiac arrhythmia in retirement community residents with coronary artery disease. Environmental Health Perspectives. 2013;121(10):1135–1141. doi: 10.1289/ehp.1205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Link MS, Luttmann-Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, et al. Acute exposure to air pollution triggers atrial fibrillation. Journal of the American College of Cardiology. 2013;62(9):816–825. doi: 10.1016/j.jacc.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanobetti A, Coull BA, Gryparis A, Kloog I, Sparrow D, Vokonas PS, et al. Associations between arrhythmia episodes and temporally and spatially resolved black carbon and particulate matter in elderly patients. Occupational and Environmental Medicine. 2014;71(3):201–207. doi: 10.1136/oemed-2013-101526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishna MT, Chauhan AJ, Frew AJ, Holgate ST. Toxicological mechanisms underlying oxidant pollutant-induced airway injury. Reviews on Environmental Health. 1998;13:59–71. [PubMed] [Google Scholar]
- 11.Pham H, Bonham AC, Pinkerton KE, Chen CY. Central neuroplasticity and decreased heart rate variability after particulate matter exposure in mice. Environmental Health Perspectives. 2009;117(9):1448–1453. doi: 10.1289/ehp.0900674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang HC, Hsueh TW, Chang CC, Hwang JS, Chuang KJ, Yan YH, et al. Nickel-regulated heart rate variability: The roles of oxidative stress and inflammation. Toxicology and Applied Pharmacology. 2013;266(2):298–306. doi: 10.1016/j.taap.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Rowan WH, 3rd, Campen MJ, Wichers LB, Watkinson WP. Heart rate variability in rodents: Uses and caveats in toxicological studies. Cardiovascular Toxicology. 2007;7:28–51. doi: 10.1007/s12012-007-0004-6. [DOI] [PubMed] [Google Scholar]
- 14.Corey LM, Baker C, Luchtel DL. Heart-rate variability in the apolipoprotein E knockout transgenic mouse following exposure to Seattle particulate matter. Journal of Toxicology and Environmental Health Part A. 2006;69:953–965. doi: 10.1080/15287390500362105. [DOI] [PubMed] [Google Scholar]
- 15.Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88:927–934. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]
- 16.Fauchier L, Babuty D, Melin A, Bonnet P, Cosnay P, Paul Fauchier J. Heart rate variability in severe right or left heart failure: The role of pulmonary hypertension and resistances. European Journal of Heart Failure. 2004;6(2):181–185. doi: 10.1016/j.ejheart.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Anselme F, Loriot S, Henry JP, Dionnet F, Napoleoni JG, Thuillez C, et al. Inhalation of diluted diesel engine emission impacts heart rate variability and arrhythmia occurrence in a rat model of chronic ischemic heart failure. Archives of Toxicology. 2007;81(4):299–307. doi: 10.1007/s00204-006-0147-4. [DOI] [PubMed] [Google Scholar]
- 18.Elder A, Couderc JP, Gelein R, Eberly S, Cox C, Xia X, et al. Effects of on-road highway aerosol exposures on autonomic responses in aged, spontaneously hypertensive rats. Inhalation Toxicology. 2007;19(1):1–12. doi: 10.1080/08958370600985735. [DOI] [PubMed] [Google Scholar]
- 19.Chuang KJ, Chan CC, Chen NT, Su TC, Lin LY. Effects of particle size fractions on reducing heart rate variability in cardiac and hypertensive patients. Environmental Health Perspectives. 2005;113:1693–1697. doi: 10.1289/ehp.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz H, Harder V, Ibald-Mulli A, Khandoga A, Koenig W, Krombach F, et al. Cardiovascular effects of fine and ultrafine particles. Journal of Aerosol Medicine. 2005;18:1–22. doi: 10.1089/jam.2005.18.1. [DOI] [PubMed] [Google Scholar]
- 21.Vallejo M, Ruiz S, Hermosillo AG, Borja-Aburto VH, Cárdenas M. Ambient fine particles modify heart rate variability in young healthy adults. Journal of Exposure Science & Environmental Epidemiology. 2006;16:125–130. doi: 10.1038/sj.jea.7500447. [DOI] [PubMed] [Google Scholar]
- 22.Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, et al. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60:455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godleski JJ, Verrier RL, Koutrakis P, Catalano P, Coull B, Reinisch U, et al. Mechanisms of morbidity and mortality from exposure to ambient air particles. Research Report/Health Effects Institute. 2000;91:5–88. [PubMed] [Google Scholar]
- 25.Tankersley CG, Campen M, Bierman A, Flanders SE, Broman KW, Rabold R. Particle effects on heart-rate regulation in senescent mice. Inhalation Toxicology. 2004;16:381–390. doi: 10.1080/08958370490439551. [DOI] [PubMed] [Google Scholar]
- 26.Jia X, Song X, Shima M, Tamura K, Deng F, Guo X. Effects of fine particulate on heart rate variability in Beijing: A panel study of healthy elderly subjects. International Archives of Occupational and Environmental Health. 2012;85(1):97–107. doi: 10.1007/s00420-011-0646-3. [DOI] [PubMed] [Google Scholar]
- 27.Farkas A, Dempster J, Coker SJ. Importance of vagally mediated bradycardia for the induction of torsade de pointes in an in vivo model. British Journal of Pharmacology. 2008;154:958–970. doi: 10.1038/bjp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gralinski MR. The dog’s role in the preclinical assessment of QT interval prolongation. Toxicologic Pathology. 2003;31:11–16. doi: 10.1080/01926230390174887. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds EB, Seda G, Ware JC, Vinik AI, Risk MR, Fishback NF. Autonomic function in sleep apnea patients: Increased heart rate variability except during REM sleep in obese patients. Sleep Breathing. 2007;11:53–60. doi: 10.1007/s11325-006-0083-9. [DOI] [PubMed] [Google Scholar]
- 30.Eguchi K, Schwartz JE, Pickering TG, Hoshide S, Ishikawa J, Shimada K, et al. Increased heart rate variability during sleep is a predictor for future cardiovascular events in patients with type 2 diabetes. Hypertension Research. 2010;33:737–742. doi: 10.1038/hr.2010.61. [DOI] [PubMed] [Google Scholar]
- 31.Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE, Cast Investigators. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. Journal of Cardiovascular Electrophysiology. 2005;16(1):13–20. doi: 10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 32.Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology. 2003;8(3):291–301. doi: 10.1046/j.1440-1843.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 33.Coleridge HM, Coleridge JC, Poore ER, Roberts AM, Schultz HD. Aortic wall properties and baroreceptor behaviour at normal arterial pressure and in acute hypertensive resetting in dogs. Journal of Physiology. 1984;350:309–326. doi: 10.1113/jphysiol.1984.sp015203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environmental Health Perspectives. 2001;109:579–584. doi: 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respiratory Physiology & Neurobiology. 2011;178(3):406–413. doi: 10.1016/j.resp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sann H, Pierau FK. Efferent functions of C-fiber nociceptors. Zeitschrift fur Rheumatologie. 1998;57:8–13. doi: 10.1007/s003930050226. [DOI] [PubMed] [Google Scholar]
- 37.Rhoades R, Bell D. Medical physiology—Principles of clinical medicine. Philadelphia, PA: Lippincott Williams and Wilkins; 2013. Lung and chest wall reflexes; pp. 386–399. [Google Scholar]
- 38.Ganong WF. Regulation of respiration. In: Taylor C, editor. Review of medical physiology. New York, NY: McGraw Hill; 2010. pp. 625–639. [Google Scholar]
- 39.Hazari MS, Rowan WH, Winsett DW, Ledbetter AD, Haykal-Coates N, Watkinson WP, et al. Potentiation of pulmonary reflex response to capsaicin 24 h following whole-body acrolein exposure is mediated by TRPV1. Respiratory Physiology & Neurobiology. 2008;160:160–171. doi: 10.1016/j.resp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Lee LY, Beck ER, Morton RF, Kou YR, Frazier DT. Role of bronchopulmonary C-fiber afferents in the apneic response to cigarette smoke. Journal of Applied Physiology. 1987;63:1366–1373. doi: 10.1152/jappl.1987.63.4.1366. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Xu F. Role of neurogenic substance P in overexpression of alveolar macrophages’ neurokinin 1 receptor in mice exposed to cigarette smoke. Experimental Lung Research. 2010;36(4):243–254. doi: 10.3109/01902140903398275. [DOI] [PubMed] [Google Scholar]
- 42.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Grace MS, Belvisi MG. TRPA1 receptors in cough. Pulmonary Pharmacology & Therapeutics. 2011;24(3):286–288. doi: 10.1016/j.pupt.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, et al. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environmental Health Perspectives. 2011;119(7):951–957. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barth K, Kasper M. Membrane compartments and purinergic signalling: Occurrence and function of P2X receptors in lung. FEBS Journal. 2009;276(2):341–353. doi: 10.1111/j.1742-4658.2008.06795.x. [DOI] [PubMed] [Google Scholar]
- 46.Lai CJ, Ruan T, Kou YR. The involvement of hydroxyl radical and cyclooxygenase metabolites in the activation of lung vagal sensory receptors by circulatory endotoxin in rats. Journal of Applied Physiology. 2005;98(2):620–628. doi: 10.1152/japplphysiol.00539.2004. [DOI] [PubMed] [Google Scholar]
- 47.Kappagoda CT, Ravi K. The rapidly adapting receptors in mammalian airways and their responses to changes in extravascular fluid volume. Experimental Physiology. 2006;91(4):647–654. doi: 10.1113/expphysiol.2006.033209. [DOI] [PubMed] [Google Scholar]
- 48.Coleridge HM, Coleridge JCG. Pulmonary reflexes: Neural mechanisms of pulmonary defense. Annual Review of Physiology. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- 49.Sant’Ambrogio G, Widdicombe J. Reflexes from airway rapidly adapting receptors. Respiration Physiology. 2001;125:33–45. doi: 10.1016/s0034-5687(00)00203-6. [DOI] [PubMed] [Google Scholar]
- 50.Lee LY, Widdicombe JG. Modulation of airway sensitivity to inhaled irritants: Role of inflammatory mediators. Environmental Health Perspectives. 2001;109:585–589. doi: 10.1289/ehp.01109s4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pys-Roberts C, Greene LT, Meloche R, Foex P. Studies of anesthesia in relation to hypertension. II: Haemodynamic consequences of induction and endotracheal intubation. British Journal of Anaesthesia. 1971;43:531–547. doi: 10.1093/bja/43.6.531. [DOI] [PubMed] [Google Scholar]
- 52.Mearns BM. Heart failure: Air pollution linked with heart-failure-related hospitalization and mortality. Nature Reviews Cardiology. 2013;10:551. doi: 10.1038/nrcardio.2013.112. [DOI] [PubMed] [Google Scholar]
- 53.Ravi K, Kappagoda T. Rapidly adapting receptors in acute heart failure and their impact on dyspnea. Respiratory Physiology & Neurobiology. 2009;167:107–115. doi: 10.1016/j.resp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respiratory Physiology & Neurobiology. 2006;152(3):223–242. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Taha BH, Simon PM, Dempsey JA, Skatrud JB, Iber C. Respiratory sinus arrhythmia in humans: An obligatory role for vagal feedback from the lungs. Journal of Applied Physiology. 1995;78:638–645. doi: 10.1152/jappl.1995.78.2.638. [DOI] [PubMed] [Google Scholar]
- 56.Hayano J, Yasuma F, Okada A, Mukai S, Fujinami T. Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation. 1996;94:842–847. doi: 10.1161/01.cir.94.4.842. [DOI] [PubMed] [Google Scholar]
- 57.Zeidan-Shwiri T, Aronson D, Atalla K, Blich M, Suleiman M, Marai I, et al. Circadian pattern of life-threatening ventricular arrhythmia in patients with sleep-disordered breathing and implantable cardioverter-defibrillators. Heart Rhythm. 2011;8:657–662. doi: 10.1016/j.hrthm.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 58.Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, et al. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46:714–718. doi: 10.1161/01.HYP.0000179581.68566.7d. [DOI] [PubMed] [Google Scholar]
- 59.Cortelli P, Lombardi C, Montagna P, Parati G. Baroreflex modulation during sleep and in obstructive sleep apnea syndrome. Autonomic Neuroscience. 2012;169(1):7–11. doi: 10.1016/j.autneu.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Walgenbach SC, Shepherd JT. Role of arterial and cardiopulmonary mechanoreceptors in the regulation of arterial pressure during rest and exercise in conscious dogs. Mayo Clinic Proceedings. 1984;59:467–475. doi: 10.1016/s0025-6196(12)60435-2. [DOI] [PubMed] [Google Scholar]
- 61.Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation: Mechanisms and potential for hypertension therapy. Hypertension. 2011;57:880–886. doi: 10.1161/HYPERTENSIONAHA.108.119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kougias P, Weakley SM, Yao Q, Lin PH, Chen C. Arterial baroreceptors in the management of systemic hypertension. Medical Science Monitor. 2010;16:1–8. [PMC free article] [PubMed] [Google Scholar]
- 63.Johansson M, Gao SA, Friberg P, Annerstedt M, Carlström J, Ivarsson T, et al. Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. Journal of Hypertension. 2007;25(1):163–168. doi: 10.1097/01.hjh.0000254377.18983.eb. [DOI] [PubMed] [Google Scholar]
- 64.Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, et al. Arterial baroreflex modulation of heart rate in chronic heart failure: Clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96(10):3450–3458. doi: 10.1161/01.cir.96.10.3450. [DOI] [PubMed] [Google Scholar]
- 65.La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: Measurement and clinical implications. Annals of Noninvasive Electrocardiology. 2008;13(2):191–207. doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Struyker-Boudier HA, Evenwel RT, Smits JF, Van Essen H. Baroreflex sensitivity during the development of spontaneous hypertension in rats. Clinical Science (London) 1982;62(6):589–594. doi: 10.1042/cs0620589. [DOI] [PubMed] [Google Scholar]
- 67.Fuks K, Moebus S, Hertel S, Viehmann A, Nonnemacher M, Dragano N, et al. Long-term urban particulate air pollution, traffic noise, and arterial blood pressure. Environmental Health Perspectives. 2011;119:1706–1711. doi: 10.1289/ehp.1103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong GH, Qian ZM, Xaverius PK, Trevathan E, Maalouf S, Parker J, et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension. 2013;61(3):578–584. doi: 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- 69.Schwartz J, Alexeeff SE, Mordukhovich I, Gryparis A, Vokonas P, Suh H, et al. Association between long-term exposure to traffic particles and blood pressure in the Veterans Administration Normative Aging Study. Occupational and Environmental Medicine. 2012;69(6):422–427. doi: 10.1136/oemed-2011-100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuks KB, Weinmayr G, Foraster M, Dratva J, Hampel R, Houthuijs D, et al. Arterial blood pressure and long-term exposure to traffic-related air pollution: An analysis in the European Study of Cohorts for Air Pollution Effects (ESCAPE) Environmental Health Perspectives. 2014 doi: 10.1289/ehp.1307725. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Babisch W, Wolf K, Petz M, Heinrich J, Cyrys J, Peters A. Associations between traffic noise, particulate air pollution, hypertension, and isolated systolic hypertension in adults: The KORA study. Environmental Health Perspectives. 2014;122(5):492–498. doi: 10.1289/ehp.1306981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hazari MS, Griggs J, Winsett DW, Haykal-Coates N, Ledbetter A, Costa DL, et al. A single exposure to acrolein desensitizes baroreflex responsiveness and increases cardiac arrhythmias in normotensive and hypertensive rats. Cardiovascular Toxicology. 2014;14(1):52–63. doi: 10.1007/s12012-013-9228-9. [DOI] [PubMed] [Google Scholar]
- 73.Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. Journal of the American Society of Hypertension. 2009;3:332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Huang W, Zhu T, Pan X, Hu M, Lu SE, Lin Y, et al. Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: Interactions of systemic inflammation, overweight, and gender. American Journal of Epidemiology. 2012;176(2):117–126. doi: 10.1093/aje/kwr511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Routledge HC, Manney S, Harrison RM, Ayres JG, Townend JN. Effect of inhaled sulphur dioxide and carbon particles on heart rate variability and markers of inflammation and coagulation in human subjects. Heart. 2006;92(2):220–227. doi: 10.1136/hrt.2004.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valenti VE, Abreu LC, Saldiva PH, Carvalho TD, Ferreira C. Effects of sidestream cigarette smoke exposure on baroreflex components in spontaneously hypertensive rats. International Journal of Environmental Health Research. 2010;20(6):431–437. doi: 10.1080/09603123.2010.491852. [DOI] [PubMed] [Google Scholar]
- 77.Legramante JM, Valentini F, Magrini A, Palleschi G, Sacco S, Iavicoli I, et al. Cardiac autonomic regulation after lung exposure to carbon nanotubes. Human and Experimental Toxicology. 2009;28:369–375. doi: 10.1177/0960327109105150. [DOI] [PubMed] [Google Scholar]
- 78.Bartoli CR, Wellenius GA, Diaz EA, Lawrence J, Coull BA, Akiyama I, et al. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environmental Health Perspectives. 2009;117:361–366. doi: 10.1289/ehp.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lai CJ, Yang CCH, Hsu YY, Lin YN, Kuo TBJ. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. Journal of Applied Physiology. 2006;100(6):1974–1982. doi: 10.1152/japplphysiol.01051.2005. [DOI] [PubMed] [Google Scholar]
- 80.La Rovere MT, Gnemmi M, Vaccarini C. Baroreflex sensitivity. Italian Heart Journal Supplement. 2001;2:472–477. [PubMed] [Google Scholar]
- 81.Vanoli E, Schwartz PJ. Sympathetic–parasympathetic interaction and sudden death. Basic Research in Cardiology. 1990;85:305–321. doi: 10.1007/978-3-662-11038-6_25. [DOI] [PubMed] [Google Scholar]
- 82.Liu JL, Murakami H, Zucker IH. Effects of NO on baroreflex control of heart rate and renal nerve activity in conscious rabbits. American Journal of Physiology. 1996;270:1361–1370. doi: 10.1152/ajpregu.1996.270.6.R1361. [DOI] [PubMed] [Google Scholar]
- 83.Spieker LE, Corti R, Binggeli C, Luscher TF, Noll G. Baroreceptor dysfunction induced by nitric oxide synthase inhibition in humans. Journal of the American College of Cardiology. 2000;36:213–218. doi: 10.1016/s0735-1097(00)00674-4. [DOI] [PubMed] [Google Scholar]
- 84.Sithu SD, Srivastava S, Siddiqui MA, Vladykovskaya E, Riggs DW, Conklin DJ, et al. Exposure to acrolein by inhalation causes platelet activation. Toxicology and Applied Pharmacology. 2010;248:100–110. doi: 10.1016/j.taap.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Toole TE, Zheng YT, Hellmann J, Conklin DJ, Barski O, Bhatnagar A. Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicology and Applied Pharmacolog. 2009;236:194–201. doi: 10.1016/j.taap.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Queiroz TM, Monteiro MM, Braga VA. Angiotensin-II-derived reactive oxygen species on baroreflex sensitivity during hypertension: New perspective. Frontiers in Physiology. 2013;105:1–6. doi: 10.3389/fphys.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kara T, Narkiewicz K, Somers VK. Chemoreflexes—Physiology and clinical implications. Acta Physiologica Scandinavica. 2003;177(3):377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- 88.Roux JC, Peyronnet J, Pascual O, Dalmaz Y, Pequignot JM. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. Journal of Physiology. 2000;522:493–501. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Q, Kim J, Cinotte J, Homolka P, Wong-Riley MT. Carotid body denervation effect on cytochrome oxidase activity in pre-Botzinger complex of developing rats. Journal of Applied Physiology. 2003;94:1115–1121. doi: 10.1152/japplphysiol.00765.2002. [DOI] [PubMed] [Google Scholar]
- 90.López-Barneo J, Ortega-Sáenz P, Pardal R, Pascual A, Piruat JI. Carotid body oxygen sensing. European Respiratory Journal. 2008;32:1386–1398. doi: 10.1183/09031936.00056408. [DOI] [PubMed] [Google Scholar]
- 91.DeMeo DL, Zanobetti A, Litonjua AA, Coull BA, Schwartz J, Gold DR. Ambient air pollution and oxygen saturation. American Journal of Respiratory and Critical Care Medicine. 2004;170:383–387. doi: 10.1164/rccm.200402-244OC. [DOI] [PubMed] [Google Scholar]
- 92.Pope CA, Dockery DW, Kanner RE, Villegas GM, Schwartz J. Oxygen saturation, pulse rate, and particulate air pollution: A daily time-series panel study. American Journal of Respiratory and Critical Care Medicine. 1999;159:365–372. doi: 10.1164/ajrccm.159.2.9702103. [DOI] [PubMed] [Google Scholar]
- 93.Volpino P, Tomei F, La Valle C, Tomao E, Rosati MV, Ciarrocca M, et al. Respiratory and cardiovascular function at rest and during exercise testing in a healthy working population: Effects of outdoor traffic air pollution. Occupational Medicine (London) 2004;54:475–482. doi: 10.1093/occmed/kqh102. [DOI] [PubMed] [Google Scholar]
- 94.Gong H, Linn WS, Terrell SL, Anderson KR, Clark KW, Sioutas C, et al. Exposures of elderly volunteers with and without chronic obstructive pulmonary disease (COPD) to concentrated ambient fine particulate pollution. Inhalation Toxicology. 2004;16:731–744. doi: 10.1080/08958370490499906. [DOI] [PubMed] [Google Scholar]
- 95.Gong H, Linn WS, Clark KW, Anderson KR, Geller MD, Sioutas C. Respiratory responses to exposures with fine particulates and nitrogen dioxide in the elderly with and without COPD. Inhalation Toxicology. 2005;17:123–132. doi: 10.1080/08958370590904481. [DOI] [PubMed] [Google Scholar]
- 96.Adgent MA. Environmental tobacco smoke and sudden infant death syndrome: A review. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2006;77:69–85. doi: 10.1002/bdrb.20068. [DOI] [PubMed] [Google Scholar]
- 97.Hoppenbrouwers T, Calub M, Arakawa K, Hodgman JE. Seasonal relationship of sudden infant death syndrome and environmental pollutants. American Journal of Epidemiology. 1981;113:623–635. doi: 10.1093/oxfordjournals.aje.a113141. [DOI] [PubMed] [Google Scholar]
- 98.Wang T, Lang GD, Moreno-Vinasco L, Huang Y, Goonewardena SN, Peng YJ, et al. Particulate matter induces cardiac arrhythmias via dysregulation of carotid body sensitivity and cardiac sodium channels. American Journal of Respiratory Cell and Molecular Biology. 2012;46:524–531. doi: 10.1165/rcmb.2011-0213OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perez CM, Hazari MS, Ledbetter AD, Haykal-Coates N, Carll AP, Cascio WE, Winsett DW, Costa DL, Farraj AK. Acrolein inhalation alters arterial blood gases and triggers carotid body mediated cardiovascular responses in hypertensive rats. doi: 10.3109/08958378.2014.984881. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iturriaga R, Rey S, Del Rio R. Cardiovascular and ventilatory acclimatization induced by chronic intermittent hypoxia: A role for the carotid body in the pathophysiology of sleep apnea. Biological Research. 2005;38:335–340. doi: 10.4067/s0716-97602005000400004. [DOI] [PubMed] [Google Scholar]
- 101.Quan SF, Gersh BJ National Center on Sleep Disorders Research, National Heart Lung and Blood Institute. Cardiovascular consequences of sleep-disordered breathing: Past, present and future: Report of a workshop from the National Center on Sleep Disorders Research and the National Heart, Lung, and Blood Institute. Circulation. 2004;109:951–957. doi: 10.1161/01.CIR.0000118216.84358.22. [DOI] [PubMed] [Google Scholar]
- 102.Halliwill JR, Morgan BJ, Charkoudian N. Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. Journal of Physiology. 2003;552(Pt 1):295–302. doi: 10.1113/jphysiol.2003.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. Journal of Applied Physiology. 1988;65(4):1548–1552. doi: 10.1152/jappl.1988.65.4.1548. [DOI] [PubMed] [Google Scholar]
- 104.Halliwill JR, Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. Journal of Applied Physiology. 2002;93(3):857–864. doi: 10.1152/japplphysiol.01103.2001. [DOI] [PubMed] [Google Scholar]