Abstract
A unique intervention combining mindfulness meditation with cognitive behavioral therapy for insomnia (CBT-I) has been shown to have acute benefits at post-treatment in an open label study. The aim of the present study was to examine the long-term effects of this integrated intervention on measures of sleep and sleep-related distress in an attempt to characterize the natural course of insomnia following this treatment and to identify predictors of poor long-term outcome. Analyses were conducted on 21 participants who provided follow-up data at 6 and 12 months post treatment. At each time point, participants completed one week of sleep and meditation diaries and questionnaires related to mindfulness, sleep, and sleep-related distress, including the Pre-Sleep Arousal Scale (PSAS), Glasgow Sleep Effort Scale (GSES), Kentucky Inventory of Mindfulness Skills (KIMS), and the Insomnia Episode Questionnaire. Analyses examining the pattern of change across time (baseline, end-of-treatment, 6 month, and 12 month) revealed that several sleep-related benefits were maintained during the 12-month follow-up period. Participants who reported at least one insomnia episode (≥ 1 month) during the follow-up period had higher scores on the PSAS (p < .05) and GSES (p < .05) at end-of-treatment compared to those with no insomnia episodes. Correlations between mindfulness skills and insomnia symptoms revealed significant negative correlations (p < .05) between mindfulness skills and daytime sleepiness at each of the three time points but not with nocturnal symptoms of insomnia. These results suggest that most sleep-related benefits of an intervention combining CBT-I and mindfulness meditation were maintained during the 12-month follow-up period with indications that higher pre-sleep arousal and sleep effort at end-of-treatment constitute a risk for occurrence of insomnia during the 12 months following treatment.
INTRODUCTION
Chronic insomnia is estimated to impact approximately one out of ten adults and has significant negative implications on quality of life (1). Cognitive-Behavioral Therapy for insomnia (CBT-I) has demonstrated efficacy and is currently recommended as the first line treatment for chronic insomnia (2). One notable advantage of CBT-I is its long-term effects beyond the completion of treatment, especially when compared to sedative-hypnotic medication. Long term superiority of CBT-I was established relative to a variety of medications for different length of follow up periods and different target outcomes. In a seminal study using a 24-month follow-up period, CBT-I was found to have the most durable sleep benefits when compared to temazepam, a combination of CBT-I and temazepam, and placebo (3). CBT-I was also found to be superior to zolpidem for reducing sleep onset latency, with these gains maintained during a 12-month follow-up period (4). Finally, CBT-I was more effective in improving sleep efficiency when compared to zopiclone at post-treatment and at 6-month follow-up (5). Although these findings are impressive, these studies only evaluated sleep measures at each follow-up time point and did not provide information about patients’ sleep between follow-up visits. Importantly, these studies provided a snapshot of patients’ sleep at the follow-up visits and did not evaluate the rates of occurrence of insomnia episodes during the follow-up period. Thus, the natural course of the insomnia disorder following treatment has not been well characterized and risk factors associated with occurrence of future episodes of insomnia have not been identified.
Mindfulness meditation is a self-regulation practice that has several health benefits when taught in a short-term group program known as mindfulness-based stress reduction (MBSR). Similar to CBT-I, the MBSR program has been found to have long-term benefits that extend beyond the end of treatment. In a three-year follow-up study of individuals diagnosed with anxiety disorders, improvements in symptoms of anxiety and depression achieved at post-treatment were maintained at the three-year follow-up with 56% of the participants maintaining a meditation practice (6). MBSR has also been found to have durable effects on symptoms of depression, anxiety, and pain during a three-year follow-up of individuals with fibromyalgia (7). Mindfulness-Based Cognitive Therapy (MBCT) is a similar mindfulness-based program that is specifically designed to prevent the relapse of depression. Over a 60-week period, MBCT was found to significantly reduce the risk of relapse compared to treatment as usual among individuals who reported at least three previous episodes of depression (8). It has been hypothesized that the emphasis on self-regulation and the application of the principles of mindfulness meditation into participants’ daily lives might account for the long-term benefits of a mindfulness-based program (6, 7).
The practice of mindfulness meditation has been previously linked to sleep improvements however its effects on people seeking treatment for chronic insomnia are not entirely clear. The MBSR program has been found to improve sleep among cancer patients (9, 10) and adolescents with substance abuse history (11). However, Winbush and colleagues (12) conducted a systematic review of the effects of MBSR on sleep disturbances and identified several methodological limitations in the literature that precluded conclusions about the efficacy of MBSR for disturbed sleep We recently reported that a unique intervention integrating mindfulness meditation with CBT-I had benefits at post-treatment on both sleep and sleep-related distress for individuals with psychophysiological insomnia (13). We reported statistically and clinically significant improvements in several nighttime symptoms of insomnia as well as statistically significant reductions in pre-sleep arousal, sleep effort, and dysfunctional sleep-related cognitions. The long-term benefits of MBSR and CBT-I on their targeted symptoms suggests that combing the two could potentially improve the long-term management of chronic insomnia by leading to sustained improvements in both sleep and associated daytime distress.
The purpose of this paper is to report on the 12-month naturalistic follow-up period following an open trial of a treatment of chronic insomnia that combines mindfulness meditation and CBT for insomnia. While evidence of the acute treatment effects and the feasibility and acceptability of the treatment have been reported (13, 14), the specific aims of this paper were to: (1) examine the long-term effects of the intervention at 6 and 12-months post treatment on indices of insomnia severity; (2) document the occurrence of insomnia episodes during the follow-up period; and (3) explore the relationship between insomnia symptoms, mindfulness skills, and meditation practice during the follow-up period. We hypothesized the integrated intervention would show long-term benefits on indices of insomnia consistent with previous non-pharmacological treatments for insomnia.
METHODS
The present study examined a sub-group of participants who completed a Stage I treatment-development study which followed the guidelines for developing behavior treatments (15). Given this early stage of testing, the focus was on developing the intervention and examining possible effects of treatment rather than testing treatment efficacy. Therefore, no control condition was employed and participants were assessed on a variety of measures to examine possible treatment effects and to guide the design of future studies in this area.
Participants
Participants included adults between 18–65 years who met research diagnostic criteria for Psychophysiological Insomnia (16). They were recruited via advertisements posted on bulletin boards, internet listings, and referrals from other clinics and research studies within the local institution. The screening process consisted of a brief telephone screen to determine general eligibility followed by an in-person interview, which consisted of a semi-structured interview to assess the inclusion and exclusion criteria (see below) and completion of baseline questionnaires. Participants who met all criteria for the study were then contacted to schedule the first treatment session. Participants were excluded if they reported symptoms suggestive of another sleep disorder (e.g., excessive daytime sleepiness, loud snoring), untreated mood, anxiety, or psychotic disorder, frequent use of alcohol within two hours before bedtime, or were experiencing a current medical condition requiring treatment not provided in this study. A total of 30 participants met inclusion and exclusion criteria and began the intervention (please see procedures below) and 27 completed the treatment program. Out of these 27 participants, 21 individuals provided follow-up data. For the present study, analyses were conducted on these 21 participants. The study protocol was approved by the local Institutional Review Board and all participants provided written informed consent during the screening interview. Please see (13) for further details on screening and intervention procedures of the treatment phase of the study.
Measures
Daily sleep and meditation diaries
Self-reports of sleep patterns were completed each morning for one week at the end of treatment (EOT) and at 6 and 12 months post-treatment using sleep diaries. The sleep variables derived from the diary included: (a) total sleep time (TST), (b) total wake time (TWT), which is the sum of sleep onset latency (SOL), wake time after sleep onset (WASO), and terminal wakefulness (TWAK), (c) time in bed (TIB), and (d) number of awakenings (NWAK). Using this data, sleep efficiency (SE) was calculated as (TST / TIB) × 100. Ratings of sleep quality, daytime sleepiness, and daytime tiredness were also assessed using a 10-point Likert scale (1 to 10) with higher numbers reflecting greater sleep quality, sleepiness, and tiredness, respectively. In addition to the sleep items, prospective self-report data on the frequency and duration of meditation activity were completed daily for one week at EOT, and at 6 and 12 months post-treatment. These data were collected to monitor the extent of daily practice of mindfulness meditation. The means and standard deviations across the week at each time point (EOT, 6, 12 month follow-up) were calculated for each sleep and meditation variable.
Insomnia Severity Index (ISI)
The Insomnia Severity Index (ISI) is a brief 5-item scale that has been used as both a screening and outcome measure in insomnia treatment research (17). It provides an index of the severity of insomnia over the past week, taking into account both nighttime and daytime symptoms. The scale has adequate internal consistency (Cronbach’s alpha = .74 to .78) with evidence supporting concurrent, predictive, and content validity (17).
Kentucky Inventory of Mindfulness Skills (KIMS)
The KIMS is a 39-item self-report measure developed by Baer and colleagues (18) to measure mindfulness skills. This scale has adequate to good internal consistency (coefficient alpha = .83 to .91) and evidence for validity was supported (18). Factor analysis revealed four factors: 1) observe, 2) describe, 3) act with awareness, and 4) accept without judgment.
Pre-Sleep Arousal Scale (PSAS)
The PSAS is a 16-item self-report measure that assesses somatic and cognitive arousal in the period prior to sleep. The scale is organized into two subscales (somatic and cognitive arousal) and a 5-point Likert scale (1 “not at all” to 5 “extremely”) is used to rate the extent to which each item is experienced. Adequate internal consistency has been reported for the PSAS (alpha = .76 and .81 for the somatic and cognitive scales respectively) and elevated PSAS scores have been found to be associated with sleep-onset difficulties (19). In this study, a time frame of the past week was used.
Glasgow Sleep Effort Scale (GSES)
The GSES is a 7-item self-report measure of sleep effort during the past week scored on a 3-point Likert scale (0, 1, 2). The scale has adequate internal consistency (Cronbach’s alpha = .77) with evidence discriminating insomnia patients from good sleepers (20).
Positive and Negative Affect Schedule (PANAS)
The PANAS consists of a 10-item positive affect (PA) scale and a 10-item negative affect (NA) scale used as a brief assessment of mood (21). Participants are asked to rate the extent to which they have experienced each of the items on a 5-point Likert scale (1 “Very little/not at all” to 5 “Extremely”). The two scales have high internal consistency (PA alpha = .86 to .90; NA alpha = .84 to .87), are stable over a 2-month period, and are largely uncorrelated.
Insomnia Episode Questionnaire (IEQ)
The IEQ is a questionnaire developed for this study to determine whether an insomnia episode occurred during the six months preceding the assessment. It provides a clinically meaningful measure of the long-term effects of treatment. The IEQ consists of a 6-month timeline in weekly intervals and was given at both follow-up assessments. For each weekly interval, participants retrospectively recorded whether or not they experienced insomnia symptoms, defined as SOL > 30 minutes or WASO > 30 minutes at least 3 times that week. These criteria follow the recommendations for the quantitative criteria of insomnia (22). Subsequently, the pattern of insomnia symptoms over the 6-month time frame was examined for insomnia episodes, defined as four consecutive weeks of having insomnia symptoms described above. This criteria follows recommendations for duration of psychophysiological insomnia (16).
Procedures
The 6-week group intervention consisted of an integration of the mindfulness training and exercises from the MBSR program (23) and group CBT-I (24, 25). A novel aspect of this intervention was the dual emphasis placed on alleviating the nocturnal symptoms of insomnia and decreasing emotional and physiological arousal (e.g., coping with sleep-related distress). The intervention was conducted in 2-hour weekly sessions over a 6 week period. Starting from the second session, formal meditation practice (body scan, sitting and walking meditation) was taught for approximately 30 minutes followed by a discussion of the application of mindfulness principles to real-world situations. Participants were instructed to engage in meditation practice for at least 30 minutes per day, five days per week between sessions. The second half of the session was spent discussing behavioral changes focusing on nocturnal symptoms (sleep restriction, stimulus control, sleep education, and sleep hygiene) that are taught in most CBT for insomnia treatment packages.
Following the intervention, participants were sent a follow-up questionnaire packet at 6 and 12 months post treatment. In the packets, participants were instructed to first complete the sleep and meditation diary for one week and then complete the questionnaires to synchronize the time frame between the responses on the questionnaires with the sleep and meditation diaries. The packet included a self-addressed, pre-stamped envelope for participants to return. Reminders were sent to participants who did not return the packets within one week of the follow-up date. No financial compensation was offered for completing the follow-up data.
Data Analysis
Given the aims of treatment-development research and the small sample size in this pilot study, non-parametric statistics were conducted for all analyses. First, a series of Friedman Tests, a non-parametric alternative to repeated measures ANOVA, were conducted comparing baseline, EOT, 6-month follow-up, and 12-month follow-up data. Dependent variables were selected to capture a wide range of effects and included TWT, TST, SE, sleep quality, daytime sleepiness, daytime tiredness, ISI, PSAS (total), GSES, KIMS (total), and PANAS (positive and negative scales). The last observation was carried forward to replace any missing follow-up data (6 or 12 months). For significant findings, post-hoc comparisons were conducted to examine the pattern of change across time. Following recommendations by Sheldon and colleagues (26), the following formula was used to determine significance: |Rj – Ri |≥ z √ (Nk(k+1) / 6) where Rj – Ri is the difference in the sums of ranks being compared (e.g., baseline – 6 month follow-up), z = 2.64 (the z score from the normal curve corresponding to α = .05 adjusted for multiple comparisons), N is the sample size, and k is the number of measurements (k=4). Second, a series of Mann Whitney-U tests were conducted to identify which EOT variables distinguished between those who did and those who did not experience an insomnia episode during the 12-month follow up period. Each model included occurrence (n = 7) versus non-occurrence (n = 11) as the independent variable and an EOT variable (same as above) as the dependent variable. To explore the relationship between mindfulness meditation and sleep, Spearman’s Rho correlations (ρ) were computed separately at each of the three time point (EOT, 6-month follow-up, 12-month follow-up) between two mindfulness variables (KIMS total sore, total minutes of meditation practice during past week) and six sleep-related variables (ISI, TWT, TST, sleep quality, sleepiness, tiredness) for a total of 12 ρ correlations at each time point. An α level = .05 was selected for all analyses (except post-hoc comparisons) to balance both Type I and Type II errors as recommended in pilot studies (27).
RESULTS
Demographics
The average age of the participants was 38.7 years (SD = 13.6; range = 21–62 years) and 52% were female. The ethnic distribution was as follows: 66.7% Caucasian, 23.8% Asian, and 9.5% other. All participants had a high school education or above, with an average of 17.6 years of education (range = 14–22 years). With regards to marital status, 52.4% of the participants were single and 47.6% were married.
Long-Term Effects
The means and standard deviations of the sleep and daytime variables collected at baseline, EOT, 6-month follow-up, and 12-month follow-up are presented in Table 1. Friedman tests conducted on the dependent variables selected above revealed significant results on TWT (Friedman χ2 (3) = 21.37, p < .005), SE (Friedman χ2 (3) = 24.84, p < .005), sleep quality (Friedman χ2 (3) = 7.97, p < .05), daytime tiredness (Friedman χ2 (3) = 11.35, p < .05), PSAS (Friedman χ2 (3) = 17.10, p < .01), GSES (Friedman χ2 (3) = 19.68, p < .005), and ISI (Friedman χ2 (3) = 35.53, p < .005). Post-hoc tests revealed that baseline levels were significantly different than EOT, 6-month, and 12-month follow-up for TWT, SE, GSES, and ISI, indicating that treatment gains were present at EOT and maintained throughout the follow-up period. For PSAS, baseline levels were significantly different than EOT and 6-month follow-up but not the 12-month follow-up. For sleep quality and daytime tiredness, baseline levels were only significantly different than 6-month follow-up. No significant results were found on TST, daytime sleepiness, KIMS, or PANAS.
Table 1.
Long-term Effects of Treatment
| Measures | Baseline | End of Treatment |
6 Month Follow-up |
12 Month Follow-up |
||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Sleep Diaries | ||||||||
| TST (min) | 384.19 | 63.76 | 382.41 | 59.39 | 390.33 | 72.13 | 380.32 | 109.00 |
| TWT (min) | 100.21 | 60.84 | 42.56* | 32.93 | 49.23* | 34.64 | 47.65* | 31.69 |
| SOL (min) | 33.15 | 23.69 | 15.58 | 10.13 | 20.37 | 15.03 | 20.16 | 19.56 |
| WASO (min) | 42.94 | 45.32 | 14.04 | 12.80 | 17.01 | 16.23 | 20.50 | 17.90 |
| TWAK (min) | 24.12 | 21.76 | 12.93 | 16.93 | 11.85 | 17.19 | 11.50 | 16.11 |
| TIB (min) | 483.41 | 78.14 | 424.97 | 53.03 | 439.36 | 75.81 | 422.76 | 116.86 |
| SE (%) | 79.97 | 10.17 | 89.93* | 7.97 | 88.93* | 7.95 | 89.91* | 10.28 |
| NWAK | 2.37 | 1.82 | 1.28 | 1.33 | 0.87 | 0.62 | 1.40 | 1.31 |
| Sleep Quality | 5.85 | 1.09 | 6.49 | 1.13 | 6.79* | 1.30 | 6.70 | 1.23 |
| Daytime Sleepiness | 4.18 | 1.84 | 3.97 | 2.04 | 3.54 | 1.88 | 3.53 | 1.89 |
| Daytime Tiredness | 4.21 | 1.63 | 4.23 | 1.75 | 3.49* | 1.79 | 3.46 | 2.02 |
| PSAS Total | 32.90 | 8.04 | 25.14* | 5.84 | 27.33* | 8.06 | 27.57 | 6.55 |
| Cognitive | 19.86 | 5.65 | 14.76 | 5.20 | 16.05 | 5.81 | 16.24 | 4.41 |
| Somatic | 13.05 | 3.69 | 10.38 | 2.27 | 11.29 | 3.29 | 11.33 | 3.06 |
| GSES | 6.70 | 2.92 | 3.00* | 2.17 | 3.05* | 3.05 | 2.81* | 3.14 |
| ISI | 14.65 | 4.18 | 8.29* | 3.38 | 8.02* | 4.40 | 7.10* | 3.55 |
| KIMS Total | 132.48 | 11.79 | 135.52 | 15.80 | 136.24 | 13.84 | 136.95 | 14.85 |
| Observe | 34.33 | 5.78 | 36.52 | 7.30 | 36.43 | 5.91 | 39.76 | 7.15 |
| Describe | 29.10 | 5.71 | 29.81 | 6.51 | 29.38 | 5.97 | 29.43 | 6.19 |
| Awareness | 30.67 | 5.56 | 31.57 | 6.19 | 31.62 | 6.60 | 31.19 | 5.61 |
| Accept | 34.19 | 5.10 | 33.48 | 6.87 | 34.95 | 7.03 | 35.48 | 6.40 |
| PANAS | ||||||||
| Positive | 33.71 | 6.01 | 34.95 | 6.08 | 32.57 | 6.45 | 34.33 | 6.61 |
| Negative | 17.14 | 4.97 | 15.52 | 4.07 | 16.05 | 4.19 | 15.52 | 4.07 |
Note. Analyses were conducted on selected variables of interest. Asterisk (*) indicates levels that were significantly different from baseline on post-hoc comparisons. Sleep quality ranges from 1 (very poor) to 10 (excellent). Daytime sleepiness and tiredness are rated from 1 to 10 with higher numbers reflecting higher levels of sleepiness / tiredness respectively. Baseline means and standard deviations for ISI and GSES are based on n = 20 due to incomplete data for one participant.
Episodes of Insomnia
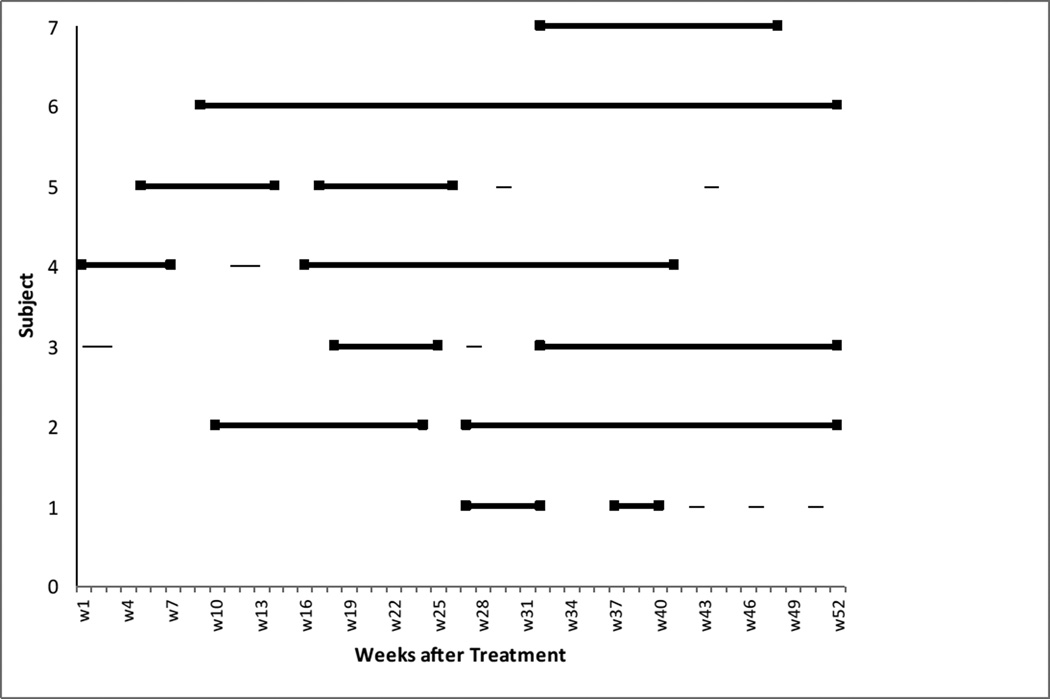
Data from the IEQ revealed that 7 out of the 18 participants (38.9%) who completed the questionnaire had an episode of insomnia (as defined above) during the 12-month follow-up period (see Figure 1). Only one of these 7 participants met criteria for insomnia symptoms (SOL > 30 minutes or WASO > 30 minutes at least 3 times that week) during the first month following EOT. The other 6 developed an insomnia episode later in the follow up period. The average length of an episode for this group lasted 16.3 weeks (SD = 11.5 weeks). The means and standard deviations of sleep and mindfulness related constructs for those who experienced an insomnia episode and those who did not are summarized in Table 2. Comparisons between the two groups revealed that participants with an occurrence had significantly higher scores on the PSAS at EOT (M = 29.14, SD = 6.62) compared with those with no occurrence (M = 22.64, SD = 3.91), Mann-Whitney U = 16.00, p < .05. In addition, those with an occurrence had significantly higher scores on the GSES at EOT (M = 4.86, SD = 2.41) compared with those with no occurrence (M = 2.18, SD = 1.40), Mann-Whitney U = 13.00, p < .05. No other significant differences were found between the two groups at EOT.
Figure 1.
Occurrence of insomnia episodes (n = 7) across the 52 weeks of follow-up based on data from the IEQ. An episode of insomnia consisted of at least four consecutive weeks with difficulty initiating or maintaining sleep for at least three nights each week. The beginning and end point of each episode is marked by a solid square. Brief periods of insomnia not meeting criteria for an episode do not have solid squares.
Table 2.
End-of-Treatment Data for Presence or Absence of Insomnia Episodes.
| Measures at End-of Treatment |
Occurrence of Insomnia |
No Occurrence of Insomnia |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Sleep Diaries | ||||
| TST (min) | 369.18 | 49.98 | 405.51 | 52.69 |
| TWT (min) | 31.02 | 11.01 | 39.10 | 17.86 |
| SOL (min) | 12.96 | 5.04 | 16.39 | 10.98 |
| WASO (min) | 8.88 | 7.11 | 12.77 | 10.43 |
| TWAK (min) | 9.18 | 7.64 | 9.95 | 7.04 |
| TIB (min) | 400.20 | 45.76 | 444.61 | 58.74 |
| SE (%) | 92.14 | 3.02 | 91.24 | 3.56 |
| NWAK | 0.86 | 0.91 | 1.27 | 1.12 |
| Sleep Quality | 6.51 | 1.26 | 6.72 | 0.98 |
| Daytime Sleepiness | 4.41 | 2.07 | 3.73 | 2.16 |
| Daytime Tiredness | 4.61 | 1.85 | 4.02 | 1.85 |
| PSAS Total* | 29.14 | 6.62 | 22.64 | 3.91 |
| Cognitive | 18.29 | 6.47 | 12.64 | 2.62 |
| Somatic | 10.86 | 1.77 | 10.00 | 2.79 |
| GSES* | 4.86 | 2.41 | 2.18 | 1.40 |
| ISI | 10.14 | 3.48 | 7.45 | 3.24 |
| KIMS Total | 141.14 | 18.77 | 131.82 | 15.40 |
| Observe | 40.29 | 7.70 | 35.00 | 6.59 |
| Describe | 29.86 | 6.74 | 30.09 | 7.33 |
| Awareness | 31.43 | 7.76 | 31.82 | 6.01 |
| Accept | 35.14 | 5.93 | 31.00 | 6.51 |
| PANAS | ||||
| Positive | 36.14 | 6.57 | 35.00 | 6.37 |
| Negative | 17.86 | 5.40 | 14.27 | 2.94 |
| Total Weeks with Insomnia | 30.71 | 11.01 | 1.91 | 2.81 |
Note. Analyses were conducted on selected variables of interest. Asterisk (*) indicates significant difference between groups. Sleep quality ranges from 1 (very poor) to 10 (excellent). Daytime sleepiness and tiredness are rated from 1 to 10 with higher numbers reflecting higher levels of sleepiness / tiredness respectively.
Relationship between Mindfulness Meditation and Sleep Diary Variables
Spearman’s Rho correlations between mindfulness and sleep-related variables at the EOT revealed significant negative correlations between KIMS total score and daytime sleepiness (ρ = -.65, p < .01, n = 20) and between the KIMS total score and daytime tiredness (ρ = −.66, p < .01, n = 20). At the 6-month follow-up, significant negative correlations were found between KIMS and daytime sleepiness (ρ = −.71, p < .01, n = 16) and the KIMS and daytime tiredness (ρ = −.62, p < .05, n = 16). At the 12-month follow-up, a significant negative correlations was again found between KIMS and daytime sleepiness (ρ = −.54, p < .05, n = 15) and a significant positive correlation was found between TST and the amount of meditation during the past week (ρ = .54, p < .05, n = 17). No other significant correlations were found.
DISCUSSION
This study examined the naturalistic follow-up over a twelve month period of a novel intervention combining mindfulness meditation and CBT for insomnia. The three main findings from this study are: (1) benefits of this combined intervention were generally maintained during the 12-month follow-up period in terms of symptom severity at each follow-up time point and the course of insomnia symptoms across the 12-month period; (2) higher pre-sleep arousal and sleep effort at EOT were associated with worse long-term outcome (experience of an insomnia episode during the follow up period); (3) mindfulness skills were negatively associated with perceived daytime sleepiness across each time point.
In general, the observed long-term benefits of this intervention are consistent with previous findings of the durability of sleep benefits following CBT-I. Most notably, total wake time, sleep efficiency, and overall insomnia severity demonstrated significant improvements at the end of treatment and at both follow-up assessments relative to baseline. The maintenance in decreased total wake time during follow-up is comparable to previous studies examining CBT-I (3, 4). Furthermore, sleep efficiency was relatively stable across the follow-up time points and well within the normal range for good sleepers (16). Long-term gains were also found on measures of pre-sleep arousal and sleep effort, although the gains in pre-sleep arousal were no longer significant at the 12-month follow-up. The reduction in arousal is consistent with the hypothesized mechanism of this combined intervention (13). In contrast to the sleep benefits, no significant changes were found on the KIMS and PANAS, suggesting that the current intervention might not have been adequately developed to provide the full benefits of mindfulness meditation that has been reported in other long-term follow-ups (6, 7)
In addition to traditional follow-up measures in sleep, the present study employed the IEQ, a new measure developed for this project to track the longitudinal course of insomnia episodes across the follow-up period. Data from the IEQ revealed that 61% of participants who provided data reported no occurrence of insomnia during the follow-up period. To our knowledge, this is the first study to report on the occurrence of insomnia episodes following treatment. Still, despite equivalent mean scores of the sleep variables at EOT and follow-up assessment, 39% of the sample reported at least one episode of insomnia during the follow-up period. This suggests that snapshot evaluations of sleep at discrete follow-up time points (e.g., 3-months) might not be sufficiently sensitive for capturing the natural course of insomnia following treatment. Given the lack of guidelines in the insomnia literature for determining long-term outcomes of treatment, development of standard definitions for response, remission, relapse, and recurrence, such as that proposed for depression (28) would aid future research in characterizing the course of insomnia following treatment.
Higher pre-sleep arousal and sleep effort at EOT were observed in those who experienced an occurrence of an insomnia episode during the follow-up period compared with those who did not. This suggests that residual sleep effort and arousal at the end of treatment might constitute a risk for future occurrence of insomnia episodes. This finding is consistent with the psychophysiological model of insomnia (29), which posits that elevated arousal is a perpetuating factor in the development of chronic insomnia. Moreover, the temporal relationship of the data in this study indicates that hyperarousal is a cause rather than an epiphenomenon of sleep disturbance. The preliminary findings here provide evidence for future hypothesis-testing studies to examine changes in pre-sleep arousal and sleep effort in relation to long-term outcomes.
Interestingly, we found a general pattern of perceived improvement in daytime functioning that was associated with more self-reported mindfulness skills. There was a significant decrease in perceived daytime tiredness at 6 months with only a modest 8-minute increase in total sleep time, indicating that increased sleep time alone was unlikely to account for the improvement in daytime tiredness. We also found a negative relationship between mindfulness skills and daytime tiredness at the end of treatment and 6 months follow-up and a negative relationship between mindfulness skills and daytime sleepiness across all three time points. These findings might be explained within Jon Kabat-Zinn’s conceptual framework of mindfulness as a state of becoming “awakened” (30). In this context, mindfulness consists of the awareness that arises through intentionally attending to the present moment in an open, accepting, and curious way (31). This heightened state of awareness is often described as an experience of being awake, alive, and alert - states that are unlikely to coincide with sleepiness and tiredness. Using this framework, one explanation for the present findings is that increases in mindfulness skills contributed to the improvement in daytime functioning. The increase in the Accept without Judgment factor of the KIMS at 12 months follow-up suggests that increased acceptance of tiredness during the day might lead to reduced distress about it and thus to lower perceived tiredness. For example, instead of expending energy judging or reacting to the perception of tiredness one can note it and attend to it directly. Further, the accepting quality of the attention may have a beneficial impact on daytime tiredness by changing one's relationship to the perception of tiredness. Given the bi-directional nature of correlations, an alternative interpretation is that higher levels of sleepiness may lead to a reduction in mindfulness skills. Taken together, these findings indicate that mindfulness skills and elevated sleepiness appear to be incompatible states. Investigating the hypothesis that cultivating mindfulness skills could decrease daytime sleepiness merits direct testing in future research and could have important clinical implications. This is especially important given that the present data did not find a clear relationship between the length and frequency of mindfulness meditation practice and insomnia symptoms.
Several limitations of this study are inherent in its design as an uncontrolled treatment development study and should be noted. First, the absence of a control group and the small sample limit our ability to conclude that the observed long-term effects can be attributed to this treatment. Further, conclusions regarding the specific benefits attributable to mindfulness meditation cannot be determined from these data as the present design precludes teasing apart the relative contribution of mindfulness training and CBT. Second, the number of comparisons in the analyses could lead to Type I errors. As with other uncontrolled pilot studies, the present findings were meant to stimulate and guide the design of subsequent hypothesis-testing studies, which should be conducted before clinical applications can be recommended. Another limitation of this study is its reliance on self-report questionnaires that are subject to recall bias and / or social desirability. This was particularly notable for the IEQ, which required retrospective week-by-week recall of insomnia-related symptoms for the past six months. This limitation is mitigated, in part, by the fact that participants did not have access to previous data and were not paid for completing the follow-up questionnaires.
Despite these limitations, the results suggest that a novel integrated treatment for insomnia has significant benefits which continue up to one year after treatment. Based on these findings, we suggest four specific areas for future study: 1) testing the long term effect of this intervention and the specific benefits of mindfulness meditation in a randomized controlled trial; 2) the inclusion of occurrence of insomnia episodes as a clinically meaningful measure of long-term outcome; 3) further exploration of the relationship between mindfulness and daytime functioning; and 4) further investigation of residual hyperarousal and sleep effort as a risk of future occurrence of insomnia. Future research building upon these findings will enhance the understanding and treatment of chronic insomnia.
Acknowledgments
This project was supported in part by a National Institutes of Health, National Research Service Award (MH 19938) awarded to the first author. Portions of the data from this study were presented at the 2008 meeting for the Associated Professional Sleep Societies in Baltimore, MD.
REFERENCES
- 1.National Institutes of Health State of the Science Conference Statement. Manifestations and Management of Chronic Insomnia in Adults. [June 13–15, 2005];Sleep. 2005 28(9):1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 2.Stepanski E. Hypnotics should not be considered for the initial treatment of chronic insomnia. Journal of Clinical Sleep Medicine. 2005;1(2):125–128. [PubMed] [Google Scholar]
- 3.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. Jama. 1999 Mar 17;281(11):991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. 2004 Sep 27;164(17):1888–1896. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- 5.Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. Jama. 2006 Jun 28;295(24):2851–2858. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 6.Miller JJ, Fletcher K, Kabat-Zinn J. Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. Gen Hosp Psychiatry. 1995 May;17(3):192–200. doi: 10.1016/0163-8343(95)00025-m. [DOI] [PubMed] [Google Scholar]
- 7.Grossman P, Tiefenthaler-Gilmer U, Raysz A, Kesper U. Mindfulness Training as an Intervention for Fibromyalgia: Evidence of Postintervention and 3-Year Follow-Up Benefits in Well-Being. Psychother Psychosom. 2007;76(4):226–233. doi: 10.1159/000101501. [DOI] [PubMed] [Google Scholar]
- 8.Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000 Aug;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 9.Carlson LE, Garland SN. Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. Int J Behav Med. 2005;12(4):278–285. doi: 10.1207/s15327558ijbm1204_9. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro SL, Bootzin RR, Figueredo AJ, Lopez AM, Schwartz GE. The efficacy of mindfulness-based stress reduction in the treatment of sleep disturbance in women with breast cancer: An exploratory study. Journal of Psychosomatic Research. 2003;(54):85–91. doi: 10.1016/s0022-3999(02)00546-9. [DOI] [PubMed] [Google Scholar]
- 11.Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clin Psychol Rev. 2005 Jul;25(5):629–644. doi: 10.1016/j.cpr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Winbush NY, Gross CR, Kreitzer MJ. The effects of mindfulness-based stress reduction on sleep disturbance: a systematic review. Explore (NY) 2007 Nov-Dec;3(6):585–591. doi: 10.1016/j.explore.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Ong JC, Shapiro SL, Manber R. Combining mindfulness meditation with cognitive-behavior therapy for insomnia: a treatment-development study. Behav Ther. 2008 Jun;39(2):171–182. doi: 10.1016/j.beth.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong JC, Shapiro SL, Manber R. Combining Mindfulness Meditation with Cognitive-Behavior Therapy for Insomnia: A Treatment-Development Study. Behavior Therapy. doi: 10.1016/j.beth.2007.07.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: Getting started and moving on from stage I. Clinical Psychology: Science and Practice. 2001 May;8(2):133–142. [Google Scholar]
- 16.Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004 Dec 15;27(8):1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 17.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001 Jul;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 18.Baer RA, Smith GT, Allen KB. Assessment of Mindfulness by Self-Report: The Kentucky Inventory of Mindfulness Skills. Assessment. 2004 Sep;11(3):191–206. doi: 10.1177/1073191104268029. [DOI] [PubMed] [Google Scholar]
- 19.Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23(3):263–271. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- 20.Broomfield NM, Espie CA. Towards a valid, reliable measure of sleep effort. J Sleep Res. 2005 Dec;14(4):401–407. doi: 10.1111/j.1365-2869.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 21.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988 Jun;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 22.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003 Apr;41(4):427–445. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 23.Kabat-Zinn J, Santoreli S. Mindfulness-Based Stress Reduction Professional Training. Center for Mindfulness in Medicine, Health Care, and Society. 2004 [Google Scholar]
- 24.Manber R, Kuo TF. Cognitative-behavioral therapies for insomnia. In: Lee-Choing L, Sateia J, Carskadon MA, editors. Sleep Medicine. Philadelphia: Hanley & Belfus; 2002. pp. 177–185. [Google Scholar]
- 25.Morin CM. Insomnia: Psychological Assessment and Management. New York: The Guilford Press; 1993. [Google Scholar]
- 26.Sheldon MR, Fillyaw MJ, Thompson WD. The use and interpretation of the Friedman test in the analysis of ordinal-scale data in repeated measures designs. Physiother Res Int. 1996;1(4):221–228. doi: 10.1002/pri.66. [DOI] [PubMed] [Google Scholar]
- 27.Schoenfeld D. Statistical considerations for pilot studies. Int J Radiat Oncol Biol Phys. 1980 Mar;6(3):371–374. doi: 10.1016/0360-3016(80)90153-4. [DOI] [PubMed] [Google Scholar]
- 28.Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006 Sep;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 29.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987 Dec;10(4):541–553. [PubMed] [Google Scholar]
- 30.Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Delacorte Press; 1990. [Google Scholar]
- 31.Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of Mindfulness. Journal of Clinical Psychology. 2006 Mar;62(3):373–386. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]