Abstract
Pain-related functional impairment and behavioral depression are diagnostic indicators of pain and targets for its treatment. Nesting is an innate behavior in mice that may be sensitive to pain manipulations and responsive to analgesics. The goal of this study was to develop and validate a procedure for evaluation of pain-related depression of nesting in mice. Male ICR mice were individually housed and tested in their home cages. On test days, a 5cm × 5cm Nestlet™ was subdivided into six pieces, the pieces were evenly distributed on the cage floor, and Nestlet consolidation was quantified during 100-min sessions. Baseline nesting was stable within and between subjects, and nesting was depressed by two commonly used inflammatory pain stimuli [intraperitoneal injection of dilute acid; intraplantar injection of complete Freund’s adjuvant (CFA)]. Pain-related depression of nesting was alleviated by drugs from two classes of clinically effective analgesics (the nonsteroidal anti-inflammatory drug ketoprofen and the mu opioid receptor agonist morphine) but not by a drug from a class that has failed to yield effective analgesics (the centrally acting kappa opioid agonist U69,593). Neither ketoprofen nor morphine alleviated depression of nesting by U69,593, suggesting that ketoprofen and morphine effects were selective for pain-related depression of nesting. In contrast to ketoprofen and morphine, the kappa opioid receptor antagonist JDTic blocked depression of nesting by U69,593 but not by acid or CFA. These results support utility of this procedure to assess expression and treatment of pain-related depression in mice.
INTRODUCTION
Pain is an unpleasant subjective state commonly assessed in humans with verbal reports [26]. However, pain is also associated with nonverbal changes in behavior that serve both as diagnostic indicators of pain and as targets for its treatment. For example, pain is often associated with functional impairment and depression of behavior that can be assessed in humans with instruments such as the Brief Pain Inventory [7; 11]. Preclinical research has also begun to assess “pain-depressed behaviors,” which can be defined as behaviors that decrease in rate, frequency or intensity after delivery of a putative pain stimulus [33]. For example, noxious stimuli have been reported to decrease feeding [18; 43], locomotor activity [8; 25; 27; 44], burrowing [1; 17; 38], and positively reinforced operant responding in rodents [24; 30]. This work signals a growing interest in use of preclinical pain measures that more closely model clinical pain measures and that might improve translation of preclinical research on the mechanisms and treatment of pain [46]. Procedures to study pain-related depression of behavior in mice could benefit this effort because mice are especially suitable for research on genetic as well as pharmacologic manipulations [12; 28].
Nest building is an innate behavior in mice that may serve as one source of useful dependent measures in this species [10; 14; 16]. Accordingly, the main goal of this study was to develop and validate a procedure for evaluation of pain-depressed nesting in mice. The study proceeded in three steps. First, we developed a metric of nesting that could be objectively quantified on a ratio scale amenable to parametric statistics [42]. Second, we evaluated sensitivity of nesting to depression by intraperitoneal administration of dilute acid and intraplantar administration of complete Freund’s adjuvant (CFA). These two noxious stimuli produce other pain-related behaviors that can be alleviated by the two major classes of clinical analgesics: nonsteroidal anti-inflammatory drugs (NSAIDs) and mu opioid receptor agonists [8; 25; 30]. Finally, we evaluated sensitivity of acid- and CFA-induced depression of nesting to treatment with the NSAID ketoprofen and the mu agonist morphine. The kappa opioid agonist U69,593 was evaluated as a negative control, because centrally acting kappa agonists often produce apparent analgesic effects in conventional preclinical assays, but they have not succeeded as effective analgesics in humans [32].
A secondary goal of this study was to evaluate potential antinocicetive effects of the kappa opioid receptor antagonist JDTic [6]. It has recently been suggested that pain-related depression of behavior and mood may involve activation of an endogenous kappa opioid system in which signaling is mediated by the endogenous opioid peptide dynorphin acting at kappa opioid receptors [4]. This hypothesis predicts that kappa antagonists may alleviate signs of pain-related behavioral depression, but recent neurochemical and behavioral studies from our laboratory did not support this hypothesis [21; 22]. The present study extended our evaluation of this hypothesis to this assay of pain-depressed nesting. Specifically, we evaluated effectiveness of JDTic to produce an analgesic-like blockade of acid- and CFA-induced depression of nesting in mice.
METHODS
Subjects
Subjects were adult male ICR mice (Harlan, Frederick, MD) that were 8 weeks old and weighed 27–48 g upon arrival in the laboratory. Mice were individually housed in plastic cages (31.75 cm long × 23.50 cm wide × 15.25 cm deep) supplied with corncob bedding (Harlan Laboratories Item 7092), one 5 × 5 cm “Nestlet”™ composed of pressed virgin cotton (Ancare, Bellmore, NY), and ad libitum access to food (Teklad LM-485 Mouse/Rat Diet, Harlan Laboratories Item 7012) and water. Cages were mounted in a RAIR HD Ventilated Rack (Lab Products, Seaford, DE) in a temperature-controlled room (21–23°C), and lights in the housing room were maintained on a 12-h light/dark cycle with lights on from 6:00 AM to 6:00 PM. Testing was conducted during the light phase (8:00 AM –4:00 PM) and was initiated no sooner than 48hr after arrival from the vendor and assignment to a home cage. These methods of single housing and home-cage acclimation were employed because pilot studies found that nesting occurred more quickly and reliably in singly housed mice tested in their own home cage with familiar (≥2-day old) bedding than in single- or group-housed mice tested in a novel cage with fresh bedding. Mice were euthanized by CO2 exposure followed by cervical dislocation after completion of the nesting tests described below. Animal-use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and complied with the National Research Council Guide for the Care and Use of Laboratory Animals [29].
Nesting Procedure
Basic parameters of the nesting procedure were established during pilot studies conducted by Dr. Miller, Dr. Altarifi, and Mr. Leitl under the supervision of Dr. Negus, and results for these pilot studies are not shown. A new investigator (Mr. Neddenriep) was then trained to operate the procedure under the supervision of Drs. Altarifi and Negus, and he conducted all nesting studies and collected all data reported here. He was not blinded to experimental treatments, but as a new graduate student, he was not familiar with the pharmacology of the test drugs and declared no explicit expectations. On test days, home cages were removed from the housing rack in the animal facility and transported to a windowless procedure room illuminated by fluorescent ceiling lights (~500 lux at benchtop level), and mice had access to food and water in their home cages throughout test sessions. At the start of each test session, home cages containing the singly housed mice were placed on a lab bench for a 10 min acclimation to the testing room. Subsequently, treatments to be delivered on that day were administered by removing the mouse from its home cage, administering the treatment, immediately returning the mouse to its home cage, and replacing the cage lid. After expiration of the pretreatment times, each mouse was briefly (<1 min) transferred from its home cage to a transfer cage, any existing nest was removed from the home cage, and a new Nestlet was placed into the home cage. The Nestlet was cut into six roughly equal pieces (~1.7 × 2.5 cm), and these Nestlet pieces were placed in the cage according to the configuration illustrated in Figure 1. The mouse was then returned to the home cage for a 100 min nesting period.
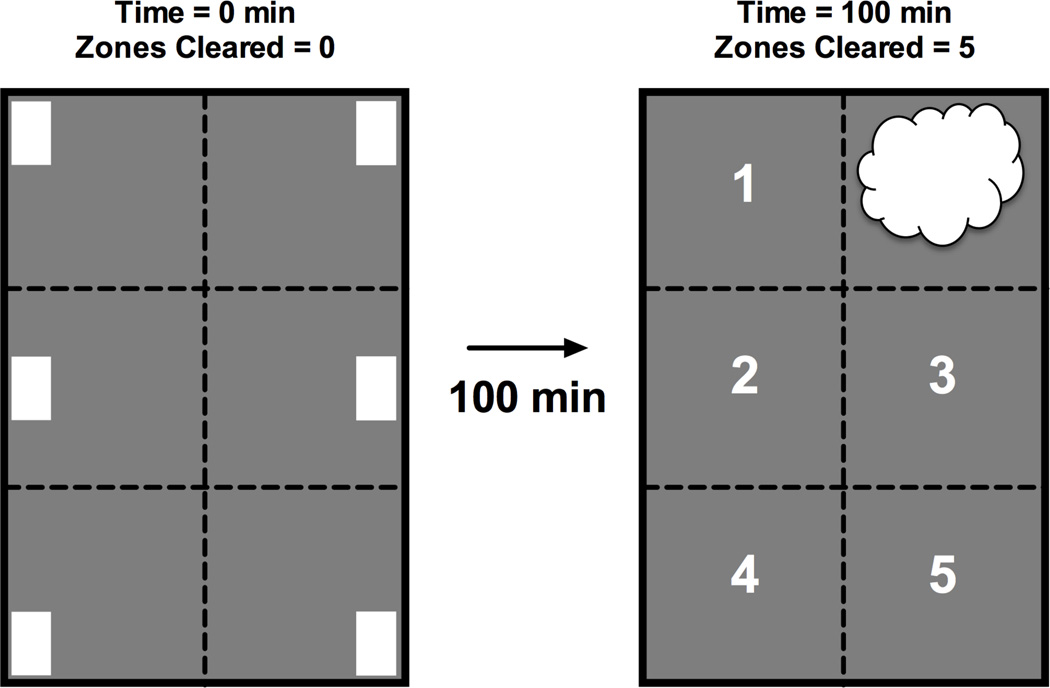
Figure 1.
Diagram of Nestlet configuration in the home cage before nesting (left, 0 minutes) and the typical location of nesting material after 100 min (right, 100 min). For purposes of data analysis, the floor space of the home cage was treated as six contiguous zones, and dotted lines show the boundaries between zones. A Nestlet was placed into each zone at the start of the nesting period (location indicated by white rectangles), and untreated mice typically consolidated these Nestlets into one zone and began to shred the Nestlets into a fluffier consistency (location indicated by white cloud shape). The primary dependent measure was “Number of Zones Cleared” of its Nestlet. At the start of the nesting period, 0 zones were cleared. After 100 min, untreated mice usually cleared 5 zones, as indicated by the numbers in empty zones in the right diagram.
Noxious Stimuli and Pharmacological Treatments
Mice were randomly assigned to treatment groups of six to nine mice per group (specific group sizes are described below), and there were no exclusion criteria. For each manipulation, the goal was to manipulate treatment levels from low levels that produced no effect to high levels that produced a maximal effect, and effects of noxious stimuli and pharmacological treatments were determined as follows. First, a concentration-effect curve for i.p. lactic acid (0–0.32%, 5 min pretreatment) was evaluated using a within-subjects, repeated-measures design in a group of six mice. The sequence of lactic acid concentrations was randomized using a Latin-square design, and tests were separated by at least 48 hr. Second, the time courses of effects produced by vehicle and 0.32% lactic acid were determined using pretreatment times of 5, 20 or 60 min. Different groups of six mice each were used for each type of injection and pretreatment time. Third, the time course of effects produced by intraplantar CFA was determined. On Day 0, four groups of mice were briefly anesthetized with isoflurane. One group received no further treatment, and the other three groups received bilateral intraplantar 30 µl injections of (1) vehicle in both rear paws, (2) CFA in one paw and vehicle in the other paw, or 3) CFA in both paws. Nesting was then evaluated daily on Days 1–7 after treatment. This experiment was conducted in an initial set of three mice for each treatment, then confirmed in a subsequent experiment with six mice for each treatment. Data from all nine mice receiving each treatment were grouped for subsequent display and analysis.
In studies with test drugs, effects of the NSAID ketoprofen (0.01–10 mg/kg), the mu opioid receptor agonist morphine (0.01–10 mg/kg) and the kappa opioid receptor agonist U69,593 (0.1–1.0 mg/kg) were evaluated in mice treated with 0.32% lactic acid or unilateral CFA or in mice that received no other treatment. In studies with lactic acid, test drugs were administered 30 min before initiation of nesting, lactic acid was administered 5 min before nesting, and each dose of each drug was tested in a group of six mice. In studies with CFA, CFA was administered 24 hr before nesting, test drugs were administered 30 min before nesting, and each dose of each drug was tested in nine mice because of the smaller and more variable effects of CFA on nesting. In studies of drugs administered alone, test drugs were administered 30 min before nesting, and each dose of each drug was tested in six mice.
U69,593 administered alone produced a dose-dependent decrease in nesting without alleviating either acid- or CFA-induced depression of nesting (see Results). Consequently, two follow-up studies were conducted. First, ketoprofen (0.1–1.0 mg/kg) and morphine (0.1–1.0 mg/kg) were evaluated for their effectiveness to block U69,593-induced depression of nesting. For these studies, ketoprofen or morphine was administered 30 min before nesting, 1.0 mg/kg U69,593 was administered 15 min before nesting, and each set of conditions was tested in a group of six mice. Second, the kappa antagonist JDTic was evaluated for its effectiveness to block depression of nesting by 1.0 mg/kg U69,593, 0.32% lactic acid or unilateral CFA. For these studies, 20 mg/kg JDTic or its vehicle was administered 48 hr before nesting to accommodate the slow onset and long duration of selective kappa antagonist effects [6]. U69,593, lactic acid or CFA was administered 15 min, 5 min or 24 hr before nesting, respectively, and each condition was tested in a group of eight mice.
Data Analysis
For the purposes of data analysis, the floor of the home cage was treated as 6 contiguous zones as diagrammed in Figure 1. At the start of the nesting period, all six zones contained one Nestlet. The first step in nest construction is consolidation of Nestlets into one region, and Nestlet consolidation was quantified as the number of zones cleared of their Nestlet. This value was always “0” at the start of the nesting period (i.e. all zones contained a Nestlet, no zones cleared; see Figure 1 left panel), and the maximum score was “5” (i.e. all Nestlets consolidated into one zone, 5 zones cleared; see Figure 1 right panel). Data for “Number Zones Cleared” were averaged across mice, evaluated either by one- or two-way ANOVA as appropriate, and a significant ANOVA was followed by a Dunnett or Tukey post hoc test (Graphpad Prism 6.0f for Mac OSX; LaJolla, CA). The criterion for significance was p<0.05 for all analyses.
To permit calculation and comparison of test drug potencies, ED50 values and 95% confidence limits were calculated as follows. Drug effects on acid- and CFA-depressed nesting were transformed in each mouse to % Maximum Possible Effect (%MPE) using the equation [(Test-Baseline)/(5-Baseline)] * 100, where “Test” was the number of zones cleared after test drug + noxious stimulus in a given mouse, “Baseline” was the mean number of zones cleared by mice treated with drug vehicle + noxious stimulus, and “5” was the maximum possible number of zones that could be cleared. Similarly, drug effects on nesting in the absence of a noxious stimulus were transformed in each mouse to % Maximum Possible Suppression using the equation [(Baseline-Test)/Baseline] * 100, where “Baseline” was the mean number of zones cleared by vehicle-treated mice, and “Test” was the number of zones cleared after a drug dose. ED50 values and associated 95% confidence limits were determined by linear regression using points from the linear portion of the dose-effect curve. In cases were a drug produced a biphasic effect (i.e. morphine in CFA-treated mice), the ED50 value was determined for the ascending limb of the dose-effect curve. ED50 values were considered to differ if 95% confidence limits did not overlap.
Drugs
Lactic acid (Sigma-Aldrich, St. Louis, MO) was diluted in sterile saline for i.p. injection. Complete Freund’s adjuvant (1.0 mg/ml; Sigma-Aldrich) was administered into the plantar aspect of the hind paw(s). Ketoprofen propionate (Spectrum Chemical Co., Gardena, CA), morphine sulfate and U69,593 (National Institute on Drug Abuse Drug Supply Program, Bethesda, MD), and JDTic ((3R)-7-Hydroxy-N-{(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl}-1,2,3,4-tetrahydro-3-isoquinoline-carboxamide; synthesized by F.I. Carroll) were dissolved in sterile saline and injected s.c.
RESULTS
Overview
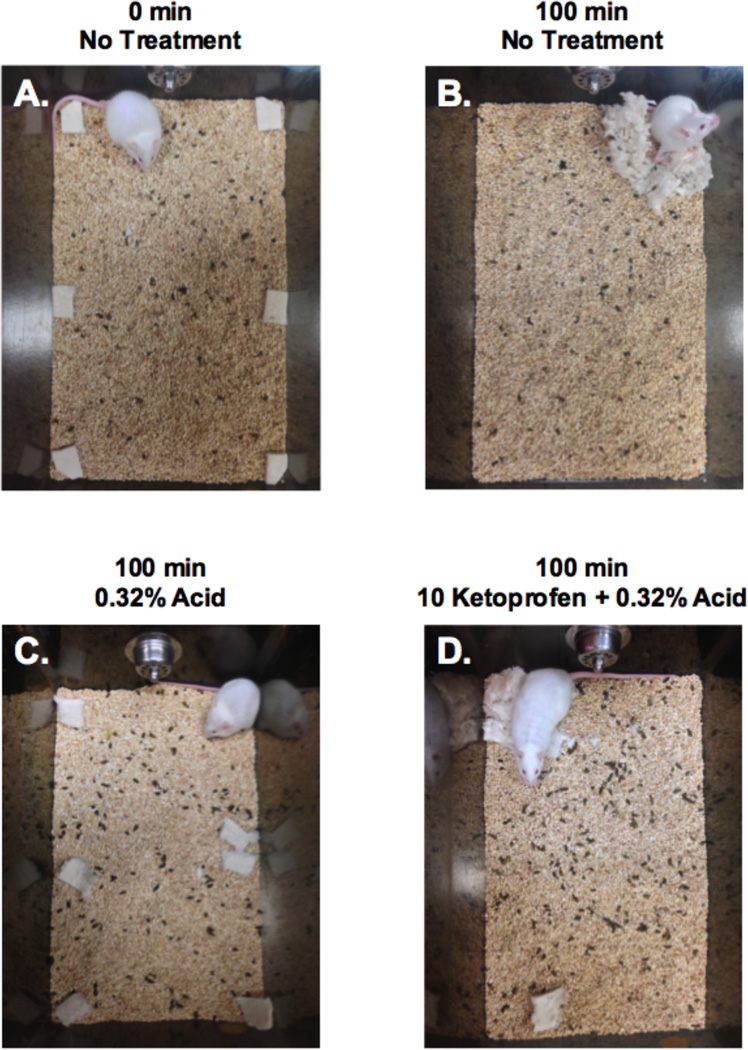
Figure 2 shows representative photographs of selected outcomes. Figure 2A shows Nestlet configuration at the start of the nesting period, and Figure 2B shows Nestlet consolidation after 100 min in a mouse that received no treatment. The numbers of zones cleared for these two conditions were 0 and 5, respectively, as diagrammed in Figure 1. Figure 2C shows nesting after treatment with 0.32% i.p. lactic acid. Only one zone was cleared, and the mouse occupied the cleared region rather than a region containing consolidated Nestlets. This is an example of pain-related depression of nesting. Figure 2D shows nesting in a mouse treated with 10 mg/kg ketoprofen as a pretreatment to 0.32% i.p. lactic acid. Four zones were cleared, and the mouse occupied the zone with consolidated Nestlets. This is an example of antinociception in this procedure.
Figure 2.
Photographs of Nestlet configurations under different treatment conditions. Panels A and B show Nestlet configuration before and 100 min after nesting by untreated mice, and “Zones Cleared” scores were 0 and 5, respectively. These panels are analogous to the diagrams in Figure 1. Note that mice have been habituated to the cage and bedding for 48 hr before testing, and consequently, bedding contains fecal pellets. Panels C and D show Nestlet configuration after treatment with 0.32% i.p. lactic acid alone (C) or after pretreatment with 10 mg/kg ketoprofen (D), and “Zones Cleared” scores were 1 and 4 in these examples.
Baseline Nesting
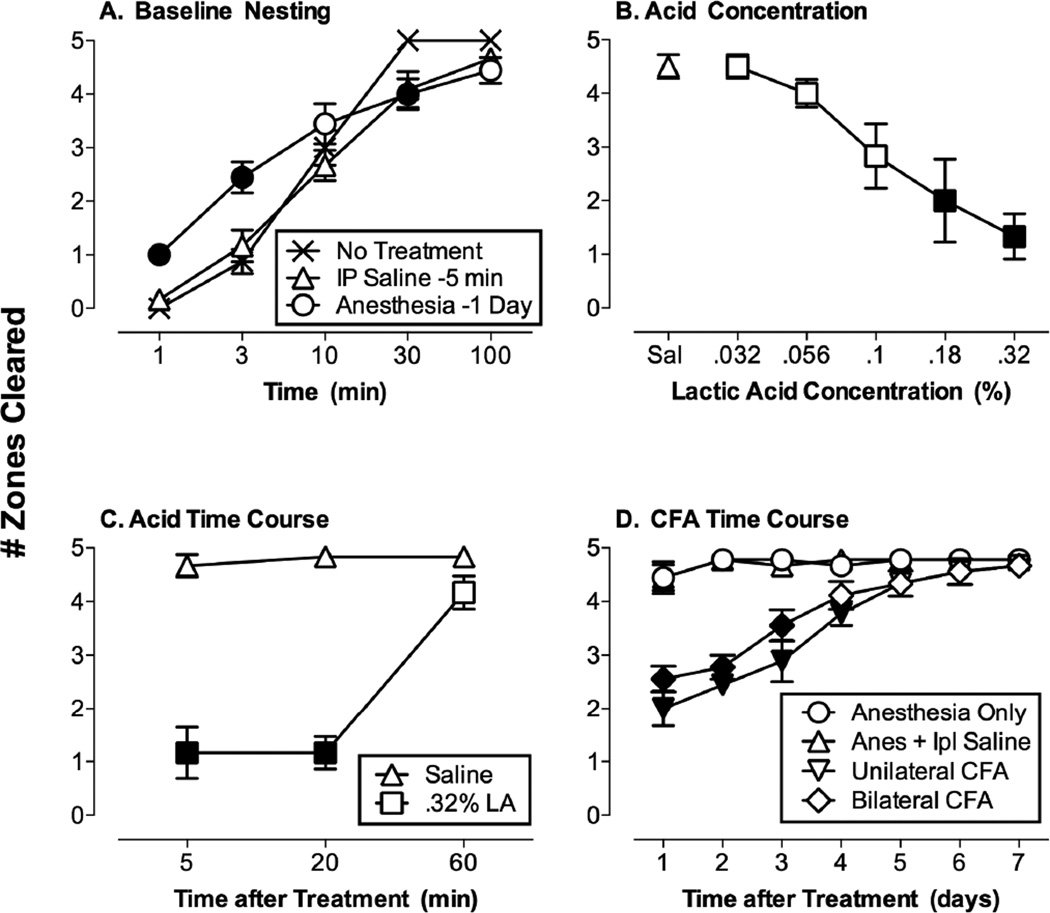
Figure 3A shows the rate of baseline Nestlet consolidation in the absence of treatment or after control treatment with either i.p. saline (5 min before testing) or brief isoflurane anesthesia (24 hr before testing). In the absence of treatment, mice usually cleared five zones and consolidated all Nestlets into one zone within 30 min, and this consolidation was retained at 100 min. Exposure to control i.p. saline injections (−5 min) or brief isoflurane anesthesia (−24 hr) produced significant but small changes in rates of nesting during the first 30 min, but all groups showed equivalent Nestlet consolidation by 100 min [2-way ANOVA: main effects of time (p<0.001) and treatment (p=0.016) and a significant interaction (p<0.001)]. Consequently, the 100 min nesting period was used for all subsequent studies.
Figure 3.
Nest consolidation under control conditions and after treatment with noxious stimuli. Panel A shows Number of Zones Cleared from 1 to 100 min after no treatment (N=8) or after control treatments for noxious stimuli (i.p. saline administered 5 min before nesting, N=12; brief isolflurane anesthesia 24 hr before nesting, N=9). Treatment was a between-subjects factor, and time was a within-subjects factor. Filled points indicate significantly different from “No Treatment” at a given time point (p<0.05 for this and all other post hoc comparisons after a significant 1- or 2-way ANOVA). Panel B shows effects of i.p. lactic acid concentration (0–0.32%, N=6). Acid concentration was a within-subjects factor, such that all mice received all acid concentrations. Filled points indicate significantly different from saline treatment (Sal). Panel C shows the time course of effects produced by i.p. saline or 0.32% lactic acid (LA). Both treatment and time were between-subjects factors, and N=6 for conditions. Filled points indicate significantly different from saline at a given time point. Panel D shows the time course of effects produced by anesthesia alone or anesthesia in combination with bilateral intraplantar saline, unilateral CFA (with saline injected in the opposite paw), or bilateral CFA. Treatment was a between-subjects factor and time was a within-subjects factor, and N=9 for all conditions. Filled points indicate significantly different from the anesthesia only control at a given time point.
Effects of Noxious Stimuli on Nesting
Figure 3B shows that i.p. administration of dilute lactic acid (0–0.32%) produced a concentration-dependent depression of Nestlet consolidation, and significant depression was observed with 0.18 and 0.32% acid (1-way ANOVA: p<0.001). A higher concentration of 0.56% acid caused lethality in some mice and was not tested further. Figure 3C shows that depression of Nestlet consolidation by 0.32% acid was observed after pretreatment times of 5 or 20 min but was no longer significant after 60 min (2-way ANOVA: main effects of treatment and time and a significant interaction, all p<0.001). Figure 3D shows effects of unilateral or bilateral intraplantar treatment with CFA (2-way ANOVA: main effects of treatment and time and a significant interaction, all p<0.001). After pretreatment with anesthesia alone or in combination with bilateral intraplantar saline, mice displayed high and stable levels of Nestlet consolidation throughout the seven-day testing period. Mice treated with either unilateral or bilateral CFA showed depressed Nestlet consolidation for up to four days after treatment, and there were no significant differences between unilateral and bilateral treatment. In view of these results, subsequent studies with test drugs were conducted using either (1) 0.32% i.p. lactic acid administered 5 min before testing, or (2) unilateral intraplantar CFA administered 24 hr before testing.
Effects of Test Drugs on Depressed Nesting
Test drugs were evaluated for their effectiveness to either (1) block acid-induced depression of nesting when administered as pretreatments to i.p. lactic acid, or (2) reverse CFA-induced depression of nesting when administered 23.5 hr after CFA but before evaluation of nesting. Figure 4A shows that both ketoprofen and morphine produced a dose-dependent blockade of acid-induced depression of nesting (1-way ANOVA, both p<0.001), whereas U69,593 had no significant effect. ED50 values are shown in Table 1, and morphine was significantly more potent than ketoprofen. Figure 4B shows that both ketoprofen and morphine also produced a dose-dependent reversal of CFA-induced depression of nesting (1-way ANOVA, both p<0.001). Ketoprofen effectiveness plateaued across a broad, 30-fold dose range (0.32–10 mg/kg), whereas the morphine dose-effect curve displayed an inverted-U shape (peak effects at 0.32–1.0 mg/kg). The potencies of ketoprofen and morphine to reverse CFA-induced depression of nesting did not differ, and ketoprofen was significantly more potent to reverse CFA effects than to block acid effects (Table 1). U69,593 was again without significant effect. Figure 4C shows effects of ketoprofen, morphine and U69,593 on nesting in the absence of treatment. Ketoprofen had no significant effect on nesting at doses up to 10 mg/kg, whereas both morphine and U69,593 dose-dependently decreased nesting (1-way ANOVA, both p<0.001). The potency of morphine to depress nesting was approximately 10-fold weaker than its potency to alleviate pain-depressed nesting (Table 1). Finally, Figure 4D shows that ketoprofen and morphine failed to alleviate U69,593-induced depression of nesting at doses that blocked and reversed acid- and CFA-induced depression of nesting, respectively.
Figure 4.
Effects of ketoprofen, morphine and U69,593 on nesting depressed by i.p. treatment with 0.32% lactic acid (Panel A), nesting depressed by unilateral I.pl. treatment with CFA (Panel B), or control nesting in the absence of any other treatment (Panel C). Panel D shows effects of ketoprofen and morphine on nesting depressed by treatment with U69,593. For each panel, the abscissa shows drug dose in mg/kg (log scale, V=vehicle), and the ordinate shows nesting expressed as Number of Zones Cleared. Different groups of 6–9 mice were used to determine each point. Filled points indicate significantly different from the associated vehicle treatment for that drug under that condition as determined by a significant 1-way ANOVA followed by the Dunnett’s post hoc test (p<0.05).
TABLE 1.
ED50 values in mg/kg (95% CL) of ketoprofen, morphine and U69,593 to alter nesting under the conditions shown.
| Acid-Depressed | CFA-Depressed | Control | U69,593-Depressed | |
|---|---|---|---|---|
| Drug | Nesting | Nesting | Nesting | Nesting |
| Ketoprofen | 2.05 (1.46–2.89) | 0.18 (0.10–0.34) | Inactive | Inactive |
| Morphine | 0.72 (0.52–1.00) | 0.38 (0.22–0.65) | 5.37 (4.48–6.45) | Inactive |
| U69,593 | Inactive | Inactive | 0.55 (0.36–0.83) | Not Tested |
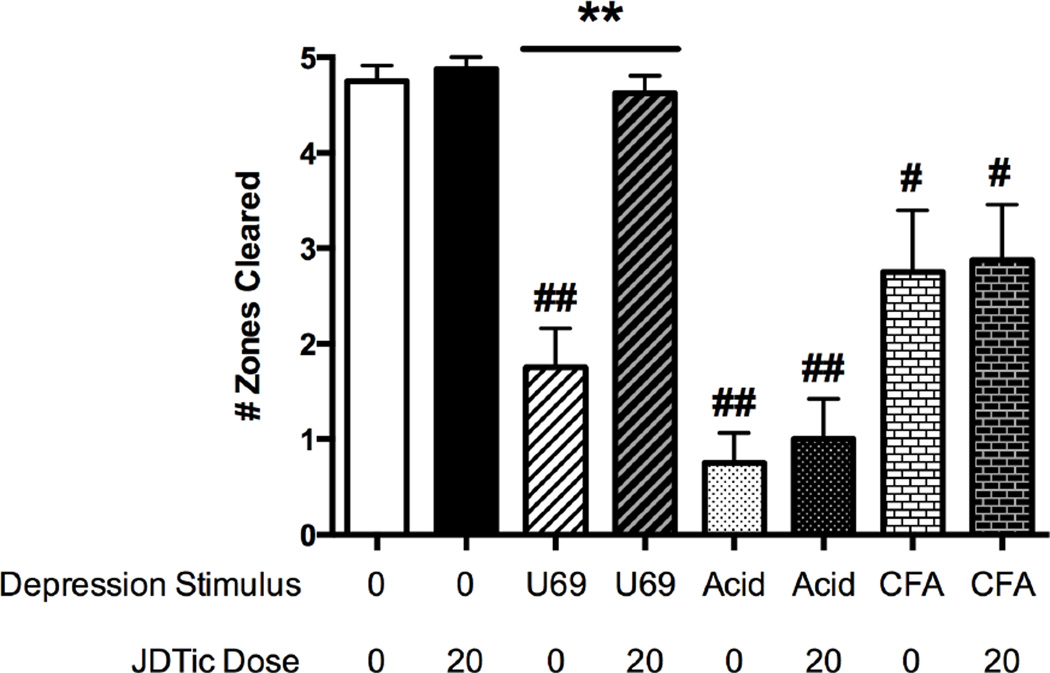
Figure 5 shows effects of the kappa opioid antagonist JDTic (20 mg/kg) on nesting under various conditions. Two-way ANOVA indicated main effects of JDTic dose and the associated co-treatment (no treatment, U69,593, i.p. acid, or i.pl. CFA) and a significant interaction (all p<0.01). After pretreatment with JDTic vehicle, nesting was significantly depressed by 1.0 mg/kg U69,593, 0.32% i.p. acid, and unilateral i.pl. CFA. JDTic pretreatment did not alter control nesting and fully blocked depression of nesting by U69,593; however, JDTic failed to alter pain-related depression of nesting produced either by i.p. acid or by i.pl. CFA.
Figure 5.
Effects of the kappa opioid receptor antagonist JDTic (20 mg/kg) on control nesting or nesting depressed by U69,593, i.p. acid, or i.pl. CFA. Treatments are shown on the abscissa, and the ordinate shows nesting expressed as Number of Zones Cleared. Data were analyzed by two-way ANOVA followed by the Tukey post hoc test. Asterisks indicate a significant effect of JDTic dose within a given depression stimulus: ** p<0.01. Number signs indicate a significant effect of the depression stimulus within a given JDTic dose: # p<0.05, ## p<0.01.
DISCUSSION
The aim of this study was to develop and validate a procedure for evaluation of pain-related depression of nesting in mice. The results suggest that this procedure is a simple, sensitive, selective, and quantitative experimental tool for research on expression and treatment of pain-related functional impairment and depression of behavior in mice.
Nesting as a target behavior for research on pain-depressed behavior
The present results are consistent with previous reports that nesting in mice is sensitive to depression by pain states and useful as a target behavior in research on pain-depressed behavior [10; 14; 16; 37]. In particular, nesting offers at least three advantages as a source of dependent measures. First, it is an innate behavior that can be evaluated in standard home-cage environments with commonly available husbandry materials and without reliance on additional equipment to generate or measure behavioral endpoints. As a result, nesting is amenable to relatively low-cost and high-throughput evaluation of effects produced by experimental manipulations, including manipulations intended to model pain states. Second, effective nesting is an adaptive behavior that promotes self-preservation in mice [19]. As such, it may serve as a model for research on functional impairment of adaptive behavior by pain. Third, behavioral measures of nesting require little interaction between the experimental subject and either the experimenter or novel experimental environments that can modify pain-related behaviors and confound evaluation of candidate analgesics, for example by producing stress-induced analgesia that can interact with drug effects [3; 41].
The present study built on previous research in part by seeking to develop a procedure for rapid and quantitative assessment of nesting in mice. Previous research on nesting has relied primarily on ordinal scales of nest quality, although other more quantitative dependent measures have also been reported [10; 14; 16]. The procedure described here includes several features that contributed to stability of baseline behavior and sensitivity to noxious stimuli. First, mice were individually housed with one Nestlet upon arrival in the laboratory, and mice were tested in their home cages no sooner than 48 hr later. This period of acclimation to the home cage and bedding was suitable to engender stable nesting (e.g. Figure 3A), and preliminary studies suggested that shorter acclimation periods or use of fresh bedding reduced the speed and stability of nesting (data not shown). Second, during test sessions, nesting material was supplied as one new Nestlet divided into six pieces and distributed throughout the home cage. Nestlets are convenient as a type of nesting material because of their low cost, widespread availability, and standardized size, content and consistency. The configuration of Nestlet presentation (as six distributed pieces rather than as a single whole Nestlet) enabled assessment of Nestlet consolidation as an early phase of nesting behavior that could be reliably assessed during a 100-min nesting period. Finally, Nestlet consolidation was scored by counting the number of zones cleared of their Nestlet during the nesting period. This is an objective and easily quantified variable on a ratio rather than ordinal scale, and it proved sensitive to the manipulations tested here and suitable for quantitative pharmacology. Finally, although other measures of nesting were not recorded in this study, the measure of Nestlet consolidation could be easily supplemented with other measures, such as amount of Nestlets shredded or a score for quality of an eventual nest [10].
The depression of nesting produced by i.p. lactic acid and i.pl. CFA is consistent with previous studies that reported depression by these noxious stimuli of other behaviors, including liquid food consumption, locomotor activity, and wheel running in mice [8; 9; 27; 43] and burrowing, wheel running, and operant responding for electrical brain stimulation in rats [1; 15; 21; 22]. Nesting may be advantageous relative to these other behaviors as a dependent measure for studies of pain-depressed behavior for several reasons. First, it requires no explicit training or habituation to experimental equipment. By comparison, wheel running is typically assessed only after multiple days of exposure to the running wheel to reach stable baselines [9; 27], and operant responding for food or electrical brain stimulation can take several weeks to train to stability [31]. Second, it appears to be especially sensitive to depression by noxious stimuli. For example, nesting was depressed by unilateral i.pl. CFA, but bilateral CFA was required to reliably depress wheel running in mice [8], and even bilateral CFA produced only a weak effect on operant responding for electrical brain stimulation in rats [22]. Lastly, pain-related depression of nesting may be more sensitive than pain-related depression of some other behaviors to analgesic drugs (see below).
Sensitivity and selectivity of pain-depressed nesting for evaluation of candidate analgesics
Both the NSAID ketoprofen and the mu opioid analgesic morphine produced dose-dependent blockade of acid-depressed nesting and reversal of CFA-depressed nesting. These results provide evidence for sensitivity of this procedure to the two main classes of clinically effective analgesics for treatment of pain associated with inflammation. These results also agree with other evidence for the potency and effectiveness of NSAIDs and mu agonists to block or reverse other examples of inflammatory pain-related depression of behavior in rodents [9; 25; 30]. For example, depression of wheel running induced by bilateral CFA in mice was also reversed by morphine and a range of NSAIDs [8]. This nesting procedure may also offer enhanced sensitivity to clinically effective analgesics relative to procedures that target some other pain-depressed behaviors. For example, morphine was more potent and/or effective to block acid-induced depression of nesting in this study than to block acid-induced depression of locomotor activity or feeding in mice [27; 43; 44].
The ineffectiveness of U69,593 to block or reverse pain-related depression of nesting provides evidence for the selectivity of this nesting procedure for clinically effective analgesic drugs. Centrally acting kappa agonists like U69,593 reliably produce antincoception in conventional assays that measure pain-stimulated behaviors, such as writhing stimulated by i.p. acid or hypersensitive withdrawal responses to thermal stimuli elicited by i.pl. CFA [2; 39; 40]. However, centrally acting kappa agonists have not succeeded as clinically effective analgesics [34; 35; 45], suggesting that apparent antinociception by kappa agonists in conventional preclinical procedures is a false-positive effect not predictive of clinical outcomes. The failure of U69,593 to produce antinociception in the present study agrees both with the poor clinical analgesic profile of centrally acting kappa agonists and with other evidence for poor effectiveness of kappa agonists to restore pain-depressed behaviors in preclinical studies [21; 32]. Taken together these results support the potential for preclinical assays of pain-depressed behavior to complement more conventional assays in efforts to characterize candidate analgesics and identify compounds with clinical potential.
The present study also used U69,593 as an experimental manipulation to depress nesting by a putative non-pain manipulation. The failure of either ketoprofen or morphine to block U69,593-induced depression of nesting provides evidence for selectivity of these drugs to alleviate depression of nesting by pain-related stimuli but not by non-pain stimuli.
Lack of evidence for a role of the endogenous kappa opioid system in pain-depressed nesting
One potential application of this assay of pain-depressed nesting is evaluation of novel candidate analgesics drugs, and this study examined the kappa opioid receptor antagonist JDTic. A growing body of research indicates that stress-induced activation of endogenous dynorphin/kappa opioid receptor signaling in brain may produce behavioral signs of depression, and kappa opioid receptor antagonists have emerged as a novel class of candidate antidepressant drugs [5]. It has also been suggested that this endogenous kappa opioid system may be activated by pain states to mediate pain-related signs of depressed behavior and mood [4]. Thus, while centrally acting kappa agonists have failed as clinically effective analgesics, it now appears that kappa antagonists are emerging as alternative candidates. However, the present results do not support the potential of kappa antagonists as analgesics, because JDTic failed to produce an analgesic-like alleviation of acid- or CFA-induced depression of nesting at a dose that was sufficient to block depression of nesting by the exogenous kappa agonist U69,593. These results agree with the failure of another kappa antagonist, norbinaltorphimine, to block pain-related depression of intracranial self-stimulation or of microdialysis measures of mesolimbic dopamine release in rats [21; 22]. It is possible that kappa antagonists may have antinociceptive efficacy under other conditions or on other behavioral endpoints; however, the present and previous studies [21; 22] have identified a range of conditions under which clinically effective analgesics are effective to alleviate pain-related depression of behavior, but kappa antagonists are not.
Limitations of the present study
This manuscript reports a new approach to evaluation of pain-related depression of nesting in mice. Although we have been able to replicate and systematically extend key aspects of the procedure in our laboratory, nesting is subject to many independent variables that were not explicitly manipulated here (e.g. sex, strain, source and age of mice [20; 37]; single- vs. group-housing [23; 37]; environmental conditions [13]; neuropathic rather than inflammatory pain stimuli [36]). Future studies will be required to investigate the roles of these and other independent variables as determinants of Nestlet consolidation.
Acknowledgments
Research reported in this publication was supported by the National Institute on Neurological Disorders and Stroke and the National Institute on Drug Abuse of the National Institutes of Health under award numbers R01NS070715, R01DA030404 and T32DA007027.
Dr. Negus declares that his research has been funded by NIH. During the past 3 years, he has received compensation as a consultant for or collaborator with Alkermes and Grunenthal for projects related to abuse potential assessment.
Dr. Carroll is an inventor of US patents claiming the composition of JDTic owned by the Research Triangle Institute.
Footnotes
Mr. Neddenriep, Dr. Altarifi, Mr. Leitl and Dr. Miller declare no conflict of interest.
REFERENCES
- 1.Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Pheby T, Huang W, Burgess G, Machin I, Rice AS. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. European journal of pain (London, England) 2012;16(4):485–495. doi: 10.1016/j.ejpain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115(3):311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- 3.Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88(3):184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Cahill CM, Taylor AM, Cook C, Ong E, Moron JA, Evans CJ. Does the kappa opioid receptor system contribute to pain aversion? Frontiers in pharmacology. 2014;5:253. doi: 10.3389/fphar.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll FI, Carlezon WA., Jr Development of kappa opioid receptor antagonists. J Med Chem. 2013;56(6):2178–2195. doi: 10.1021/jm301783x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, Pollard GT, Aceto MD, Harris LS. Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol. 2004;501(1–3):111–119. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 8.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153(4):876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobos EJ, Portillo-Salido E. "Bedside-to-Bench" Behavioral Outcomes in Animal Models of Pain: Beyond the Evaluation of Reflexes. Current neuropharmacology. 2013;11(6):560–591. doi: 10.2174/1570159X113119990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1(3):1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Elmer GI, Pieper JO, Negus SS, Woods JH. Genetic variance in nociception and its relationship to the potency of morphine-induced analgesia in thermal and chemical tests. Pain. 1998;75(1):129–140. doi: 10.1016/S0304-3959(97)00215-7. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson DR, Bailey MM. Reproductive performance of mice in disposable and standard individually ventilated cages. Journal of the American Association for Laboratory Animal Science : JAALAS. 2013;52(3):228–232. [PMC free article] [PubMed] [Google Scholar]
- 14.Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR. Nest building as an indicator of health and welfare in laboratory mice. Journal of visualized experiments : JoVE. 2013;(82):51012. doi: 10.3791/51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grace PM, Strand KA, Maier SF, Watkins LR. Suppression of Voluntary Wheel Running in Rats Is Dependent on the Site of Inflammation: Evidence for Voluntary Running as a Measure of Hind Paw-Evoked Pain. J Pain. 2013 doi: 10.1016/j.jpain.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods. 2014;234:139–146. doi: 10.1016/j.jneumeth.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. Burrowing behavior as an indicator of post-laparotomy pain in mice. Frontiers in behavioral neuroscience. 2010;4:165. doi: 10.3389/fnbeh.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwilasz AJ, Negus SS. Dissociable Effects of the Cannabinoid Receptor Agonists {Delta}9-Tetrahydrocannabinol and CP55940 on Pain-Stimulated Versus Pain-Depressed Behavior in Rats. J Pharmacol Exp Ther. 2012;343(2):389–400. doi: 10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latham N, Mason G. From house mouse to mouse house: the behavioral biology of free-living Mus musculus and its implications in the laboratory. App Anim Behav Sci. 2004;86:261–289. [Google Scholar]
- 20.Lee CT. Genetic analyses of nest-building behavior in laboratory mice (Mus musculus) Behav Genet. 1973;3(3):247–256. doi: 10.1007/BF01067601. [DOI] [PubMed] [Google Scholar]
- 21.Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Jr, Banks ML, Negus SS. Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous kappa-opioids. Neuropsychopharmacology. 2014;39(3):614–624. doi: 10.1038/npp.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA, Jr, Negus SS. Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund's adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol Pain. 2014;10:62. doi: 10.1186/1744-8069-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin LJ, Hathaway G, Isbester K, Mirali S, Acland EL, Niederstrasser N, Slepian PM, Trost Z, Bartz JA, Sapolsky RM, Sternberg WF, Levitin DJ, Mogil JS. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr Biol. 2015;25(3):326–332. doi: 10.1016/j.cub.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101(1):191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320(1):194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- 26.Melzack R, Katz J. Pain assessment in adult patients. In: McMahon LR, Koltzenburg M, editors. Textbook of Pain. London: Elsevier; 2006. pp. 291–304. [Google Scholar]
- 27.Miller LL, Picker MJ, Schmidt KT, Dykstra LA. Effects of morphine on pain-elicited and pain-suppressed behavior in CB1 knockout and wildtype mice. Psychopharmacology (Berl) 2012;215(3):455–465. doi: 10.1007/s00213-011-2232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80(1–2):67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 29.National_Research_Council. Guide for the Care and Use of Laboratory Animals. 8th. Washington DC: National Academy Press; 2011. [Google Scholar]
- 30.Negus SS. Expression and treatment of pain-related behavioral depression. Lab animal. 2013;42(8):292–300. doi: 10.1038/laban.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negus SS, Bilsky EJ, Pereira Do Carmo G, Stevenson GW. Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. In: Szallasi A, editor. Methods in Molecular Biology: Analgesia. New York: Humana Press; 2010. pp. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 2010;210(2):149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical Assessment of Candidate Analgesic Drugs: Recent Advances and Future Challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 34.Pande AC, Pyke RE, Greiner M, Cooper SA, Benjamin R, Pierce MW. Analgesic efficacy of the kappa-receptor agonist, enadoline, in dental surgery pain. Clin Neuropharmacol. 1996;19(1):92–97. doi: 10.1097/00002826-199619010-00009. [DOI] [PubMed] [Google Scholar]
- 35.Pande AC, Pyke RE, Greiner M, Wideman GL, Benjamin R, Pierce MW. Analgesic efficacy of enadoline versus placebo or morphine in postsurgical pain. Clin Neuropharmacol. 1996;19(5):451–456. doi: 10.1097/00002826-199619050-00009. [DOI] [PubMed] [Google Scholar]
- 36.Percie du Sert N, Rice AS. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br J Pharmacol. 2014;171(12):2951–2963. doi: 10.1111/bph.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rock ML, Karas AZ, Rodriguez KB, Gallo MS, Pritchett-Corning K, Karas RH, Aronovitz M, Gaskill BN. The time-to-integrate-to-nest test as an indicator of wellbeing in laboratory mice. Journal of the American Association for Laboratory Animal Science : JAALAS. 2014;53(1):24–28. [PMC free article] [PubMed] [Google Scholar]
- 38.Rutten K, Schiene K, Robens A, Leipelt A, Pasqualon T, Read SJ, Christoph T. Burrowing as a non-reflex behavioural readout for analgesic action in a rat model of sub-chronic knee joint inflammation. European journal of pain (London, England) 2014;18(2):204–212. doi: 10.1002/j.1532-2149.2013.00358.x. [DOI] [PubMed] [Google Scholar]
- 39.Schepers RJ, Mahoney JL, Gehrke BJ, Shippenberg TS. Endogenous kappa-opioid receptor systems inhibit hyperalgesia associated with localized peripheral inflammation. Pain. 2008;138(2):423–439. doi: 10.1016/j.pain.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seguin L, Le Marouille-Girardon S, Millan MJ. Antinociceptive profiles of non-peptidergic neurokinin1 and neurokinin2 receptor antagonists: a comparison to other classes of antinociceptive agent. Pain. 1995;61(2):325–343. doi: 10.1016/0304-3959(94)00194-J. [DOI] [PubMed] [Google Scholar]
- 41.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nature methods. 2014;11(6):629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 42.Stevens SS. On the Theory of Scales of Measurement. Science. 1946;103(2684):677–680. [PubMed] [Google Scholar]
- 43.Stevenson GW, Bilsky EJ, Negus SS. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain. 2006;7:408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- 44.Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: Drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci. 2009;85:309–315. doi: 10.1016/j.lfs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadenberg ML. A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev. 2003;9(2):187–198. doi: 10.1111/j.1527-3458.2003.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whiteside GT, Pomonis JD, Kennedy JD. An industry perspective on the role and utility of animal models of pain in drug discovery. Neurosci Lett. 2013 doi: 10.1016/j.neulet.2013.08.033. [DOI] [PubMed] [Google Scholar]