Abstract
The role of complement components in traumatic brain injury is poorly understood. Here we show that secondary damage after acute cryoinjury is significantly reduced in C3−/− or C5−/− mice or in mice treated with C5a receptor antagonist peptides. Injury sizes and neutrophil extravasation were compared. While neutrophil density increased following traumatic brain injury in wild type (C57BL/6) mice, C3-deficient mice demonstrated lower neutrophil extravasation and injury sizes in the brain. RNase protection assay indicated that C3 contributes to the induction of brain inflammatory mediators, MIF, RANTES (CCL5) and MCP-1 (CCL2). Intracranial C3 injection induced neutrophil extravasation in injured brains of C3−/− mice suggesting locally produced C3 is important in brain inflammation. We show that neutrophil extravasation is significantly reduced in both C5−/− mice and C5a receptor antagonist treated cryoinjured mice suggesting that one of the possible mechanisms of C3 effect on neutrophil extravasation is mediated via downstream complement activation products such as C5a. Our data indicates that complement inhibitors may ameliorate traumatic brain injury.
Keywords: Inflammation, Neutrophil, Cryoinjury, Trauma, CNS, Cytokines, Chemokines
1. Introduction
The complement system is a protein cascade involved in the innate immune response. Complement components have been implicated in exacerbation of inflammatory injury in several different tissues, including the central nervous system (CNS). Within the CNS, complement has been shown to be involved in traumatic brain injury using several animal models as well as studying human trauma patients (Stahel et al., 2001). It was suggested that the complement system might contribute to secondary damage caused by inflammation of the CNS. The magnitude and mechanism of this contribution have yet to be elucidated, due in part to the multifunctionality of the complement system.
Possible results of complement activation in the CNS that might contribute to inflammatory damage include opsonization and phagocytosis via C3b deposition, recruitment and activation of immune cells, especially neutrophils, from the peripheral blood by the C5a anaphylatoxin, and direct destruction of tissue by formation of pore-forming membrane attack complexes (MAC) leading to further inflammation. All of these mechanisms have been suggested to play a role in CNS inflammatory diseases and trauma. For example, experimentally induced global ischemia affects the biosynthesis of C1q, the recognition subcomponent of the classical complement activation pathway, in the CNS (Schafer et al., 2000). Rancan et al. (2003) has shown that transgenic mice with astrocyte targeted expression of the soluble complement inhibitor sCrry have reduced neurologic impairment and more normal blood–brain barrier function following closed head injury compared with wild type C57BL/6 control littermates. Studies suggest that elevated levels of complement proteins in CSF of patients with severe traumatic brain injury may contribute to secondary damage, (Kossmann et al., 1997) and it has been demonstrated that there is complement C3 accumulation at the site of traumatic brain injury (Kossmann et al., 1997). There is further evidence that the complement system may play an important role in neurodegenerative conditions such as Alzheimer’s disease (AD) (Bergamaschini et al., 1999; Bradt et al., 1998; Cooper et al., 2000; Fischer et al., 1995; Shen et al., 1998; Veerhuis et al., 1995, 1996; Walker and McGeer, 1992; Wyss-Coray et al., 2002; Yasojima et al., 1999). The above examples illustrate the harmful role of complement in nervous tissue. However, the beneficial role of complement components in brain has also been suggested by demonstration of a neuroprotective role for C5a against glutamate-mediated neurotoxicity, in AD, and in experimental autoimmune encephalomyelitis (EAE) (Mukherjee and Pasinetti, 2000; Niculescu et al., 2004; Osaka et al., 1999).
Regarding the cellular sources of complement components in these processes, it has been suggested that the majority of complement in CNS after traumatic brain injury derives from the peripheral blood after breakdown of the blood–brain barrier (Kossmann et al., 1997). We must also consider that cells native to CNS can also produce complement components and even increase production of these components in response to ischemic injury (Schafer et al., 2000; Van Beek et al., 2000). The functional relevance of local production of complement components to traumatic brain injury has yet to be determined.
To clarify the pathophysiologic role of complement components in the CNS, we performed traumatic brain cryoinjury on wild type (C57BL/6) mice, component C3 deficient (C57BL/6-C3−/−) mice, component C5 deficient (C57BL/10-C5−/−) mice, or wild type mice treated with C5a receptor antagonist. Following induction of traumatic injury, we compared traumatic injury size, neutrophil infiltration and inflammatory mediator production as measures of tissue damage in these experimental animals.
We demonstrate that neutrophil extravasation is significantly reduced in complement deficient animals. In parallel, the size of traumatic injury and the level of tissue trauma in the injury sites are also reduced as analyzed by immunohistochemistry and RNase protection assays (RPA). RPA showed significant induction of IL-12p35, IL-6 and eotaxin in C57BL/6-C3−/− mice with lower increase of RANTES (CCL5) and MCP-1 chemokines. There was no detectable increase in MIF mRNA expression in C3-deficient animals. When wild type animals were analyzed, we found significantly increased levels of IL-12 p35, IL6, MIF, RANTES, eotaxin and MCP-1 mediators demonstrating inflammation in the injured brains. MIF and MCP-1 were more highly expressed in the C57BL/6 controls throughout the 7-day timecourse. These results suggest that C3 contributes to the inflammatory damage of traumatic brain injury.
We also administered purified murine C3 intracerebrally to genetically deficient mice (C57BL/6-C3−/−) and quantified neutrophil infiltration as an indicator of inflammation. We demonstrate that intracranial injection of C3 leads to significant restoration of neutrophil extravasation in the cryoinjured brains of C57BL/6-C3−/− mice suggesting a role for locally produced complement components in traumatic brain injury. We also analyzed the role of the downstream complement pathway components in traumatic injury in CNS using C57BL/10-C5−/− mice and using a C5a receptor antagonist in C57BL/6 mice. We demonstrate that neutrophil influx is significantly reduced but not completely blocked in both cases compared with C57BL/6 animals.
Altogether these data show the important role of local complement C3 and C5a components in traumatic processes in the CNS and suggest that treatment with complement inhibitors may ameliorate the extent of traumatic brain injury. These results, combined with an understanding of the contributions of individual complement components, will lead to better tools for modulating the inflammatory response in the CNS after traumatic injury.
2. Materials and methods
2.1. Animals
Wild type (C57BL/6) and C5 deficient (C57BL/10-C5−/−) mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Mice deficient in C3 component, C57BL/6-C3−/− were obtained from the Department of Pediatrics, Washington University School of Medicine (St. Louis, MO, USA). All mice were maintained in the University of Wisconsin-Madison Animal Care Facility in specific pathogen-free facilities. Mice were used at 5–7 weeks of age. Three independent experiments in each experimental group were performed, using three to five mice per group. All protocols used in these experiments were approved by the Committee on Animal Care at the University of Wisconsin-Madison.
2.2. Traumatic brain cryoinjury
This injury model is a very well characterized traumatic injury model described previously (Cancilla et al., 1972, 1992, 1979; Cancilla and DeBault, 1980; Fee et al., 2003; Swartz et al., 2001). In this model, the injury is readily reproducible and allows for comparison of affected and unaffected hemispheres. Mice were anesthetized by intra-peritoneal injection of ketamine/xylazine and placed in a Stoelting (Wood Dale, IL) stereotactic apparatus with a mouse and neonatal rat adaptor, to ensure consistent injury size and placement. A sagittal incision was made in the scalp and a 3 mm diameter steel probe cooled in liquid nitrogen was applied to the skull for 6 s. Incisions were sutured and mice recovered from anesthesia. Brains were harvested at 1 h, 1 day, 4 days and 7 days post injury time points.
2.3. Tissue harvesting, hematoxylin and eosin (H&E) staining, and injury size determination
Under deep anesthesia, mice were briefly perfused through the left cardiac ventricle with physiologic saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH = 7.4). Brains were immediately removed, fixed overnight in the same solution, and processed for paraffin embedding. The injury was visually evident at the time of brain harvest as a hemorrhagic area on the surface of the parietal cortex. For confocal analyses, harvested brains were immediately placed into an OCT-laden boat (Tissue Tek® OCT compound, Sakura Finetek USA, Torrance, CA, USA; Cryomold® standard disposable vinyl molds, Miles, Elkhart, IN, USA) for snap freezing over dry ice. Ten micron thick coronal sections were cut using a Reich-art-Jung 1800 Fridge Cut cryostat (Leica, Solms, Germany), and placed on glass slides (Fisherbrand® Superfrost®/Plus, Fisher Scientific, Pittsburgh, PA, US). Coronal sectioning allowed for simultaneous evaluation of injured and uninjured hemispheres. Formalin fixed tissue sections, 8–10 μm thick, were stained with H&E for evaluation under light microscopy and injury areas were photographed on an Olympus BX40 microscope (Olympus America, Melville, NY) using a CMOS Pro 1000 series digital camera (Sound Vision, Wayland, MA, USA). Images were stored as Adobe Photoshop files and imported into the Scion image program (a version of NIH image, Scion, Frederick, MD) for calculation of the area of the circumscribed injury site on each slide. The three largest areas from 10 central slides of each injury were used for comparison of injury sizes.
2.4. Immunohistochemistry
Paraffin embedded tissue specimens were obtained as described above. Eight-micron sections were deparaffinized using Hemo-D and rehydrated in decreasing concentrations of ethanol. Neutrophils were detected using rat anti-mouse neutrophil antibody (Serotec, Raleigh, NC) and biotinylated anti-rat secondary antibody (Vector, Burlingame, CA), both at 1:100 dilution, followed by development with Vectastain ABC reagents (Vector). Negative controls included incubation with isotype-matched antibodies as described previously (Fee et al., 2000; Hofstetter et al., 2003; Swartz et al., 2001) or omission of primary antibodies (data not shown). After injury areas were determined, stained extravascular neutrophils were counted in the entire injury area using a microscope with an ocular grid. Five mice per group per time-point were counted. Slides were coded so that the counter was blind to the identity of the slides being counted. Three independent experiments were performed. Statistical analysis to analyze data for significance included repeated Analysis of Variance (ANOVA) measurements to compare groups and Student’s t-test statistics (JMP 3.1 program, SAS Institute, Cary, NC).
2.5. C5a receptor antagonist
The C5a receptor antagonist consisted of the cyclic hexapeptide AcF, synthesized as previously described (Finch et al., 1999; Mastellos et al., 2001). Wild type C57BL/6 mice were injected i.p. immediately prior to cryoinjury with C5a receptor antagonist peptides (1 mg/kg body weight in 200 μl sterile PBS). Control mice received equal volume of sterile PBS with or without cryoinjury. Mice were euthanized at 24 h post injury and brains were harvested for evaluation of injury sites. Maximal injury cross-sectional areas were determined for each mouse and density of infiltrating neutrophils was determined as described above. We have chosen this concentration to study the effect of C5aR blocking in the extravasation of neutrophils in the acute phase of traumatic injury in the brain based on previous reports indicating that the half life of the C5aRa peptide in serum is about 6 h and sufficient amount of this blocker can be detected in the acute phase of injuries (Walport, 2001).
2.6. Purification of murine C3 complement component and intracerebral injection
Native mouse complement component C3 was prepared using fractionated precipitation by polyethylene glycol 6000 (Fluka, Buchs, Switzerland) followed by Mono Q anion-exchange FPLC chromatograpy on a type HR 5/5 column (Pharmacia, Uppsala, Sweden) as previously described (Van den Berg et al., 1989). All fractions were tested for hemolytic C3 activity and for immunoreactivity by double immunodiffusion. The hemolytic C3 containing fractions were then applied to Superose 12 gelfiltration. The C3-containing fractions were pooled and analyzed on SDS-PAGE under reducing and non-reducing conditions. Only minor contaminations, but not C3 split products were detectable. No C5 was detected by double immunodiffusion. The possible contamination of the C3-preparation by anaphylatoxic peptides was tested by mast cell degranulation assay, using mouse bone-marrow derived and peritoneal mast cells, the rat RBL-2H3 and the human HMC-1 cell lines. No activation of any of these cells was observed, even when the C3-preparation was used at a concentration of 1 mg/ml. A 20 μl purified C3 at 1 μg/μl was injected intracerebrally (20 μg total) into C3-deficient animals following a procedure established previously (Ling et al., 2003).
2.7. RNase protection assay (RPA)
Total RNA was purified from homogenized brains of PBS-perfused animals using Trizol (Life Technologies) following the manufacturer’s instructions. Multiprobe DNA templates for chemokines (lymphotactin (LN), RANTES, eotaxin, MIP-1β, MIP-1α, MIP-2, IP-10, MCP-1, and TCA-3), cytokines (IL12p35, IL12p40, IL10, IL1α, IL1β, IL1Ra, IL18, IFNγ, MIF), and housekeeping genes, L32 and GAPDH (PharMingen, San Diego, CA) were used for RPA according to the manufacturer’s protocol. Briefly, the DNA templates were used to synthesize anti-sense ribop-robes, which were labeled with [32P]-UTP (DuPont-NEN Research Products, Guelph, Canada) using T7 polymerase. Labeled probes were hybridized with 20 μg of total RNA at 56 °C for 16 h. Samples were then digested with RNase A and T1, and treated with proteinase K. The remaining RNase-protected RNA duplexes were extracted with phenol/chloroform/isoamyl alcohol (Life Technologies) and resolved on 5% denaturing polyacrylamide gels. Undigested labeled probes included as size markers and controls. Dried gels were visualized by autoradiography or PhosphorImager (Molecular Dynamics, Sunnyvale, CA) after an exposure of 12–48 h for chemokines and 4–7 days for cytokines. Bands were quantitated and compared with housekeeping genes using ImageQuant 5.0 software (University of Virginia).
3. Results
3.1. Less severe traumatic injury in C3 deficient mice following cerebral cryoinjury
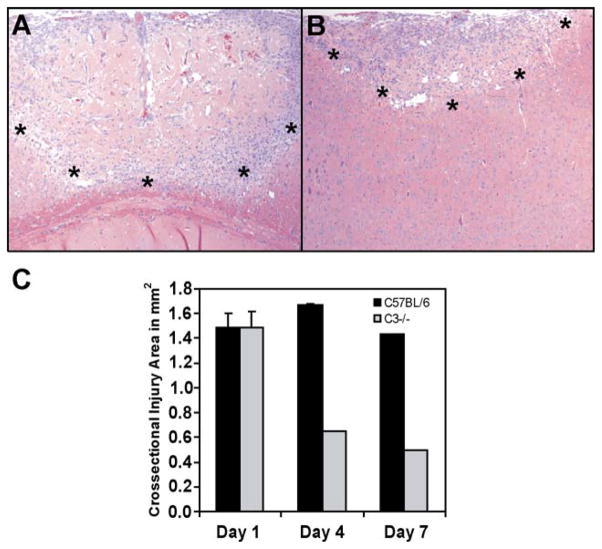
To study the role of C3 in traumatic brain injury we used wild type and C3-deficient mice. Comparison of C57BL/6 and C57BL/6-C3−/− H&E stained sections of brains harvested 1, 4 and 7 days after injury revealed distinct differences (Fig. 1 and data not shown). As early as day 1, in the C3-complement deficient mouse, the injured area showed fewer infiltrating cells, less hemorrhage, and better preservation of cytoplasm compared to injured wild type brains (data not shown). Fig. 1 shows dramatic lessening in the size of the area and severity of tissue injury visible at 7 days in C3 deficient mice. These data suggest that C3 is critical to secondary tissue damage following traumatic brain injury. When the cross-sectional injury areas were analyzed, a significantly decreased area of damage was detected in C3-deficient mice at both four and 7 days following the induction of traumatic injury. The early damaged areas (1 day following traumatic injury) were similar in both wild type and C3-deficient animals (Fig. 1C).
Fig. 1.
Tissue morphology and injury size in the brain of C3 deficient mice are less severe following traumatic brain injury. (A) H&E stained sections of C57BL/6 and (B) C57BL/6/C3−/− mouse brains at 7 days after traumatic injury. At day 7, C3−/− injury sites present a significantly smaller area of leukocyte extravasation with total resolution of the central area of acelluar necrosis, compared to the C57BL/6 brain tissues. (*) represents the edge of the injury site. Images were taken at 100 × magnification. (C) Quantitation of injury areas at 1, 4 and 7 days post injury. Ten H&E stained, coronal sections from the center of injuries of each mouse were photographed, and injury area on each slide was quantified using Scion Image as described in Materials and methods. The three largest areas from each injury site were averaged as maximal cross sectional area. Error bars represent standard error from four mice per group.
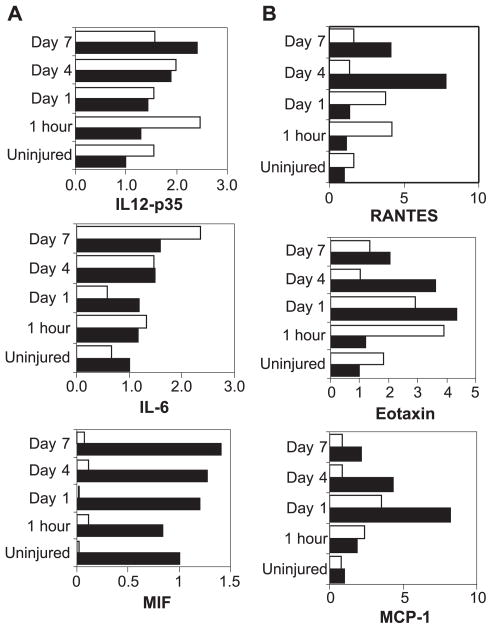
To further analyze the level of inflammation induced by tissue trauma in C3-deficient mice compared to wild type animals, we performed RPA. We analyzed the gene expression of pro-inflammatory cytokines and chemokines that influence tissue inflammation and leukocyte invasion by virtue of their ability to selectively attract and activate subsets of leukocytes. As expected, a very low level of chemokine mRNA was observed in the perfused CNS of naive mice, while multiple chemokine mRNA transcripts were up-regulated following traumatic injury in all mice (Fig. 2). In injured tissue of C57BL/6 wild type mice, four- to eight-fold induction of RANTES (CCL5), eotaxin (CCL11) and MCP-1 (CCL2) transcripts predominate over other chemokines (Fig. 2B), including lymphotactin, MIP-2, and TCA-3 (not shown). Mice with deficiency in C3, however, display a chemokine gene expression profile distinct from that of wild type mice. In general, the RANTES (CCL5), eotaxin (CCL11) and MCP-1 (CCL2) chemokines demonstrated lower fold induction (two-to four-fold), earlier peak levels and earlier return to baseline levels (Fig. 2B). Similarly, insignificant amounts of MIP-1α (CCL3), MIP1-β (CCL4), and MIP-2 mRNA were detected in C3−/− mice (not shown). When inflammatory cytokines were analyzed, prominent expression of MIF was seen in the injured brain of wild type mice (Fig. 2A), however, nearly undetectable expression of MIF was demonstrated both pre and post-traumatic injury in C3 deficient mice. Overall, expression of IL12-p35 and IL6 was not significantly different between the two groups.
Fig. 2.
RPA analysis of inflammatory mediators, and chemokine mRNA expression in brains of C57BL/6 and C57BL6/C3−/− mice. (A) Quantitative inflammatory mediator mRNA expression and (B) chemokine mRNA expression in C57BL/6 or in C57BL6/C3−/− mouse brain injury sites at 1 h, 1 day, 4 days and 7 days following traumatic injury. These images are representative of three experiments. Expression of IL-12p35, IL-6, MIF, Rantes, Eotaxin and MCP-1 were normalized to L32 expression and compared between C57BL/6 wild type mouse brains (black bars) and C3−/− mouse brains (white bars) at 5 time points, uninjured, and 1 h, 1 day, 4 days and 7 days post traumatic brain cryoinjury. Expression level of cytokines and chemokines in uninjured C57BL/6 was arbitrarily set to one with fold change from this level displayed throughout the time course.
Together, these results show that genetically deficient C57BL/6-C3−/− mice have a distinct phenotype following traumatic brain injury. This phenotype is characterized by decreased cellular infiltrates, preserved parenchymal cytoplasm, and decreased expression of selected chemokines and pro-inflammatory mediators.
3.2. Neutrophil extravasation is inhibited in the absence of complement C3 component following brain cryoinjury
Neutrophil extravasation has been shown to play an important role in inflammatory injury. Upon extravasation into injured tissues in response to chemotactic signals, neutrophils become capable of an oxidative burst, which can destroy microorganisms and healthy cells alike. The typical pattern of neutrophil recruitment and accumulation over time begins just a few hours after injury and peaks at 1 day post injury. This is followed by a rapid decline, such that few remaining neutrophils are detectable 4 days after injury. Because of the similarity of this timing to the altered events visible in Fig. 1 we hypothesized that neutrophils contribute to the differences in acute damage from traumatic brain injury of C3 deficient mice. In our next experiments, we focused on the level of neutrophil infiltration into acute injury sites in brains of wild type and C3-deficient mice 24 h following traumatic injury.
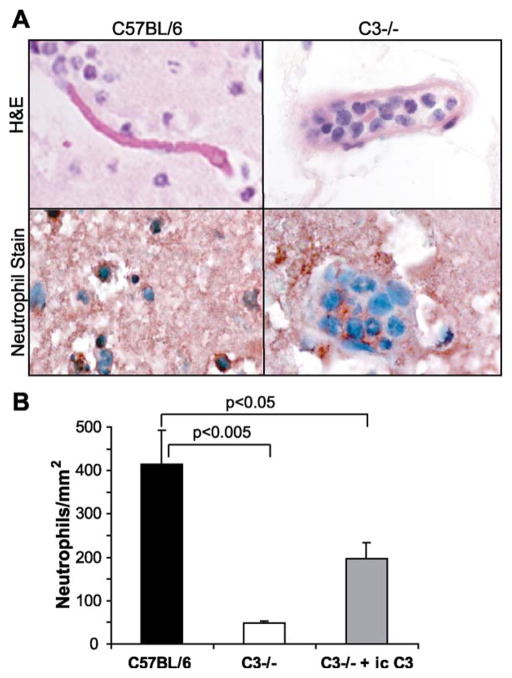
We found that in addition to less hemorrhage and more intact vasculature visible in H&E-stained sections of brain injury sites of C57BL/6-C3−/− mice (Fig. 1), neutrophil extravasation into injury sites was significantly inhibited in C3-deficient mice in the early (24 h) phase of injury. (Fig. 3A, right panels). Staining with anti-neutrophil antibody confirmed that neutrophils are highly concentrated in vessels at the injury sites, but they are not able to extravasate into tissues adjacent to the injured tissues to the same extent as in wild type animals (Fig. 3A, right panels). In the wild type animals, neutrophils are distributed relatively normally, both within the lumina of blood vessels and extravascularly throughout the brain parenchyma within and surrounding the injury (Fig. 3A, left panels). This difference was quantified by measuring injury areas and counting extravasated neutrophils in the injury sites. Calculated neutrophil densities were compared in the wild type and complement deficient mice (Fig. 3B). When compared with wild type, extravascular neutrophil density was decreased by ~90% in the C57BL/6-C3−/− mice ( p < 0.005 by Student’s t-test). This data supports hypothesis that complement can play an important role in the early extravasation of neutrophils following traumatic brain injury.
Fig. 3.
Neutrophil infiltration in the brain of mice following traumatic cryoinjury. (A) Injury sites 1 day post injury from wild type C57BL/6 and C3−/− brains were stained with H&E, upper panels, and an anti-neutrophil antibody, lower panels. Neutrophils, identified by characteristic segmented nuclei on H&E sections and red staining with anti-neutrophil antibody, are distributed throughout the parenchyma of the injury site in wild type mice and are not contained within vessel lumina (left panels). In complement deficient mouse brain injury sites, the parenchyma contain many fewer neutrophils (right panels). Many neutrophils remain in the luminae of large vessels and fail to extravasate into the parenchyma (right panels). (B) Extravascular neutrophil density per square millimeter of injured tissue was calculated and found to be reduced by ~90% in the absence of complement C3 ( p < 0.005 by Student’s t-test). C3 was administered by intra-cerebral injection of purified mouse C3 (20 μg/injection in 20 μl) into the injury site of C3−/− mice at the time of injury and again 2 h before harvest of 1 day post injury tissue punctuation. Intracerebral administration of C3 partially restored neutrophil extravastion into the injury (n = 4 mice/group, error bars indicate S.D.).
3.3. Intracerebral reconstitution of C3 in C3-deficient mice can restore neutrophil extravasation in acutely injured brains
To further evaluate the dependence of neutrophil extravasation in response to injury on complement C3 component, we reconstituted C3 in the brains of genetically deficient, C57BL/6-C3−/− mice. Purified murine C3 protein (20 μg/injection) was injected into the injury site at the time of injury and again 2 h before tissue harvest (22 h post-injury). In these mice, the injury site was histologically more similar to the wild type mice (data not shown), and neutrophil density was increased as compared to non-reconstituted mice (Fig. 3B).
These data suggest that in situ C3 in the brain is critical for neutrophil extravasation in response to traumatic brain injury.
3.4. Neutrophil extravasation is significantly reduced in both C5−/− deficient mice and in C57BL/6 mice treated with a C5a receptor antagonist
C3 activates a number of different effector pathways in the complement system (most notably C5a) that are important for neutrophil extravastation. Therefore, we investigated the role of downstream component C5 in neutrophil extravasation following traumatic injury in the brain. We performed aseptic cryoinjury using component C5 deficient mice, and found that injury sites were morphologically intermediate between the wild type and C3−/− phenotypes (data not shown). Quantification of the neutrophil density as an indicator of degree of acute inflammation revealed a significant decrease, approximately 65%, in extravasation in C5 deficient mice compared to genetically normal controls (Fig. 4). However, the neutrophil extravasation was not inhibited to the extent that was seen in C3 deficient mice (approximately 90% inhibition). This suggests that the majority of complement dependent neutrophil extravasation is due to the contribution of component C5, but does not exclude the role of C3 activation products or other downstream elements of the complement system in this process.
Fig. 4.
C5 deficiency and C5aR antagonists protect mice from acute brain injury. Quantification of extravascular neutrophil density within the injury site as an indicator of severity of inflammation was performed using C5 deficient mice and wild type mice treated with C5aR antagonist. The neutrophil density resulting from the administration of C5aR antagonist prior to cryoinjury of wild type mice (right) was not significantly different from the C5−/− (*p>0.8). In both C5−/− and C5aR antagonist peptide injected wild type mice, extravascular neutrophil density was higher than in C3−/− animals ( p < 0.03, n = 9, mean ± S.D.).
To further dissect potential effects of the anaphylatoxin C5a in traumatic brain injury, we employed C5a receptor antagonist. C5a receptor antagonist has been shown to block the acute phase of injuries in several models including antiphospholipid syndrome, sepsis and liver regeneration following toxic injury (Girardi et al., 2003; Huber-Lang et al., 2002; Strey et al., 2003). When the antagonist injected intraperitoneally into C57BL/6 wild type animals, the cryoinjury site in the brain was histologically similar to (data not shown), and percent inhibition of neutrophil extravastion was not statistically different from, genetically deficient C5−/− animals ( p>0.8) (Fig. 4). These data suggest that C5a complement component accounts for the majority, but not the all of the neutrophil extravasation in the injured CNS.
4. Discussion
Local CNS production of complement components and their contributing role in traumatic brain injury were suggested previously (Barnum, 1995; Kossmann et al., 1997; Rancan et al., 2003). Increased C3 and complement activation have also been detected in models of traumatic injury, endotoxemia, ischemia, viral encephalitis, and in human brain injury patients (Nadeau and Rivest, 2001; Schafer et al., 2000; Speth et al., 2001; Van Beek et al., 2000). These data demonstrate activation of complement during acute inflammatory processes, yet reveal very little of the contribution of complement components to the mechanism of CNS inflammatory damage.
We demonstrate diminished tissue damage associated with decreased neutrophil infiltration in the brain following traumatic cryoinjury in C3 or C5-deficient mice. Lower fold induction of several inflammatory molecules, such as MIF, Rantes (CCL5) and MCP-1 (CCL2), in C3-deficient mice compared to wild type animals following traumatic brain cryoinjury correlates with our morphologic findings indicating a lower level of inflammation in the absence of complement C3. At this time, the exact mechanism by which complement components C3 and C5 contribute to chemokine and cytokine mediator upregulation at traumatic injury sites in the brain is not known. Several of these chemokines such as RANTES (CCL5) and its receptor, CCR5 have been implicated as critical contributing factors in the trafficking of leukocytes into CNS of mice with EAE and MHV infections (Glass et al., 2004, 2001; Lane et al., 2000). In our studies we have demonstrated the upregulation of these chemokines following traumatic injury in brain. The underlying mechanism linking regulation of chemokine and cytokine expression with C3 or C5 expression following traumatic injury in the brain is a focus of ongoing study in our laboratory. Although this is not a major focus of this paper, these differences can offer an approach to the study of how complement interferes with the local inflammatory processes in CNS.
Our data from C3 deficient mice demonstrate that extra-vascular neutrophil density is reduced to approximately 10% of the wild type, and that extravasation of neutrophils within the injury site is impaired. The complement component C3 is therefore necessary for normal neutrophil extravasation in response to aseptic cryoinjury of the mouse cortex. These observations suggest C3 as a potential target candidate for therapeutic modulation of inflammatory injury in the CNS.
Some studies of complement depletion and complement inhibition have been carried out in animal models of brain injury. A model of thromboembolic stroke in rabbits showed no significant effect of complement depletion on the outcome of stroke induced injury (Lew et al., 1999; Rancan et al., 2003). In contrast, a rat model of traumatic brain injury showed a 41% decrease in neutrophil myeloperoxidase activity after systemic C3 inhibition by soluble complement receptor 1 (sCR1) (Kaczorowski et al., 1995). These differing results of complement modification could be due to the different injurious processes or to the different animals used in these models. Our data is most similar to the rat model. We demonstrate that in mouse, complement deficiency inhibits neutrophil extravasation and ameliorates acute inflammatory damage due to traumatic injury. The results of our reconstitution experiments support the possibility of complement activation in situ following traumatic injury. C3 administered locally is able to restore some neutrophil extravasation in the genetically deficient mice (Fig. 3). The level of neutrophil extravasation/mm2 in the brain of C3 i.c. reconstituted C3-deficient animals does not reach the level of extravasation in wild type mice. This data indicates that either the local concentration of C3 in the brain is not sufficiently high or that a systemic C3 source is also needed to induce optimal neutrophil extravasation. The fact that control mice receiving C3 intracranially without cryoinjury, or PBS with cryoinjury did not develop a detectable lesion or neutrophil infiltration demonstrates that the needle induced trauma alone is not sufficient to induce neutrophil extravasation (data not shown). This model would imply that a good therapeutic C3 inhibitor should readily cross an intact blood–brain barrier or be administered directly into the cerebrospinal fluid of victims of traumatic brain injury.
We also demonstrated that complement component C5 deficiency alone could decrease neutrophil infiltration to approximately 35% of wild type. This indicates that most but not all of the complement dependent neutrophil extravastion is induced by C5. C3a might also contribute to this process based on the fact that recent studies have demonstrated increased C3aR mRNA and protein during acute inflammatory processes of endotoxemia, focal ischemia, multiple sclerosis, and bacterial meningitis (Gasque et al., 1998; Nadeau and Rivest, 2001; Van Beek et al., 2000).
Furthermore, we also characterized the specific contribution of activated C5 using a cyclic hexapeptide with C5a receptor (C5aR) antagonist activity. Our data showed that C5a receptor antagonist activity was sufficient to obtain the same decrease in neutrophil extravasation as genetic deficiency of C5. This is the first use of a C5aR antagonist in a brain injury model. Previously, it was demonstrated that C5aR expression is increased on cortical endothelia in response to ischemic damage in the mouse (Van Beek et al., 2000). Expression of this receptor has also been reported on neurons and astrocytes (Gasque et al., 1995; Van Beek et al., 2000). The dual role of C5 in enhancement of inflammatory demyelination in acute EAE and promoting remyelination in recovery in the CNS has been recently proposed (Weerth et al., 2003). Furthermore, it was shown that the C5 complement component can protect oligodendrocytes from apoptosis in the recovery phase of EAE (Niculescu et al., 2004). These data altogether indicate that C5 can be therapeutically used either through blocking its activity in the acute phase of traumatic brain injury, or by inducing its activity in chronic CNS inflammations. The importance of component C5 and its activation products is further emphasized by the observation that C5 mRNA is constitutively expressed in the mouse brain by both neuronal and non-neuronal cells and to a much higher degree than C3 mRNA (Nadeau and Rivest, 2001). In conclusion, these data indicate that a C5aR antagonist might be an effective therapeutic tool for decreasing the acute inflammation of traumatic brain injury without compromising systemic complement activities.
Acknowledgments
The authors thank L. Spruce for excellent technical assistance, Toshi Kinoshita for tissue processing, Thomas Jacques for immunohistochemistry and Dr. Laura Hogan for critical reading of the manuscript and valuable discussions.
Footnotes
This work was supported by National Institute of Health, Grant RO1-NS 37570-01A2 to Z. Fabry and National Institutes of Health grants GM-62135 and ÁGM-30040 to J.D. Lambris.
References
- Barnum SR. Complement biosynthesis in the central nervous system. Crit Rev Oral Biol Med. 1995;6:132–146. doi: 10.1177/10454411950060020301. [DOI] [PubMed] [Google Scholar]
- Bergamaschini L, Canziani S, Bottasso B, Cugno M, Braidotti P, Agostoni A. Alzheimer’s beta-amyloid peptides can activate the early components of complement classical pathway in a C1q-independent manner. Clin Exp Immunol. 1999;115:526– 533. doi: 10.1046/j.1365-2249.1999.00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer’s disease beta-peptide. J Exp Med. 1998;188:431– 438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancilla PA, DeBault LE. Freeze injury and repair of cerebral microvessels. Adv Exp Med Biol. 1980;131:257– 269. doi: 10.1007/978-1-4684-3752-2_20. [DOI] [PubMed] [Google Scholar]
- Cancilla PA, Baker RN, Pollack PA, Frommes SP. The reaction of pericytes of the central nervous system to injury. Lab Invest. 1972;26:376– 383. [PubMed] [Google Scholar]
- Cancilla PA, Frommes SP, Kahn LE, DeBault LE. Regeneration of cerebral microvessels: a morphologic and histochemical study after local freeze-injury. Lab Invest. 1979;40:74– 82. [PubMed] [Google Scholar]
- Cancilla PA, Bready J, Berliner J, Sharifi-Nia H, Toga A, Santori EH, Scully S, de Vellis J. Expression of mRNA for glial fibrillary acidic protein after experimental cerebral injury. J Neuropathol Exp Neurol. 1992;51:560– 565. doi: 10.1097/00005072-199209000-00011. [DOI] [PubMed] [Google Scholar]
- Cooper NR, Bradt BM, O’Barr S, Yu JX. Focal inflammation in the brain: role in Alzheimer’s disease. Immunol Res. 2000;21:159–165. doi: 10.1385/IR:21:2-3:159. [DOI] [PubMed] [Google Scholar]
- Fee D, Grzybicki D, Dobbs M, Ihyer S, Clotfelter J, Macvilay S, Hart MN, Sandor M, Fabry Z. Interleukin 6 promotes vasculogenesis of murine brain microvessel endothelial cells. Cytokine. 2000;12:655– 665. doi: 10.1006/cyto.1999.0599. [DOI] [PubMed] [Google Scholar]
- Fee D, Crumbaugh A, Jacques T, Herdrich B, Sewell D, Auerbach D, Piaskowski S, Hart MN, Sandor M, Fabry Z. Activated/effector CD4(+) T cells exacerbate acute damage in the central nervous system following traumatic injury. J Neuroimmunol. 2003;136:54– 66. doi: 10.1016/s0165-5728(03)00008-0. [DOI] [PubMed] [Google Scholar]
- Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965– 1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- Fischer B, Schmoll H, Riederer P, Bauer J, Platt D, Popa-Wagner A. Complement C1q and C3 mRNA expression in the frontal cortex of Alzheimer’s patients. J Mol Med. 1995;73:465–471. doi: 10.1007/BF00202265. [DOI] [PubMed] [Google Scholar]
- Gasque P, Chan P, Fontaine M, Ischenko A, Lamacz M, Gotze O, Morgan BP. Identification and characterization of the complement C5a anaphylatoxin receptor on human astrocytes. J Immunol. 1995;155:4882– 4889. [PubMed] [Google Scholar]
- Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol. 1998;160:3543– 3554. [PubMed] [Google Scholar]
- Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, Lambris JD, Holers VM, Salmon JE. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644– 1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Liu MT, Kuziel WA, Lane TE. Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology. 2001;288:8– 17. doi: 10.1006/viro.2001.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Hickey MJ, Hardison JL, Liu MT, Manning JE, Lane TE. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J Immunol. 2004;172:4018–4025. doi: 10.4049/jimmunol.172.7.4018. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Sewell DL, Liu F, Sandor M, Forsthuber T, Lehmann Z, Fabry Z. Autoreactive T cells promote post-traumatic healing in the central nervous system. J Neuroimmunol. 2003;134:25–34. doi: 10.1016/s0165-5728(02)00358-2. [DOI] [PubMed] [Google Scholar]
- Huber-Lang MS, Riedeman NC, Sarma JV, Younkin EM, McGuire SR, Laudes IJ, Lu KT, Guo RF, Neff TA, Padgaonkar VA, Lambris JD, Spruce L, Mastellos D, Zetoune FS, Ward PA. Protection of innate immunity by C5aR antagonist in septic mice. FASEB J. 2002;16:1567– 1574. doi: 10.1096/fj.02-0209com. [DOI] [PubMed] [Google Scholar]
- Kaczorowski SL, Schiding JK, Toth CA, Kochanek PM. Effect of soluble complement receptor-1 on neutrophil accumulation after traumatic brain injury in rats. J Cereb Blood Flow Metab. 1995;15:860–864. doi: 10.1038/jcbfm.1995.107. [DOI] [PubMed] [Google Scholar]
- Kossmann T, Stahel PF, Morganti-Kossmann MC, Jones JL, Barnum SR. Elevated levels of the complement components C3 and factor B in ventricular cerebrospinal fluid of patients with traumatic brain injury. J Neuroimmunol. 1997;73:63– 69. doi: 10.1016/s0165-5728(96)00164-6. [DOI] [PubMed] [Google Scholar]
- Lane TE, Liu MT, Chen BP, Asensio VC, Samawi RM, Paoletti AD, Campbell IL, Kunkel SL, Fox HS, Buchmeier MJ. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew SM, Gross CE, Bednar MM, Russell SJ, Fuller SP, Ellenberger CL, Howard D. Complement depletion does not reduce brain injury in a rabbit model of thromboembolic stroke. Brain Res Bull. 1999;48:325–331. doi: 10.1016/s0361-9230(99)00004-0. [DOI] [PubMed] [Google Scholar]
- Ling C, Sandor M, Fabry Z. In situ processing and distribution of intracerebrally injected OVA in the CNS. J Neuroimmunol. 2003;141:90– 98. doi: 10.1016/s0165-5728(03)00249-2. [DOI] [PubMed] [Google Scholar]
- Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479– 2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Pasinetti GM. The role of complement anaphylatoxin C5a in neurodegeneration: implications in Alzheimer’s disease. J Neuroimmunol. 2000;105:124– 130. doi: 10.1016/s0165-5728(99)00261-1. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. The complement system is an integrated part of the natural innate immune response in the brain. FASEB J. 2001;15:1410– 1412. doi: 10.1096/fj.00-0709fje. [DOI] [PubMed] [Google Scholar]
- Niculescu T, Weerth S, Niculescu F, Cudrici C, Rus V, Raine CS, Shin ML, Rus H. Effects of complement C5 on apoptosis in experimental autoimmune encephalomyelitis. J Immunol. 2004;172:5702–5706. doi: 10.4049/jimmunol.172.9.5702. [DOI] [PubMed] [Google Scholar]
- Osaka H, Mukherjee P, Aisen PS, Pasinetti GM. Complement-derived anaphylatoxin C5a protects against glutamate-mediated neuro-toxicity. J Cell Biochem. 1999;73:303–311. [PubMed] [Google Scholar]
- Rancan M, Morganti-Kossmann MC, Barnum SR, Saft S, Schmidt OI, Ertel W, Stahel PF. Central nervous system-targeted complement inhibition mediates neuroprotection after closed head injury in transgenic mice. J Cereb Blood Flow Metab. 2003;23:1070– 1074. doi: 10.1097/01.WCB.0000084250.20114.2C. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Schwaeble WJ, Post C, Salvati P, Calabresi M, Sim RB, Petry F, Loos M, Weihe E. Complement C1q is dramatically up-regulated in brain microglia in response to transient global cerebral ischemia. J Immunol. 2000;164:5446– 5452. doi: 10.4049/jimmunol.164.10.5446. [DOI] [PubMed] [Google Scholar]
- Shen Y, Sullivan T, Lee CM, Meri S, Shiosaki K, Lin CW. Induced expression of neuronal membrane attack complex and cell death by Alzheimer’s beta-amyloid peptide. Brain Res. 1998;796:187– 197. doi: 10.1016/s0006-8993(98)00346-1. [DOI] [PubMed] [Google Scholar]
- Speth C, Stockl G, Mohsenipour I, Wurzner R, Stoiber H, Lass-Florl C, Dierich MP. Human immunodeficiency virus type 1 induces expression of complement factors in human astrocytes. J Virol. 2001;75:2604– 2615. doi: 10.1128/JVI.75.6.2604-2615.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahel PF, Morganti-Kossmann MC, Perez D, Redaelli C, Gloor B, Trentz O, Kossmann T. Intrathecal levels of complement-derived soluble membrane attack complex (sC5b-9) correlate with blood–brain barrier dysfunction in patients with traumatic brain injury. J Neurotrauma. 2001;18:773–781. doi: 10.1089/089771501316919139. [DOI] [PubMed] [Google Scholar]
- Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913– 923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz KR, Liu F, Sewell D, Schochet T, Campbell I, Sandor M, Fabry Z. Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res. 2001;896:86– 95. doi: 10.1016/s0006-8993(01)02013-3. [DOI] [PubMed] [Google Scholar]
- Van Beek J, Bernaudin M, Petit E, Gasque P, Nouvelot A, MacKenzie ET, Fontaine M. Expression of receptors for complement anaphylatoxins C3a and C5a following permanent focal cerebral ischemia in the mouse. Exp Neurol. 2000;161:373– 382. doi: 10.1006/exnr.1999.7273. [DOI] [PubMed] [Google Scholar]
- Van den Berg CW, Van Dijk H, Capel PJ. Rapid isolation and characterization of native mouse complement components C3 and C5. J Immunol Methods. 1989;122:73– 78. doi: 10.1016/0022-1759(89)90336-0. [DOI] [PubMed] [Google Scholar]
- Veerhuis R, van der Valk P, Janssen I, Zhan SS, Van Nostrand WE, Eikelenboom P. Complement activation in amyloid plaques in Alzheimer’s disease brains does not proceed further than C3. Virchows Arch. 1995;426:603–610. doi: 10.1007/BF00192116. [DOI] [PubMed] [Google Scholar]
- Veerhuis R, Janssen I, Hack CE, Eikelenboom P. Early complement components in Alzheimer’s disease brains. Acta Neuropathol (Berl) 1996;91:53–60. doi: 10.1007/s004019570001. [DOI] [PubMed] [Google Scholar]
- Walker DG, McGeer PL. Complement gene expression in human brain: comparison between normal and Alzheimer disease cases. Brain Res Mol Brain Res. 1992;14:109– 116. doi: 10.1016/0169-328x(92)90017-6. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- Weerth SH, Rus H, Shin ML, Raine CS. Complement C5 in experimental autoimmune encephalomyelitis (EAE) facilitates remyelination and prevents gliosis. Am J Pathol. 2003;163:1069– 1080. doi: 10.1016/S0002-9440(10)63466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc Natl Acad Sci USA. 2002;99:10837– 10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, McGeer PL. Up-regulated production and activation of the complement system in Alzheimer’s disease brain. Am J Pathol. 1999;154:927– 936. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]