Abstract
Imbibitional chilling injury during germination causes agricultural losses but this can be overcome by osmopriming. It remains unknown how membranes reorganize during germination. Herein, we comparatively profiled changes of membrane lipids during imbibition under normal and chilling temperatures in chilling-tolerant and -sensitive soybean seeds. We found three patterns of dynamic lipid remodelling during the three phases of germination. Pattern 1 involved a gradual increase in plastidic lipids during phases I and II, with an abrupt increase during phase III. This abrupt increase was associated with initiation of photosynthesis. Pattern 3 involved phosphatidic acid (PA) first decreasing, then increasing, and finally decreasing to a low level. Pattern 1 and 3 were interrupted in chilling-sensitive seeds under low temperature, which lead a block in plastid biogenesis and accumulation of harmful PA respectively. However, they were rescued and returned to their status under a normal temperature after polyethylene glycol (PEG) osmopriming. We specifically inhibited phospholipase D (PLD)-mediated PA formation in chilling-sensitive seeds of soybean, cucumber, and pea and found their germination under low temperature was significantly improved. These results indicate that membranes undergo specific and functional reorganization of lipid composition during germination and demonstrate that PLD-mediated PA causes imibibitional chilling injury.
Keywords: germination, imbibitional chilling injury, membrane lipid remodelling, osmopriming, phospholipase D, soybean seeds, membrane lipids, dynamic remodelling, plastid biogenesis
INTRODUCTION
As the first essential step of the plant life cycle, seed germination is an important part of crop growth. Depending on the rate of water uptake, the time course of germination and subsequent growth is divided into three phases. In phase I, which is also called imbibition, water uptake is rapid; in phase II, water uptake is much slower and reaches a plateau; in phase III (post-germination), there is an increase in water uptake (Bewley, 1997, Weitbrecht, Muller & Leubner-Metzger, 2011). Imbibition is negatively affected by high rates of water uptake and low temperatures. Low temperatures reduce the speed and/or rate of germination through imbibitional chilling injury. Imbibitional chilling injury is a common problem in agriculture (Bramlage, Leopold & Parrish, 1978, Cheng, Li & He, 2009) and has been reported for a wide range of crops, including bean and lima bean (Pollock, 1969), cotton (Christiensen, 1967), pea (Powell & Matthews, 1978), cucumber (Makeen, Normah, Dussert & Clyde, 2006, Posmyk, Balabusta, Wieczorek, Sliwinska & Janas, 2009), and corn and soybean (Hobbs & Obendorf, 1972). An attempt to introduce guar bean in Beijing in 1970 failed because this bean is very sensitive to imbibitional chilling (Barba-Espin, Hernandez & Diaz-Vivancos, 2012). The yield of soybean production in the US decreased by up to 25% due to imbibitional injury in 1972 (Hobbs & Obendorf, 1972). Imbibition has attracted wide and long-lasting interest from researchers. In agricultural practice, imbibitional chilling injury is often overcome by osmopriming pre-treatment (Li, Yang, Zhang & Wang, 2010, Sun, Li, Wang, Wu & Wang, 2011, Zhuo, Wang, Lu, Sen & Wang, 2009). However, many fundamental questions about imbibition remain to be explored (Weitbrecht et al., 2011).
The biological events that take place during germination were recently revealed by transcriptomic (Bhardwaj, Anand & Nagarajan, 2012, Demarsy, Buhr, Lambert & Lerbs-Mache, 2012, Holdsworth, Bentsink & Soppe, 2008, Parrish & Leopold, 1977) and proteomic analyses (Cheng, Gao, Li, Shi, Javeed, Jing, Yang & He, 2010, Han, Yin, He & Yang, 2013, Law, Narsai, Taylor, Delannoy, Carrie, Giraud, Millar, Small & Whelan, 2012, Pagnussat, Burbach, Baluska & de la Canal, 2012, Swigonska & Weidner, 2013, Yang, Liu, Liu, Chen, Chen & Shen, 2009), and can basically be classified into two types. The first involves the metabolic quiescence of dry seeds being reversed after imbibition. For example, triacylglycerols in the oil bodies are hydrolysed by lipases to provide energy and carbon during late germination and early seedling growth (Han et al., 2013, Yang et al., 2009), pre-existing mRNA is translated (Parrish & Leopold, 1977), and the high abscisic acid levels in dry seeds decrease during phases I and II (Weitbrecht et al., 2011). The second type involves events that depend on de novo synthesis after hydration, such as the synthesis of gibberellin during germination and its induction of the expression of alpha-amylase (Kim, Kang, Wang, Kim, Hwang & Kang, 2008). Control of timing is a prominent feature for the events during germination. For example, the repair of DNA damage starts early in phase I and ends in phase II; the onset of the mobilization of nutrient reserves occurs early in phase II; and cytoplasmic leakage must stop at the end of phase I, or else germination may fail to occur (Bewley, 1997, Weitbrecht et al., 2011). Owing to the fact that organelles are not fully differentiated in mature dried seeds, important occurrences during germination also include organelle formation and tissue development. For example, vacuoles start to develop during phase II (Tan-Wilson & Wilson, 2012), mitochondria start to develop early in phase I (Ventura, Dona, Macovei, Carbonera, Buttafava, Mondoni, Rossi & Balestrazzi, 2012), and the radicle elongates early in phase III (Bewley, 1997).
The most important event during imbibition is probably membrane reorganization, given that it occurs before other events and is a precondition for most cellular process (Simon, 1974). Adoption of the hexagonal II phase of membrane (Ishibashi, Koda, Zheng, Yuasa & Iwaya-Inoue, 2013, Verkleij, De Maagd, Leunissen-Bijvelt & De Kruijff, 1982) contributes to substantial cytoplasmic leakage upon hydration (Bramlage et al., 1978, Lyons, 1973). Membranes must quickly reorganize from a hexagonal II phase to a lamellar phase in order to restore normal function and to terminate leakage of cellular components(Villa-Hernandez, Dinkova, Aguilar-Caballero, Rivera-Cabrera, Sanchez de Jimenez & Perez-Flores, 2013). Against this background, the widely accepted model for imbibitional chilling injury is that a low temperature disrupts membrane reorganization. However, it remains unclear whether and how the lipid composition changes during membrane reorganization, particularly how the lipids change during phase I, II, and III. The model for imbibitional chilling injury has not been tested yet, at least at the level of the lipid composition of membranes.
Plant membranes mainly consist of glycerolipids, including six classes of phospholipids: phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidic acid (PA), and phosphatidylglycerol (PG), and two classes of galactolipids: monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), which are plastidic lipids. In comparison with the lipid composition in leaves, seed membranes have low levels of both MGDG and DGDG, and high levels of PA (Crowe & Crowe, 1992). The low levels of MGDG and DGDG result from the lack of plastids in seeds. The high level of PA probably results from the drying during seed maturation because desiccation usually induces its formation(Devaiah, Roth, Baughman, Li, Tamura, Jeannotte, Welti & Wang, 2006, Gasulla, Dorp, Dombrink, Zahringer, Gisch, Dormann & Bartels, 2013); moreover, a high level of PA in membranes favours formation of the hexagonal II phase (Verkleij et al., 1982). Besides its specific role in membrane structure, PA is also a central intermediary (PA pool) in lipid metabolism pathways for both storage and membrane lipids (Gasulla et al., 2013). Given the importance of storage lipid catalysis, the PA pool could have critical functions during germination. Under conditions of dehydration and low temperature, PA is derived mainly from phospholipase D-mediated hydrolysis of phospholipids (Zhang, Wang, Zhang, Tao & Li, 2013). However, it is largely unknown whether and how the composition of membrane lipids changes and functionally contributes to germination.
Soybean is an important crop and its seed is a good experimental model to study germination (Cheng et al., 2010, Han et al., 2013, Hobbs & Obendorf, 1972, Lee, Welti, Schapaugh & Trick, 2011). Using a lipidomics approach based on electrospray ionization mass spectrometry (ESI-MS/MS) (Li, Wang, Li, Li, Wang, Welti & Wang, 2008, Welti, Li, Li, Sang, Biesiada, Zhou, Rajashekar, Williams & Wang, 2002, Welti, Shah, Li, Li, Chen, Burke, Fauconnier, Chapman, Chye & Wang, 2007), we profiled the changes of lipid molecular species and found distinctive patterns of lipid changes during phases I, II, and III in a chilling-tolerant soybean cultivar. We then examined the alteration of the patterns in a chilling-sensitive cultivar and thus showed the association between the patterns of lipid changes and imbibitional chilling injury. We verified the association by rescuing the chilling-sensitive with osmopriming in the chilling-sensitive seeds and analysing their lipid changes. We finally demonstrated that pattern 3 was the cause, at least partly, for chilling sensitivity by specific pharmaceutical effects on its formation. The patterns of extraplastidic lipids and their quantitative and temporal effects on plastid biogenesis in seeds are presented. We provide detailed descriptions of lipid changes during membrane reorganization and propose a novel model for imbibitional chilling injury.
MATERIALS AND METHODS
Seed materials
Soybean seeds of nine lines (Liaoxin, Tiefeng, G1005, 95-1, 91-1, 8157, Taiwan25, Taiwan292, and Riben 5) were bought in 2008 from commercial supplier. The seeds were equilibrated to 10% moisture over a saturated solution of LiCl (53% relative humidity) for one week and stored at 15 °C until subsequent experiments.
Water content measurements
Seeds were dried in an oven at 105 °C for 17 h to determine their water contents. Moisture content was calculated on a dry-weight basis. Five replicates were used for the lipid analysis (five seeds per replicate); the results are presented on a dry-weight basis.
Seed germination and sensitivity to imbibitional chilling injury
Seeds of the nine lines were tested for sensitivity to imbibitional chilling. The seeds were subjected to imbibition for 24 h by submersion in distilled water at 25 °C or 4 °C hydration of 10% moisture seed. After 24 h of imbibition, the seeds were transferred to Petri dishes (12 cm) that each contained three layers of filter paper moistened with 9 ml of distilled water, and were germinated in an HPG-280 controlled environment chamber at 25 °C with a 12 h photo-period at 80 µmol m−2 s−1. Forty seeds for each of four replications were used. Five-day-old germinated seeds were counted. Germination rate was measured to determine the sensitivity to imbibitional chilling.
Seed priming
Dry seeds R5 were subjected to imbibition in 33% PEG-6000 solution for three days at 15 °C. After this treatment, the seeds were washed three times in distilled water and then dried for three days in air at room temperature. After this re-drying, the moisture content of the osmoconditioned seeds was 10.3% on average (dry weight basis), similar to that of control untreated seeds. The control seeds and the primed seeds were then imbibed at 4 °C for 24 h (chilling treatment). Untreated seeds were used as controls.
N-Acylethanolamine and butanol treatments
For seed germination assays, thirty dry seeds were subjected to imbibition at 4 °C and then placed at 25 °C in individual plates that contained water along with n-butanol, isobutyl alcohol, s-butyl alcohol, t-butanol, NAE 12:0, and an equivalent volume of DMSO (used as a solvent for NAE). The seeds (soybean, pea, cucumber) were subjected to NAE and butanol treatments from different times during germination. S-butyl alcohol and t-butanol served as inactive isomer controls (Blancaflor, Hou & Chapman, 2003, Gardiner, Collings, Harper & Marc, 2003, Jia, Tao & Li, 2013, Motes, Pechter, Yoo, Wang, Chapman & Blancaflor, 2005).
Electrolyte leakage measurements
To measure electrolyte leakage from seeds during imbibitional chilling, the seeds were placed in 15 ml of distilled water for various times at 4 °C. Leakage was determined after the distilled water increases to 25 °C to record the data. The conductivity of the medium was measured with a conductivity meter (K220 Consort) after three hours. The results are expressed as the percentages of the control total leakage from seeds boiled for 15 min in water.
Chlorophyll fluorescence analysis
Chlorophyll fluorescence was analysed using an imaging chlorophyll fluorometer, MAXI-Imaging Pulse-Amplitude (PAM) (Walz, Germany). The maximal quantum yield of photosystem II (PS II) photochemistry Fv / Fm was measured in dark-adapted (20 minutes) samples on the basis of the initial fluorescence level (F0) and the maximal fluorescence level (Fm), and expressed as Fv/Fm = (Fm −F0)/ Fm.
Lipid extraction
Samples were harvested at the indicated time and the embryonic axes were ground into powder in liquid nitrogen. To inhibit lipolytic activity, were transferred immediately into 3 ml of isopropanol with 0.01% butylated hydroxytoluene in a 75 °C water bath for 15 min. The tissue was extracted three times with chloroform/methanol (2:1), with a week of agitation each time. The remaining plant tissue was dried overnight at 105 °C for 18 h and weighed to give the dry weight of the tissue. The combined extracts were washed once with 1 ml of 1 M KCl and once with 2 ml of water. The lipid extract solvent was evaporated under nitrogen.
Thin Layer Chromatography (TLC) analysis
The dried lipid samples were dissolved in chloroform, and then spotted onto a TLC plate (silica gel G) by the normalization. The plate was developed with chloroform: methanol: NH4OH (6.5:3.5:0.6). The spots of PA were monitored by UV colorimetric analysis after spraying with the color-developing agent primuline (Li, Wang, Yu, Yu & Li, 2014). The measurements were repeated twice.
ESI-MS/MS analysis and data processing
ESI-MS/MS analysis and quantification were performed as described previously, with minor modifications (Kansas Lipidomics Research Center, http://www.k-state.edu/lipid/lipidomics). Lipid samples of the embryonic axes were analysed using a triple quadrupole MS/MS equipped for ESI. Data processing was performed as described previously (Welti et al., 2002). The lipids in each class were quantified in comparison to two internal standards for that class. Five replicates of each treatment for each genotype were analysed. The Q-test was performed on the total amount of lipid in each class, and data from discordant samples were removed. The data were subjected to one-way analysis of variance with SPSS 13.0. Statistical significance was tested by Fisher’s least significant difference (LSD) method. Hierarchal clustering analysis was performed using Genespring version 7.2 (Silicon Genetics). Principal component analysis (PCA) was performed using Statistical Product and Service Solutions (SPSS) version 16. The diagram of the proposed model of PA activity was made by Microsoft Excel.
RESULTS AND DISCUSSION
Germination and imbibitional chilling injury in the seeds of soybean cultivars
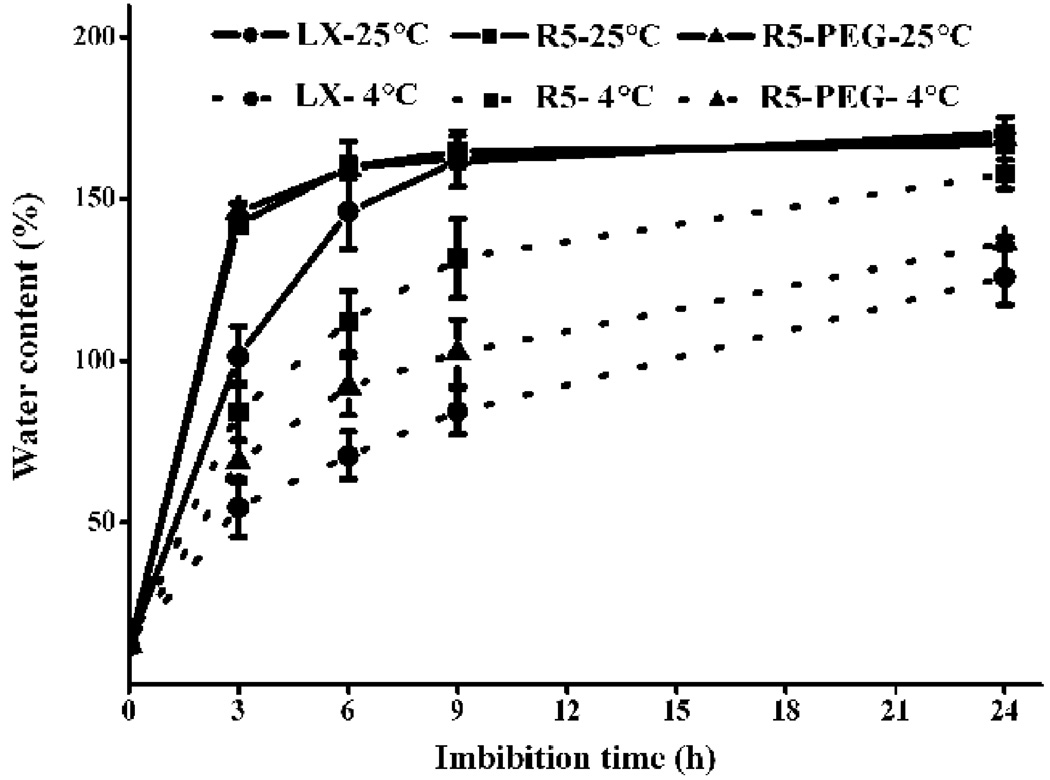
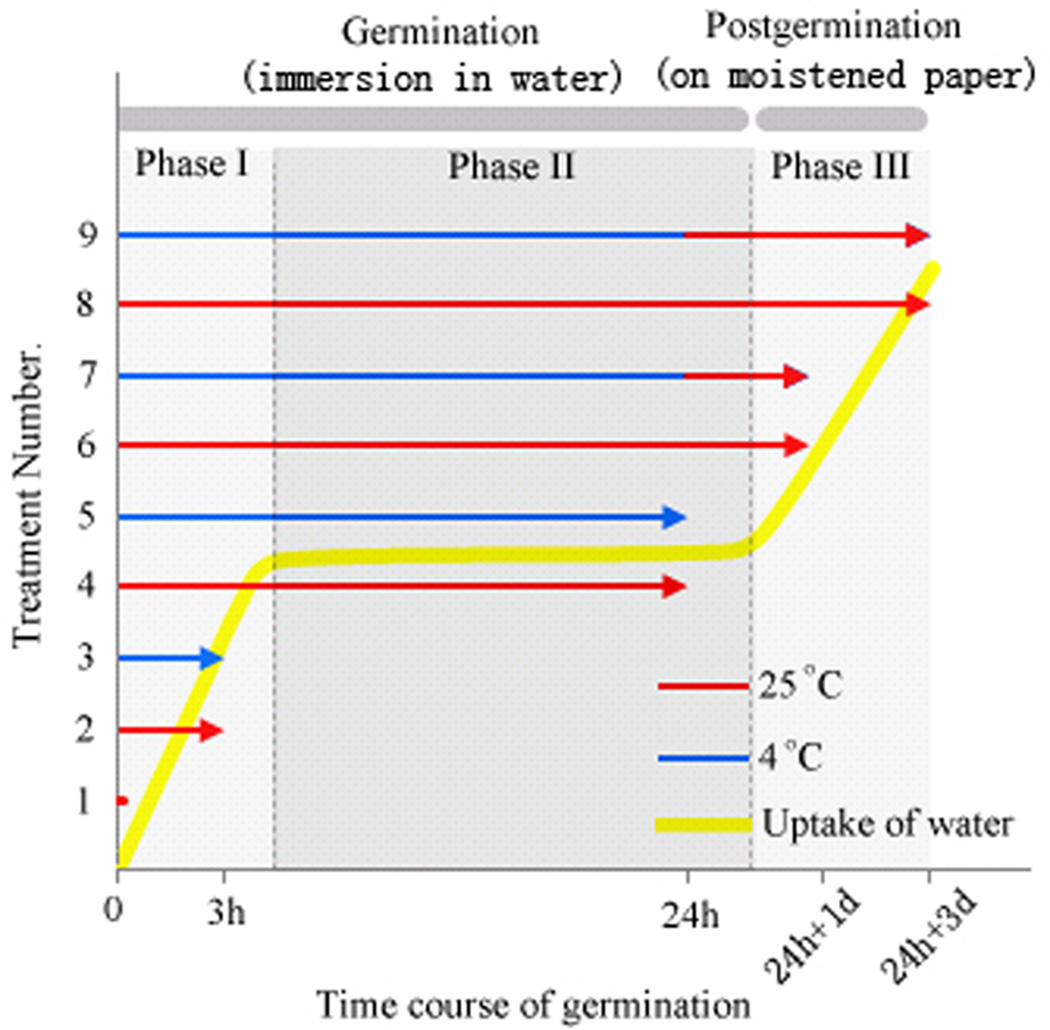
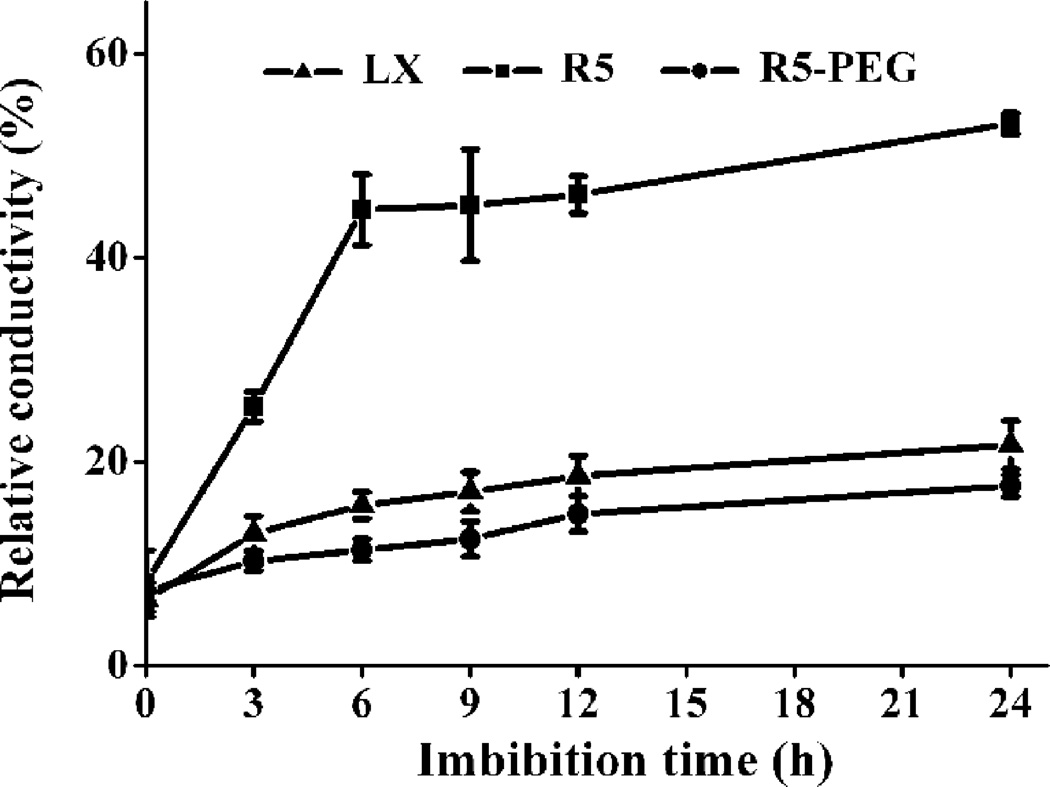
We first investigated the germination rates of nine soybean cultivars, randomly chosen from a commercial supplier, under high and low rates of water uptake, as well as under normal (25 °C) and low (4 °C) temperatures (Fig. S1). We found Liaoxin (LX) and Riben 5 (R5) were chilling-tolerant and -sensitive cultivars, respectively (Fig. S1). We used them as two genotypes of same species with different traits for further comparative investigations of imbibition. We then examined the changes of water content in LX and R5 seeds in order to define the duration of their imbibitional phases accurately and thus to determine the optimal sampling points for subsequent investigation (Fig. 1). We found that radicle elongation of LX and R5 seeds was shown to occur after 24 h of immersion in water at 25 °C, indicating that phase III started after 24 h. At all sampling times during 0 – 24 h, the rate of water uptake of the chilling-sensitive cultivar R5 is higher than that of the chilling-resistant cultivar LX. The water content increased rapidly during the first 6 h and then remained unchanged or increased slowly thereafter (Fig. 1). These lines of evidence show three clear phases of water uptake, as previously reported (Bewley, 1997, Han et al., 2013). Therefore, we used five sampling points as follows: dry seed for control, upon 3 h of immersion in water (I-W) for phase I, 24 h I-W for phase II, one day after 24 h I-W for early post-germination (P-G), and 3 d after 24 h I-W for later P-G, in the subsequent experiment. Concerning the two genotypes, LX and R5, and the temperature conditions of 25 and 4 °C, all of our sampling strategies are shown in Fig. 2. We also examined changes in water content upon pre-treatment with polyethylene glycol (PEG) in R5 (Fig. 1), the results for which are analysed below. The measurements of seed conductivity indicate that the intensity of membrane disorder in R5 seeds was much greater than that in LX seeds (Fig. 3). We also examined the membrane leakage from R5 seeds pre-treated with PEG (Fig. 3), and showed that the level of leakage was as low as that from LX seeds.
Fig. 1. Water uptake pattern in soybean seeds under room and chilled temperatures.
Seeds were immersed in water at 25 °C or 4 °C. LX, Liaoxin; R5, Riben 5; R5-PEG, Riben 5 pre-treated with PEG. Data are shown as mean ± SE (n = 4).
Fig. 2. Diagram of the sampling strategy of nine seed treatments.
We harvested the embryonic axes of LX and R5 seeds after the following treatments: 1, immersion in water for 0 h (control, dry seeds); 2, immersion in water at 25 °C for 3 h; 3, immersion in water at 4 °C for 3 h; 4, immersion in water at 25 °C for 24 h; 5, immersion in water at 4 °C for 24 h; 6, immersion in water at 25 °C for 24 h, then placement on moistened paper at 25 °C for one day; 7, immersion in water at 4 °C for 24 h, then placement on moistened paper at 25 °C for one day; 8, immersion in water at 25 °C for 24 h, then placement on moistened paper at 25 °C for three days; and 9, immersion in water at 4 °C for 24 h, then placement on moistened paper at 25 °C for three days.
Fig. 3. Effects of chilling imbibition on the relative conductivity of soybean seeds.
Dry seeds were immersed in water at 4 °C for the indicated time and then measured for conductivity at 25 °C. LX, Liaoxin; R5, Riben 5; R5-PEG, Riben 5 pre-treated with PEG. Data are shown as mean ± SE (n = 4).
Profiling the molecular species of membrane lipids during germination
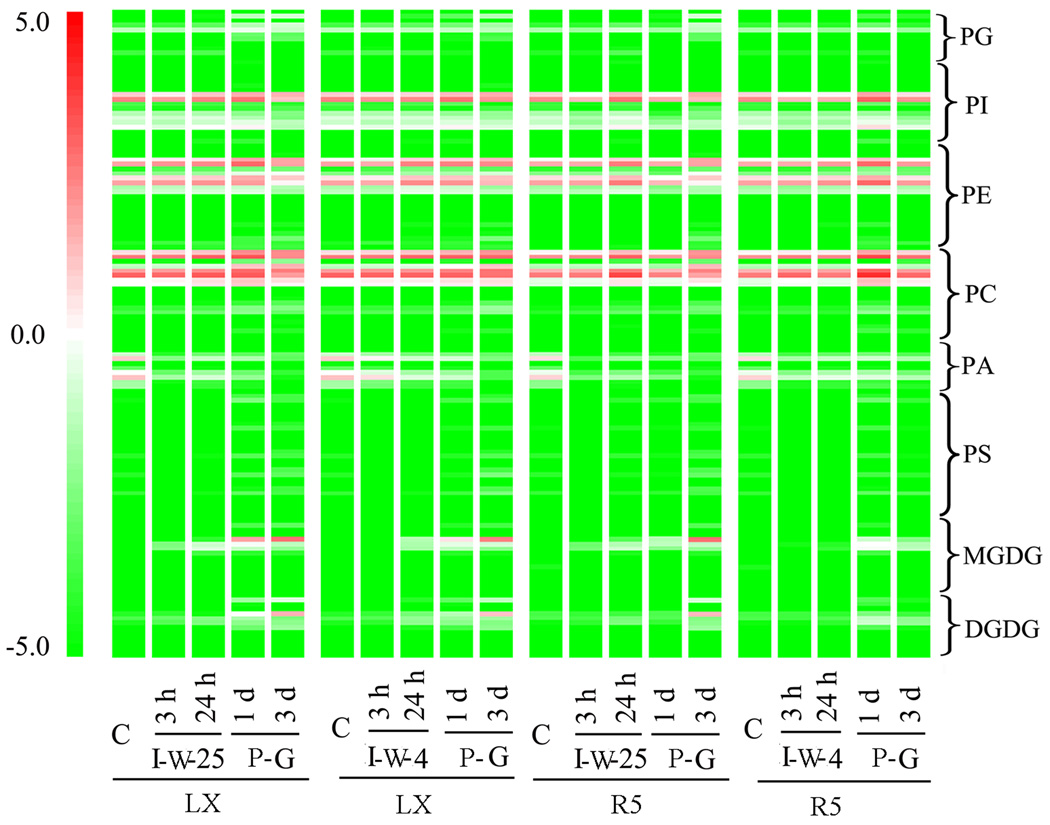
Embryonic axes are pivotal for seedling growth and are usually used for studies of seed imbibition (Obendorf, Zimmerman, Ortiz, Taylor & Schnebly, 2008, Posmyk et al., 2009, Stewart & Bewley, 1981, Torres-Franklin, Gigon, De Melo, Zuily-Fodil & Pham-Thi, 2007, Yin, Sun, Xin, Qin, Liang & Jing, 2009). We thus harvested the embryonic axes of LX and R5 seeds for lipidomics analysis (Fig. S2). We quantitatively identified more than 140 molecular species of eight glycerolipid classes in soybean embryonic axes of both cultivars. We visualized the composition of lipid molecular species during germination and post-germination and with treatments of either normal or chilled temperature in Fig. 4, which showed that extensive dynamic changes occurred through phases I, II, and III.
Fig. 4. Changes in lipid molecular species from dry seeds to post-germination stage.
Each coloured bar within a column represents a lipid molecular species in the indicated cultivars and treatments. The colour of each bar represents the level of the corresponding lipid species. Data are expressed as log2 lipid amount (nM/mg dry weight). A total of 140 lipid species in the indicated lipid classes were organised in terms of class. The dry weight is dry weight minus lipid (i.e. dry weight after lipid extraction). “I-W-25”, immersion in water at 25 °C; “I-W-4”, immersion in water at 4 °C; “P-G”, post-germination, on moistened paper at 25 °C.
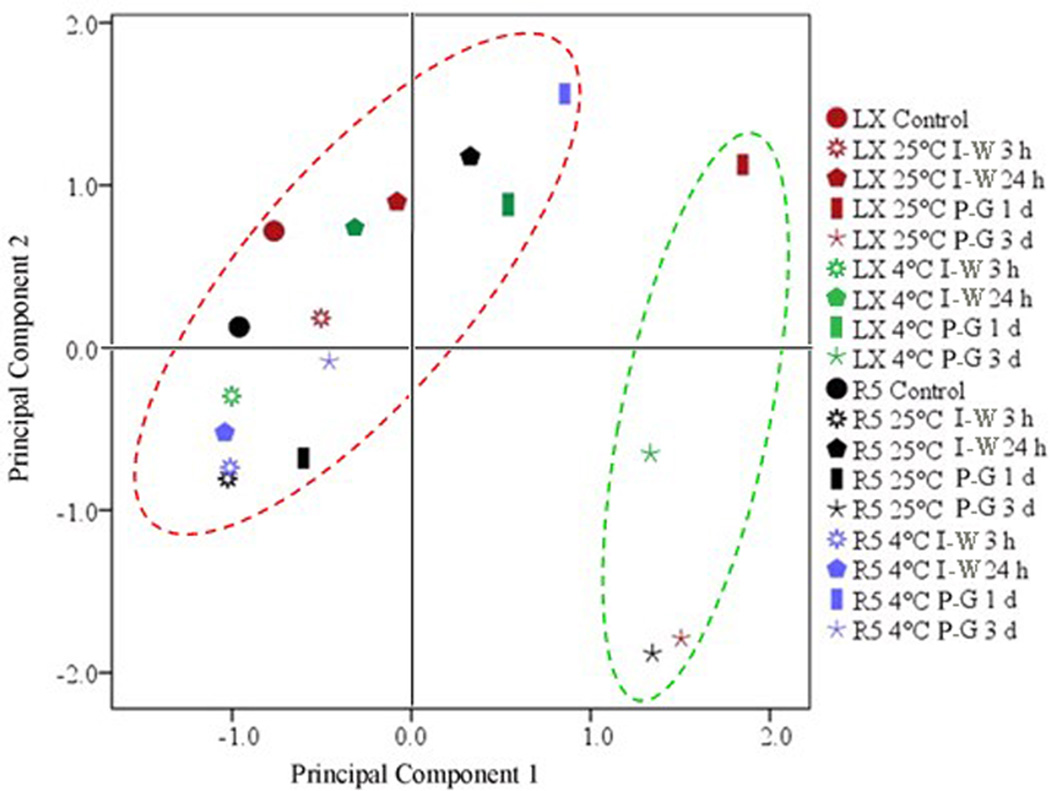
To reveal the general patterns of lipid changes, we calculated the average levels of the eight classes (Table 1) and conducted principal component analysis (PCA) on them to extract three principal components (Table S2 and Table S3). Principal component 1 explained 58% of the variance, with its main contributions being from MGDG, DGDG, PG, and PS. Principal component 2 explained 25% of the variance, with its main contributions being from PC, PE, and PI. Principal component 3 explained 11% of the variance, mainly representing PA. Statistically, principal component 3 could be replaced by PA, the original variable, because its eigenvalue was less than 1. From these results, the changes of the eight classes of membrane lipids were clustered into three principal components during and after germination. The biological interpretation of these three principal components is explored in later sections. Given that principal components 1 and 2 explained 83% of the variance overall, we used their score plots to show the similarities and differences of lipid compositions between LX and R5 seeds and between imbibition at room temperature and under chilling conditions. Basically, 18 plotted points of lipid composition of all cultivars and conditions were clearly separated into two groups (Fig. 5, red and green circles). The first group included 14 points, most of which were associated with LX and R5 seeds during phases I and II (Fig. 5, red cycle); the second group included only four points, all of which were associated with phase III. This implies that there are two distinct sets of lipid composition during seed germination.
Table 1. Amount of lipid in each class and total lipids in LX and R5 during warm or chilling imbibition and germination.
“I-W”, immersion in water. “P-G”, post-germination. C, dry seeds. Data are shown as mean ± SE (n = 4 or 5). Values in the same row with different letters are significantly different (P < 0.05).
| Lipid class |
Genotype and treatment during phases I and II |
Lipids/dry weight (nmol mg−1) |
|||||
|---|---|---|---|---|---|---|---|
| C | I-W 3 h | I-W 24 h | P-G 1 d | P-G 3 d | |||
| PG | LX | 25 °C | 0.69 ± 0.11c | 0.70 ± 0.08c | 0.93 ± 0.13b | 1.96 ± 0.22a | 2.14 ± 0.26a |
| 4 °C | 0.69 ± 0.11c | 0.82 ± 0.10bc | 0.71 ± 0.07c | 0.96 ± 0.04b | 1.28 ± 0.12a | ||
| R5 | 25 °C | 0.52 ± 0.03c | 0.43 ± 0.07c | 0.98 ± 0.14b | 0.58 ± 0.07c | 1.88 ± 0.19a | |
| 4 °C | 0.52 ± 0.03c | 0.72 ± 0.04b | 0.42 ± 0.06c | 0.92 ± 0.12a | 0.47 ± 0.06c | ||
| PI | LX | 25 °C | 10.17 ± 0.67b | 7.80 ± 0.75c | 10.39 ± 0.51b | 12.69 ± 1.04a | 6.44 ± 0.96c |
| 4 °C | 10.17 ± 0.67b | 6.36 ± 0.59d | 9.19 ± 0.77bc | 12.05 ± 0.56a | 8.97 ± 1.09c | ||
| R5 | 25 °C | 8.65 ± 0.75a | 4.78 ± 0.60b | 10.45 ± 1.12a | 6.30 ± 0.75b | 6.12 ± 0.66b | |
| 4 °C | 8.65 ± 0.75b | 5.12 ± 0.37c | 4.56 ± 0.36c | 10.76 ± 1.05a | 7.76 ± 0.93b | ||
| PE | LX | 25 °C | 10.27 ± 1.02c | 12.34 ± 1.15bc | 13.93 ± 1.21b | 19.00 ± 1.09a | 11.94 ± 1.17bc |
| 4 °C | 10.27 ± 1.02b | 8.28 ± 1.10b | 14.77 ± 1.22a | 15.85 ± 0.80a | 13.88 ± 1.15a | ||
| R5 | 25 °C | 10.36 ± 0.63bc | 9.87 ± 0.74c | 16.13 ± 1.15a | 9.76 ± 0.60c | 11.67 ± 0.74b | |
| 4 °C | 10.36 ± 0.63bc | 8.66 ± 0.80c | 10.24 ± 1.06bc | 18.21 ± 1.40a | 10.92 ± 1.28b | ||
| PC | LX | 25 °C | 22.29 ± 2.04c | 23.43 ± 2.87bc | 27.94 ± 2.85b | 37.95 ± 3.57a | 25.51 ± 3.42bc |
| 4 °C | 22.29 ± 2.04c | 21.93 ± 1.80c | 24.79 ± 1.87bc | 27.04 ± 2.69ab | 28.05 ± 0.62a | ||
| R5 | 25 °C | 15.69 ± 1.59c | 16.42 ± 1.48c | 32.30 ± 2.09a | 18.36 ± 1.05c | 23.75 ± 1.77b | |
| 4 °C | 15.69 ± 1.59c | 18.88 ± 1.69bc | 19.62 ± 0.87bc | 40.03 ± 5.28a | 22.70 ± 2.72b | ||
| PA | LX | 25 °C | 6.38 ± 1.06a | 0.77 ± 0.08c | 1.36 ± 0.17b | 0.95 ± 0.11c | 0.42 ± 0.07d |
| 4 °C | 6.38 ± 1.06a | 3.47 ± 0.41b | 2.21 ± 0.31c | 1.15 ± 0.18d | 0.48 ± 0.05e | ||
| R5 | 25 °C | 5.10 ± 0.64a | 0.46 ± 0.06c | 0.85 ± 0.08b | 0.58 ± 0.07c | 0.47 ± 0.04c | |
| 4 °C | 5.10 ± 0.64a | 1.17 ± 0.21c | 2.10 ± 0.16b | 1.15 ± 0.22c | 1.15 ± 0.08c | ||
| PS | LX | 25 °C | 0.37 ± 0.02b | 0.16 ± 0.032c | 0.26 ± 0.04c | 0.83 ± 0.12a | 0.82 ± 0.04a |
| 4 °C | 0.37 ± 0.02c | 0.08 ± 0.01e | 0.21 ± 0.04d | 0.50 ± 0.06b | 0.99 ± 0.13a | ||
| R5 | 25 °C | 0.36 ± 0.03b | 0.11 ± 0.01c | 0.40 ± 0.06b | 0.39 ± 0.05b | 0.82 ± 0.04a | |
| 4 °C | 0.36 ± 0.03b | 0.06 ± 0.006c | 0.15 ± 0.03c | 0.54 ± 0.08a | 0.38 ± 0.05b | ||
| MGDG | LX | 25 °C | 0.05 ± 0.01d | 0.37 ± 0.05c | 0.28 ± 0.04c | 5.30 ± 0.31b | 7.61 ± 0.86a |
| 4 °C | 0.05 ± 0.01d | 0.06 ± 0.01d | 0.57 ± 0.05c | 2.86 ± 0.24b | 5.87 ± 0.34a | ||
| R5 | 25 °C | 0.12 ± 0.03d | 0.46 ± 0.03c | 1.07 ± 0.12b | 1.28 ± 0.12b | 7.82 ± 0.79a | |
| 4 °C | 0.12 ± 0.03c | 0.11 ± 0.02c | 0.13 ± 0.02c | 2.52 ± 0.37a | 0.90 ± 0.04b | ||
| DGDG | LX | 25 °C | 0.23 ± 0.03d | 0.30 ± 0.06d | 0.49 ± 0.06c | 2.06 ± 0.14b | 4.19 ± 0.56a |
| 4 °C | 0.23 ± 0.03d | 0.23 ± 0.05d | 0.53 ± 0.08c | 1.27 ± 0.13b | 3.43 ± 0.07a | ||
| R5 | 25 °C | 0.26 ± 0.03c | 0.24 ± 0.04c | 0.47 ± 0.07b | 0.40 ± 0.07b | 4.11 ± 0.47a | |
| 4 °C | 0.26 ± 0.03c | 0.22 ± 0.04c | 0.24 ± 0.04c | 1.02 ± 0.12a | 0.67 ± 0.05b | ||
Fig. 5. Principal components analysis score plot of the dependence of germination on the levels of change in lipid classes.
The red group had Fv/Fm fluorescence and the green group did not.
Dynamic changes of membrane lipids in LX and R5 seeds after imbibition at room temperature
To improve our understanding of the three patterns of membrane lipid change during germination, we first characterized the membrane lipid composition phase by phase from dry seeds to the post-germination stage in LX seeds under 25 °C imbibition. The dry seeds had very low levels of galactolipids (Fig. 4 and Table 1). This confirmed that plastids rarely developed in the dry seeds. The PA level was 6.38 nmol/mg in dry seeds (Table 1), which was much higher than that previously reported in leaves (Crowe & Crowe, 1992, Li, Wang, Liu, Cui, Chen, Zhang, Jiang, Xu, Li, Li, Zhao & Chen, 2013, Welti et al., 2002). PA levels in leaves are usually around 1 nmol/mg, but can increase 2–10-fold under stress conditions, such as freezing and desiccation (Li et al., 2008, Pagnussat et al., 2012, Welti et al., 2002). The higher PA levels in dry seeds are consistent with previous data(Devaiah et al., 2006) and support the hypothesis that their cellular membranes are commonly in the hexagonal II phase, which is characteristic of disordered membranes (Simon, 1974). This is supported by the observation that the most ion leakage occurred during the initial phase of imbibition (Fig. 3).
Membrane lipids changed remarkably during the first 3 h after the start of imbibition (Table 1). Levels of PI, PA, and PS decreased significantly. Levels of PA decreased by 5.61 nmol (from 6.38 to 0.77 nmol/mg) in LX seeds. The decrease in PA accounted for a loss of 11% of the total lipid content prior to imbibition. These results indicate that the largest change of membrane lipids involved a decrease in levels of PA during phase I of germination. This suggests that PA plays an essential role in membrane reorganization during this phase. In contrast, levels of MGDG increased significantly after 3 h (for example, by 7.4-fold in LX seeds; Fig. 4; Table 1); this suggests that plastid biogenesis started at this time. The levels of other lipids (PC, PE, PG, and DGDG) remained the same during phase I of germination.
During phase II of germination, the levels of PG, PE, PC, and MGDG remained the same as those in phase I; however, the levels of PA, PI, PS, and DGDG increased significantly (Table 1). The increase in the level of DGDG also suggests that plastid biogenesis continued during phase II. A surprising finding is that, following the remarkable decrease in levels of PA during phase I, there was a transient increase in levels of PA during phase II. This is unlike the trends for other lipids, the levels of which maintained the same trends during both phase I and phase II. These complex dynamic changes of PA level imply that there are multiple factors that control its level and that this molecule might have specific roles during phase II.
During phase III, when seeds were germinated at 25 °C for one and three days, levels of PG increased only slightly, whereas levels of MGDG and DGDG increased dramatically (Table 1). Levels of MGDG increased 27-fold (from 0.28 to 7.61 nmol/mg) and levels of DGDG increased 19-fold (from 0.49 to 4.19 nmol/mg). This suggests that plastid biogenesis had basically achieved at this stage. This was physiologically verified by the observation of photosynthetic activity in the hypocotyls from LX seeds, as indicated by Fv/Fm, the potential photochemical quantum efficiency, of photosystem II (PS II) (Fig. 6, upper left panel). Fv/Fm fluorescence of hypocotyls was not observed during phases I and II, but were observed in hypocotyls during phase III of germination. During phase III, PA once again declined to a level below 1 nmol/mg, which was the same as previously reported in leaves. This means that the level of PA was restored to the level found in the membranes of regular cells. PC, PE, and PI showed interesting patterns in phase III, with increases at the first day after the beginning of imbibition and reductions back to background levels by day three.
Fig. 6. The effects of imbibitional chilling on Fv/Fm fluorescence of hypocotyls.
Assessment of Fv/Fm as a measure of the photochemical quantum efficiency of PS II. The colour bar on the right indicates the Fv/Fm values.
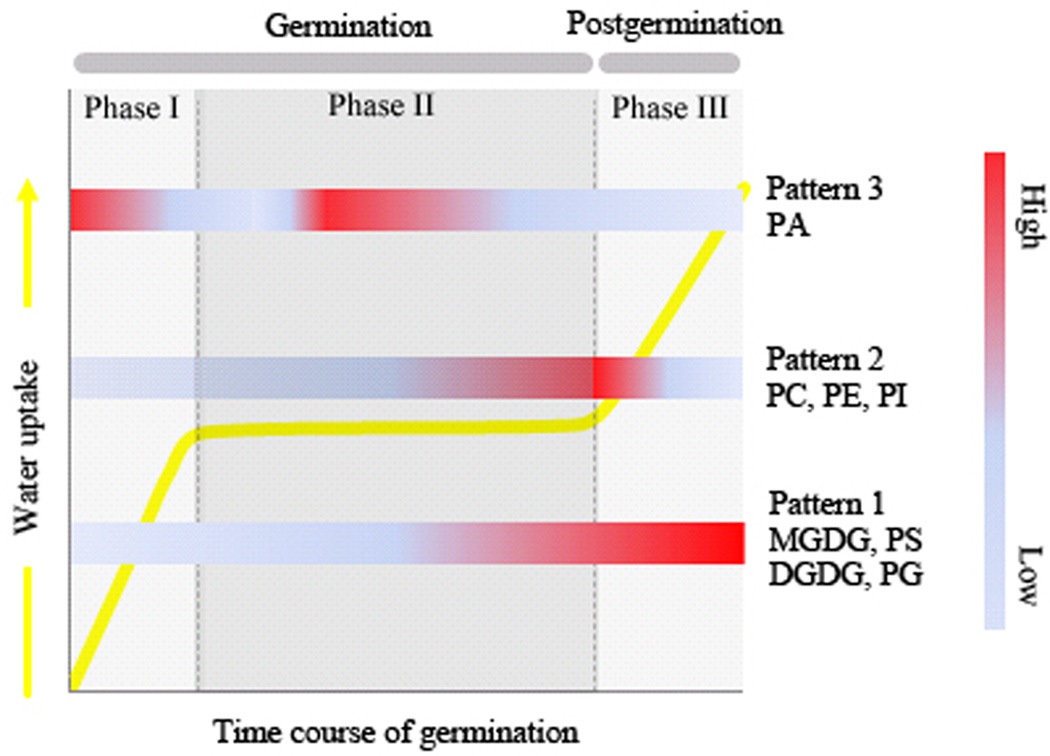
Detailed lipid analysis indicated remarkable changes in lipid classes consistent with the PCA results; the three principal components represented three distinct patterns of dynamic change during normal germination and post-germination. For principal component 1, levels of PS, PG, MGDG, and DGDG increased dramatically and the increases of MGDG and DGDG were associated with photosynthetic activity in the hypocotyl. For principal component 2, the levels of PC, PE, and PI were basically maintained, except for a transient increase that occurred during the late stage of imbibition or the first day of post-germination. For principal component 3, the level of PA mostly declined, but a transient increase occurred during phase II. The three principal components explained the fundamental patterns of membrane lipid changes during germination, as presented in Fig. 7. Besides the association of the MGDG and DGDG increases with photosynthetic activity, the potential biological significance of the other patterns is explored below.
Fig. 7. Working model of three patterns of lipid change during non-stress germination in LX and R5 seeds.
Pattern 1: principal component 1, MGDG/DGDG/PG/PS. Pattern 2: principal component 2, PC/PE/PI. Pattern 3: principal component 3, PA.
The effects of chilling on the membrane lipids in chilling-tolerant seeds during germination
Here, we compared the three patterns of change in lipid composition between imbibition at 25 °C and at 4 °C in LX seeds to determine if and how the chilling temperature affects the seed membranes. For pattern 1, there were increases of PS, PG, MGDG, and DGDG after imbibition at 4 °C , but these increases were significantly smaller than those after imbibition at 25 °C (Table 1). For example, the levels of MGDG and DGDG after 4 °C imbibition were only half of those at 25 °C at one day of germination (Table 1). The suppression of MGDG and DGDG synthesis suggested that chilling temperature inhibited the biosynthesis of plastidic lipids and thus might delay plastid biogenesis. This was verified by the delay of photosynthetic activity in the hypocotyls of LX seeds after imbibition at 4 °C (Fig. 6, top panel). Fv/Fm fluorescence of hypocotyls occurred by one day of germination after imbibition at 25 °C, but was not observed until three days of germination after imbibition at 4 °C. The changes for pattern 2-namely, the transient increases of PC, PE, and PI at 25 °C (Fig. 7) -were not observed at 4 °C during the late stage of phase II or the early stage of phase III (Table 1). The levels of PC, PE, and PI were essentially maintained after 4 °C imbibition (Table 1). For pattern 3, PA decreased by 2.91 nmol/mg from 6.38 to 3.47 nmol/mg during phase I under chilled conditions, which was significantly less than the decrease of 5.61 nmol/mg at room temperature (Table 1). The continuing presence of PA might delay the restoration of membrane function because PA levels are usually very low in normal membranes, as described above.
The above results indicate that chilling had a slight effect on the patterns of lipid changes in LX seeds during germination. Such effect was also seen in LX seeds, for which germination decreased from 94% with imbibition at room temperature to 83% after imbibition at a chilling temperature (Fig. S1). We therefore hypothesized that the three patterns of lipid composition were required for normal germination, and that their disruption might cause failure of germination.
The effects of chilling on the lipid composition in chilling-sensitive seeds during germination
To test the hypothesis raised above, we analysed the changes in lipid composition in R5 seeds under chilling conditions (Fig. 4). For pattern 1, the levels of PG and PS were basically maintained (Table 1), such that levels after three days of germination were very close to those in dry seeds. The levels of MGDG and DGDG increased transiently at the first day of post-germination, followed by decreases at the third days of post-germination (Table 1). In contrast, LX seeds that underwent imbibition at 25 °C exhibited continual increases of PG, PS, MGDG, and DGDG (Fig. 7). The features of the lipid compositions in R5 seeds after 4 °C imbibition could also be clearly seen in the PCA score plot (Fig. 5). The plotted points associated with the first and third day of post-germination for R5 seeds after 4 °C imbibition are shown in red, and are separate from those in green along principal component 1. This means that R5 seeds after 4 °C imbibition did not develop the membrane lipids required for photosynthesis and therefore no Fv/Fm fluorescence was observed (Fig. 6). This conclusion was supported by the detailed analysis of the contents of MGDG and DGDG. The highest levels of MDGD and DGDG in R5 after 4 °C imbibition were only 2.52 and 1.52 nmol/mg, respectively, and did not reach the minimum levels required for photosynthetic activity, as described above.
For pattern 2, cold-imbibed R5 seeds showed transient increases in PI, PC, and PE at the first day of post-germination; these levels were similar to those in LX seeds (Fig. 4 and Table 1). For pattern 3, PA levels dramatically decreased during phase I, transiently increased during phase II, and were then maintained above those of LX and R5 with normal germination during phase III (Table 1). Specifically, during phase III, PA levels of R5 after 4 °C imbibition (1.15 nmol/mg) were approximately 2.5-fold higher than those of the other three conditions (0.42–0.48 nmol/mg, Table 1). Considering the high rates of cytoplasmic leakage (Fig. 3) and absence of germination (Fig. S1) in the R5 seeds, the results suggest that the high PA levels induced by chilling might contribute to imbibitional chilling injury. All of the evidence above indicates that the lipid compositions of R5 seeds after 4 °C imbibition differed markedly from those of LX seeds with normal germination, and suggests that the disruption of the three phases of lipid composition-particularly patterns 1 and 3 might contribute to a reduced rate of germination.
PEG osmopriming causes slight remodelling of membrane lipid composition in chilling-sensitive seeds
Osmopriming of chilling-sensitive seeds by PEG can markedly improve their germination when they are subsequently imbibed at low temperature (Heydecker, Higgins & Gulliver, 1973, Lu, Liu, Wang, Jing & Lin, 2006, Posmyk, Corbineau, Vinel, Bailly & Come, 2001, Sun et al., 2011, Yang, Wang, Zheng, Jing & Lin, 2003, Zhuo et al., 2009). Here, we pre-treated R5 seeds with 33% PEG for three days at 15 °C and then desiccated them for three days at 25 °C back to 10% water content, which was close to that of the original dry seeds (Table S1). We then check PEG pre-treated seeds (Fig. S3 left half) to germinate at 25 °C with and without prior imbibition at 4 °C (Fig. S3 panel 2). Physiologically, the PEG pre-treatment (Fig. 1 and 3) caused significantly lower rates of water uptake and membrane leakage. The germination rate of cold-imbibed R5 seeds increased from 0% for non-osmoprimed to 91% for osmoprimed seeds, which was similar to that of LX control seeds (Fig. S1). These results proved that imbibitional chilling injury was overcome by PEG pre-treatment in R5 seeds.
To investigate whether PEG osmopriming affects the lipid composition of R5 seeds, we compared the lipid compositions among dry, PEG pre-treated, and PEG pre-treated after 3 h of chilling imbibition (Fig. S3 panel 1; Table 2 panel 1). In primed seeds, the levels of PG, PI, PC, and PE increased significantly. However, MGDG and DGDG remained unchanged at very low levels, whereas PA levels remained high (6.85 nmol/mg) after drying back to 10% water content. This means that PEG pre-treatment did not directly change the membrane lipid composition and thus might not directly change membrane structure from the hexagonal phase (in dry seeds) to the lamellar phase (in imbibed seeds). Interestingly, after chilling imbibition, PEG-pre-treated R5 seeds had a lipid composition (Table 2 panel 1) that differed from that of R5 seeds at 4 °C, but was similar to that in LX and R5 seeds imbibed at 25 °C (Table 1). Specifically, PA levels dramatically decreased to 0.53 nmol/mg, and levels of both MGDG and DGDG increased significantly (Table 2 panel 1). These findings suggest that effects of PEG osmopriming occurred during phases I, II, and III.
Table 2. Amount of lipid in each class and total lipids in PEG-treated R5 seeds during chilling imbibition and germination.
Data are shown as mean ± SE (n = 4 or 5). Values in the same row with different letters are significantly different (P < 0.05). Panel 1 is the membrane lipid composition in control PEG-treated R5 seeds. Panel 2 is the membrane lipid composition of PEG-treated R5 seeds immersed in water (I-W) at 4 °C for 3 and 24 h and then placed on moistened paper at 25 °C for one and three days of post-germination (P-G). Values in the same row with different letters are significantly different (P < 0.05).
| Lipid class |
Panel 1, lipid/dry weight (nmol mg−1) |
Panel 2, lipid/dry weight (nmol mg−1) |
|||||
|---|---|---|---|---|---|---|---|
| Control | Dried seeds | I-W 3 h | I-W 3 h | I-W 24 h | P-G 1 d | P-G 3 d | |
| PG | 0.73 ± 0.09c | 1.48 ± 0.12a | 1.22 ± 0.10b | 0.75 ± 0.08b | 0.68 ± 0.09bc | 1.02 ± 0.13a | 0.58 ± 0.08c |
| PI | 8.55 ± 0.71c | 13.21 ± 1.49a | 10.53 ± 1.16b | 6.39 ± 1.21b | 7.27 ± 1.06b | 9.99 ± 1.10a | 6.59 ± 0.25b |
| PE | 3.99 ± 0.32c | 6.98 ± 0.72b | 8.90 ± 0.48a | 9.11 ± 1.03b | 10.64 ± 0.94b | 14.74 ± 1.22a | 10.68 ± 1.00b |
| PC | 10.00 ± 0.93c | 17.33 ± 1.74b | 22.02 ± 0.89a | 18.66 ± 1.43c | 23.16 ± 1.19b | 41.88 ± 2.20a | 22.11 ± 2.27b |
| PA | 8.19 ± 0.52a | 6.85 ± 0.78b | 0.53 ± 0.06c | 0.63 ± 0.08a | 0.44 ± 0.05b | 0.40 ± 0.08b | 0.39 ± 0.04b |
| PS | 0.27 ± 0.03a | 0.23 ± 0.04ab | 0.18 ± 0.02b | 0.11 ± 0.01c | 0.17 ± 0.02b | 0.55 ± 0.06a | 0.60 ± 0.09a |
| MGDG | 0.16 ± 0.03b | 0.12 ± 0.02b | 0.38 ± 0.12a | 0.06 ± 0.01d | 0.43 ± 0.08c | 2.21 ± 0.28b | 3.69 ± 0.63a |
| DGDG | 0.37 ± 0.02a | 0.39 ± 0.05a | 0.61 ± 0.40a | 0.11 ± 0.02d | 0.17 ± 0.01c | 0.71 ± 0.03b | 2.05 ± 0.32a |
Effects of PEG osmopriming on lipid composition in chilling-sensitive seeds during phases II and III
To examine if and how the effects of PEG osmopriming occur during phases II and III, we analysed the membrane lipid composition in PEG pre-treated R5 seeds immersed in 4 °C water for 3 and 24 h and then placed on moistened paper at 25 °C for one and three days (Fig. S3 panel 2; Table 2 panel 2). For pattern 1, levels of MGDG, DGDG, and PS continually increased. For pattern 2, levels of PI, PE, and PC increased first, peaked at the first day of post-germination, and then declined. These patterns were the same as those in LX and R5 seeds germinated at 25 °C. In particular, the contents of MGDG and DGDG were 3.69 and 2.05 nmol/mg, respectively, after germination for three days, which fulfilled the minimal requirement for photosynthetic activity as proposed above. For pattern 3, PA decreased continually and reached levels as low as those in LX seeds (Table 2). In particular, there was no transient increase in PA during phase II. This implies that the inhibition of PA formation might favour normal germination.
These results indicate that PEG osmopriming rescues the resistance of R5 seeds to imbibitional chilling injury mainly through remodelling their lipid composition to one that resembles that of LX seeds. By a combined analysis of the patterns of change in MGDG, DGDG, and PA in both LX and R5 seeds under normal or chilled temperature treatments and in PEG-pre-treated R5 seeds, our evidences strongly suggested that three specific patterns of lipid change are required for germination. Moreover, chilling-induced block of MGDG and DGDG formation and increases in PA might be the causes for imbibitional chilling injury.
Inhibition of PLD-mediated PA during phase II attenuates imbibitional chilling injury
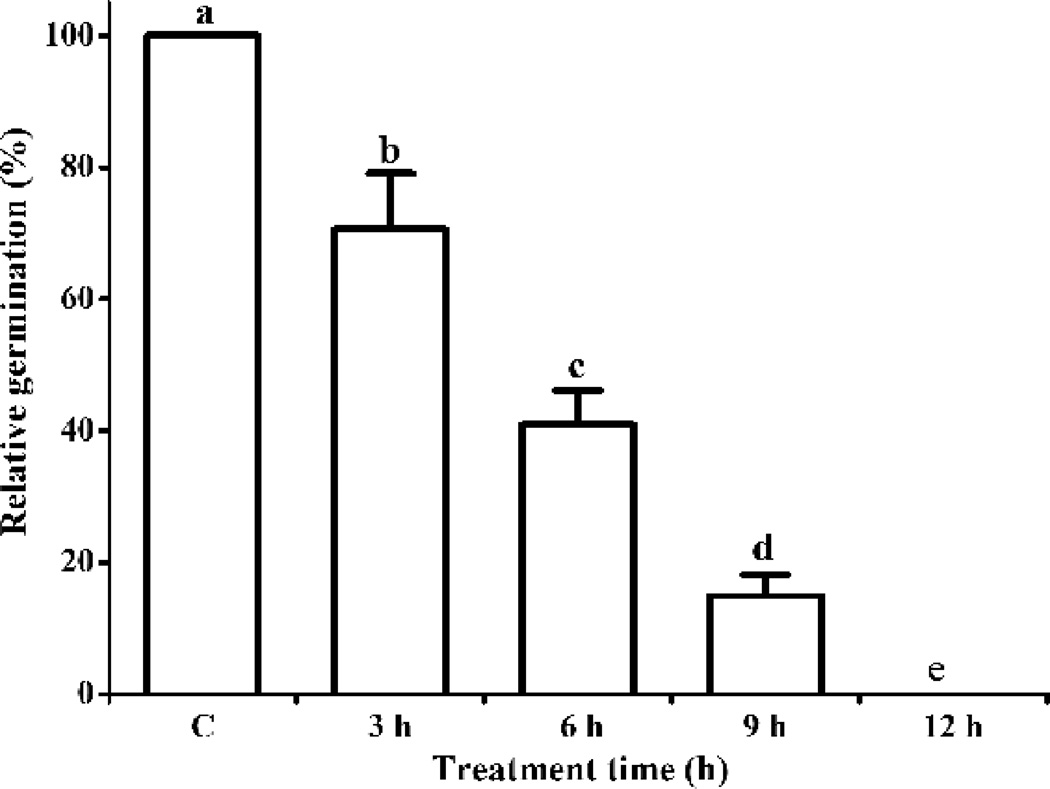
We further tested whether suppression of PA levels is required to overcome imbibitional chilling injury and figured out what causes the transient increase of PA during phase II in chilling-sensitive seeds. Given that PLD is mainly responsible for PA formation in plants at low temperature, we hypothesized that the transient increase in PA is due to PLD-mediated hydrolysis. To test this hypothesis, we first examined the contribution of chilling effects during phase II to the imbibitional chilling injury in R5 seeds. R5 seeds were immersed in water at 4 °C for 3, 6, 9, and 12 h and then placed on moistened paper for germination at 25 °C (Fig. 8). The germination rate of R5 seeds decreased with increasing duration of chilling treatment and reached 0% after 12 h. Relative germination decreased by 30% after 3 h and was 70.0% from 3 to 12 h. These results indicate that major chilling injury occurred during phase II.
Fig. 8. Effect of the duration of chilling imbibition on the viability of Riben 5 soybean seeds (germination rate).
Data are shown as mean ± SE (n = 4 replicates of 40 seeds each). Values in the same row with diff4erent letters are significantly different (P < 0.05).
Secondly, we tested the effects of PLD-mediated PA accumulation during phase II on imbibitional chilling injury. Whereas n-butanol specifically inhibits the cellular response induced by PLD-mediated PA, neither s- nor t-butanol have any effect (Gardiner et al., 2003, Jia et al., 2013, Motes et al., 2005). We applied n-, s-, and t-butanol to R5 seeds and assessed their effects on the germination rate. Each of the three butanol isomers was applied at 0, 3, and 24 h after seeds had been immersed in 4 °C water and then placed for one day on moistened paper at 25 °C. Germination was significantly improved when n-butanol was present in the chilling imbibition stage (0 and 3 h), but was not affected when it was present in phase III (24 h (I-W) and 1 d (P-G), Table 3). In contrast, chilling-reduced germination was not attenuated by the application of s- and t-butanol. Chilling imbibition of R5 seeds under n-butanol actually produced less PA than that under water or t-butanol (Fig. S4). These results indicate that the suppression of PLD-mediated PA formation during phase II was able to attenuate chilling injury. This suggests that the transient increase of PA during phase II was derived from PLD hydrolysis and was the major cause of imbibitional chilling injury.
Table 3. The effects of butanol on imbibitional chilling injury in soybean seeds.
n-, s-, and t-butanol were applied to R5 seeds and their effects on the germination rate were assessed. Each of the three butanol isoforms was applied to the seeds immersed in water (I-W) at 4 °C for 0, 3, and 24 h and then they were transferred to moistened paper at 25 °C for one day of post-germination (P-G). Data are shown as mean ± SE (n = 4 replicates of 40 seeds each). Values in the same row with an asterisk are significantly different from that of control (Water) (P < 0.05).
| Reagent | Concentration (%) |
Germination (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| C | 3 h (I-W) | 24 h (I-W) | 1 d (P-G) | |||||
| Water | 0 | 0 | 0 | 0 | 0 | |||
| n-butanol | 0.01 | 10.8 ± 2.2* | 10.4 ± 2.0* | 0 | 0 | |||
| 0.05 | 15.1 ± 3.9* | 12.3± 3.1* | 0 | 0 | ||||
| 0.08 | 13.1 ± 2.1* | 10.6± 3.0* | 0 | 0 | ||||
| s-butanol | 0.01 | 0 | 0 | 0 | 0 | |||
| 0.05 | 0 | 0 | 0 | 0 | ||||
| 0.08 | 0 | 0 | 0 | 0 | ||||
| t-butanol | 0.01 | 0 | 0 | 0 | 0 | |||
| 0.05 | 0 | 0 | 0 | 0 | ||||
| 0.08 | 0 | 0 | 0 | 0 | ||||
Phospholipase Dα1 (PLDα1) is the most abundant membrane component of the PLD family and is responsible for most PA formation at low temperature (Li et al., 2008). N-Acylethanolamine (NAE) 12:0 is a specific inhibitor of PLDα1 activity (Blancaflor, Kilaru, Keereetaweep, Khan, Faure & Chapman, 2014, Chapman, 2004, Jia et al., 2013, Motes et al., 2005). To investigate further the role of a transient increase of PA during phase II in imbibitional chilling injury, we applied NAE 12:0 to R5 seeds and assessed its effects on germination. NAE in dimethyl sulfoxide (DMSO) solvent was applied at 0, 3, and 24 h after imbibition at 4 °C, and the first day of post-germination at 25 °C. Germination was significantly improved when NAE 12:0 was present during the chilling imbibition stage (0 h and 3 h), but was not affected when it was present in the post-germination stage (24 h (I-W) and 1 d (P-G), Table 4), whereas DMSO alone had no effects under the same conditions. These results suggest that inhibition of PLDα1-mediated PA formation during imbibition attenuates chilling injury by blocking the increase of PA during phase II derived from PLDα1 hydrolysis. This mechanism appears to be the major cause of imbibitional chilling injury.
Table 4. The effects of NAE on imbibitional chilling injury in soybean seeds.
NAE 12:0 was applied to R5 seeds and assessed for its effects on germination. NAE in DMSO solvent was applied to the seeds immersed in water (I-W) at 4 °C for 0, 3, and 24 h, which were then placed on moistened paper at 25 °C for one day of post-germination (P-G). Data are shown as mean ± SE (n = 4 replicates of 40 seeds each). Values in the same row with an asterisk are significantly different from that of control (Water) (P < 0.05).
| Reagent | Concentration (µmol) |
Germination (%) |
|||
|---|---|---|---|---|---|
| C | 3 h (I-W) | 24 h (I-W) | 1 d (P-G) | ||
| Water | 0 | 0 | 0 | 0 | 0 |
| DMSO | 5 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | |
| 25 | 0 | 0 | 0 | 0 | |
| NAE | 5 | 28.5 ± 4.3* | 24.1 ± 3.9* | 0 | 0 |
| 10 | 17.4 ± 3.1* | 15.3 ± 4.1* | 0 | 0 | |
| 25 | 15.0 ± 5.0* | 10.2 ± 2.8* | 0 | 0 | |
Thirdly, to test the universal role of PLD-mediated PA in germination, we applied n- and t-butanol and NAE to seeds of cucumber (Cucumis sativus Linn.) and pea (Pisum sativum Linn.). The respective germination rates of cucumber and pea were 84% and 86% at 25 °C, and 38% and 19% after imbibitional chilling at 4 °C (Table 5). Although the application of t-butanol did not affect their germination, n-butanol and NAE significantly improved it (Table 5). These results indicate that inhibition of PLDα1-mediated PA formation alleviates imbibitional chilling injury in cucumber and pea seeds and suggest that the role of PLDα1-mediated PA in chilling imbibition is universal.
Table 5. The effects of exogenous NAE and butanol on germination of cucumber and pea seeds during imbibitional chilling.
NAE in DMSO solvent and n- and t-butanol were applied to the seeds immersed in water at 4 °C for 24 h, which were then transferred to moistened paper at 25 °C and scored for their relative germination rate at seven days. Data are shown as mean ± SE (n = 4 replicates of 40 seeds each). Values in the same row with an asterisk are significantly different from that of control (Water) (P < 0.05).
| Reagent | Concentration | Germination (%) |
|
|---|---|---|---|
| Cucumber | Pea | ||
| Water | 0 | 37.78 ± 4.69 | 19.44 ± 2.81 |
| DMSO | 5 µmol | 35.16 ± 6.73 | 18.91 ± 3.39 |
| 10 µmol | 34.72 ± 5.91 | 20.64 ± 4.24 | |
| 25 µmol | 37.26 ± 6.02 | 20.98 ± 3.51 | |
| NAE | 5 µmol | 57.55 ± 8.42* | 49.30 ± 7.36* |
| 10 µmol | 58.72 ±9.38* | 43.39 ± 8.75* | |
| 25 µmol | 60.19 ±10.45* | 40.29 ± 9.42* | |
| n-butanol | 0.01% | 49.57 ± 5.42* | 32.70 ± 4.64* |
| 0.05% | 47.48 ± 7.41 | 30.74 ± 5.38* | |
| 0.08% | 48.82 ± 8.33 | 32.48 ± 5.36* | |
| t-butanol | 0.01% | 35.92 ± 6.47 | 20.05 ± 3.18 |
| 0.05% | 34.07 ± 7.42 | 21.95 ± 3.38 | |
| 0.08% | 33.39 ± 4.19 | 22.35 ± 4.05 | |
CONCLUSIONS
Owing to its major importance in plant science and agriculture, germination has been studied extensively (Bewley, 1997, Simon, 1974, Weitbrecht et al., 2011). Previous studies focused primarily on the morphology, physiology, primary metabolism, and phytohormonal regulation of germination. However, recent studies that involved transcriptomic and proteomic approaches have provided new insight into the molecular mechanisms of germination (Bhardwaj et al., 2012, Demarsy et al., 2012, Holdsworth et al., 2008, Law et al., 2012, Pagnussat et al., 2012, Parrish & Leopold, 1977, Swigonska & Weidner, 2013). Although the essential role of membranes in germination is widely accepted, the concept that describes membrane reorganization is built on simple and in vitro biophysical models (Simon, 1974). This study presented, for the first time, to the best of our knowledgment, the dynamic changes of membrane lipid composition during germination and osmopriming. In comparison of lipid changes among soybean seeds with, without, and rescuing chilling resistance by osmopriming, we provided experimental demonstration that the lipid composition caused imbibitional chilling injury. Taking advantage of the specific pharmaceutical effects of butanol isomers and NAE on PLD in chilling sensitive seeds of multiple species, we revealed not only determinate but also conserved mechanistic roles of PLD in the imbibition. Our results also show that n-butanol and NAE 12:0 have potential application to improve the germination of crop seeds.
Our major conclusions are as follows:
Dynamic remodelling of membrane lipid composition occurred through three patterns during germination and post-germination (Fig. 7). Pattern 1: increases in MGDG, DGDG, PG, and PS. If the increases of MGDG and DGDG are delayed or blocked, germination will in turn be delayed or blocked. Pattern 2: increases and then decreases in PC, PE, and PI. These components peak at the end of phase II. Pattern 3: a decrease, an increase, and then a decrease in level of PA during phases I, II, and III, respectively. If a significant decrease during phase III does not occur, the membrane will suffer cytoplasm leakage and the seed will fail to germinate.
The increase of PA is derived, at least in part, from PLD-mediated phospholipid hydrolysis. In chilling-sensitive seeds, low temperature promotes PA formation and inhibition of PA formation enhances chilling resistance.
Plastid biogenesis starts early in phase I, and this organelle has largely formed by early in phase III.
Given that patterns 1 and 3 cannot occur for chilling-sensitive seeds at low temperature, plastid biogenesis cannot occur and cytoplasm leakage cannot be restored during phase III.
For chilling-sensitive seeds at low temperature, PEG osmopriming does not directly transform the membrane structure from the hexagonal to the lamellar phase, but causes patterns 1 and 3 to occur, which overcomes chilling injury and facilitates germination.
PLD-mediated PA formation during phase II is the main cause of imbibitional chilling injury.
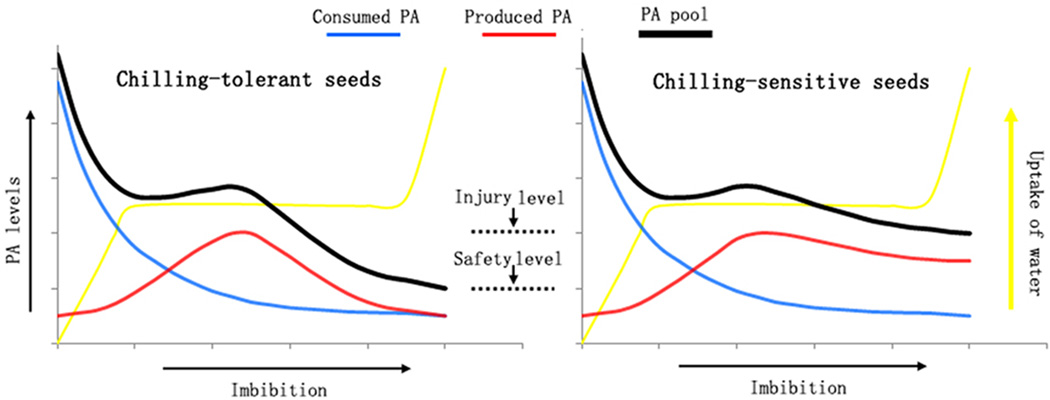
We propose a working model for imbibitional chilling injury (Fig. 9). The size of the PA pool of seeds (black line in Fig. 9) changes dynamically during germination and is determined by two opposing factors. The first is the level of PA consumed (blue line in Fig. 9), which continually decreases. The second is the level of PA produced (red line in Fig. 9), which is derived from PLD hydrolysis and is promoted by low temperature. During phase I, the level of consumed PA is high and the level of produced PA is small, which causes the size of the PA pool to decrease. During phase II, the level of PA consumed is equal to that of the level of PA produced, which causes the size of the PA pool to be maintained or increase slightly. During phase III, the low level of consumption of PA means that the size of the PA pool is mainly determined by the level of PA synthesis. For chilling-tolerant seeds (left panel in Fig. 9), the smaller PLD-mediated increase in PA levels decreases the size of the PA pool to a low level that is safe for membrane function. By contrast, in chilling-sensitive seeds (right panel in Fig. 9), sustained levels of PLD-mediated PA synthesis sustains a large PA pool, which damages membranes and delays or prevents germination.
Fig. 9. Diagram of the proposed model of PA activity during imbibitional chilling injury.
Supplementary Material
Summary Statement.
The reorganization of membrane lipids is the most important event during the imbibition of seed germination. This study finds three patterns of dynamic lipid remodelling during the germination in soybean seeds. Two patterns are interrupted in chilling-sensitive seeds under low temperature and this may result in a block in plastid biogenesis and accumulation of harmful phosphatidic acid. Osmopriming rescues the interruption and inhibition of phospholipase D-mediated phosphatidic acid increases the germination of chilling sensitive-seeds.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (30670471 and 30870571), Kunming Institute of Botany (KSCX2-EW-J-24), the Germplasm Bank of Wild Species and the CAS Innovation Program of Kunming Institute (540806321211), as well as the 100-Talents Program of CAS. The authors would like to thank Drs. Kent Chapman for kindly providing NAE 12:0, Hugh Pritchard and Ruth Welti for their critical reading of this paper, and Mary Roth for the acquisition and processing of the ESI-MS/MS data at the Kansas Lipidomics Research Center Analytical Laboratory, where instrument acquisition and lipidomics method development was supported by National Science Foundation (EPS 0236913, MCB 0920663, DBI 0521587, DBI1228622), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20GM103418), and Kansas State University.
Abbreviations
- DGDG
digalactosyldiacylglycerol
- DMSO
dimethyl sulfoxide
- ESI-MS/MS
electrospray ionization tandem mass spectrometry
- I-W
immersion in water
- LX
Liaoxin
- MGDG
monogalactosyldiacylglycerol
- NAE
N-Acylethanolamine
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PCA
principal components analysis
- PE
phosphatidylethanolamine
- PEG
polyethylene glycol
- PG
phosphatidylglycerol
- P-G
post-germination
- PI
phosphatidylinositol
- PLD
phospholipase D
- PLDα1
phospholipase Dα1
- PS
phosphatidylserine
- R5
Riben 5
REFERENCES
- Barba-Espin G, Hernandez JA, Diaz-Vivancos P. Role of H2O2 in pea seed germination. Plant signaling & behavior. 2012;7:193–195. doi: 10.4161/psb.18881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj J, Anand A, Nagarajan S. Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiology and Biochemistry. 2012;57:67–73. doi: 10.1016/j.plaphy.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Blancaflor E, Hou G, Chapman K. Elevated levels of N-lauroylethanolamine, an endogenous constituent of desiccated seeds, disrupt normal root development in Arabidopsis thaliana seedlings. Planta. 2003;217:206–217. doi: 10.1007/s00425-003-0985-8. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Kilaru A, Keereetaweep J, Khan BR, Faure L, Chapman KD. N-Acylethanolamines: lipid metabolites with functions in plant growth and development. Plant Journal. 2014;79:568–583. doi: 10.1111/tpj.12427. [DOI] [PubMed] [Google Scholar]
- Bramlage WJ, Leopold AC, Parrish DJ. Chilling stress to soybeans during imbibition. Plant Physiology. 1978;61:525–529. doi: 10.1104/pp.61.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KD. Occurrence, metabolism, and prospective functions of N-acylethanolamines in plants. Progress In Lipid Research. 2004;43:302–327. doi: 10.1016/j.plipres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cheng L, Gao X, Li S, Shi M, Javeed H, Jing X, Yang G, He G. Proteomic analysis of soybean [Glycine max (L.) Meer.] seeds during imbibition at chilling temperature. Molecular Breeding. 2010;26:1–17. [Google Scholar]
- Cheng LB, Li SY, He GY. Isolation and expression profile analysis of genes relevant to chilling stress during seed imbibition in soybean [Glycine max (L.) Meer.] Agricultural Sciences in China. 2009;8:521–528. [Google Scholar]
- Christiensen Mn. Periods of sensitivity to chilling in germinating cotton. Plant Physiology. 1967;42:431–433. doi: 10.1104/pp.42.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM. Membrane integrity in anhydrobiotic organisms: toward a mechanism for stabilizing dry seeds. In: Somero GN, Osmond CB, Bolis CL, editors. Water and Life. Berlin: Spinger-Verlag; 1992. pp. 87–103. [Google Scholar]
- Demarsy E, Buhr F, Lambert E, Lerbs-Mache S. Characterization of the plastid-specific germination and seedling establishment transcriptional programme. Journal of Experimental Botany. 2012;63:925–939. doi: 10.1093/jxb/err322. [DOI] [PubMed] [Google Scholar]
- Devaiah SP, Roth MR, Baughman E, Li M, Tamura P, Jeannotte R, Welti R, Wang X. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a phospholipase Dalpha1 knockout mutant. Phytochemistry. 2006;67:1907–1924. doi: 10.1016/j.phytochem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Gardiner J, Collings DA, Harper JDI, Marc J. The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organisation in Arabidopsis . Plant and Cell Physiology. 2003;44:687–696. doi: 10.1093/pcp/pcg095. [DOI] [PubMed] [Google Scholar]
- Gasulla F, Dorp K, Dombrink I, Zahringer U, Gisch N, Dormann P, Bartels D. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: a comparative approach. The Plant Journal. 2013;75:726–741. doi: 10.1111/tpj.12241. [DOI] [PubMed] [Google Scholar]
- Han C, Yin XJ, He DL, Yang PF. Analysis of proteome profile in germinating soybean seed, and its comparison with rice showing the styles of reserves mobilization in different crops. Plos One. 2013;8:e56947. doi: 10.1371/journal.pone.0056947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydecker W, Higgins J, Gulliver RL. Accelerated germination by osmotic seed treatment. Nature. 1973;246:42–44. [Google Scholar]
- Hobbs PR, Obendorf RL. Interaction of initial seed moisture and imbibitional temperature on germination and productivity of soybean. Crop Science. 1972;12:664–667. [Google Scholar]
- Holdsworth MJ, Bentsink L, Soppe WJJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytologist. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Koda Y, Zheng S-H, Yuasa T, Iwaya-Inoue M. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Annals Of Botany. 2013;111:95–102. doi: 10.1093/aob/mcs240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Tao F, Li W. Lipid Profiling Demonstrates That Suppressing Arabidopsis Phospholipase D delta Retards ABA-Promoted Leaf Senescence by Attenuating Lipid Degradation. Plos One. 2013;8:e65687. doi: 10.1371/journal.pone.0065687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Kang SY, Wang Y, Kim SG, Hwang DH, Kang KY. Analysis of embryonic proteome modulation by GA and ABA from germinating rice seeds. PROTEOMICS. 2008;8:3577–3587. doi: 10.1002/pmic.200800183. [DOI] [PubMed] [Google Scholar]
- Law SR, Narsai R, Taylor NL, Delannoy E, Carrie C, Giraud E, Millar AH, Small I, Whelan J. Nucleotide and RNA Metabolism Prime Translational Initiation in the Earliest Events of Mitochondrial Biogenesis during Arabidopsis Germination. Plant Physiology. 2012;158:1610–1627. doi: 10.1104/pp.111.192351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Welti R, Schapaugh WT, Trick HN. Phospholipid and triacylglycerol profiles modified by PLD suppression in soybean seed. Plant Biotechnology Journal. 2011;9:359–372. doi: 10.1111/j.1467-7652.2010.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Wang D, Yu B, Yu X, Li W. Maintenance or collapse: responses of extraplastidic membrane lipid composition to desiccation in the resurrection plant Paraisometrum mileense . Plos One. 2014;9:e103430. doi: 10.1371/journal.pone.0103430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Wang L, Liu X, Cui D, Chen T, Zhang H, Jiang C, Xu C, Li P, Li S, Zhao L, Chen H. Deep sequencing of maize small RNAs reveals a diverse set of microRNA in dry and imbibed seeds. PLoS One. 2013;8:e55107. doi: 10.1371/journal.pone.0055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang R, Li M, Li L, Wang C, Welti R, Wang X. Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in Arabidopsis thaliana. Journal of Biological Chemistry. 2008;283:461–468. doi: 10.1074/jbc.M706692200. [DOI] [PubMed] [Google Scholar]
- Li X, Yang YQ, Zhang M, Wang XF. Effect of PEG priming on plasma membrane H+-ATPase activities and mitochondrium function in soybean seeds. Seed Science and Technology. 2010;38:49–60. [Google Scholar]
- Lu Y, Liu X-P, Wang X-F, Jing X-M, Lin J. Effects of osmoconditioning on membrane lipid components and fatty acid content of cold-sensitive soybean seeds. Zhiwu Shengli yu Fenzi Shengwuxue Xuebao. 2006;32:225–230. [PubMed] [Google Scholar]
- Lyons JM. Chilling injury in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1973;24:445–466. [Google Scholar]
- Makeen AM, Normah MN, Dussert S, Clyde MM. Seed moisture characteristics in relation to total lipid content of five Citrus taxa using an equilibrium dehydration protocol. Seed Science and Technology. 2006;34:453–464. [Google Scholar]
- Motes CM, Pechter P, Yoo CM, Wang YS, Chapman KD, Blancaflor EB. Differential effects of two phospholipase D inhibitors, 1-butanol and N-acylethanolamine, on in vivo cytoskeletal organization and Arabidopsis seedling growth. Protoplasma. 2005;226:109–123. doi: 10.1007/s00709-005-0124-4. [DOI] [PubMed] [Google Scholar]
- Obendorf RL, Zimmerman AD, Ortiz PA, Taylor AG, Schnebly SR. Imbibitional chilling sensitivity and soluble carbohydrate composition of low raffinose, low stachyose soybean seed. Crop Science. 2008;48:2396–2403. [Google Scholar]
- Pagnussat L, Burbach C, Baluska F, de la Canal L. Rapid endocytosis is triggered upon imbibition in Arabidopsis seeds. Plant signaling and behavior. 2012;7:416–421. doi: 10.4161/psb.19669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish DJ, Leopold AC. Transient changes during soybean imbibition. Plant Physiology. 1977;59:1111–1115. doi: 10.1104/pp.59.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock BM. Imbibition temperature sensitivity of lima bean seeds controlled by initial seed moisture. Plant Physiology. 1969;44:907–911. doi: 10.1104/pp.44.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posmyk MM, Balabusta M, Wieczorek M, Sliwinska E, Janas KM. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. Journal of Pineal Research. 2009;46:214–223. doi: 10.1111/j.1600-079X.2008.00652.x. [DOI] [PubMed] [Google Scholar]
- Posmyk MM, Corbineau F, Vinel D, Bailly C, Come D. Osmoconditioning reduces physiological and biochemical damage induced by chilling in soybean seeds. Physiologia Plantarum. 2001;111:473–482. doi: 10.1034/j.1399-3054.2001.1110407.x. [DOI] [PubMed] [Google Scholar]
- Powell AA, Matthews S. The damaging effect of water on dry pea embryos during imbibition. Journal of Experimental Botany. 1978;29:1215–1229. [Google Scholar]
- Simon EW. Phospholipids and plant membrane permeability. New Phytologist. 1974;73:377–420. [Google Scholar]
- Stewart RRC, Bewley JD. Protein-synthesis and phospholipids in soybean axes in response to imbibitional chilling. Plant Physiology. 1981;68:516–518. doi: 10.1104/pp.68.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Li L, Wang X, Wu S, Wang X. Ascorbate-glutathione cycle of mitochondria in osmoprimed soybean cotyledons in response to imbibitional chilling injury. Journal of Plant Physiology. 2011;168:226–232. doi: 10.1016/j.jplph.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Swigonska S, Weidner S. Proteomic analysis of response to long-term continuous stress in roots of germinating soybean seeds. Journal of Plant Physiology. 2013;170:470–479. doi: 10.1016/j.jplph.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Tan-Wilson AL, Wilson KA. Mobilization of seed protein reserves. Physiologia Plantarum. 2012;145:140–153. doi: 10.1111/j.1399-3054.2011.01535.x. [DOI] [PubMed] [Google Scholar]
- Torres-Franklin ML, Gigon A, De Melo DF, Zuily-Fodil Y, Pham-Thi A-T. Drought stress and rehydration affect the balance between MGDG and DGDG synthesis in cowpea leaves. Physiologia Plantarum. 2007;131:201–210. doi: 10.1111/j.1399-3054.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- Ventura L, Dona M, Macovei A, Carbonera D, Buttafava A, Mondoni A, Rossi G, Balestrazzi A. Understanding the molecular pathways associated with seed vigor. Plant Physiology and Biochemistry. 2012;60:196–206. doi: 10.1016/j.plaphy.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Verkleij A, De Maagd R, Leunissen-Bijvelt J, De Kruijff B. Divalent cations and chlorpromazine can induce non-bilayer structures in phosphatidic acid-containing model membranes. Biochimica et Biophysica Acta-Biomembranes. 1982;684:255–262. doi: 10.1016/0005-2736(82)90014-1. [DOI] [PubMed] [Google Scholar]
- Villa-Hernandez JM, Dinkova TD, Aguilar-Caballero R, Rivera-Cabrera F, Sanchez de Jimenez E, Perez-Flores LJ. Regulation of ribosome biogenesis in maize embryonic axes during germination. Biochimie. 2013;95:1871–1879. doi: 10.1016/j.biochi.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Weitbrecht K, Muller K, Leubner-Metzger G. First off the mark: early seed germination. Journal of Experimental Botany. 2011;62:3289–3309. doi: 10.1093/jxb/err030. [DOI] [PubMed] [Google Scholar]
- Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou H-E, Rajashekar CB, Williams TD, Wang X. Profiling membrane lipids in plant stress responses. Journal of Biological Chemistry. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- Welti R, Shah J, Li W, Li M, Chen J, Burke JJ, Fauconnier M-L, Chapman K, Chye M-L, Wang X. Plant lipidomics: discerning biological function by profiling plant complex lipids using mass spectrometry. Frontiers Bioscience. 2007;12:2494–2506. doi: 10.2741/2250. [DOI] [PubMed] [Google Scholar]
- Yang MF, Liu YJ, Liu Y, Chen H, Chen F, Shen SH. Proteomic analysis of oil mobilization in seed germination and postgermination development of Jatropha curcas. Journal of Proteome Research. 2009;8:1441–1451. doi: 10.1021/pr800799s. [DOI] [PubMed] [Google Scholar]
- Yang Y-Q, Wang X-f, Zheng G-H, Jing X-M, Lin J. Osmoconditioning improves soybean seeds vigor. Zhiwu Shengli yu Fenzi Shengwuxue Xuebao. 2003;29:555–560. [Google Scholar]
- Yin GK, Sun HM, Xin X, Qin GZ, Liang Z, Jing XM. Mitochondrial damage in the soybean seed axis during Imbibition at chilling temperatures. Plant and Cell Physiology. 2009;50:1305–1318. doi: 10.1093/pcp/pcp074. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang R, Zhang F, Tao F, Li W. Lipid profiling and tolerance to low-temperature stress in Thellungiella salsuginea in comparison with Arabidopsis thaliana . Biologia Plantarum. 2013;57:149–153. [Google Scholar]
- Zhuo JJ, Wang WX, Lu Y, Sen W, Wang XF. Osmopriming-regulated changes of plasma membrane composition and function were inhibited by phenylarsine oxide in soybean seeds. Journal of Integrative Plant Biology. 2009;51:858–867. doi: 10.1111/j.1744-7909.2009.00861.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.