Abstract
Thanks to numerous technological advances, the production of recombinant proteins in mammalian cell lines has become an increasingly routine task that is no longer viewed as a heroic enterprise. While production in prokaryotic or lower eukaryotic systems may be more rapid and economical, the advantages of producing large amounts of protein that closely resembles the native form is often advantageous and may be essential for the realization of functionally active material for biological studies or biopharmaceuticals. The correct folding, processing and post-translational modifications conferred by expression in a mammalian cell is relevant to all classes of proteins, including cytoplasmic, secreted or integral membrane proteins. Therefore considerable efforts have focused on the development of growth media, cell lines, transformation methods and selection techniques that enable the production of grams of functional protein in weeks, rather than months. This review will focus on a plethora of methods that are broadly applicable to the high yield production of any class of protein (cytoplasmic, secreted or integral membrane) from mammalian cells.
Cell type
The workhorse of mammalian protein expression in an a pharmaceutical company is Chinese Hamster Ovary cells (CHO), due to their relative ease of use and long history of regulatory acceptance for the production of biopharmaceuticals [1]. The top selling biologic in 2012 was Humira, a monoclonal antibody made in CHO cells, directed against tumor necrosis factor alpha for the treatment of rheumatoid arthritis, with sales of close to 9 billion dollars [2]. In development and laboratory settings, Human Embryonic Kidney 293 (HEK) cells are commonly used and, other novel cell lines may provide even more desirable properties for protein production. These include the human retina-derived Per.C6 and amniocyte-derived Cap-T lines, which are capable of very high-density growth (~5–15*107 cells/ml) that supports concomitant increases in protein yield and decreased media costs (reviewed in [3, 4]). Cell types may also be chosen, or engineered, to alter the extent of post-translational modifications such as glycosylation, lipidation, sulphation etc., which can modulate the activity of the target protein (e.g., 10–20 fold in the case of an anti-CD20 antibody) [5, 6]. Unfortunately these post-translational modifications, while essential for function, may be inversely correlated with the success of structural studies, as increased heterogeneity can adversely affect crystal packing [7]; but numerous options are available for the mitigation of these challenges. Several investigators have attempted to limit or homogenize glycosylation by the use of processing-deficient strains, such as the N-acetylglucosaminyl transferase I (GnTI) deficient HEK293S GnTI(−) line in the production of the hormone glucagon. Alternatively, inhibitors of glycosyl processing enzymes, such as kifunensine (that targets mannosidase 1) or swainsonine (that targets mannosidase 2), can be added to the growth media, which result in modifications that are more amenable to enzymatic removal of sugar moieties post-production. [8, 9].
Cell growth conditions
Advances in serum free media formulation allow for high-density cell growth (>1*106 cells/ml) in the absence of serum, which simplifies downstream purification and eliminates animal-based components, which alleviates some regulatory hurdles. Media has been optimized for protein production using design of experiment (DOE) approaches or metabolic analysis to derive optimized media, with the goal of increasing the protein yield per cell or volumetric yield (e.g., optimized CHO cell media supporting the ten-fold greater production of Tumor Necrosis factor fusion protein over yields from unoptimized basal media [10–12]). Additives can be used to supplement growth media such as histone deactylase inhibitors (e.g. valproic acid or sodium butyrate) to de-condense chromatin and increase the transcriptional activity of integrated genes with a concomitant enhancement in protein yield (e.g., four-fold increase in yield for an antibody produced in HEK293E cells after valproic acid addition). Proprietary feed solutions (HyClone Cell Boost, Thermo Scientific Inc) have been shown to increase yields and growth times by supplementing essential components that have become depleted in conditioned media; for example, doubling of the lifetime of a batch culture of CHO cells producing tissue plasminogen activator [13, 14]. Growth factors can also be added to the media. For example, the LONG R3 IGF-I engineered peptide appears to show culture enhancing properties, such as doubling cell viability over a 12 day experiment for some cells, including CHO, HEK293 and PER.C6, when compared to more routinely used insulin additives [15–17]. Growth factors and cell cycle regulators may also be introduced by co-transfection of expression plasmids (such as acidic fibroblast growth factor as demonstrated in Backliwal et al, [18]), rather than by addition of purified proteins, with a resulting savings in cost.
Cell growth and protein yields may also be made more robust by the use of carefully controlled growth environments (such as bioreactors) or by providing microenviroments via the use of micro or macrocarriers (Cytodex or Cytopore from GE Lifesciences or Fibra-Cel from New Brunswick) which shield cells from harmful shear forces and can increase yields 2–5 fold [19–21]. Hypothermic shifts (37°C to 33°C) have also been found to be advantageous for increasing cell specific productivity (e.g., at least two-fold for a variety of test proteins) and in particular for CHO cells [20, 22]. Growth vessels for cell culture have been improved by the introduction of convenient disposables for high-density growth in suspension such as the Tubespin bioreactor and the Optimum Growth™ flask (Thompson Instrument Company), which allows high-density growth with a smaller footprint than fernbach flasks [23]. For scale up (i.e., lab scale), many variants of ‘wave’ reactors (GE Lifesciences) are available including newer versions that rock in two rather than one direction, giving rise to more efficient mixing and mass transfer and potentially high cell viabilities (XRS Bioreactors from Pall Corporation); other platforms include hollow fiber bioreactors (FiberCell Systems Inc) which provides very high cell densities of cells in low volumes (up to 1*108/ml), various air lift bioreactors (Cellexus Inc), and even those with merry-go-round type waterwheels inside (PBS Biotech Inc); many of these technologies are relatively novel (especially in the disposable format), so head-to-head comparisons of utility are currently unavailable.
Transforming the cells and expression vectors
Large-scale transient transfection, which is useful for very rapid (days) protein production, is now an effective option, with more than 50 reported successful attempts to produce useful proteins, and various optimizations have lead to simpler operational procedure at even the hundred liter scale, giving rise to gram levels per liter of production [23–26]. In a highly optimized large-scale transient transfection protocol, grams per liter of recombinant antibodies have been generated by engineering the expression vector to utilize a human CMV promoter, an artificial intron and woodchuck post-transcriptional regulatory element (WPRE) [18]. This result highlights, not surprisingly, that the choice of promoter and other vector elements are critical factors in determining protein expression yields. This study also utilized high cell density transient transfection mediated by the economical transfection reagent, polyethylenimine (PEI), along with a series of plasmids expressing growth factors and cell cycle regulators. The importance of different 5′UTR and polyadenylation sequences in expression vectors has been investigated in other studies and they have been found to be of utility in particular cases. [27–29].
Design of experiment has also been utilized to optimize various parameters for large-scale transient gene expression and has shown that DNA concentration may be held at a relatively low level whist achieving good protein expression yields. This work is interesting as the production of large amounts of high quality (i.e. endotoxin free) DNA could be a severely limiting factor [30]. Relatively inexpensive, yet effective transfection reagents, in particular PEI, allow for efficient transfection at both small (sub milliliter) and large-scale (hundreds of liters) [24, 31, 32].
Piggybac transposons offer a method of integrating many copies of the transgene into the genome of the cell, leading to high levels of stable protein production (i.e., months) from clonally sorted or polyclonal populations. [33, 34], The highly active piggybac transposase specifically targets transposition into active areas of the mammalian genome (not silenced by chromatin structure) resulting in greater levels of protein expression. PiggyBac systems have been used to produce some highly interesting and active complexes (such as human gamma secretase) and also come in a single plasmid version that confers the ability to exploit inducible expression without extra modification of the host cell type (e.g., in contrast to the T-rex system [35–37].
Multiple systems are available for gene delivery and protein expression based on viral vectors. The optimized “Daedalus” lentiviral protein production system [38] has demonstrated the effectiveness of using lentivirus to rapidly (within a few weeks) generate stable cell lines for the production of mammalian proteins at up to the 100mg/L scale. This system employs a unique and minimized Ubiquitous Chromatin Opening Element (UCOE) of only 0.7kb to help maintain stable protein expression over several months. This optimized UCOE fragment supports the long-term culture of transduced cell lines in shake flasks or bioreactors for maximizing protein yield. Inducible systems are also available that may benefit from the absence of negative selection due to expression of toxic gene products during viral amplification, which could reduce titer [39].
Other popular systems are the BacMam system, in which insect cell viruses are generated in insect cells and subsequently used at high multiplicities of infection (MOI) to transduce mammalian cells [40]. Dukkipati et al. have used BacMam viruses to express GPCR-protein ligand complexes for structural studies in mammalian cells; the virus was engineered to encode a promoter (CMV) that is only active in mammalian cells, and not the insect cell. Another viral system derived from Semliki forest virus has been utilized for protein production after optimization to reduce cell cytopathic effects leading to more stable expression over time [41], with yields as high as 2mg/L/day.
Selection methods for high yields
Stable cell lines have a long history of being generated by integrate-and-select approaches, which while effective, are slowly being superseded by more rapid and facile technologies (i.e., a couple of weeks rather than ~6 months) [7]. Several selection (and amplification) techniques have exploited the ability of the expression plasmid to rescue the growth of an auxotropic strain in media deficient for a crucial compound, with the severity of auxotrophy being augmented (or produced) by supplementation of the growth media with inhibitory compounds that block key biosynthetic pathways. A notable example is dihydrofolate reducatase (DHFR)-enabled gene amplification, which can be used in DHFR-deficient cells in conjunction with inhibition of residual DHFR enzyme by the drug methotrexate, leading to amplification of the gene of interest that is supplied in an expression vector physically linked to the rescuing DHFR gene. Selection and amplification via this system has enabled the production of gram levels of protein (often antibodies) per liter in CHO cells, and improvements are being developed to increase the rapidity of amplification. Strategies to increase the speed of gene amplification include weakening the selection marker (such as via fusion of the murine ornithine decarboxylase PEST protease targeting sequence) and the use of DNA sequences that lead to spontaneous gene amplification in transformed cells (such as the cassette of the mammalian replication initiation region (IR) matrix attachment region (MAR)). [42–45]. In highly analogous systems, selection by the introduction of the glutamate synthase gene into cells that are deficient in this vital enzyme, or rendered so by the inhibition using methionine sulfoximine, is frequently used for protein production (maximal antibody yields of 5g/L, with a 20–25 weeks timeline from cloning to large-scale fermentation) and is offered commercially by Lonza, Inc [46]. Another system, called OSCAR™, uses a cell line that is deficient for purine synthesis. Cells are transfected with a rescue vector that supplies the gene of interest and a partially disabled hypoxanthine phosporibosyltransferase gene thus enabling selection for cell survival and amplification of the gene of interest in a single step [47]. Upon transformation and selection, a polyclonal pool of cells is established and further selection to a pure clonal line may be desirable to tease out highest producing cell lines. This often involves limited dilution to raise a single cell population. To aid in these efforts, flow cytometry has been successfully employed to enrich for high-level expressors via selection of cells that are fluorescently marked, either by co-expression of a fluorescent protein, or by a fluorescent protein fusion [48–50]. An intriguing selection method is Laser Enabled Analysis and Processing (LEAP), which involves imaging live adherent cells in bright field and fluorescent mode, with the degree of fluorescence being a marker of target protein expression [51]. The cells with zero or low target protein expression are destroyed via a laser pulse, leaving all other cells untouched. This process can be repeated at time intervals leaving a high expressing population of cells that have remained unmolested by the rigors of flow cytometric processing.
Discussion
It is an exciting time for the production of increasingly complex proteins and assemblies in greater yields and with ever decreasing timescales. Enormous attention continues to focus on the generation of mammalian expression systems to support academic and biopharma efforts. Overall, there are many effective paths for generating proteins and optimizing yields; perhaps lacking at this point are objective comparative studies that accurately evaluate the benefits (and weaknesses) of the many system with respect to one another. Despite this shortcoming, it is clear that the production of eukaryotic proteins via mammalian cell culture is becoming a more routine, rapid and successful route to realizing material for structural, functional and therapeutic use.
Figure 1.
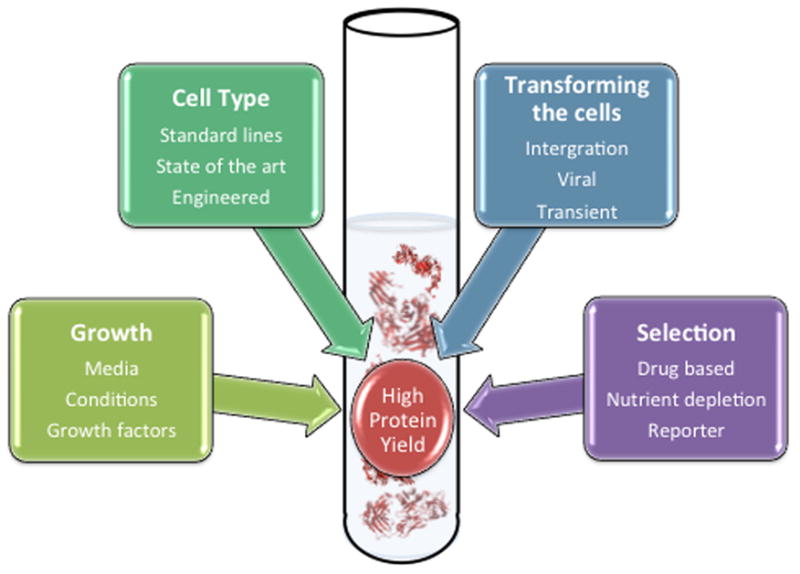
Options for generating and selecting cell lines to produce high yields of functional proteins.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants U54-GM094662 and U01-GM094665.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhu J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol Adv. 2012;30(5):1158–70. doi: 10.1016/j.biotechadv.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Huggett B. Public biotech 2012--the numbers. Nat Biotechnol. 2013;31(8):697–703. doi: 10.1038/nbt.2653. [DOI] [PubMed] [Google Scholar]
- 3**.Swiech K, Picanco-Castro V, Covas DT. Human cells: new platform for recombinant therapeutic protein production. Protein Expr Purif. 2012;84(1):147–53. doi: 10.1016/j.pep.2012.04.023. A review of novel cell types for protein production. [DOI] [PubMed] [Google Scholar]
- 4.Fischer S, et al. Transient recombinant protein expression in a human amniocyte cell line: the CAP-T(R) cell system. Biotechnol Bioeng. 2012;109(9):2250–61. doi: 10.1002/bit.24514. [DOI] [PubMed] [Google Scholar]
- 5.Wilke S, et al. Streamlining homogeneous glycoprotein production for biophysical and structural applications by targeted cell line development. PLoS One. 2011;6(12):e27829. doi: 10.1371/journal.pone.0027829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies J, et al. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol Bioeng. 2001;74(4):288–94. [PubMed] [Google Scholar]
- 7.Chaudhary S, et al. Overexpressing human membrane proteins in stably transfected and clonal human embryonic kidney 293S cells. Nat Protoc. 2012;7(3):453–66. doi: 10.1038/nprot.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unson CG. Expression of glucagon receptors in tetracycline-inducible HEK293S GnT1- stable cell lines: an approach toward purification of receptor protein for structural studies. Biopolymers. 2008;90(3):287–96. doi: 10.1002/bip.20951. [DOI] [PubMed] [Google Scholar]
- 9*.Chang VT, et al. Glycoprotein structural genomics: solving the glycosylation problem. Structure. 2007;15(3):267–73. doi: 10.1016/j.str.2007.01.011. A useful paper setting out the problems and solutions of working with glycoproteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham PL, et al. Large-scale transient transfection of serum-free suspension-growing HEK293 EBNA1 cells: peptone additives improve cell growth and transfection efficiency. Biotechnol Bioeng. 2003;84(3):332–42. doi: 10.1002/bit.10774. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Lee GM. Development of serum-free medium supplemented with hydrolysates for the production of therapeutic antibodies in CHO cell cultures using design of experiments. Appl Microbiol Biotechnol. 2009;83(4):639–48. doi: 10.1007/s00253-009-1903-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, et al. Rational development of a serum-free medium and fed-batch process for a GS-CHO cell line expressing recombinant antibody. Cytotechnology. 2013;65(3):363–78. doi: 10.1007/s10616-012-9488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Backliwal G, et al. Valproic acid: a viable alternative to sodium butyrate for enhancing protein expression in mammalian cell cultures. Biotechnol Bioeng. 2008;101(1):182–9. doi: 10.1002/bit.21882. HDAC inhbitors boosting protein yields. [DOI] [PubMed] [Google Scholar]
- 14.Sauer PW, et al. A high-yielding, generic fed-batch cell culture process for production of recombinant antibodies. Biotechnol Bioeng. 2000;67(5):585–97. [PubMed] [Google Scholar]
- 15.Voorhamme D, Yandell CA. LONG R3IGF-I as a more potent alternative to insulin in serum-free culture of HEK293 cells. Mol Biotechnol. 2006;34(2):201–4. doi: 10.1385/mb:34:2:201. [DOI] [PubMed] [Google Scholar]
- 16.Tsao YS, et al. Development and improvement of a serum-free suspension process for the production of recombinant adenoviral vectors using HEK293 cells. Cytotechnology. 2001;37(3):189–98. doi: 10.1023/A:1020555310558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King R, et al. Production and characterization of recombinant insulin-like growth factor-I (IGF-I) and potent analogues of IGF-I, with Gly or Arg substituted for Glu3, following their expression in Escherichia coli as fusion proteins. J Mol Endocrinol. 1992;8(1):29–41. doi: 10.1677/jme.0.0080029. [DOI] [PubMed] [Google Scholar]
- 18**.Backliwal G, et al. Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/l by transient transfection under serum-free conditions. Nucleic Acids Res. 2008;36(15):e96. doi: 10.1093/nar/gkn423. The authors investigate many factors leading to very high protein yeilds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tharmalingam T, et al. Enhanced production of human recombinant proteins from CHO cells grown to high densities in macroporous microcarriers. Mol Biotechnol. 2011;49(3):263–76. doi: 10.1007/s12033-011-9401-y. [DOI] [PubMed] [Google Scholar]
- 20.Nam JH, Ermonval M, Sharfstein ST. The effects of microcarrier culture on recombinant CHO cells under biphasic hypothermic culture conditions. Cytotechnology. 2009;59(2):81–91. doi: 10.1007/s10616-009-9196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knibbs RN, et al. Sustained high-yield production of recombinant proteins in transiently transfected COS-7 cells grown on trimethylamine-coated (hillex) microcarrier beads. Biotechnol Prog. 2003;19(1):9–13. doi: 10.1021/bp020092r. [DOI] [PubMed] [Google Scholar]
- 22*.Al-Fageeh MB, et al. The cold-shock response in cultured mammalian cells: harnessing the response for the improvement of recombinant protein production. Biotechnol Bioeng. 2006;93(5):829–35. doi: 10.1002/bit.20789. Hypothermic shifts as a method to increase productivity. [DOI] [PubMed] [Google Scholar]
- 23.Hacker DL, et al. Polyethyleneimine-based transient gene expression processes for suspension-adapted HEK-293E and CHO-DG44 cells. Protein Expr Purif. 2013;92(1):67–76. doi: 10.1016/j.pep.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Backliwal G, et al. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol Bioeng. 2008;99(3):721–7. doi: 10.1002/bit.21596. [DOI] [PubMed] [Google Scholar]
- 25.Tuvesson O, et al. Development of a generic transient transfection process at 100 L scale. Cytotechnology. 2008;56(2):123–36. doi: 10.1007/s10616-008-9135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vink T, et al. A simple, robust and highly efficient transient expression system for producing antibodies. Methods. 2014;65(1):5–10. doi: 10.1016/j.ymeth.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Mariati, et al. Evaluating post-transcriptional regulatory elements for enhancing transient gene expression levels in CHO K1 and HEK293 cells. Protein Expr Purif. 2010;69(1):9–15. doi: 10.1016/j.pep.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Wijesuriya SD, Cotter RL, Horwitz AH. Functional premature polyadenylation signals and aberrant splicing within a recombinant protein coding sequence limit expression. Protein Expr Purif. 2013;92(1):14–20. doi: 10.1016/j.pep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Goodman DB, Church GM, Kosuri S. Causes and effects of N-terminal codon bias in bacterial genes. Science. 2013;342(6157):475–9. doi: 10.1126/science.1241934. [DOI] [PubMed] [Google Scholar]
- 30*.Bollin F, Dechavanne V, Chevalet L. Design of Experiment in CHO and HEK transient transfection condition optimization. Protein Expr Purif. 2011;78(1):61–8. doi: 10.1016/j.pep.2011.02.008. Showing the utility of DOE methods for protein production. [DOI] [PubMed] [Google Scholar]
- 31.Kadlecova Z, et al. DNA delivery with hyperbranched polylysine: a comparative study with linear and dendritic polylysine. J Control Release. 2013;169(3):276–88. doi: 10.1016/j.jconrel.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Kadlecova Z, et al. Comparative study on the in vitro cytotoxicity of linear, dendritic, and hyperbranched polylysine analogues. Biomacromolecules. 2012;13(10):3127–37. doi: 10.1021/bm300930j. [DOI] [PubMed] [Google Scholar]
- 33.Matasci M, et al. Rapid recombinant protein production from pools of transposon-generated CHO cells. BMC Proc. 2011;5(Suppl 8):P34. doi: 10.1186/1753-6561-5-S8-P34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matasci M, et al. The PiggyBac transposon enhances the frequency of CHO stable cell line generation and yields recombinant lines with superior productivity and stability. Biotechnol Bioeng. 2011;108(9):2141–50. doi: 10.1002/bit.23167. [DOI] [PubMed] [Google Scholar]
- 35.Xiao S, et al. Transient and stable expression of the neurotensin receptor NTS1: a comparison of the baculovirus-insect cell and the T-REx-293 expression systems. PLoS One. 2013;8(5):e63679. doi: 10.1371/journal.pone.0063679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Alattia JR, et al. Highly efficient production of the Alzheimer’s gamma-secretase integral membrane protease complex by a multi-gene stable integration approach. Biotechnol Bioeng. 2013;110(7):1995–2005. doi: 10.1002/bit.24851. Production of a highly important membrane protein complex. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, et al. Simple piggyBac transposon-based mammalian cell expression system for inducible protein production. Proc Natl Acad Sci U S A. 2013;110(13):5004–9. doi: 10.1073/pnas.1218620110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandaranayake AD, et al. Daedalus: a robust, turnkey platform for rapid production of decigram quantities of active recombinant proteins in human cell lines using novel lentiviral vectors. Nucleic Acids Res. 2011;39(21):e143. doi: 10.1093/nar/gkr706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaillet B, et al. High-level recombinant protein production in CHO cells using lentiviral vectors and the cumate gene-switch. Biotechnol Bioeng. 2010;106(2):203–15. doi: 10.1002/bit.22698. [DOI] [PubMed] [Google Scholar]
- 40*.Dukkipati A, et al. BacMam system for high-level expression of recombinant soluble and membrane glycoproteins for structural studies. Protein Expr Purif. 2008;62(2):160–70. doi: 10.1016/j.pep.2008.08.004. An insect virus mediated method of protein production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casales E, et al. A novel system for the production of high levels of functional human therapeutic proteins in stable cells with a Semliki Forest virus noncytopathic vector. N Biotechnol. 2010;27(2):138–48. doi: 10.1016/j.nbt.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 42*.Ng SK. Generation of high-expressing cells by methotrexate amplification of destabilized dihydrofolate reductase selection marker. Methods Mol Biol. 2012;801:161–72. doi: 10.1007/978-1-61779-352-3_11. A commonly used gene amplification system and it’s improvement. [DOI] [PubMed] [Google Scholar]
- 43.Cacciatore JJ, Chasin LA, Leonard EF. Gene amplification and vector engineering to achieve rapid and high-level therapeutic protein production using the Dhfr-based CHO cell selection system. Biotechnol Adv. 2010;28(6):673–81. doi: 10.1016/j.biotechadv.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Noguchi C, et al. Fusion of the Dhfr/Mtx and IR/MAR gene amplification methods produces a rapid and efficient method for stable recombinant protein production. PLoS One. 2012;7(12):e52990. doi: 10.1371/journal.pone.0052990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cacciatore JJ, Leonard EF, Chasin LA. The isolation of CHO cells with a site conferring a high and reproducible transgene amplification rate. J Biotechnol. 2012;164(2):346–53. doi: 10.1016/j.jbiotec.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Knox R, et al. A streamlined implementation of the glutamine synthetase-based protein expression system. BMC Biotechnol. 2013;13:74. doi: 10.1186/1472-6750-13-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melton DW, et al. A one-step gene amplification system for use in cultured mammalian cells and transgenic animals. Transgenic Res. 2001;10(2):133–42. doi: 10.1023/a:1008951732020. [DOI] [PubMed] [Google Scholar]
- 48.Brezinsky SC, et al. A simple method for enriching populations of transfected CHO cells for cells of higher specific productivity. J Immunol Methods. 2003;277(1–2):141–55. doi: 10.1016/s0022-1759(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 49.Meng YG, et al. Green fluorescent protein as a second selectable marker for selection of high producing clones from transfected CHO cells. Gene. 2000;242(1–2):201–7. doi: 10.1016/s0378-1119(99)00524-7. [DOI] [PubMed] [Google Scholar]
- 50*.Carroll S, Al-Rubeai M. The selection of high-producing cell lines using flow cytometry and cell sorting. Expert Opin Biol Ther. 2004;4(11):1821–9. doi: 10.1517/14712598.4.11.1821. Cell sorting by fluorescent marker. [DOI] [PubMed] [Google Scholar]
- 51.Koller MR, et al. High-throughput laser-mediated in situ cell purification with high purity and yield. Cytometry A. 2004;61(2):153–61. doi: 10.1002/cyto.a.20079. [DOI] [PubMed] [Google Scholar]


