Abstract
One of the most consistent findings in children with ADHD is increased moment-to-moment variability in reaction time (RT). The source of increased RT variability can be examined using ex-Gaussian analyses that divide variability into normal and exponential components and Fast Fourier transform (FFT) that allow for detailed examination of the frequency of responses in the exponential distribution. Prior studies of ADHD using these methods have produced variable results, potentially related to differences in task demand. The present study sought to examine the profile of RT variability in ADHD using two Go/No-go tasks with differing levels of cognitive demand. A total of 140 children (57 with ADHD and 83 typically developing controls), ages 8–13 years, completed both a “simple” Go/No-go task and a more “complex” Go/No-go task with increased working memory load. Repeated measures ANOVA of ex-Gaussian functions revealed for both tasks children with ADHD demonstrated increased variability in both the normal/Gaussian (significantly elevated sigma) and the exponential (significantly elevated tau) components. In contrast, FFT analysis of the exponential component revealed a significant task × diagnosis interaction, such that infrequent slow responses in ADHD differed depending on task demand (i.e., for the simple task, increased power in the 0.027–0.074 Hz frequency band; for the complex task, decreased power in the 0.074–0.202 Hz band). The ex-Gaussian findings revealing increased variability in both the normal (sigma) and exponential (tau) components for the ADHD group, suggest that both impaired response preparation and infrequent “lapses in attention” contribute to increased variability in ADHD. FFT analyses reveal that the periodicity of intermittent lapses of attention in ADHD varies with task demand. The findings provide further support for intra-individual variability as a candidate intermediate endophenotype of ADHD.
Keywords: Ex-Gaussian, Fast Fourier transform, Response preparation, Response inhibition, Response selection, Supplementary motor area, “pre-SMA”
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most commonly diagnosed childhood disorders. The current diagnostic criteria for ADHD emphasize observable behaviors from two core domains, symptoms of hyperactivity/impulsivity and symptoms of inattention, most notably decreased ability to sustain attention when required to complete non-preferred tasks (APA, 2000). Although the etiology of ADHD is not known, recent research clearly identifies ADHD as a neurobiological disorder (Buitelaar, Montgomery, & van Zwieten-Boot, 2003; Denckla, 2003; Durston, 2003; Tannock, 1998). Because ADHD is clinically heterogeneous, it is unlikely to have a single neurobiological etiology. Despite the possibility of multiple etiologies, the cardinal symptoms of hyperactivity, impulsivity, and cognitive dysfunction may emanate from closely related disturbances in cerebral function, which once understood, could serve as biomarkers that help to guide diagnosis and treatment and which can be used as “intermediate” endophenotypes in studies of genetic and environmental etiologies (Gottesman & Gould, 2003; Rommelse et al., 2007).
One particularly fruitful line of research has been the characterization of performance of children with ADHD on tasks assessing components of controlled responding. Response control tasks utilize a number of formats including Go/No-go (i.e., responding to one or more proscribed stimuli while withholding response to another), stop signal tasks (i.e., responding in an ongoing manner until cued by a separate signal not to do so), choice reaction time (i.e., responding differentially based on external stimuli, e.g., flanker task), simple reaction time (i.e., responding quickly to an external stimulus) and self-generated responding (e.g., tapping continuously). These paradigms can be manipulated by changing sensory modality or by altering the cognitive complexity of the tasks, for example, by increasing demands of working memory or changing the complexity of the stimuli themselves.
For many years there was emphasis on measures of inhibitory failure in ADHD, stemming in part from clinical observations suggesting that impaired inhibitory control contributes to excessive impulsivity, hyperactivity, and distractibility (Barkley, 1997). In practice, however, the evidence supporting deficits in inhibitory control as an endophenotype of ADHD have been mixed; some studies find that children with ADHD show high rates of inhibitory failures (commission errors) compared to typically developing (TD) children (e.g., Johnson et al., 2007b; Wodka et al., 2007) while others find no differences in errors between groups (Schulz et al., 2004). In addition, children with other developmental disabilities have been shown to demonstrate deficits in response inhibition on some tasks, indicating that this may not be a characteristic specific to children with ADHD (e.g., Johnson et al., 2007a). Given the inconsistency of the inhibitory findings, other indices have been considered as potential intermediate behavioral endophenotypes.
There has been accumulating evidence in recent years that other aspects of response control are affected in ADHD. In particular, several studies find that children with ADHD show increased intra-subject variability (ISV) in their response time when compared to TD children (Castellanos et al., 2005; Johnson et al., 2007b; Klein, Wendling, Huettner, Ruder, & Peper, 2006; Williams, Strauss, Hultsch, Hunter, & Tannock, 2007; Wodka et al., 2007; Suskauer, Simmonds, Fotedar et al., 2008).
Several pieces of evidence suggest that increased ISV may be a good candidate as an intermediate endophenotype of ADHD (Castellanos & Tannock, 2002, Castellanos, Sonuga-Barke, Milham, & Tannock, 2006). First, increased variability in responding has been demonstrated to correlate with impulsive responding and self-report of inattention to tasks (Rommelse et al., 2007; Simmonds et al., 2007; Strandburg et al., 1996), suggesting that variability in responding is a contributing factor to expression of diagnostic characteristics of ADHD. Further, several studies have demonstrated that close family members of individuals with ADHD demonstrate increased variability in responding, including, siblings sharing an ADHD diagnosis, discordant dizygotic twins, and siblings who do not meet criteria for diagnosis of ADHD (Bidwell, Willcutt, DeFries, & Pennington, 2007; Rommelse et al., 2007). This pattern of results suggests a genetic mechanism for expression of the phenotype. Analyses characterizing ISV in ADHD has revealed a pattern of occasional responses with unusually long reaction time, with the majority of responses being comparable to comparison groups (Castellanos et al., 2005; Hervey et al., 2006; Leth-Steensen, King Elbaz, & Douglas, 2000).
Statistical analyses utilizing comparison of group means and variability (i.e., standard deviation), may mask such responses by treating them as outliers or by treating them as “noise” that becomes averaged with other responses (Hervey et al., 2006). This likely explains why reaction time differences have not been a consistent finding in all studies as their group mean is differentially affected by outliers. As such, researchers have more recently moved toward utilizing statistical methodologies allowing measurement and comparison of intra-individual variability in addition to inter-individual variability in order to more fully evaluate the significance of variability in responding as it relates to ADHD.
Use of the ex-Gaussian distributional model provides a more appropriate framework in which to evaluate ISV. This model posits that the distribution of reaction times can be represented as the sum of a normal (Gaussian) distribution of response times and an independent exponentially distributed variable (Leth-Steensen et al., 2000). The ex-Gaussian distribution is composed of three primary components, mu, a measure of central tendency often closely related to the mean of the normal distribution, sigma a measure of the variation of the normal distribution, and tau, a measure of the mean of the exponential component of the distribution (Hervey et al., 2006; Leth-Steensen et al., 2000). In analysis of response times, the values of mu and sigma represent the distribution of faster responses while the value of tau provides a measure of increased intra-individual variability in the form of infrequent but long response times.
Two studies have applied ex-Gaussian analyses to children with ADHD (Hervey et al., 2006; Leth-Steensen et al., 2000). In the first, subjects performed a simple choice response task with minimal demands on response control; children with ADHD showed increased variability in the exponential component (increased tau), but not the normal portion of the distribution. The authors posited that this may have been the result of occasional lapses in attention leading to unusually long response times for some trials (Leth-Steensen et al., 2000). In a more recent study using a Go/No-go (“continuous performance”) task designed to assess response (including inhibitory) control, investigators found ADHD was associated with abnormalities in both the normal (increased sigma) and exponential (increased tau) components of reaction time distribution (Hervey et al., 2006). The discrepancy in findings between these two studies may be related to differential task demands, with the increased need for response control in the Go/No-go task unmasking ADHD-associated increases in variable responding throughout the task.
Based on the evaluation of the ex-Gaussian distribution, it is not clear if the increased ISV occurs randomly or whether it is more predictable and periodic in nature. Periodicity in neural firing has been observed in organized, distributed and independent brain networks. Analysis of patterns of periodicity in behavioral responding may implicate inefficient or impaired functioning within specific brain networks. Fast Fourier transform (FFT) is a method utilized to identify such periodicity in responding. This method utilizes logarithmic transformation to measure the power of periodic responding at various temporal frequency bands. In this way, increased variability can also be identified for particular temporal frequency bands, potentially identifying sources of increased variability in ADHD (Johnson et al., 2007b).
In prior FFT studies of ADHD (Castellanos et al., 2005; Di Martino et al., 2008; Johnson et al., 2007b) variability has been evaluated using choice response tasks with relatively minimal demands for motor response control. These studies found increases in spectrum in specific frequency bands (e.g., 0.027–0.074 Hz) for children with ADHD compared to controls. In a separate study, Johnson et al. (2007a) used FFT to compare performance of ADHD and TD children on two versions of a Sustained Attention to Response Task (SART). Both tasks utilized a “Go/No-go” format using 9 digits, with children responding to all of the digits but one with a button push. In one version of the task the order of the stimuli was entirely predictable (repeating throughout the task), while in the other they were randomized (consistent with the format typically used in Go/No-go tasks). For both tasks, children with ADHD demonstrated both increased fast (i.e., moment-to-moment) and slower (i.e., over the course of the task) variability. The authors attributed this pattern of performance to deficits in “top-down” phasic response control (i.e., fronto-parietal circuits) and more tonic (i.e., basal ganglia circuits and cerebral hemodynamic mechanisms), respectively.
In the current study we were interested in examining response variability in ADHD using two different Go/No-go tasks, both of which had relatively high demands on motor response (inhibitory) control, but which differed in working memory demand. One task used a “simple” Go/No-go format in which working demand was minimized using a well-ingrained stimulus-response association (green = go; red = no-go). The second “complex” Go/No-go task involved increased working memory demand necessary to guide inhibitory control (i.e., responding to all green objects, but requiring subject to count sequential stimuli in order to determine whether or not to respond to red objects).
Functional imaging findings using these two tasks indicate that the neural substrates of response control are, in some respects, dependent on task demand (Mostofsky et al., 2003). For both the simple and the complex Go/No-go tasks, no-go-associated activation was observed in the rostral supplementary motor area (“pre-SMA”), a region important for response selection and control (Isoda & Hikosaka, 2007). Dorsolateral prefrontal cortex activation was only observed in the complex Go/No-go task in which working memory was necessary to guide inhibitory control. This pattern of findings was confirmed in a recent meta-analysis of studies using simple and complex GNG tasks (Simmonds, Pekar, & Mostofsky, 2008).
Examination of children with ADHD using these simple and complex Go/No-go tasks revealed that, compared with typically developing children (TD) children, impaired inhibitory control (i.e., higher commission error rate) on the simple Go/No-go task was as robust as that seen in the more complex task with higher cognitive demand (Wodka et al., 2007). In other words, children with ADHD did not demonstrate increased impairment in inhibitory function when the task was made more cognitively demanding by increasing working memory load. Impairment in inhibitory control, then, appears to be a stable cognitive feature associated with ADHD that is independent of functioning in other cognitive/executive domains. Consistent with this, fMRI analysis using the simple GNG task reveals that, compared to TD children, children with ADHD show decreased pre-SMA activation (Suskauer, Simmonds, Fotedar et al., 2008) as well as anomalous association between pre-SMA activation and ISV (Suskauer, Simmonds, Caffo et al., 2008).
Given the increasing recognition of the importance of ISV as a potential biomarker of ADHD, in the present study, ex-Gaussian and FFT methods were used to examine the effect of task demand on ISV in an expanded sample to children with ADHD and TD children who performed both the simple and complex Go/No-go tasks. Consistent with the commission error rate findings reported in Wodka et al. (2007), it was hypothesized that analyses of ISV using standard, ex-Gaussian and FFT approaches would show that children with ADHD would show similar patterns of increased variability irrespective of task demands. Specifically, first it was hypothesized that children with ADHD would demonstrate similar increases in ISV on both tasks compared to controls. Second, consistent with the one previous ex-Gaussian evaluation of Go/No-go performance, children with ADHD would show increases in both the normal and exponential components of variability and that these elevations would be similar across both the simple and complex Go/No-go tasks. Finally, FFT analysis of the frequency spectra of Go/No-go RT would reveal greater relative spectrum in the 0.02–0.07 Hz band (14–40 s) as seen in prior studies using choice response task (Castellanos et al., 2005; Di Martino et al., 2008), as well as in faster frequencies reflecting more global variability in responding; furthermore, these differences would be similar across both the simple and complex Go/No-go tasks.
2. Method
2.1. Participants
A total of 140 children, ages 8–13 years, were included in the present study [ADHD n = 57 (inattentive subtype n = 20, combined subtype n = 37); control n = 83]. Participants were recruited from a variety of sources including outpatient clinics at Kennedy Krieger Institute, from local chapters of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder (CHADD), from local schools, pediatrician offices and services organizations (e.g., boy/girl scouts), and through fliers posted in the community.
Children were initially screened about inclusion criteria through a brief telephone interview with a parent. Children with a history of mental retardation, seizures, traumatic brain injury or other neurological illnesses were excluded from participation. Intellectual ability was then assessed using the Wechsler Intelligence Scale for Children-III (WISC-III; Wechsler, 1991) or WISC-IV (Wechsler, 2003). Children with FSIQ scores below 80 were excluded from participation.
Diagnostic status (ADHD) was established through administration of the Diagnostic Interview for Children and Adolescents-IV (DICA-IV; Reich, Welner, & Herjanic, 1997). Children meeting criteria for diagnosis of conduct, mood, generalized anxiety, separation anxiety or obsessive-compulsive disorders on DICA-IV interview were excluded. Children with oppositional defiant disorder were not excluded from participation. Parents and teachers also completed Conners' Parent and Teacher Rating Scales-Revised (CPRS-R, CTRS-R; Conners, 1997) and the ADHD Rating Scale-IV, home and school versions (ARS; DuPaul, Power, Anastopolous, & Reid, 1998). Inclusion in the ADHD group was made based on the following criteria: (1) diagnosis of ADHD and referral for participation by community clinicians; (2) DSM-IV-TR diagnosis of ADHD based on positive scores on at least one of the parent and one of the teacher rating scales (i.e., T-score of 65 or higher on scale L (DSM-IV: inattentive) or M (DSM-IV: hyperactive-impulsive) on the CPRS-R or CTRS-R or children receiving scores of 2 or 3 on at least 6/9 items on the Inattentive or Hyperactivity/Impulsivity scales of the ARS); and (3) confirmation of ADHD diagnosis by DICA-IV psychiatric interview. Diagnosis was then confirmed by a child neurologist (S.H.M.) prior to participation. Children taking psychotropic medications other than stimulant medication were excluded from participation and all children taking stimulant medication were asked to withhold medication on the day prior and day of testing. All children were also administered the Basic Reading subtest from Wechsler Individual Achievement Test (WIAT: Wechsler, 1992) or the Word Reading subtest from the WIAT-II (Wechsler, 2002) in order to rule out a learning disability in reading. Children were excluded from participation if they demonstrated a statistically significant discrepancy between FSIQ and WIAT/WIAT-II score or a Basic/Word Reading subtest score below 85.
In order to meet inclusion criteria for the control group, parent and teacher reports on CPRS-R/CPRS-T and ARS had to be below clinical cutoff scores and they could not meet diagnostic criteria for any psychiatric disorder based on DICA-IV. Like the children in the ADHD group, they could not have history of neurological disorder, be taking psychotropic medication or meet criteria for diagnosis of learning disability based on WIAT/WIAT-II scores word reading scores significantly discrepant from IQ, and had to have FSIQ scores above 80.
2.2. Measures
Participants completed the two Go/No-go tasks over a 2-day period as part of a larger battery of neuropsychological tests. Parents completed rating scales and the structured diagnostic interview at the time of the child's testing.
2.3. Simple Go/No-go task
Children were seated in front of a computer screen and instructed to push a button with their right index finger as quickly as possible when shown a green (i.e., “go”) spaceship, and to refrain from pushing the button when shown a red (i.e. “no-go”) spaceship. Cues were presented at midline once every 2300ms and remained on the screen for 300 ms. Cues were weighted toward green spaceships at a ratio of 3:1 (173 go cues; 44 no-go cues), creating a bias toward habitual “go” responses. The task duration was 8 min, 19 s.
2.4. Complex Go/No-go task
The complex Go/No-go task is described in detail in Mostofsky et al. (2003). The same green and red spaceship stimuli, presented one at a time and at midline, were used in this task as the simple task. Children were instructed to push the button as quickly as possible in response to a green spaceship and in response to a red spaceship preceded by an even number of green spaceships. They were to refrain from responding to red spaceships preceded by an odd number of green spaceships. Cues were presented once every 2300ms and remained on the screen for 300 ms. Test stimuli were weighted 3:1 green spaceships to red spaceships with 163 green spaceships, 22 red “go” stimuli (i.e., preceded by an even number of green spaceships) and 22 red “no-go” spaceships (i.e., preceded by an odd number of green spaceships). The total time of this task was 7 min, 56 s.
2.5. Data preparation and analysis
For all analyses, the last 10 trials of the simple Go/No-go task were discarded in order to match the duration and number of trials in the complex Go/No-go task. Additionally, all Go trials with reaction times less than 200ms were discarded as “anticipatory” errors.
For the standard analyses, primary variables of interest were mean reaction time, inhibitory performance (measured as percent commission errors) and ISV, measured as the coefficient of variability (CV) (standard deviation of RT/mean RT) (Stuss, Murphy, Binns, & Alexander, 2003). Ex-Gaussian analyses were performed using the RTSYS software (Heathcote, 1996), in which a maximum likelihood algorithm is employed to fit normal and exponential components to the reaction time series; these analyses only included correct responses on green Go trials.
As certain aspects of reaction time series can confound frequency analyses, the reaction time data underwent several preprocessing steps before the frequency analyses were performed. First, reaction times were log converted in order to reduce the effects of outlier reaction times. Second, due to the well-described phenomenon of post-error slowing (Garavan, Ross, Murphy, Roche, & Stein, 2002), trial-specific effects (e.g., the first Go trial after a commission error, in which responses are slower) were regressed by subtraction from the mean of all trials in the reaction time series. Third, using established procedures for FFT analyses (Castellanos et al., 2005; Di Martino et al., 2008), missing trials (red trials, omission and anticipatory errors) were interpolated by averaging the previous and following correct green Go trials, in order to obtain evenly spaced data to be used in the FFT. Finally, reaction times were “SD-normalized” (divided by the SD of the series) to obtain frequency measurements independent of inter-individual differences in reaction time variability (Di Martino et al., 2008).
After these preprocessing steps were undertaken, we carried out the frequency analyses. Spectral density of a time series of equally spaced observations is estimated using a discrete Fourier transform (Muller, Reinhard, Oehm, Hetzel, & Timmer, 2003). Given n time points (in this case, reaction times, with a stimulus onset asynchrony (SOA) of Δt, the discrete Fourier transform is defined between 2/(nΔt) (Nyquist frequency) and 1/(2Δt), in bins that are positive integer multiples of 1/(nΔt). FFT is an efficient algorithm for calculating the discrete Fourier transform and, thus, for estimating the spectral density. Since we analyzed n = 207 stimuli at Δt = 2.3-s intervals we considered that the frequency interval we could reliably study was between of 0.217 Hz (equal to 1/(2Δt) = 1/4.6 s) for the upper bound and 0.004 Hz (2/(nΔt) = 2/476.1 s) for the lower bound. Further, we discarded the three lowest frequency bins of 0.002 Hz each (1/(nΔt) = 1/476.1 s) because of their low reliability (Muller et al., 2003). Thus, we examined the range from 0.01 to 0.217 Hz. We chose to divide these frequencies into intervals similar to those used by Di Martino et al. (2008), which were based on observations that the central frequencies of neurophysiological oscillation bands follow a linear progression in the natural logarithmic scale (Penttonen & Buzsaki, 2003). We examined three frequency ranges centered around e (2.72) raised to the −2.6, −3.6 and −4.6, with each range bounded by the center plus or minus 0.5; this translated to linear ranges of 0.074–0.202 Hz (~5–15 s, or 2–5 trials), 0.027–0.074 Hz (~15–40 s, or 5–15 trials) and 0.01–0.027 Hz (~40–100 s, or 15–45 trials). Slower frequencies were not considered due low reliability of those frequencies (Muller et al., 2003).
2.6. Statistical analyses
Group means for control and ADHD groups for each of the Go/No-go tasks were analyzed using 2 × 2 mixed model analyses of variance, with diagnostic status as between subjects factor and task as within subjects factor. Additionally, for the FFT analyses, data were analyzed using a 2 × 2 × 3 mixed model analysis of variance, with diagnostic status as between subjects factor and task and frequency bands as within subjects factors.
3. Results
3.1. Demographic information
Demographic information for the sample is provided in Table 1. Participants were primarily Caucasian (75% Caucasian, 14% African American, 2% Asian, 0.7% Native American) with slightly more boys than girls (53% boys, 47% girls). Children ranged in age from 8.1 to 13.9 years (M = 10.9, SD = 1.5). Groups did not differ significantly in gender distribution (χ2 = 1.78, p = 0.18), handedness (χ2 = 0.178, p = 0.67), racial distribution (χ2 = 2.54, p = 0.47) or age (F = 0.01, p = 0.91).
Table 1.
Demographic characteristics of participants.
| Control (n = 83) |
ADHD (n = 57) |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 11 | 1.48 | 10.9 | 1.55 |
| FSIQa | 116.2b | 11.81b | 111.3b | 11.92b |
| VCIc | 119.6 | 15.04 | 115.4 | 14.14 |
| PRIc | 112.7 | 12.71 | 108.7 | 11.95 |
| Percentage | Percentage | |
|---|---|---|
| Gender | ||
| Male | 48 | 60 |
| Female | 52 | 40 |
| Handedness | ||
| Right | 87 | 84 |
| Left | 12 | 7 |
| Mixed | 1 | 9 |
| Ethnicityd | ||
| Caucasian | 84 | 77 |
| African American | 13 | 19 |
| Asian | 1 | 4 |
| Native American | 1 | 0 |
Either WISC-III or WISC-IV.
Statistically significant (p < 0.05).
WISC-IV only.
Ethnicity was unknown for 11 cases.
There was a statistically significant group difference in FSIQ, with ADHD children having lower FSIQ (M = 111.3, SD = 11.92) than children in the control group (M = 116.2, SD = 11.8) (F[1,138] = 5.65, p = 0.02). FSIQ scores include measures of working memory and processing speed. These domains are typically impaired in individuals with ADHD and it is believed that this selectively lowers FSIQ scores in children with ADHD (Mayes & Calhoun, 2006; Wilcutt, Doyle, Nigg, Faraone, & Pennington, 2005). For most children in this study (n = 125) FSIQ was assessed using the WISC-IV, which provides for Verbal Comprehension Index (VCI) and Perceptual Reasoning Index (PRI) scores that are distinct from indices of Working Memory and Processing Speed. WISC-IV VCI and PRI scores were therefore examined as more valid measures of intellectual reasoning in children with ADHD with findings revealing no statistically significant differences between groups for either PRI or VCI score (PRI: F = 3.33, p = 0.07; VCI: F = 2.55, p = 0.11). Nevertheless, all analyses were conducted both including and not including IQ as a covariate, with the pattern of results being the same for all analyses. The findings reported below are those generated without using FSIQ as a covariate.
3.2. Comparison of speed and accuracy
There were no main effects for reaction time (i.e., mean reaction time) for either diagnostic category or task (F[1,138] = 1.30, p = 0.26 and F[1,138] = 1.44, p = 0.23, respectively). There was a significant two-way interaction between task and diagnosis (F[1,138] = 3.87, p = 0.05), with children in the ADHD group performing significantly slower on the complex task (M = 422.73, SD = 112.56) compared to the simple task (M = 401.55, SD = 85.02) (t =−2.092, p < 0.001), while the children in the control group were slightly, but not significantly, faster on the complex task (M = 390.50, SD = 106.68) compared to the simple task (M = 395.62, SD = 109.47) (t = 0.594).
Analysis of percent commission errors, indicated a significant main effect for task (F[1,138] = 22.13, p < 0.001) with children across both groups showing more commission errors on the complex task than on the simple task (simple M percentage = 0.33, SD = 0.20; complex M = 0.41, SD = 0.19). There was also a significant main effect for diagnosis with children from the ADHD group making significantly more errors of commission across both tasks compared to TD children (F[1,138] = 17.04, p < 0.001). There was no significant task × diagnosis interaction (F[1,138] = 0.93, p = 0.34) (Table 2).
Table 2.
Normal and ex-Gaussian measures of performance.
| Control (n = 83) |
ADHD (n = 57) |
Significance |
|||||
|---|---|---|---|---|---|---|---|
| Simple task mean (SD) | Complex task mean (SD) | Simple task mean (SD) | Complex task mean (SD) | Group F | Task F | Group × task F | |
| Commission errors (%) | 0.29 (0.19) | 0.36 (0.18) | 0.38 (0.20) | 0.48 (0.17) | 17.04* | 22.13* | 0.93 |
| Reaction time | 395.62 (109.47) | 390.50 (106.68) | 401.55 (85.02) | 422.73 (112.56) | 1.30 | 1.44 | 3.87* |
| CV | 0.28 (0.12) | 0.34 (0.15) | 0.35 (0.13) | 0.39 (0.12) | 9.92* | 13.49* | 0.18 |
| Mu | 301.68 (79.40) | 281.34 (79.96) | 286.37 (61.83) | 290.44 (89.95) | 0.07 | 1.65 | 3.71* |
| Sigma | 52.40 (35.86) | 60.47 (45.47) | 62.72 (39.43) | 75.61 (50.93) | 4.45* | 6.12* | 0.32 |
| Tau | 94.48 (54.55) | 110.10 (59.73) | 116.24 (57.32) | 134.11 (64.01) | 6.98* | 10.45* | 0.05 |
p < 0.05.
3.3. Variability
Analysis of CV indicated a significant main effect for diagnostic status (F[1,138] = 9.92, p = 0.002) with children in the ADHD group demonstrating higher CV (M = 0.37, SD = 0.11) compared to the control group (M = 0.31, SD = 0.11) across both tasks. There was also a significant main effect for task (F[1,138] = 13.49, p < 0.001), with children across both groups showing higher CV on the complex Go/No-go task (M = 0.362, SD = 0.142) compared to the simple task (M = 0.31, SD = 0.13). Interaction between diagnostic status and task was not significant (F[1,138] = 0.18, p = 0.68).
There was no significant effect of ADHD subtype on CV for either Go/No-go task. Performance was equivalent between groups for both the simple (t[1,55] = 30.389, p = 0.699) and for the complex task (t[1,55] =−1.313, p = 0.125).
4. Gaussian and ex-Gaussian distribution of RT
Analysis of mu, a measure of the mean of the normal component of the normal portion of the distribution, yielded no significant main effect for diagnosis (F[1,138] = 0.07, p = 0.80) or task (F[1,138] = 1.65, p = 0.20). There was a nearly significant interaction between diagnosis and task (F[1,138] = 3.71, p = 0.056), with controls demonstrating decreased values for mu for the complex task, and increased values for mu for the simple task, while children in the ADHD showed slightly increased mu for the complex task compared to the simple task. This mirrors the results for mean reaction time, as would be expected.
There were no significant effect of ADHD subtype on mu in the simple task (t[1,55] = 0.40, p = 0.69). For the complex task, ADHD inattentive subtype children had significantly higher values for mu compared to ADHD combined subtype children (t[1,55] = 2.440, p = 0.02).
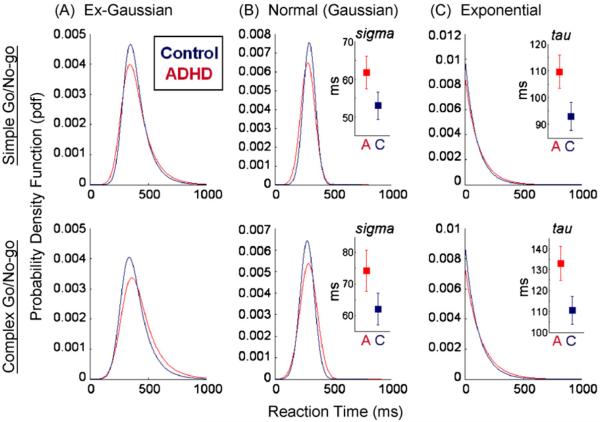
Analysis of sigma, a measure of the standard deviation of the normal distribution, revealed a similar pattern of results to those previously described for CV; there was a significant main effect for diagnosis (F[1,138] = 4.45, p = 0.04) with children in the ADHD group showing increased variability (M= 69.17, SD= 35.06) compared to controls (M= 56.44, SD = 35.07). There was also a significant main effect for task (F[1,138] = 6.11, p = 0.02) with increased variability for the complex task across groups (M= 66.64, SD = 48.17) compared to the simple task (M= 56.60, SD = 37.56). Task×diagnosis interaction was not significant (F[1,138] = 0.32, p = 0.57) and there were no significant effect of ADHD subtype on either the simple (t[1,55] = 0.49, p = 0.62) or the complex (t[1,55] = 1.19, p = 0.24).
Analysis of tau, a measure of the mean and standard deviation of the exponential component of the distribution, revealed a significant main effect for task across groups of children (F[1,138] = 10.45, p = 0.002), with increased variability in the exponential component of the distribution for the complex Go/No-go task (M= 119.88, SD = 62.42) compared to the simple task (M= 103.34, SD = 56.52). There was also a significant main effect for diagnosis (F[1,138] = 6.98, p = 0.009) with children in the ADHD group demonstrating significantly increased variability in the exponential component (M= 125.18, SD= 50.35) compared to children in the control group (M= 102.29, SD = 50.35). Task×diagnosis interaction was not significant (F[1,138] = 0.03, p = 0.855). There were no significant effect of ADHD subtype for tau on either the simple (t[1,55] =−0.35, p = 0.73) or complex task (t[1,55] = 0.63, p = 0.53) Fig. 1.
Fig. 1.
Illustration of the components of the ex-Gaussian distribution. Significant main effects for diagnostic group and task for sigma and tau. Significant task × diagnostic group for mu (p < 0.05).
4.1. Fast frequency spectral analysis
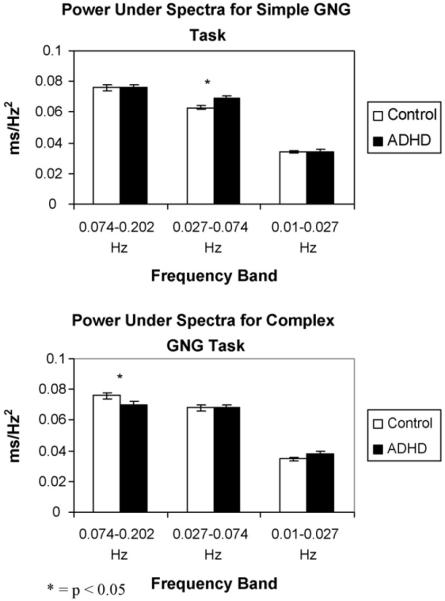
The area under the curve of the frequency spectrum obtained by FFT was calculated for each task and compared using a 2 (diagnosis)×2 (task)×3 (band) mixed-model ANOVA. The results indicated significant main effects for band (F[1,138] = 1890.24, p < 0.001). Children in both groups demonstrated increased power in the shortest wavelength bands, and reduced power across longer wavelength bands. There was also a significant two-way interaction between task and diagnosis (F[1,136] = 4.33, p = 0.04).
Follow-up one-way ANOVA indicated that for the simple Go/No-go task, there was a significant difference between the two groups in frequency band 0.027–0.074 Hz with children in the ADHD group showing significantly increased power compared to controls (F[1,138] = 6.45, p = 0.01). In contrast, one-way ANOVA of the complex task indicated a significant group difference with children in the control group showing significantly increased power compared to children in the ADHD group in the 0.074–0.202 Hz band (F[1,138] = 5.31, p = 0.02) (Fig. 2).
Fig. 2.
Illustration of the results of the FFT analyses. A significant main effect for band was seen, such that both groups of children showed increased power in the shorter wavelength bands (p < 0.001). There was also a significant task by diagnosis interaction (p = 0.04); follow-up analyses revealed that for the simple Go/No-go task, children with ADHD showed significantly increased power compared to controls in the 0.027–0.074 Hz band (p = 0.01), while in the complex task, children in the control group showed significantly increased power compared to children with ADHD in the 0.074–0.202 Hz band (p = 0.02).
5. Discussion
In this study we found that, compared to TD controls, children with ADHD showed increased variability on both the simple and complex Go/No-go tasks. The results further revealed that ADHD-associated increased variability on the simple Go/No-go task was as robust as that seen in the more complex task with higher working memory demand. In addition, evaluation of the ex-Gaussian distribution revealed that for both tasks, children with ADHD demonstrated a pattern of increased variability in both the fast (i.e., sigma) and slow (i.e., tau) portions of the distribution, despite changing working memory demands. The pattern of findings, which is consistent with that seen for commission rate (Wodka et al., 2007), suggest that increased variability in ADHD, like impaired inhibitory control, appears to be a stable feature that is independent of the effect of other cognitive/executive domains.
Our findings of increases in normal (i.e., sigma) as well as exponential (i.e., tau) variability are consistent with that seen in a prior study of ADHD in which ex-Gaussian analysis was applied to a different Go/No-go task (Hervey et al., 2006); in that study children with ADHD also showed increased variability in both the normal and exponential portions of the RT distribution. This is particularly notable given that the tasks used in both the current study and the one by Hervey and colleagues involved high demands on response control (i.e., inhibitory control). These findings are in contrast to those from a study in which ex-Gaussian analysis was applied to a simple choice response task with minimal inhibitory demands (Leth-Steensen et al., 2000); in that case, children with ADHD showed elevation in only the exponential (tau) portion of the RT distribution.
When looked at in totality, the ex-Gaussian findings generated from this and the two prior studies suggest that under conditions involving low degrees of response preparation and control (i.e., more automated responding), increased variability in ADHD is principally due to infrequent slow responses (so-called “lapses of attention”; Leth-Steensen et al., 2000). However, our findings and those generated by Hervey et al. (2006) indicate that under conditions requiring higher degrees of response control, increased variability in ADHD is present throughout the RT distribution, regardless of ADHD subtype, reflecting inefficiency in neural mechanisms critical to engaging a state of preparedness to respond.
Consistent with this, children with ADHD show abnormalities in premotor regions, in particular the supplementary motor area on anatomic (Mostofsky, Cooper, Kates, Denckla, & Kaufmann, 2002; Shaw et al., 2006) and functional (Suskauer, Simmonds, Fotedar et al., 2008) imaging studies. The SMA, in particular the rostral “pre-SMA”, has been shown to be critical to response selection (Isoda and Hikosaka, 2007) and recent fMRI findings reveal that activation in this region is predictive of lower ISV for TD children (Suskauer, Simmonds, Fotedar et al., 2008), but not children with ADHD (Suskauer, Simmonds, Caffo et al., 2008). It may be that abnormalities in pre-SMA circuits, important for response selection and preparation, contribute to ADHD-associated difficulties with response control, reflected as impaired response inhibition and increased variability in responding.
Examination of mean RT in ADHD have demonstrated mixed results, with some showing children with ADHD have significantly slower RT (e.g., Tamm, Menon, Ringel, & Reiss, 2004) while others show no differences depending on ADHD diagnostic subtype (e.g., Derefinko et al., 2008). Analysis of mean reaction time in the current study may help to explain these inconsistencies. Here we found a significant task×diagnosis effect on RT. Specifically, there was no between-group difference in RT on the simple Go/No-go task; however, children with ADHD showed significantly increased RT on the complex Go/No-go task. Given that children with ADHD show higher variability, the slower RT on the complex task may not reflect a generalized pattern of slowing but rather, occasional “outlier” slow responses.
FFT analysis provided for more detailed examination of these anomalous RT patterns demonstrated by children with ADHD. The results of this analysis indicated that children with ADHD demonstrate greater spectra in the 0.027–0.074 frequency band on the simple Go/No-go task. This is consistent with findings from two studies from the same group (Castellanos et al., 2005; Di Martino et al., 2008) using a flanker task. It appears, then, that children with ADHD show a predictable pattern of increased variability in responding occurring approximately every 15–40 s when completing tasks with increased response control (i.e., inhibitory) demands, but otherwise reduced cognitive demands. This does not appear to correlate directly to any demand of the task, and therefore likely represents inefficiency in meeting task demands.
Furthermore the TD group demonstrated increased power in the 0.074–0.202 Hz band when completing the complex Go/No-go task, corresponding to increases in variability in the 5–15 s (2–5 trials) range. It appears based on these findings that for the complex Go/No-go tasks TD children may be better able to alter their responding to meet task demands than are ADHD children. In other words, it appeared that the TD children were speeding or slowing their responding more frequently in order to maximize their efficiency and accuracy in the task. If this hypothesis were correct one might expect to see a pattern in which TD children would show more rapid responses to odd green trials and slow responses to even green trials. However, follow-up analyses failed to reveal a difference RT to odd green and even green stimuli, so that we were unable to directly demonstrate that the pattern of responding in the TD group was directly related to increasing efficiency in responding. In future, it would be useful to alter the presentation of the even stimuli to make responding even more predictable. For example, rather than occurring after any number of even numbers, the stimuli could be altered to require a response to a red target only after 4 green stimuli. The pattern of responding to this more predictable interval could then be compared across groups.
In previous studies utilizing FFT, the authors selected and compared specific frequency bands (Castellanos et al., 2005), or utilized simpler RT tasks producing greater spectra in the same frequency band (Di Martino et al., 2008). In the current study, we chose to compare the two groups across tasks with varying response control demands. In so doing we were able to demonstrate a differential pattern of variability in children with ADHD and TD controls in response to changes in task demands. Based on this analysis, it appears that FFT is a useful method by which ISV can be more closely examined. It is important to note, however, that the frequencies that can be evaluated using this method are limited by the intervals at which stimuli are presented. In this study, the shortest frequency band was 0.074–0.202 Hz, a duration of 5–15 s. As such, FFT is useful in evaluating variability in the slower portion of the RT distribution (i.e., tau), but cannot provide analysis of more rapid variability.
A strength of the present study was the use of two methods, ex-Gaussian and FFT analysis, to evaluate intra-individual variability in children with ADHD compared with TD controls during two separate tasks that required controlled responding. The results indicate that under conditions requiring at least moderate degrees of response control, children with ADHD show increased variability in both the normal Gaussian (fast”) and exponential (“slow”) components of the reaction time distribution. This pattern was observed regardless of working memory demand, suggesting that abnormalities in systems critical engaging a state of preparedness to respond may be central to the neural basis of ADHD. Some task-specific changes in variability in the slow portion of the distribution for children with ADHD were revealed using finer grained FFT analysis of the periodicity of reaction time, suggesting that neural contributions to infrequent lapses of attention in ADHD may be more task-dependent.
Taken together, it appears that ISV is a stable trait directly related to inefficient response preparation mechanisms. As such, it is a good candidate for an intermediate endophenotypic trait that can be used in future studies to further refine our understanding of the relationship between genetic and physiological mechanisms associated with response preparation and ADHD.
Acknowledgements
This research was funded by grants from the National Institutes of Health: K02 NS044850 (SHM), R01 NS048527 (SHM) and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, an NIH/NCRR CTSA Program, UL1-RR025005.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 2000. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Willcutt EG, DeFries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;62:991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitelaar JK, Montgomery SA, van Zwieten-Boot BJ. Attention deficit hyperactivity disorder: Guidelines for investigating efficacy of pharmacological intervention. European Neuropsychopharmacology. 2003;13:297–304. doi: 10.1016/s0924-977x(03)00047-6. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends in Cognitive Sciences. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A, DiMartino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nature Reviews Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Conners CK. The Conners' rating scales-revised. Multi-Health Systems Inc.; North Tonawanda, NY: 1997. [Google Scholar]
- Denckla MB. ADHD: Topic update. Brain and Development. 2003;25:383–389. doi: 10.1016/s0387-7604(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Derefinko KJ, Adams ZW, Milich R, Fillmore MT, Lorch EP, Lynam DR. Response style differences in the inattentive and combined subtypes of attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36(5):745–758. doi: 10.1007/s10802-007-9207-3. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ghaffari M, Curchack J, Reiss P, Hyde C, Vannucci M, Petkova E, Klein DF, Castellanos FX. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;64(7):607–614. doi: 10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopolous AD, Reid R. ADHD rating scale-IV. Guilford Press; New York: 1998. [Google Scholar]
- Durston S. A review of the biological bases of ADHD: What have we learned from imaging studies? Mental Retardation and Developmental Disabilities. 2003;9:184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive function in the dynamic control of behavior: Inhibition, error detection and correction. Neuroimage. 2002;17(4):1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Heathcote A. RTSYS: A DOS application for the analysis of reaction time data. Behavior Research Methods, Instruments & Computers. 1996;28:427–445. [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Arnold LE, Conners CK, et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychology. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Switching form automatic to controlled action by monkey medial frontal cortex. Nature Neuroscience. 2007;10(2):240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, et al. Response variability in attention deficit hyperactivity disorder: Evidence for neuropsychological heterogeneity. Neuropsychologia. 2007;45:630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Daibhis A, et al. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia. 2007;45:2234–2245. doi: 10.1016/j.neuropsychologia.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, King Elbaz Z, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: A response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. WISC-IV and WISC-III profiles in children with ADHD. Journal of Attention Disorders. 2006;9(3):486–493. doi: 10.1177/1087054705283616. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2002;52(8):785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JGB, Abrams MT, Goldberg MC, Flower AA, Boyce A, et al. FMRI evidence that the neural basis of response inhibition is task-dependent. Cognitive Brain Research. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Muller T, Reinhard M, Oehm E, Hetzel A, Timmer J. Detection of very low-frequency oscillations of cerebral haemodynamics is influenced by data detrending. Medical and Biological Engineering and Computing. 2003;41:69–74. doi: 10.1007/BF02343541. [DOI] [PubMed] [Google Scholar]
- Penttonen M, Buzsaki G. Natural logarithmic relationship between brain oscillators. Thalamus & Related Systems. 2003;2:145–152. [Google Scholar]
- Reich W, Welner Z, Herjanic B. The diagnostic interview for children and adolescents-IV. Multi-Health Systems; North Tonawanda, NY: 1997. [Google Scholar]
- Rommelse NNJ, Altink ME, Oosterlaan J, Beem L, Buschgens CJM, Buitelaar J, et al. Speed variability and timing of motor output in ADHD: Which measures are useful for endophenotypic research? Behavior Genetics. 2007 doi: 10.1007/s10519-007-9186-8. e-publication 12/07 in advance of publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, et al. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: An event-related fMRI study. American Journal of Psychiatry. 2004;161(9):1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of go/no-go tasks demonstrating the fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandburg RJ, Marsh JT, Brown WS, Asarnow RF, Higa J, Harper R, et al. Continuous-processing-related event-related potentials in children with attention deficit hyperactivity disorders. Biological Psychiatry. 1996;40:964–980. doi: 10.1016/0006-3223(95)00545-5. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: The frontal lobes control individual performance variability. Brain. 2003;126(11):2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Caffo BS, Denckla MB, Pekar JJ, Mostofsky SH. FMRI of intrasubject variability in ADHD: Anomalous premotor activity with prefrontal compensation. Journal of the American Academy of Child and Adolescent Psychiatry. 2008 doi: 10.1097/CHI.0b013e3181825b1f. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Fotedar S, Blankner JG, Pekar JJ, Denckla MB, et al. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: Differences in activation associated with response inhibition but not habitual motor response. Journal of Cognitive Neuroscience. 2008;20(3):478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL. Event-related fMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(11):1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- Tannock R. Attention deficit hyperactivity disorder: Advances in cognitive, neurobiological, and genetic research. Journal of Child Psychology and Psychiatry. 1998;39(1):65–99. [PubMed] [Google Scholar]
- Wechsler DL. Wechsler intelligence scale for children. 3rd ed. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler DL. Wechsler individual achievement test. The Psychological Corporation; San Antonio, TX: 1992. [Google Scholar]
- Wechsler DL. Wechsler individual achievement test-II. The Psychological Corporation; San Antonio, TX: 2002. [Google Scholar]
- Wechsler DL. Wechsler intelligence scale for children. 4th Ed. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Wilcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Williams BR, Strauss EH, Hultsch DF, Hunter MA, Tannock R. Reaction time performance in adolescents with attention deficit/hyperactivity disorder: Evidence of inconsistency in the fast and slow portions of the RT distribution. Journal of Clinical and Experimental Neuropsychology. 2007;29(3):277–289. doi: 10.1080/13803390600678020. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Gidley Larson JC, Fotedar S, Denckla MB, et al. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. 2007;29(4):345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]