Abstract
Adoptive cellular therapy, in which activated tumor-reactive T cells are transferred into murine lymphodepleted hosts, is a promising cancer treatment option. Activation of T cells decreases IL-7 responsiveness; therefore, IL-15 is generally considered the main driver of effector T cell responses in this setting. However, we found in lymphodepleted hosts that CD8+ T cells activated with IL-12 showed enhanced engraftment that was initially dependent on host IL-7, but not IL-15. Mechanistically, enhanced IL-7 responsiveness was conferred by elevated IL-7Rα expression, which was critical for anti-tumor immunity. Elevated IL-7Rα expression was achievable without IL-12, as polyclonal CD8+ T cells activated with high TCR stimulation depended on T cell IL-7Rα expression and host IL-7 for maximal engraftment. Finally, IL-12 conditioning during the activation of human CD8+ T cells, including TCR-modified T cells generated using a clinically relevant protocol, led to enhanced IL-7Rα expression. Our results demonstrate the importance of the donor IL-7Rα/host IL-7 axis for effector CD8+ T cell engraftment and suggest novel strategies to improve adoptive cellular therapy as a cancer treatment.
Keywords: adoptive cell therapy, T cells, cancer, IL-7, IL-15
Introduction
The cytokines IL-7 and IL-15 are both critical for T cell homeostasis (1–4). In the context of adoptive T cell therapy (ACT), which involves transfer of effector T cells into lymphodepleted hosts, the relative importance of each cytokine for T cell support has not been fully elucidated; however, several lines of evidence suggest IL-15 is more critical. First, activated T cells downregulate IL-7Rα (CD127) and upregulate IL-2/15Rβ (CD122), leading to a gain in IL-15 responsiveness but concomitant loss in IL-7 responsiveness (5–7). Second, IL-15 has been shown to be more important for antitumor efficacy than IL-7 in a preclinical ACT model (8). Third, memory CD8+ T cells predominantly require IL-15 for proliferation in lymphoreplete and lymphodepleted hosts (9,10). Next, multiple studies have demonstrated that IL-7 and/or IL-7Rα are not critical for the accumulation of effector CD8+ T cells at the peak of an anti-viral immune response (11–13). Finally, IL-15 more potently and specifically maintains effector CD8+ T cell numbers at the culmination of infection compared with IL-7 (14). Based on these studies, IL-15 would be predicted to more relevant than IL-7.
Priming activated T cells with the Th1/Tc1 polarizing cytokine IL-12 (15,16) dramatically improves the persistence and antitumor efficacy of CD8+ T cells after adoptive transfer (17–19). As IL-7 and IL-15 are elevated after lymphodepletion (20–22), this enhanced persistence may be due to an increase in the expression of IL-2Rβ and/or IL-7Rα induced by IL-12 (7,23). While IL-2Rβ has consistently been shown to be increased by IL-12 (24,25), data concerning IL-7Rα is conflicting. Several studies have found that IL-12 exposure decreased IL-7Rα levels (26–29), although in other settings IL-12 increased IL-7Rα on activated CD8+ T cells (24,25,30). Thus, the impact of IL-12 on the ability of CD8+ T cells to respond to the homeostatic cytokines IL-7 and IL-15 warrants further consideration.
In this study, we investigated the cytokine requirements of effector CD8+ T cells in murine lymphodepleted hosts. We initially focused on CD8+ T cells conditioned with IL-12 because these cells expand robustly in a lymphodepleted host without a requirement for exogenous cytokines or vaccination (17). This strategy revealed that activated CD8+ T cells require host IL-7, but not IL-15, for maximal initial expansion in a lymphodepleted host. Accordingly, the persistence and anti-tumor activity of these cells was dependent on IL-7Rα. These findings are generalizable and translatable, as polyclonal CD8+ T cells activated in the absence of IL-12 were also dependent on IL-7/IL-7Rα for initial engraftment, and human T cells cultured with IL-12 acquired superior IL-7 responsiveness. These findings have direct implications for the design of future adoptive cellular therapy trials for cancer therapy.
Methods
Mice
C57BL/6 (B6), B6.PL (Thy1.1), pmel-1 TCR transgenic (31), β2-microglobulin−/− (β2m−/−) and IL-7Rα−/− were obtained from Jackson Laboratory (Bar Harbor, ME). IL-15−/− mice were purchased from Taconic (Hudson, NY). H3T TCR transgenic mice were generated as previously described (32). Pmel-1 mice were maintained by crossing a pmel-1 (male) to a Thy1.1 (female) generating hemizygous offspring. IL-7Rα+/− heterozygous mice were generated by crossing a IL-7Rα−/− male to either a Thy1.1/1.1 homozygous B6 female (generating the B6 IL-7Rα+/− Thy1.1/1.2 mouse) or a pmel+/+Thy1.1/1.1 homozygous female (generating the pmel+/− IL-7Rα+/− mouse). All mice were used between 6–16 weeks of age. Mice were housed under specific pathogen-free conditions in accordance with institutional and federal guidelines at the Medical University of South Carolina.
Cell cultures
B16-F1 tumor cells were obtained from ATCC (Manassas, VA) and immediately expanded and frozen down into a large number of aliquots. Cells were verified to be mycoplasma free and one aliquot was briefly expanded for each experiment using culture conditions as previously described (17). All T cells were grown in RPMI 1640 complete media as described (17). For generation of mouse gp100-reactive T cells, pmel-1 TCR transgenic splenocytes (1×106 cells/mL) were stimulated with 1 µg/mL H-2Db-restricted human gp10025–32 peptide (KVPRNQDWL, American Peptide Company) for 3 days with or without mIL-12 (10 ng/mL, Shenandoah Biotechnology) to generate Tc1 or Tc0 T cells, respectively. For generation of mouse tyrosinase-reactive T cells, h3T TCR transgenic splenocytes were cultured with irradiated T2-A2 cells loaded with 1 µg/mL HLA-A2-restricted human tyrosinase368–376 peptide (YMDGTMSQV, American Peptide Company) for 3 days with or without mIL-12. Polyclonal stimulations were performed by adding 1 µg/mL soluble anti-CD3 mAb (145-2C11) ± 2 µg/mL anti-CD28 mAb (37.51) directly or by coating a 24 well plate with 1 µg/mL anti-CD3 ± 2 µg/mL anti-CD28 before addition of splenocytes.
Cytokine responsiveness
Cytokine responsiveness was assessed by washing cells 3× in PBS then replating cells at 0.8–1×106/mL with the indicated cytokine (mouse cytokines from Shenandoah Biotechnology). After overnight incubation, cells were either fixed/permeabilized for phosflow analysis per the manufacturer’s instructions (Phosflow; BD Bioscience, San Jose, CA) or 10 µM BrdU was added for 1 h at 37°C and cells were processed according to the manufacturer’s protocol (BrdU Flow Kit; BD Bioscience). Note that the percentage of cells that were pSTAT5+ 15 minutes after restimulation was not significantly different from values obtained after overnight incubation (data not shown).
Flow cytometry
For flow cytometric analysis, cells were processed as previously described (17) and analyzed either on an LSRII or Accuri C6 flow cytometer (BD Bioscience). Data was processed using FlowJo vX (Treestar, Ashland, OR) or C6 software (BD Bioscience). Mouse antibody clones used in this study include: CD4 (GK1.5), CD8 (53-6.7), CD25 (PC61.5), CD62L (MEL-14), CD122 (TM-β1), IL-7Rα (SB/199 or A7R34), Eomes (Dan11mag), granzyme B (GB12), IFNγ (XMG1.2), pAKT S473 (D9E), pSTAT5 (47/Stat5 pY694), pS6 (D57.2.2E), Thy1.1 (OX-7 or HIS51), TNFα (TN3-19.12) and Tbet (4B10). Human antibody clones used are CD8 (OKT8, SK1) and IL-7Rα (eBioRDR5, A019D5). These were purchased from BD Bioscience, Biolegend (San Diego, CA), Invitrogen (Carlsbad, CA), eBioscience (San Diego, CA) and/or Cell Signaling Technology (Danvers, MA).
Tumor challenge, lymphodepletion and adoptive T cell transfer
For tumor experiments, B6 mice had 2.5×105 B16-F1 tumor cells injected subcutaneously (s.c). Tumor growth was measured by an observer blinded to treatment groups with calipers 2–3 times per week and tumor surface area (mm2) was calculated as length × width. Mice were sacrificed when tumors reached > 400 mm2. Total body irradiation (TBI) was administered at 6 Gy the day before adoptive transfer. Mice were excluded from analysis if they developed i.p. tumor spread within the first 4 weeks after injection.
In vivo cytokine neutralization
All neutralizing antibodies were purchased from BioXCell (West Lebanon, NH) except for JES6-1A12 (UCSF monoclonal antibody core, San Francisco, CA). Unless otherwise indicated, the following amounts of mAb were injected i.p. on days 0, 2, 5, 8, 12 and 17 following adoptive transfer: αIL-7 (M25, 200µg), αIL-7Rα (A7R34, 500µg), αIL-2 (250 µg each of S4B6 and JES6-1A12 injected together), and mIgG2b isotype control (MPC-11, 200µg).
Measurement of IFNγ
Day 3 culture supernatants were analyzed for mIFNγ via ELISA per the manufacturer’s instructions (Biolegend).
Experiments involving human PBMCs
De-identified human PBMCs were isolated from a leukapheresis pack obtained from Research Blood Components (Boston, MA) and experiments were performed in accordance with MUSC IRB guidelines. For in vitro stimulation, cells were thawed and rested in 100 IU/mL hIL-2 overnight. The next day, 0.5 µg/mL soluble αCD3 (Okt3, NCI repository) was added to culture ± 10 ng/mL hIL-12. After 3 days of activation, cytokine responsiveness and phenotype were assessed. In some experiments, activated cells were maintained in cytokines as indicated for 2 weeks. Every 2–3 days cells were counted and given fresh cytokine-containing media to maintain a concentration of 0.8 × 106 cells/ml. For generation of TCR-modified human T cells, we used a modification of a previously described protocol (33). On day 1, human PBMCs were stimulated with soluble anti-CD3 mAb (OKT3, NCI preclinical repository) for 48 hours. Beginning on day 3, cells were cultured with hIL-2 (300 IU/ml) and hIL-15 (100 ng/ml), and maintained between 1–2×106 cells/ml. Also on day 3, activated T cells were transduced by co-culture with 50% retroviral supernatant from PG13 packaging cells transfected with the TIL1383ITCR/CD34t construct (34). Transduction was done with retronectin-coated plates and spinoculation (2000g for 2 hours at 32°C). On day 8, cells underwent a rapid expansion protocol by incubation in a G-Rex 100 flask (Wilson Wolf Manufacturing) of 1×106 transduced T cells with 2×108 irradiated (50 Gy) allogeneic feeder cells from human donors. Soluble anti-CD3 mAb (OKT3, 30ng/ml) was also added to the cultures. On REP day 14, cultures were harvested, washed and replated for IL-7Rα analysis 3 days later.
Statistics
Statistical analysis was done with GraphPad Prism 6 software. One-Way ANOVA with a Tukey multiple comparisons correction or a two-sided two-sample t-test was used to evaluate statistical significance of means between groups. When variances were unequal, Welch’s t-test was used. Data expressed on a ratio scale (e.g. fold change) was first log-transformed to normalize the distribution, then analyzed by t-test or one-way ANOVA, as appropriate. For survival data, the logrank test was used. Unless otherwise indicated, summary statistics in figures are presented as mean ± standard error of the mean (s.e.m.).
Results
The enhanced initial engraftment of IL-12–conditioned effector CD8+ T cells (Tc1) transferred into lymphodepleted hosts is dependent on IL-7 but not IL-15
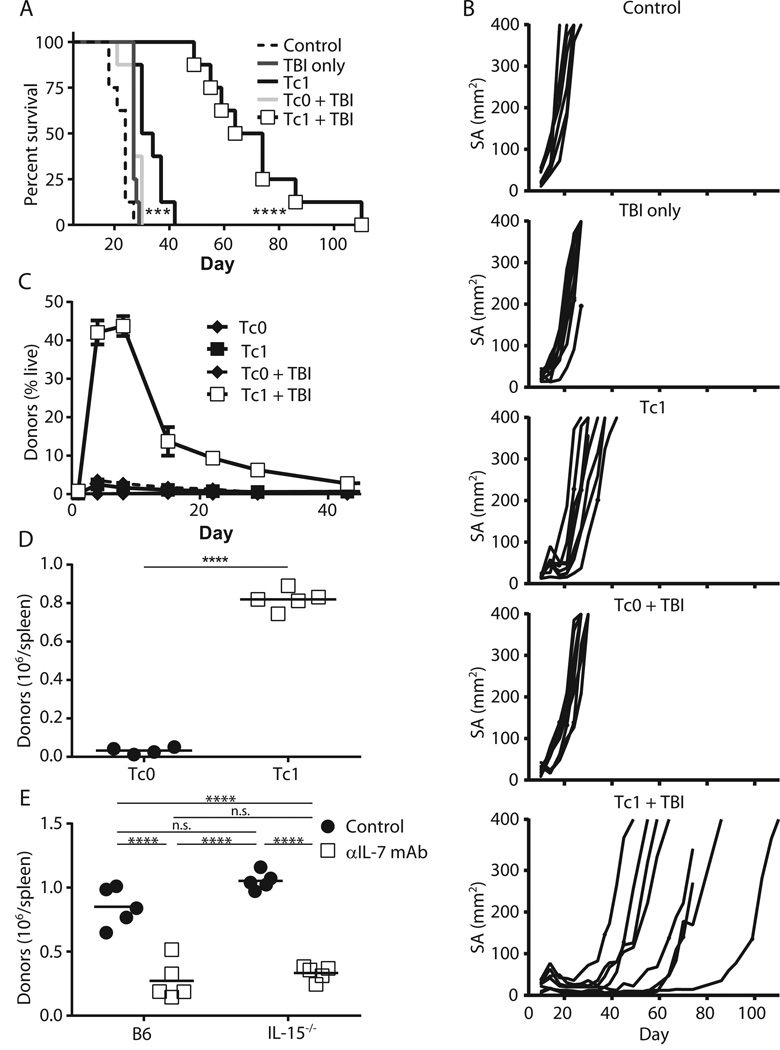
We previously demonstrated that the persistence and antitumor abilities of IL-12–conditioned pmel-1 CD8+ T (Tc1) cells were enhanced by cyclophosphamide, a lymphodepleting agent (35). Similarly, lymphodepletion with 6 Gy total body irradiation (TBI) before adoptive transfer of Tc1 significantly delayed the growth of established B16 tumors, while transfer of Tc1 alone or transfer of cells activated without IL-12 (Tc0) into irradiated hosts did not (Fig. 1A–B). The persistence of Tc1 cells was also strikingly enhanced relative to Tc0 cells, with the peak of expansion seen about 1 week after transfer (Fig. 1C–D). This enhanced persistence with multiple forms of lymphodepletion but without the need for IL-2 or vaccination establishes the feasibility of using our Tc1 model to investigate the host cytokine requirements of effector CD8+ T cells.
Figure 1. The enhanced persistence of IL-12 conditioned CD8+ T cells (Tc1) in lymphodepleted hosts is dependent on IL-7.
(A, B) B6 mice were injected with B16 melanoma tumor s.c. on day −12 and then irradiated on day −1. On day 0, mice were adoptively transferred with 2×106 3-day activated pmel-1 CD8+ T cells with IL-12 conditioning (Tc1) or without (Tc0). (A) Survival curves (n = 8, *** p = 0.001 for Tc1 vs. control, p < 0.0001 for Tc1 vs. Tc1 + TBI) and (B) individual tumor growth curves. (C, D), 5×106 Tc1 or Tc0 cells were transferred into mice with or without 6 Gy TBI and Thy1.1+ donors were tracked in the (C) peripheral blood over time (n = 5) or (D) in the spleens 7 days post-transfer (n = 5, **** p < 0.0001). (E) As in (D) except cells were transferred into WT B6 or IL-15−/− mice with or without αIL-7 neutralizing mAb (clone M25; n = 5, **** p < 0.0001). All results are representative of at least 2 independent experiments.
Because IL-7 and IL-15 are thought to be the dominant cytokines for T cell homeostatic expansion (1–3), and they are elevated post-lymphodepletion (20–22), we assessed their importance for the expansion of Tc1 cells. We transferred Tc1 cells into irradiated WT or IL-15−/− mice with or without an IL-7 neutralizing monoclonal antibody (clone M25). We then harvested spleens at day 7 post-transfer, as this correlated with the peak of their expansion (Fig. 1C). Surprisingly, Tc1 cells exhibited a significant expansion defect at day 7 in WT mice treated with IL-7 neutralizing antibodies, but not in IL-15−/− mice (Fig. 1E). Removal of both cytokines did not further decrease the engraftment of these cells (Fig. 1E). We confirmed our results by administering a blocking antibody against IL-7Rα (A7R34) (Fig. s1A). Like IL-15, IL-2 was not critical, as a combination of neutralizing IL-2 antibodies (JES6-1A12 and S4B6) (36) did not significantly affect Tc1 cell expansion (Fig. s1B). Additionally, the absence of host IL-2, IL-7 and/or IL-15 did not significantly impair the ability of Tc1 cells to secrete IFNγ and TNFα after ex vivo restimulation (Fig. s2). In summary, Tc1 cells are dependent on host IL-7 alone for their initial expansion.
Certain T cell subsets require TCR engagement for homeostatic maintenance (3,4). Because pmel-1 T cells have engineered specificity against gp100, a self-antigen, we transferred Tc1 cells into β2m−/− mice, which are devoid of MHC-I presentation. Tc1 cells persisted equally well in WT B6 and β2m−/− B6 mice, indicating that Tc1 did not require TCR engagement for effector expansion (Fig. s3A). To confirm our results in a second model, we used the h3T TCR transgenic mouse, whose T cells recognize tyrosinase in an HLA-A2-restricted manner (37). h3T T cells activated in the presence or absence of IL-12 showed similar persistence when transferred into irradiated WT B6 or HLA-A2 transgenic mice (Fig. s3B). Thus, activated Tc1 cells do not require contact with cognate MHC-I for maximal effector expansion in irradiated hosts.
IL-7 and IL-15 are required for maximal antitumor efficacy of Tc1 cells
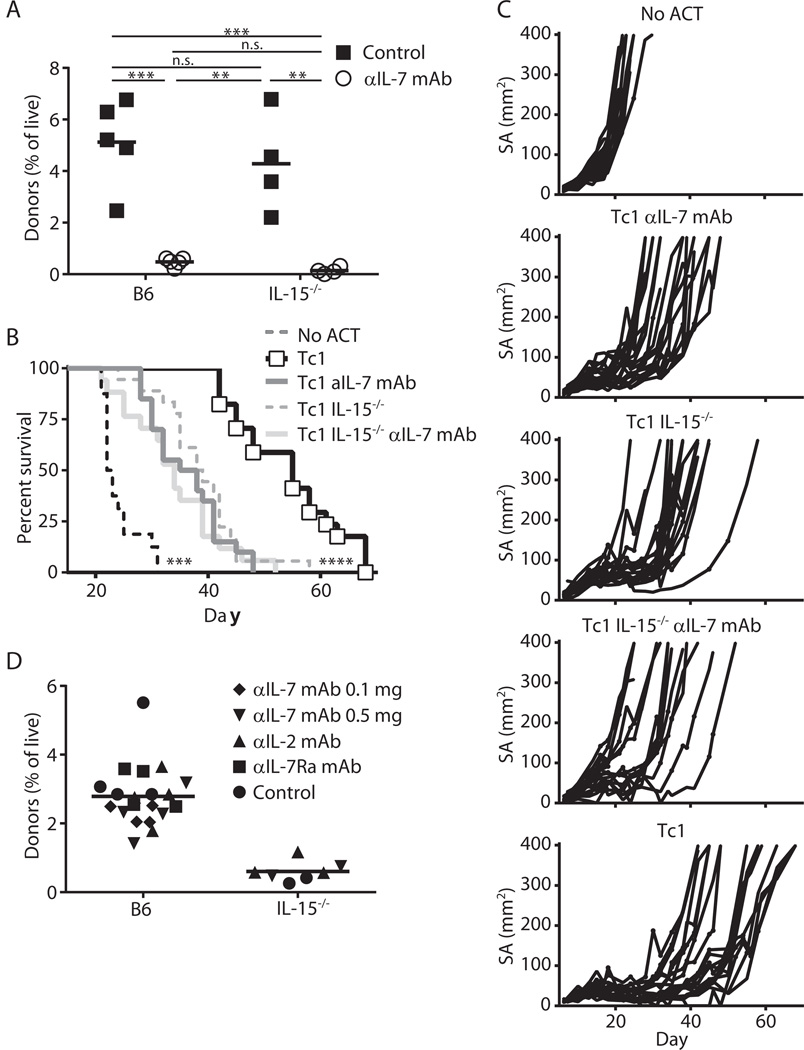
The results above were obtained in tumor-free animals. Therefore, we assessed the cytokine requirements for optimal expansion of effector CD8+ T cells adoptively transferred into B6 mice bearing 12-day established B16 tumors. In a manner similar to tumor-free mice, the initial engraftment of Tc1 cells was dependent on IL-7 but not IL-15 (Fig. 2A). Consistent with our early expansion data (Fig. 2A), Tc1 cells required IL-7 for maximum antitumor efficacy (Fig. 2B–C). In contrast to this data, Tc1 cells also needed IL-15 for maximal antitumor efficacy (Fig. 2B–C). This result is likely because IL-15 is required for the long-term persistence and memory formation of Tc1 cells (Fig. 2D), although IL-15-dependent host cells may be relevant. Thus, Tc1 cells require IL-7 for initial expansion but both IL-7 and IL-15 for maximal antitumor efficacy.
Figure 2. IL-7 and IL-15 are required for maximal anti-tumor efficacy of IL-12-conditioned CD8+ (Tc1) T cells.
(A–C) B6 mice were injected with B16 melanoma tumor s.c. on day −12 and then irradiated (6 Gy) on day −1. On day 0, mice were adoptively transferred with 2×106 Tc1 CD8+ effector T cells. (A) Donor cells in blood on day 5 (n = 4–5, ** p < 0.01, *** p < 0.001; representative of 2 independent experiments). (B) Survival data (n = 16–20, *** p < 0.001 for No ACT vs. IL-15−/− Tc1 + αIL-7 mAb and **** p < 0.0001 for Tc1 IL-15−/− vs. Tc1) and (C) tumor growth curves are pooled from 2 independent experiments of 8–10 mice. (D) 5×106 Tc1 cells were injected into irradiated WT or IL-15−/− mice with or without administration of the indicated antibodies. Anti-IL-7 mAb was given at either 100ug or 500ug per injection. After 77 days, the frequency of donor cells in the peripheral blood was measured. Results are representative of 2 independent experiments.
Tc1 cells demonstrate superior IL-7 responsiveness and elevated IL-7Rα levels in vitro
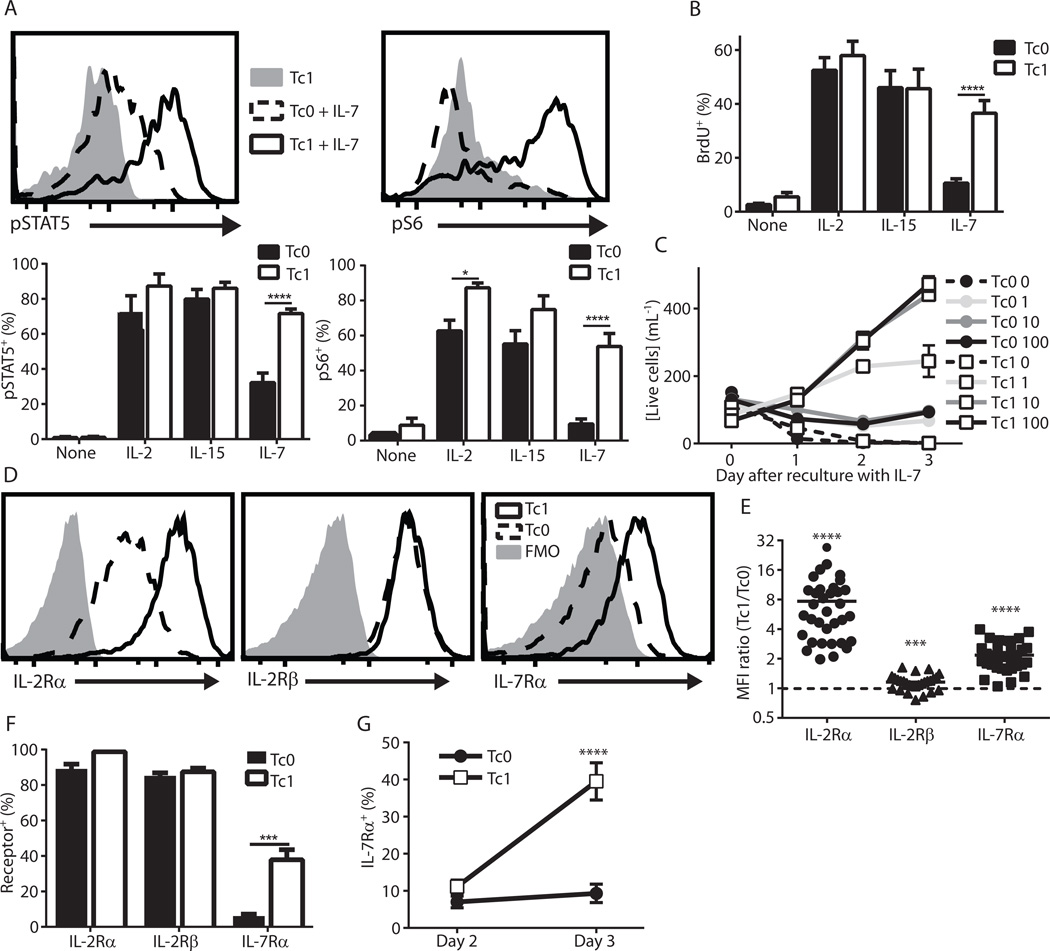
Because Tc1 cells exhibited IL-7–dependent expansion in irradiated hosts, we assessed the in vitro IL-7 responsiveness of Tc1 cells compared to Tc0 cells. We also assessed IL-2 and IL-15 signaling as controls. We first cultured Tc0 cells and Tc1 cells in high doses (100 ng/mL) of IL-2, IL-15 or IL-7 overnight and then assessed phosphorylation of STAT5 and ribosomal S6 (Fig. 3A), both of which are downstream of IL-2/7/15 cytokine signaling (4,38). As expected, IL-2 and IL-15 led to high levels of phosphorylation in both Tc0 and Tc1 cells. However, when cultured with IL-7, only Tc1 cells robustly phosphorylated STAT5 and S6 (Fig. 3A). These enhanced signaling events translated into increased proliferation of Tc1 cells after reculture in IL-7 as determined by BrdU incorporation (Fig. 3B). In contrast, Tc0 and Tc1 cells proliferated extensively in IL-2 or IL-15, as over half of the cells had incorporated BrdU in 1 h (Fig. 3B). The enhanced proliferation rate after overnight culture led to about a 5-fold expansion of Tc1 over Tc0 cells after 3 days of culture in IL-7 (Fig. 3C). Remarkably, even 100-fold lower levels of IL-7 (1 ng/mL) led to an increased concentration of Tc1 cells after 3 days, while Tc0 cells at the highest dose barely maintained their numbers (Fig. 3C). These signaling and proliferation events were inhibited by JAK-STAT and PI3K inhibitors, but not mTOR inhibitors (Fig. s4), indicating that IL-7 was engaging established pathways for cytokine-mediated T cell proliferation (39–41). In summary, these findings demonstrate the ability of IL-12 conditioning to induce IL-7 responsiveness in effector CD8+ T cells.
Figure 3. IL-12 conditioning during CD8+ T cell activation leads to elevated IL-7 responsiveness and IL-7Rα expression in vitro.
(A–C) Pmel-1 T cells were activated for 3 days with (Tc1) or without (Tc0) IL-12, washed and replated in the indicated cytokines (A, top). Representative histograms depicting pSTAT5 and pS6 levels after reculture without cytokine or with IL-7 (A, bottom). Mean pSTAT5 and pS6 levels after reculture in 100 ng/mL of the indicated cytokine (n = 4, * p < 0.05, **** p < 0.0001). (B) BrdU was added for the final hour after overnight culture in the indicated cytokine (n = 10, **** p < 0.0001). (C) Cells were counted on days 0, 1, 2 and 3 post-replate in the indicated concentration of IL-7 in ng/mL (results are from 1 experiment with 2 replicates and are representative of at least 3 independent experiments). (D–F) Tc0 and Tc1 cells were analyzed for the indicated cytokine receptors via flow cytometry. (D) Representative histograms and (E) MFI ratios (*** p < 0.001, **** p < 0.0001; p-values represent significantly different from Tc0, which is indicated by the dashed line). (F) The percentage of cells expressing each cytokine receptor are shown (n = 11 independent experiments, *** p < 0.001 via Welch’s t-test). (G) The percentage of cells expressing IL-7Rα on days 2 and 3 after stimulation (n = 7, **** p < 0.0001 for all comparisons with Tc1 Day 3, ns for others).
We next sought to delineate the mechanism(s) responsible for the enhanced IL-7 responsiveness of Tc1 cells by evaluating IL-7Rα as well as IL-2Rβ and IL-2Rα expression on Tc0 and Tc1 cells. The expression of all three receptors was increased by the addition of IL-12 (Fig. 3D–E), although the magnitude of these increases varied (Fig. 3E). When expressed as a proportion of cells staining positive for the receptor rather than the magnitude of expression, a striking difference was seen with IL-7Rα. A large proportion of Tc1 cells expressed IL-7Rα while Tc0 cells had almost none, in contrast to high levels seen with IL-2Rβ and IL-2Rα on Tc0 and Tc1 cells (Fig. 3F). We next investigated the kinetics of IL-7Rα expression. As expected, IL-7Rα was initially decreased on both cell types after T cell activation, but Tc1 cells increased expression by 72h after stimulation (Fig. 3G). Thus, IL-12 promotes IL-7Rα re-expression in Tc1 cells, a finding that may explain the enhanced IL-7-mediated persistence of effector CD8+ T cells (Tc1) cells after transfer into lymphodepleted hosts.
IL-7Rα upregulation is responsible for the enhanced IL-7 responsiveness and subsequent in vivo persistence of Tc1 cells
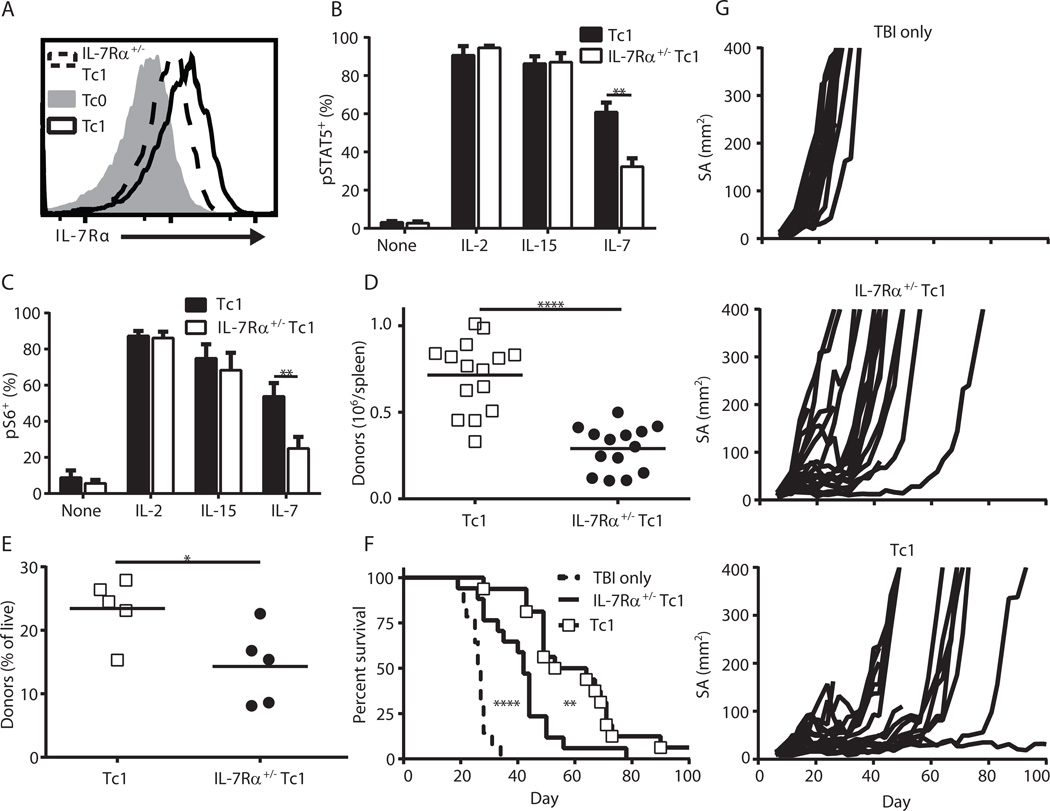
To directly test whether IL-7Rα was critical for the enhanced IL-7 responsiveness of Tc1 cells, we generated pmel-1 IL-7Rα+/− mice. As expected, Tc1 cells generated from IL-7Rα+/+ and IL-7Rα+/− pmel-1 mice expressed similar levels of IL-2Rβ, IL-2Rα, granzyme B (GrzB), Tbet, Eomes and CD62L (Fig s5A), and produced equivalent levels of IFNγ after 3 day culture (Fig. s5B). In contrast, IL-7Rα levels in the IL-7Rα+/− Tc1 cells were about half that of Tc1 cells (Fig. 4A–B). This decreased IL-7Rα expression translated to reduced IL-7-induced STAT5 and S6 phosphorylation for IL-7Rα+/− Tc1 compared to WT Tc1, despite having similar levels when maintained in IL-2 or IL-15 (Fig. 4B–C). BrdU incorporation also trended lower with IL-7 cultures of IL-7Rα+/− Tc1 relative to Tc1 (Fig. s5C).
Figure 4. IL-7Rα expression is required for maximal expansion and anti-tumor efficacy of Tc1 cells.
(A) Representative histogram of IL-7Rα levels in Tc0, Tc1 and IL-7Rα+/− Tc1 cells. (B) pSTAT5 and (C) pS6 levels of Tc1 and IL-7Rα+/− Tc1 cells after replate in 100 ng/mL of the indicated cytokine (n = 4–6, ** p < 0.01). (D) 3–5×106 pmel Tc1 or IL-7Rα+/− Tc1 cells were transferred into irradiated hosts (6 Gy), and the absolute number of donor cells in host spleens 7 days later is displayed (data is combined from 3 independent experiments, **** p < 0.0001). (E–G) Day 12 B16 tumor-bearing mice were injected with 2×106 T cells the day after irradiation. (E) The percentage of donor cells in the peripheral blood on day 8 post-transfer (* p < 0.05). (F) Survival curves (**** p < 0.0001 for TBI only vs. IL-7Rα+/− Tc1, ** p < 0.01 for IL-7Rα+/− Tc1 vs. Tc1; combined from 2 independent experiments for total n = 14–17) and (G) growth curves.
These in vitro results indicate that IL-7Rα+/− Tc1 cells can be used to evaluate the functional importance of IL-7Rα, given that they appeared identical to WT Tc1 in all aspects tested except for IL-7Rα expression and IL-7 responsiveness. Therefore, we transferred WT and IL-7Rα+/− Tc1 cells into irradiated hosts. On day 7 post-transfer into irradiated hosts, there were about half as many IL-7Rα+/− Tc1 cells as WT Tc1 cells in the spleens of recipient mice (Fig. 4D). Similar results were observed in the peripheral blood of tumor-bearing mice 7 days after transfer (Fig. 4E). Importantly, this decreased initial expansion of Tc1 cells also led to significantly reduced antitumor activity in IL-7Rα+/− Tc1 cells relative to WT pmel-1 Tc1 cells (Fig. 4F–G). Together, these results indicate that elevated IL-7Rα expression is critical for driving the initial engraftment and subsequent antitumor activity of Tc1 cells.
Host IL-7 and donor IL-7Rα are required for maximal persistence of polyclonal CD8+ T cells in lymphodepleted hosts
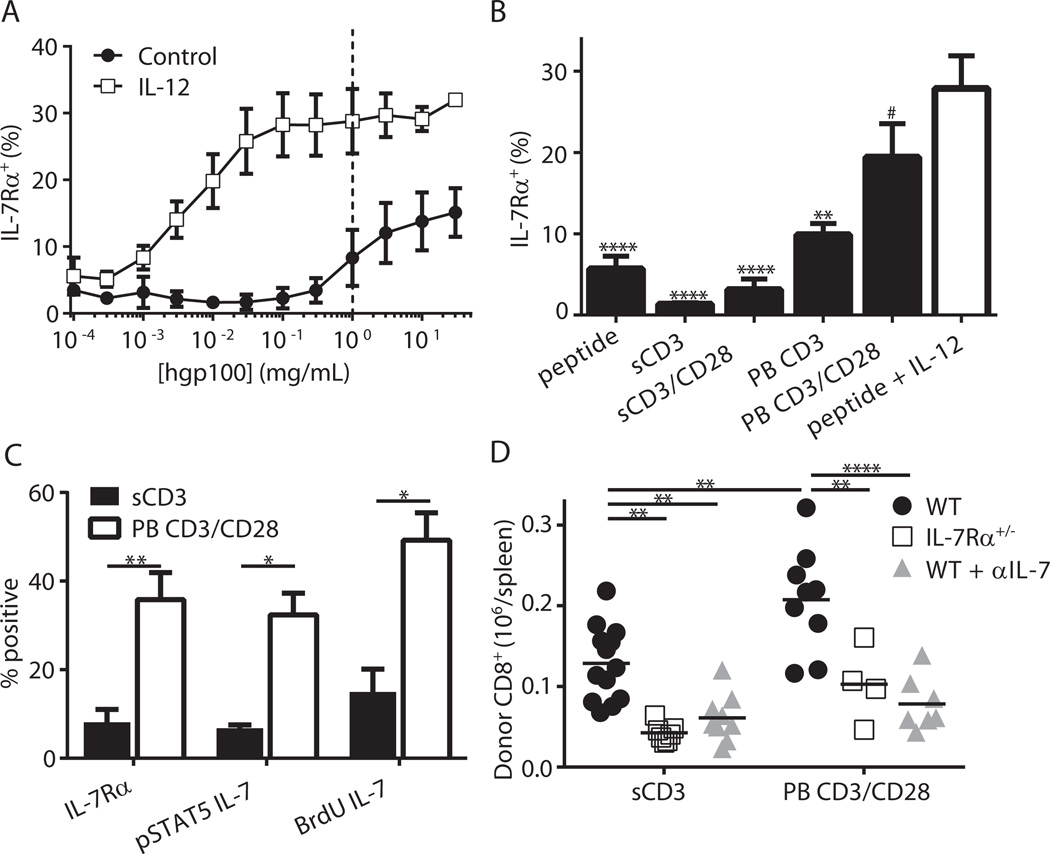
Next, we investigated the importance of IL-7Rα for the initial engraftment of effector CD8+ T cells activated without IL-12. As shown in Figs. 1C–D, pmel-1 T cells stimulated with hgp100 alone (Tc0) persisted poorly, presumably due to low IL-7Rα expression (Fig. 3F). Therefore, we sought IL-12-independent activation conditions that would elevate IL-7Rα appreciably and thereby generate effector cells capable of persisting in lymphodepleted hosts. Because TCR strength has been shown to modulate IL-7Rα levels in human CD4+ T cells (42), we activated pmel-1 T cells over a broad range of hgp100 concentrations. While higher peptide concentrations increased IL-7Rα expression, the receptor levels did not reach those achieved with IL-12 (Fig. 5A). To further increase the strength of TCR stimulation, we next activated T cells non-specifically with soluble or plate-bound anti-CD3 mAb with or without anti-CD28 mAb. Consistent with reports demonstrating elevated TCR signaling with immobilized anti-CD3 mAb (43) and co-stimulation with anti-CD28 mAb (44), IL-7Rα levels were increased in plate-bound conditions and even higher when anti-CD28 mAb was added (Fig. 5B). In fact, plate-bound anti-CD3 mAb and anti-CD28 mAb (PB CD3/CD28) were statistically indistinguishable from Tc1 cells (hgp100 + IL-12, Fig. 5B).
Figure 5. TCR strength modulates IL-7Rα expression, which dictates engraftment of activated CD8+ T cells.
(A) Pmel-1 CD8+ T cells were stimulated for 3 days ± IL-12 with titrated hgp100 peptide. (B) Pmel-1 T cells were stimulated with soluble anti-CD3 mAb (sCD3), sCD3 + soluble anti-CD28 mAb (sCD3/CD28), plate-bound anti-CD3 mAb (PB CD3), PB CD3 + plate-bound anti-CD28 mAb (PB CD3/CD28) or hgp100 peptide with or without IL-12 for 3 days and assessed for IL-7Rα expression (combined data from 4–5 independent experiments, # p > 0.05, ** p < 0.01, **** p < 0.0001 vs. hgp100 + IL-12). (C) B6 T cells were stimulated as indicated and assessed for IL-7Rα expression (n = 5, ** p < 0.01) or responsiveness to IL-7 (n = 3 for pSTAT5 and BrdU assays, * p < 0.05). (D) WT or IL-7Rα+/− mice were stimulated with soluble or plate bound antibodies then transferred into irradiated hosts. Where indicated, the IL-7 blocking antibody clone M25 was administered on days 0, 2 and 5 post-transfer. Shown are absolute numbers of donor CD8+ T cells 7 days after transfer (** p < 0.01, **** p < 0.0001, data is combined from 3 independent experiments).
Having established that higher TCR signals increase IL-7Rα expression in the pmel-1 model, we evaluated this relationship in CD8+ T cells from WT B6 mice. Like with pmel-1 T cells, PB CD3/CD28 produced the highest IL-7Rα levels in polyclonal T cells, and IL-12 further enhanced IL-7Rα expression across all TCR stimuli (Fig. s6). Next, we characterized the PB CD3/CD28 and soluble αCD3 (sCD3) conditions as they possessed the highest and lowest IL-7Rα expression, respectively (Fig. s6). As expected, sCD3 stimulated T cells had decreased IL-7 responsiveness compared to PB CD3/CD28 (Fig. 5C). When transferred into irradiated hosts, PB CD3/CD28 stimulated CD8+ T cells accumulated at significantly higher levels than cells stimulated with soluble αCD3 alone (Fig. 5D). Importantly, IL-7Rα+/− cells stimulated with either TCR strength failed to engraft as well as their WT counterparts. Finally, both WT cell types were also dependent on IL-7, as IL-7 neutralization led to significant reductions in donor CD8+ cell numbers (Fig. 5D). In sum, these data indicate that host IL-7 and donor IL-7Rα are critical for maximal accumulation of activated CD8+ effector cells transferred into lymphodepleted hosts.
Human T cells conditioned with IL-12 display enhanced IL-7Rα expression and IL-7 responsiveness
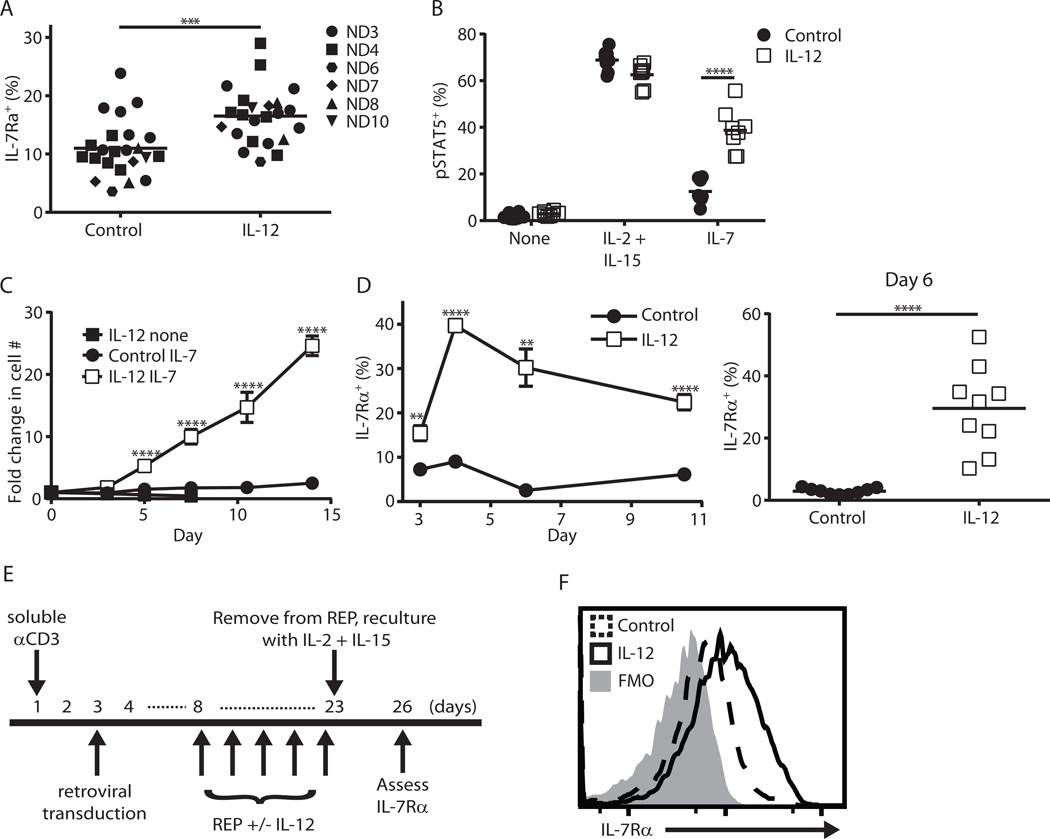
Given the importance of donor IL-7Rα and host IL-7 for the persistence of effector CD8+ T cells in mice, we next tested the ability of IL-12 to enhance IL-7Rα expression in activated human CD8+ T cells. CD8+ T cells from day 3 activated human peripheral blood mononuclear cells (PBMCs) exhibited higher IL-7Rα expression with IL-12, although the magnitude of this effect was not as large as our murine data (Fig. 6A compared to mouse data in Fig. 3D). In contrast to this small change in IL-7Rα expression, human T cells were only able to phosphorylate STAT5 robustly in response to IL-7 if they were activated with IL-12 (Fig. 6B). When these activated T cells were washed and recultured in vitro, only those activated with IL-12 expanded in the presence of IL-7 (Fig. 6C). Given the discordance between initial IL-7Rα levels (Fig. 6A) and IL-7 responsiveness (Fig. 6B–C), we assessed IL-7Rα levels after reculture of cells. We speculated that the ability to re-express IL-7Rα after withdrawal of TCR stimulation might explain the observed differences in IL-7 responsiveness. Consistent with this hypothesis, the presence of IL-12 during the first 3 days of activation led to a striking enhancement in IL-7Rα expression that lasted for at least 1 week after reculture (Fig. 6D). Finally, we sought to evaluate the translatability of our findings from 3-day cultures in a clinically relevant scenario by using the retroviral transduction protocol depicted in Figure 6E, in which IL-12 was added or withheld during the rapid expansion protocol (REP). We found that the inclusion of IL-12 did not significantly increase IL-7Rα levels at the end of the REP. Like from our 3-day cultures, however, the transduced T cells that underwent the REP in the presence of IL-12 possessed higher IL-7Rα expression 3 days after reculture (Fig. 6F). These results suggest that the addition of IL-12 to human T cell cultures during the REP is a feasible strategy to augment IL-7Rα levels and this may be applicable in a number of clinically used protocols (45–47).
Figure 6. Human T cells conditioned with IL-12 display enhanced IL-7Rα expression and IL-7 responsiveness.
(A–D) Human PBMCs were activated with soluble anti-CD3 mAb (0.5 µg/mL, Okt3 clone) with or without hIL-12 (10ng/ml) for 3 days. (A) IL-7Rα expression after 3 day activation (*** p < 0.001; “ND” is normal donor). (B, C) Day 3 activated T cells were washed then replated in the indicated cytokines (300 IU/mL IL-2 + 100 ng/mL IL-15; IL-7, 100 ng/mL). (B) pSTAT5 staining via flow cytometry after overnight culture (n = 8 from 2 independent experiments with 4 normal donors, **** p < 0.0001). (C) Cells were counted and given fresh media every 2–3 days (n = 6 from 2 independent experiments with 3 normal donors). (D) As in (C) except activated cells were recultured in IL-2 + IL-15 on day 3 then assessed for IL-7Rα expression at the indicated time points (n = 6–9 from 2 independent experiments with 4 normal donors, ** p < 0.01, **** p < 0.0001 via Welch’s t-test). (E) Overview of the clinical transduction protocol to generate TCR-transduced melanoma-reactive human T cells. Shown is the timing of IL-12 addition and three day reculture in IL-2 (300 IU/mL) + IL-15 (100 ng/mL). (F) IL-7Rα expression at day 26 of above timeline of human T cells initially grown with or without hIL-12. This result is representative of two independent experiments.
Discussion
In this study, we evaluated the host cytokines required for the initial engraftment of effector CD8+ T cells transferred into lymphodepleted hosts. Contrary to our expectations, IL-7 was initially required whereas IL-15 was not. Because multiple methodologies for the activation of CD8+ T cells, including IL-12 conditioning or strong TCR stimulation, demonstrated IL-7 and IL-7Rα dependence, our results are likely generalizable to a variety of T cell activation methodologies.
Our results indicate that transferred effector T cells should be IL-7 responsive for maximal engraftment in a lymphodepleted host without exogenously provided cytokine. In our murine models CD8+ T cells required IL-7Rα for maximal engraftment after adoptive transfer; however, in a clinical setting, expression of IL-7Rα on donor T cells was 1 of 45 markers that failed to differentiate persisting T cell clones from those that failed to engraft (48). In this prior study, T cells were not conditioned with IL-12. Our results with human T cells suggest that re-expression of IL-7Rα after cessation of TCR stimulation and extended culture corresponds most directly with IL-7 responsiveness (Fig. 6). We therefore predict that assessing IL-7Rα levels after extended reculture may have more clinical utility than determining IL-7Rα levels at the predetermined point of infusion.
An intriguing result from this work is that IL-15 does not initially play a role in the support of effector Tc1 cells. This data is in contrast to prior studies with memory phenotype CD8+ T cells transferred into lymphopenic hosts (9–11). Because IL-15 is known to be elevated in the lymphodepleted host (20), these differences are potentially explained by distinct trafficking of activated versus resting T cells.
That in vitro IL-12 priming increases IL-7Rα expression appears to be discordant with the well-described phenomenon that enhanced IL-12/inflammation during effector responses in vivo leads to more terminally differentiated CD8+ T cells with decreased IL-7Rα expression (27,29,49). A potential explanation is that the programming for terminal differentiation has not yet occurred after 3 days of activation in the presence of IL-12, a theory supported by the increased IL-7Rα and CD62L expression observed with IL-12 priming on day 3 (24). The kinetics of IL-7Rα re-expression we observed further support this idea, as IL-7Rα transcription appears to be initiated on day 2 of culture. Given that the expression of IL-7Rα is modulated by the transcription factors Gfi-1 and GABPα, the relationship between IL-12 and these transcription factors warrants further investigation (50).
In summary, our results suggest a model in which effector CD8+ T cells are dependent on host IL-7 for maximal persistence and antitumor efficacy in a lymphodepleted host. This represents a shift in the current paradigm that considers IL-15 as the critical cytokine capable of modulating effector CD8+ T cell durability and efficacy in this increasingly relevant clinical setting. In practical terms, our results demonstrate that a direct and feasible way to produce IL-7Rα expressing, IL-7 responsive effector T cells is ex vivo IL-12 conditioning.
Supplementary Material
Acknowledgments
Funding: Grant funding for this project was provided by the following grants and fellowships from the National Institutes of Health and the National Cancer Institute: P01CA54778-01, R01CA133503, and 5F30CA177208. This work was also supported in part by the Cell Evaluation & Therapy Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30CA138313).
We thank Dan Neitzke and Wern Su for critical evaluation of this manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors do not have any conflicts of interest to disclose.
- Conception and design: CBJ, DJC, MPR
- Development of methodology: CBJ, SM, DJC, MPR
- Acquisition of data: CBJ, BPR, BRM, SCG
- Analysis and interpretation of data: CBJ, BPR, BRM, SCG, GL, KSOC, EGM, SM, DJC, MPR
- Writing, review and/or revision of the manuscript: CBJ, BPR, BRM, SCG, EGM, SM, DJC, MPR
- Administrative, technical, or material support: CBJ, BPR, BRM, SCG, GL, KSOC, SM, DJC, MPR
- Study supervision: DJC, MPR
References
- 1.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annual review of immunology. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 2.Overwijk WW, Schluns KS. Functions of gammaC cytokines in immune homeostasis: current and potential clinical applications. Clinical immunology (Orlando, Fla. 2009;132(2):153–165. doi: 10.1016/j.clim.2009.03.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nature reviews Immunology. 2009;9(12):823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 5.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 6.Vera JF, Hoyos V, Savoldo B, Quintarelli C, Giordano Attianese GM, Leen AM, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(5):880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 8.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195(12):1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195(12):1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195(12):1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(28):11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. Journal of immunology. 2008;180(5):2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 14.Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112(9):3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22(3):333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Reviews Immunology. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 17.Rubinstein MP, Cloud CA, Garrett TE, Moore CJ, Schwartz KM, Johnson CB, et al. Ex vivo interleukin-12-priming during CD8(+) T cell activation dramatically improves adoptive T cell transfer antitumor efficacy in a lymphodepleted host. Journal of the American College of Surgeons. 2012;214(4):700–707. doi: 10.1016/j.jamcollsurg.2011.12.034. discussion 07–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobrzanski MJ, Reome JB, Dutton RW. Type 1 and type 2 CD8+ effector T cell subpopulations promote long-term tumor immunity and protection to progressively growing tumor. Journal of immunology. 2000;164(2):916–925. doi: 10.4049/jimmunol.164.2.916. [DOI] [PubMed] [Google Scholar]
- 19.Gerner MY, Heltemes-Harris LM, Fife BT, Mescher MF. Cutting edge: IL-12 and type I IFN differentially program CD8 T cells for programmed death 1 re-expression levels and tumor control. Journal of immunology. 2013;191(3):1011–1015. doi: 10.4049/jimmunol.1300652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergamaschi C, Bear J, Rosati M, Beach RK, Alicea C, Sowder R, et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum. Blood. 2012;120(1):e1–e8. doi: 10.1182/blood-2011-10-384362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(2 Pt 1):644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 22.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer MJ, Mahajan VS, Chen J, Irvine DJ, Lauffenburger DA. Signaling thresholds govern heterogeneity in IL-7-receptor-mediated responses of naive CD8(+) T cells. Immunology and cell biology. 2011;89(5):581–594. doi: 10.1038/icb.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, Eppolito C, Odunsi K, Shrikant PA. IL-12-programmed long-term CD8+ T cell responses require STAT4. Journal of immunology. 2006;177(11):7618–7625. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- 25.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32(1):67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammerbeck CD, Mescher MF. Antigen controls IL-7R alpha expression levels on CD8 T cells during full activation or tolerance induction. Journal of immunology. 2008;180(4):2107–2116. doi: 10.4049/jimmunol.180.4.2107. [DOI] [PubMed] [Google Scholar]
- 27.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: Role of direct IL-12 signals for cell fate decision of CD8(+) T cells. European journal of immunology. 2009;39(7):1774–1783. doi: 10.1002/eji.200839093. [DOI] [PubMed] [Google Scholar]
- 29.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. Journal of immunology. 2007;179(4):2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 30.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. The Journal of clinical investigation. 2011;121(12):4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehrotra S, Al-Khami AA, Klarquist J, Husain S, Naga O, Eby JM, et al. A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self immunity in mice. Journal of immunology. 2012;189(4):1627–1638. doi: 10.4049/jimmunol.1103271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roszkowski JJ, Lyons GE, Kast WM, Yee C, Van Besien K, Nishimura MI. Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res. 2005;65(4):1570–1576. doi: 10.1158/0008-5472.CAN-04-2076. [DOI] [PubMed] [Google Scholar]
- 34.Norell H, Zhang Y, McCracken J, Martins da Palma T, Lesher A, Liu Y, et al. CD34-based enrichment of genetically engineered human T cells for clinical use results in dramatically enhanced tumor targeting. Cancer immunology, immunotherapy : CII. 2010;59(6):851–862. doi: 10.1007/s00262-009-0810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubinstein MP, Cloud CA, Garrett TE, Moore CJ, Schwartz KM, Johnson CB, et al. Ex vivo interleukin-12-priming during CD8(+) T cell activation dramatically improves adoptive T cell transfer antitumor efficacy in a lymphodepleted host. J Am Coll Surg. 2012;214(4):700–707. doi: 10.1016/j.jamcollsurg.2011.12.034. discussion 07–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209(13):2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehrotra S, Al-Khami AA, Klarquist J, Husain S, Naga O, Eby JM, et al. A Coreceptor-Independent Transgenic Human TCR Mediates Anti-Tumor and Anti-Self Immunity in Mice. J Immunol. 2012 doi: 10.4049/jimmunol.1103271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro I, Yu A, Dee MJ, Malek TR. The basis of distinctive IL-2- and IL-15-dependent signaling: weak CD122-dependent signaling favors CD8+ T central-memory cell survival but not T effector-memory cell development. Journal of immunology. 2011;187(10):5170–5182. doi: 10.4049/jimmunol.1003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho JH, Kim HO, Kim KS, Yang DH, Surh CD, Sprent J. Unique features of naive CD8+ T cell activation by IL-2. Journal of immunology. 2013;191(11):5559–5573. doi: 10.4049/jimmunol.1302293. [DOI] [PubMed] [Google Scholar]
- 40.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38(1):13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer MJ, Mahajan VS, Trajman LC, Irvine DJ, Lauffenburger DA, Chen J. Interleukin-7 receptor signaling network: an integrated systems perspective. Cellular & molecular immunology. 2008;5(2):79–89. doi: 10.1038/cmi.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lozza L, Rivino L, Guarda G, Jarrossay D, Rinaldi A, Bertoni F, et al. The strength of T cell stimulation determines IL-7 responsiveness, secondary expansion, and lineage commitment of primed human CD4+IL-7Rhi T cells. European journal of immunology. 2008;38(1):30–39. doi: 10.1002/eji.200737852. [DOI] [PubMed] [Google Scholar]
- 43.van Lier RA, Brouwer M, Rebel VI, van Noesel CJ, Aarden LA. Immobilized anti-CD3 monoclonal antibodies induce accessory cell-independent lymphokine production, proliferation and helper activity in human T lymphocytes. Immunology. 1989;68(1):45–50. [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss A, Manger B, Imboden J. Synergy between the T3/antigen receptor complex and Tp44 in the activation of human T cells. Journal of immunology. 1986;137(3):819–825. [PubMed] [Google Scholar]
- 45.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. Journal of immunotherapy. 2008;31(8):742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. Journal of immunology. 2005;175(10):7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature medicine. 2005;11(7):748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 50.Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. Journal of immunology. 2008;180(8):5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.