Abstract
Background
Understanding the pathogenesis of osteochondrosis/osteochondritis dissecans and other developmental orthopaedic diseases that are thought to occur secondary to defects in vascular supply to growth/epiphyseal cartilage has been hampered by the inability to image the vasculature in this tissue. This is particularly true in human beings due to limitations of current imaging techniques and the lack of availability of appropriate cadaveric samples for histological studies.
Hypothesis
Susceptibility weighted imaging, an MRI sequence, allows identification of characteristic differences in the vascular architecture in species that are affected by osteochondrosis/osteochondritis dissecans on the femoral condyle (humans and pigs) versus one that is free of the disease (goat).
Study design
Descriptive laboratory study
Materials
Distal femora from cadavers of juvenile humans (n=5), pigs (n=3), and goats (n=3) were scanned in a 9.4T MRI scanner using susceptibility weighted imaging. Three-dimensional reconstructions were created and minimum intensity projections were calculated in three planes to enhance visualization of the vascular architecture.
Results
Susceptibility weighted imaging allowed clear visualization of the epiphyseal vasculature in all species. Vascular architecture, with vessels primarily arising from the perichondrium, was similar in humans and pigs, which are predisposed to osteochondrosis/osteochondritis dissecans, and was starkly different from that present in goats, a species in which there are no reports of osteochondrosis/osteochondritis dissecans. Furthermore, vessels in the distal femoral predilection site disappeared with age in a similar pattern in humans as has been reported previously in pigs.
Conclusion
Nearly identical vascular architecture at the shared primary predilection site of osteochondrosis/osteochondritis dissecans in the femoral condyles in human beings and pigs suggests that vascular failure, which is known to be central to the pathogenesis of this disease in pigs, is also important may also play a role in humans. This assumption of a shared pathogenesis is supported by the pattern of disappearance of vessels with age at the primary predilection site of osteochondritis dissecans in humans, which is essentially identical to that which has been reported in pigs. Susceptibility weighted imaging will likely help further elucidate this potential relationship in the future.
Key Terms: osteochondrosis/osteochondritis dissecans, MRI, epiphyseal cartilage, vasculature, cartilage canals, susceptibility weighted imaging
Introduction
In vertebrates, the axial and the appendicular skeleton, along with a portion of the craniofacial bones, are formed by endochondral ossification, the process in which epiphyseal (growth) cartilage is converted to bone8. Through this process the articular-epiphyseal cartilage-complex (AECC), a structure composed of articular cartilage and sub-articular epiphyseal growth cartilage that is present at the ends of growing long bones, forms the adult shape and size of joints. During endochondral ossification the epiphyseal cartilage relies on a rich vascular supply5, 20–22, 26 to deliver nutrients, supply perivascular mesenchymal cells, and maintain the secondary ossification center. Arteries, veins, and capillaries are embedded in a matrix of connective tissue within the epiphyseal cartilage, and are collectively termed cartilage canals5. The vessels forming the cartilage canals originate either from a dense vascular plexus located in the perichondrium or from the secondary center of ossification16, 22. These vessels and the epiphyseal growth cartilage that they supply disappear with age and are absent in adults, leaving only the articular cartilage covering the ends of the long bones.
Focal failure of endochondral ossification is a hallmark of the developmental orthopaedic disease known as osteochondrosis [OC] and its clinically apparent form osteochondritis dissecans [OCD], a term most frequently used in human medicine. This disease affects both humans and animals at predilection sites that are characteristic of the species, causing formation of intra articular (osteo)cartilaginous fragments. Histological studies performed in swine and horses have demonstrated that the earliest lesion of subclinical OC (OC latens) is characterized by locally extensive ischemic necrosis of the epiphyseal cartilage triggered by failure of cartilage canal blood supply2, 15, 18, 24, 26, 27. These studies also revealed that the vascular supply to the epiphyseal cartilage most often fails at the time when transition from perichondrial to medullary blood supply occurs.26 Alterations in the blood supply are also suspected to play a similarly important role in the development of human OCD, but due to limitations of current imaging techniques and the lack of tissue samples from predilection sites in healthy/subclinically affected human beings, their role has not been investigated in depth. Perhaps more importantly, subclinical disease is not recognized in human beings, as recognition of the disease depends on a patient presenting with clinical signs of joint pain.
In situ evaluation of the vascular supply to the epiphyseal cartilage in animals is most often performed ex vivo after planned euthanasia using various perfusion techniques followed by clearing, µCT, or MRI7, 17, 25, 26. However, innovative use of susceptibility weighted imaging (SWI), an MRI sequence utilizing both magnitude and phase data to generate image contrast, recently enabled the visualization of this vascular supply at various field strengths in pigs (both ex vivo and in vivo)13 and humans (ex vivo) without the use of contrast media. Results obtained with this novel method20, as well as earlier perfusion and histological studies25, also indicate that the vascular architecture in a given species is largely conserved among individuals, allowing collection of important information from a small set of samples.
Observations made during data processing in previous imaging and perfusion studies led us to hypothesize that the vascular architecture of the AECC at areas predisposed to OC/OCD is different in species with a relatively high incidence of OC/OCD (e.g., swine and humans) compared to species not affected by this disease (e.g., goats). In the study reported here, we used a refined method of processing of SWI data12 obtained using a high field (9.4 T) MR scanner. The distal femoral AECC, the primary predilection site for OC/OCD in both pigs and humans, was imaged in developing humans, pigs, and goats to compare the vascular architecture of the AECC among species with different predispositions to OC/OCD.
Methods
Three pigs aged 1, 7, and 21 days13, 20, three goats aged 1, 11, and 19 days, and five human cadaveric knee joints harvested from donors aged 1, 3, 4, 24, and 36 months were included in the study. Distal femoral specimens were harvested from pigs and goats immediately after euthanasia. Femora were cleared of soft tissues, wrapped in saline soaked paper towels and stored at −80°C until they were imaged. Human cadaveric knee joints submerged in saline solution were stored at −80 °C before being processed for MRI by thawing at room temperature and harvesting distal femora free of soft tissue attachments. MR imaging of the specimens was conducted on a 9.4 T scanner driven with VnmrJ console, using a quadrature volume transceiver coil. The specimens were immersed in perfluoropolyether for clean and susceptibility matched background. SWI datasets were acquired using a three-dimensional gradient recalled echo sequence with repetition time of 40 ms, echo time of 14 ms and receiver bandwidth of 16 kHz. The field of view and imaging matrix were set for each sample to achieve approximately 100-µm isotropic resolution13. The acquisition time ranged from 70 to 98 minutes depending on the field of view and matrix settings. Post-processing of the SWI datasets was done by solving for the underlying susceptibility distribution and using the contrast-inverted, masked susceptibility maps as the source data for visualization of the vascular architecture.12 To enhance visualization of the vascular architecture 2, 4, and 5 mm thick minimum intensity projections were calculated in the sagittal, coronal, and axial planes, respectively. Three-dimensional visualizations of the vasculature were created using OsiriX (OsiriX v.5.6, 64-bit, http://www.osirix-viewer.com/).
The study used only cadaveric specimens. No patient identifiers were utilized, nor was any information about familial relatives retrieved; therefore, no IRB or IACUC approval was necessary.
Results
Results of three-dimensional reconstruction (Fig. 1) of SWI data revealed that, in both human beings and pigs, the majority of the vascular supply to the distal femoral epiphyseal cartilage arises from the perichondrium on both the axial (central) and abaxial (peripheral) aspects of the femoral condyles and then courses towards the midline of the condyle tangentially (parallel to the articular surface). The axial and abaxial vascular beds terminate before reaching the sagittal midline of the condyle creating an avascular “watershed region”. Conversely, in goats the majority of the vascular supply to the distal femoral epiphysis arises from the secondary ossification center traversing the ossification front and entering the epiphyseal cartilage radially (perpendicular to the articular surface). Findings obtained from three-dimensional reconstructions were confirmed by evaluating minimum intensity projections reconstructed in the sagittal, axial (transverse), and coronal (dorsal) planes (Fig. 2). The vascular architecture in human beings and pigs appears highly similar in the axial (transverse) and coronal (dorsal) planes and is starkly different from that of goats. The presence of a large secondary ossification center in the coronal (dorsal) and sagittal planes in the goat imaged 1 day after birth (Fig. 2) indicates that skeletal development in this species is much more advanced at the time of birth than in either pigs or human beings. Evaluation of three-dimensional reconstructions obtained from human cadaveric samples of increasing age was consistent with earlier disappearance of the axial (central) vascular bed when compared to vessels originating from the abaxial (peripheral) aspect (Fig. 1 and 3).
Fig. 1.
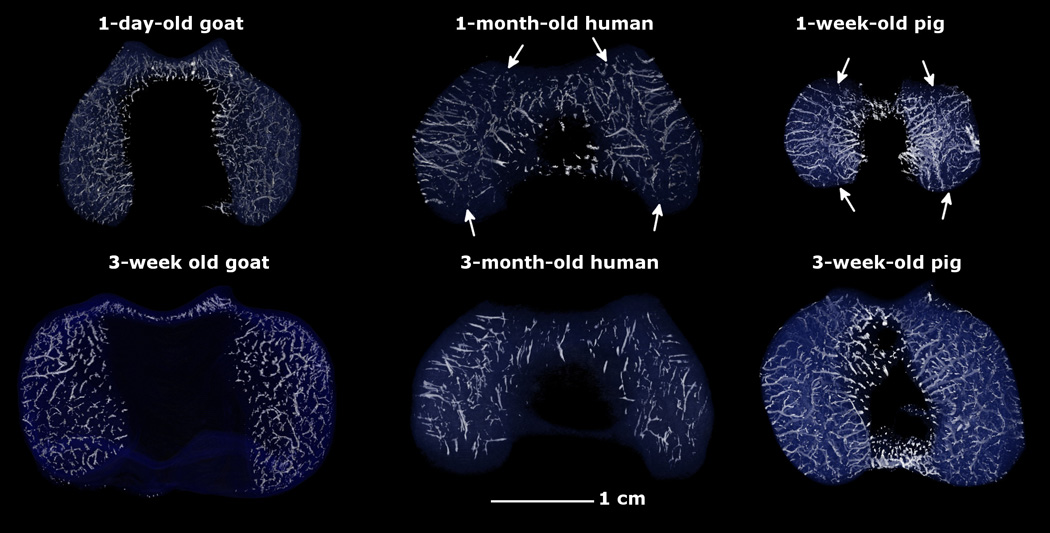
Three-dimensional reconstructions of susceptibility weighted magnetic resonance imaging data depicting the vascular architecture to the distal femoral epiphyseal cartilage in the axial plane in pigs (1 and 3 weeks old), human beings (1 and 3 months old) and goats (1 day and 3 weeks old). Note the presence of distinct axial (central) and abaxial (peripheral) vascular beds in humans and pigs with vessels oriented parallel to the articular surface creating a characteristic avascular region (white arrows) in the midsagittal plane in both condyles. In goats, the majority of the epiphyseal vessels arise from the ossification front, are shorter, and are oriented perpendicular to the articular surface. All specimens are oriented with the medial aspect towards the left and the anterior (cranial) aspect towards the top of the figure.
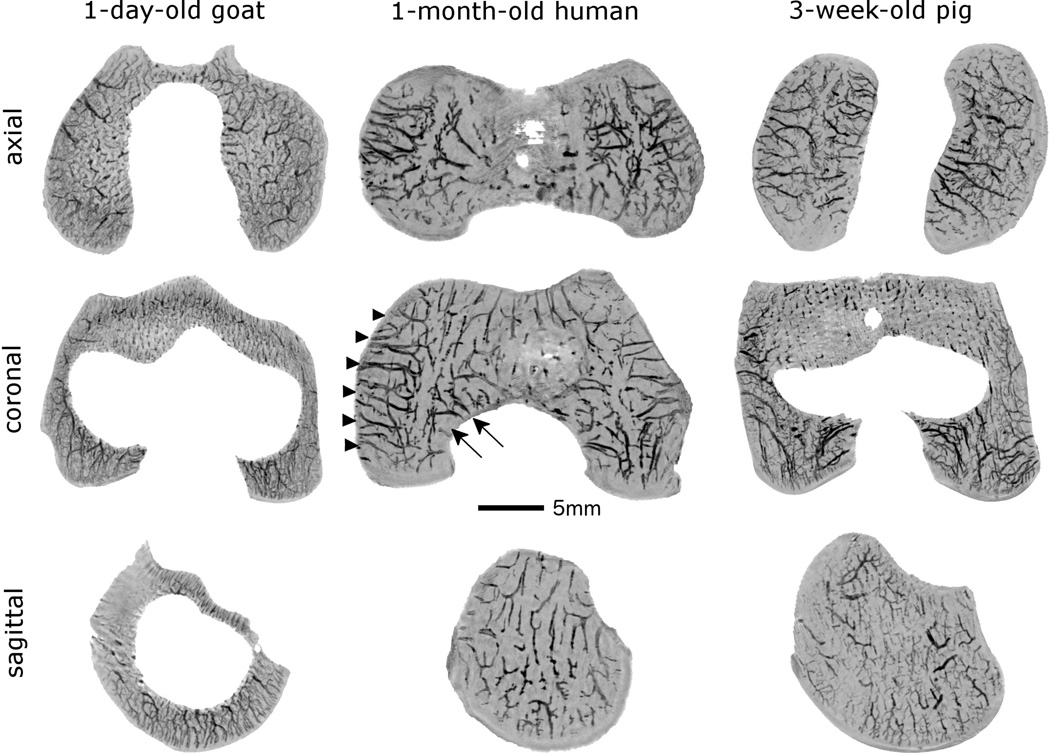
Fig. 2.
Four and five millimeter thick minimum intensity projections calculated from the susceptibility weighted imaging datasets in the coronal and axial planes demonstrate two distinct vascular beds (axial[central] and abaxial [peripheral]) in the epiphyseal cartilage of the femoral condyles in specimens obtained from a 1-month-old human and a 3-week-old pig (shown in the medial femoral condyle in the middle image, arrowheads mark abaxial, arrows mark axial vascular bed). Conversely, no separate vascular beds can be distinguished in the specimen obtained from a 1-day-old goat, where the majority of the vessels arise from the secondary ossification center providing a dense, evenly distributed vascular supply to both femoral condyles. In the two-millimeter-thick minimum intensity projection obtained in the sagittal plane the secondary ossification center is clearly apparent in the goat (white void in the middle of the image), consistent with more advanced skeletal development when compared to both the human and the porcine specimens. All specimens are oriented with the medial aspect towards the left of the figure. Red lines in the porcine specimen mark the plane for the images depicted for each species.
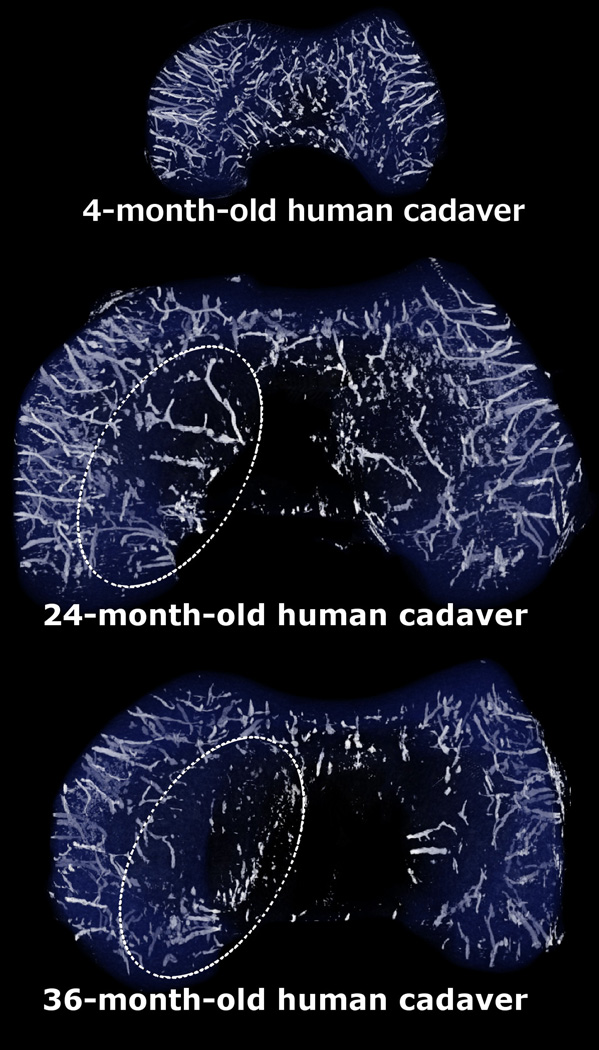
Fig. 3.
Three-dimensional reconstructions of the susceptibility weighted imaging data obtained from distal femoral specimens of 4-, 24- and 36-month-old human cadaveric donors demonstrating earlier resolution of the axial (central) vascular bed vs. the abaxial (peripheral) one. This area of decreased vascularity in the axial aspect of the femoral condyles (indicated with oval, dotted ellipses in the 24- and 36-month-old specimens) corresponds with the predilection site of osteochondrosis/osteochondritis dissecans. All specimens are oriented with the medial aspect towards the left of the figure.
Discussion
Novel application of the SWI MRI sequence enabled detailed evaluation of the vascular architecture of the distal femoral epiphyseal cartilage in humans and facilitated comparison of the vascular architecture among different species having different susceptibilities to OC/OCD in this site. This imaging technique is presently available on all clinical MRI systems, which typically operate at lower field strengths than research magnets. For in vivo application in human subjects, an MRI system of at least 3 T will be required to allow evaluation of vascular changes suspected to be associated with clinical/subclinical cases of osteochondrosis without the use of contrast media. Clinical use would also necessitate substantial shortening of the excessively long scanning times (70–98 min) used ex vivo in the 9.4T scanner. Our preliminary results suggest that 12–15 min scanning time is adequate to provide valuable data in 7 T research- and 3 T clinical-systems.
Among the several proposed etiologies of OC/OCD, including inflammation, osteonecrosis, genetics, repetitive trauma, and vascular failure,4 results of experimental and epidemiologic studies indicate that trauma and vascular failure are two of the most important factors in the development of clinically apparent OCD.1, 2, 11 Studies performed in pigs and horses described the presence of ischemic cartilage necrosis at predilection sites of OC/OCD.2, 15, 18 These areas of necrotic cartilage are considered to be the earliest lesions associated with OC/OCD and are termed as OC latens in the veterinary medical literature.24 As long bone development continues, the epiphyseal growth cartilage is gradually replaced by bone and, during this process, the ossification front reaches the areas of necrotic cartilage. At these sites, the progression of the ossification front and the replacement of the epiphyseal growth cartilage with bone cease or markedly decelerate. Thus, areas of retained necrotic cartilage become apparent as irregularities of the subchondral bone on radiographs or MR. These radiographically apparent lesions are therefore termed OC manifesta. Interestingly, OC manifesta lesions in pigs and horses show a striking resemblance to findings described as ‘ossification variants’ in MRI results from the maturing femoral condyles in humans.6 Eventually, subclinical lesions of OC (i.e., OC latens and OC manifesta) either undergo healing or, if the overlying articular cartilage is exposed to repeated trauma, as it is often the case during athletic activities, progression from subclinical (OC) to clinically-apparent disease (OCD) occurs.1, 9, 11 The cause of vascular failure at the predilection sites of OC/OCD remains to be elucidated, but it may be influenced by regional changes in the extracellular matrix resulting in structural weakness10 or could be due to the forces arising at the bone cartilage interface because of the different biomechanical characteristics of these two tissues.
In this study, SWI was used to demonstrate similarities in the vascular architecture of pigs and humans at the shared predilection site for OC/OCD in the distal femur. The results suggest that vascular changes known to be central in the pathogenesis of OC/OCD in pigs23, 26 may also play a role in the pathogenesis of OCD in humans. Vessels arising from the perichondrium and coursing axially in pigs and humans are expected to be more prone to fail than the more dense vascular supply composed of numerous shorter vessels arising from the ossification front in goats. Indeed, studies performed in pigs demonstrated that vascular failure leading to ischemic chondronecrosis is most likely to occur at the time of transition from perichondrial to medullary blood supply26, a process necessary to maintain blood supply to the ever thinning epiphyseal cartilage as the advancing ossification front overtakes the perichondrial vessels. Given the large secondary center of ossification that was already present in the 1-day-old goat, it is likely that this transition occurs in goats prior to birth. In fact, this unique blood supply is likely to contribute to the goats’ lack of susceptibility for developing clinically apparent OCD even after surgical interruption of the vascular supply to the epiphyseal cartilage19, a procedure known to trigger the development of OCD like lesions in horses14. The shared predilection site of OC/OCD along with a nearly identical vascular architecture in pigs and humans provides an intriguing argument for utilizing pigs as a naturally occurring animal model of human OCD.
Minimal intensity projections obtained from the human specimens of various ages also demonstrated that vascular supply to the axial (central) portion of the femoral condyles, the primary predilection site for OCD in humans3, diminishes sooner than that to the abaxial (peripheral) portion. This difference in the regression of the vascular supply may explain why the majority of clinically apparent OCD lesions in humans affect the axial area of the condyles. Indeed, in pigs, where subclinical OC lesions have been examined histologically, a similar pattern in vascular regression has been directly linked to the development of OC/OCD in the axial aspects of the femoral condyles, evidenced by the very common finding of necrotic cartilage and necrotic cartilage canal vessels at this site25 Interestingly, in the tarsocrural joint of horses, it has been determined that one of the predilection sites of OCD (distal end of the lateral trochlear ridge) tends to retain its vascular supply longer than does the remainder of the talus.17 Conversely, in the stifle (knee) joint of the same species, the first structure to loose its blood supply is the trochlear groove, one of the primary predilections sites of OCD in this joint. These discrepancies in the rate of disappearance of the blood supply to the epiphyseal cartilage at predilection sites of OC/OCD indicate that other factors, such as trauma along with its timing, also play a role in the development of this disease.
Although this study focused on osteochondrosis/osteochondritis dissecans, the MRI technique used here will likely have broad application to other poorly understood developmental orthopaedic diseases with a suspected/known vascular component (e.g., Legg-Calvé-Perthes disease, hip dysplasia, and others).
Our study has several limitations. First and foremost, limited availability of human cadaveric specimen forced us to draw our conclusions from a low number of observations. Previous studies in pigs25, horses14, and goats19, however, have indicated that the vascular supply to epiphyseal cartilage is highly predictable within a species at a given site and age. This limitation also prevented us from more closely matching the ages of pigs, goats, and humans. Comparisons among species were further complicated because, ideally, they should be based on developmental rather than absolute age. This drove the selection of very young goat and pig specimens to be included in the study for comparison to human cadavers that were substantially older. Clearly, a one-day-old goat is more developmentally mature (based on the size of the secondary ossification center) than a 1-month-old human. Indeed, during individual development, weeks to months in animals, some of which often reach maturity within the first year of life, often translate into years in humans. Finally, the present study is descriptive in nature. From these findings it is not possible to establish a cause and effect relationship between the early resolution of vasculature in the axial aspect of the medial femoral condyle and the development of OCD in this area in human beings.
In conclusion, differences in the cartilage canal blood supply of the distal femoral epiphysis have been identified using susceptibility weighted magnetic resonance imaging in species with different predispositions to OC/OCD. The well-established role that vascular failure plays in the development of OC/OCD in pigs and the similarities of the vascular supply between pigs and humans at their shared predilection site of OC/OCD could imply that vascular failure contributes to the development of human OCD.
What is known about the subject
In animal species (pigs and horses) it is established that osteochondrosis/osteochondritis dissecans develops after vascular supply to the epiphyseal growth cartilage fails and ischemic chondronecrosis develops. In contrast, the pathogenesis of osteochondrosis/osteochondritis dissecans in human beings is poorly understood due to factors mentioned above, and because it is only studied in the osteochondritis dissecans stage of the disease.
What this study adds to existing knowledge
As far as we are aware, this is the first study in which the vascular architecture of the epiphyseal cartilage in humans has been demonstrated at any site in the body without the administration of intravenous contrast agents. The similarity in the vascular architecture at the primary predilection site of OCD in humans and pigs, as demonstrated in this work, suggests that the pathogenesis of OCD may be similar in these two species. These findings provide indirect evidence that humans, like pigs, develop vascular failure and subsequent ischemic chondronecrosis at specific predilection sites of OC/OCD as a result of a vascular architecture where vessels initially arise from the perichondrium. Susceptibility weighted imaging is a promising tool to help develop future studies to further elucidate the pathogenesis of OCD in humans.
Footnotes
Conflict of Interest:
The authors have declared that no conflict of interest exists.
References
- 1.Cahill BR. Osteochondritis Dissecans of the Knee: Treatment of Juvenile and Adult Forms. J Am Acad Orthop Surg. 1995;3(4):237–247. doi: 10.5435/00124635-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Carlson CS, Meuten DJ, Richardson DC. Ischemic necrosis of cartilage in spontaneous and experimental lesions of osteochondrosis. J Orthop Res. 1991;9(3):317–329. doi: 10.1002/jor.1100090303. [DOI] [PubMed] [Google Scholar]
- 3.Crawford DC, Safran MR. Osteochondritis dissecans of the knee. J Am Acad Orthop Surg. 2006;14(2):90–100. doi: 10.5435/00124635-200602000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Edmonds EW, Polousky J. A Review of Knowledge in Osteochondritis Dissecans: 123 Years of Minimal Evolution from Konig to the ROCK Study Group. Clinical orthopaedics and related research. 2012 doi: 10.1007/s11999-012-2290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haines RW. Cartilage Canals. J Anat. 1933;68(Pt 1):45–64. [PMC free article] [PubMed] [Google Scholar]
- 6.Jans LB, Jaremko JL, Ditchfield M, Huysse WC, Verstraete KL. MRI differentiates femoral condylar ossification evolution from osteochondritis dissecans. A new sign. Eur Radiol. 2011;21(6):1170–1179. doi: 10.1007/s00330-011-2058-x. [DOI] [PubMed] [Google Scholar]
- 7.Jaramillo D, Villegas-Medina OL, Doty DK, et al. Age-related vascular changes in the epiphysis, physis, and metaphysis: normal findings on gadolinium-enhanced MRI of piglets. AJR Am J Roentgenol. 2004;182(2):353–360. doi: 10.2214/ajr.182.2.1820353. [DOI] [PubMed] [Google Scholar]
- 8.Jochmann K, Bachvarova V, Vortkamp A. Reprint of: Heparan sulfate as a regulator of endochondral ossification and osteochondroma development. Matrix Biol. 2014;35:239–247. doi: 10.1016/j.matbio.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Laverty S, Girard C. Pathogenesis of epiphyseal osteochondrosis. Vet J. 2013;197(1):3–12. doi: 10.1016/j.tvjl.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Lecocq M, Girard CA, Fogarty U, Beauchamp G, Richard H, Laverty S. Cartilage matrix changes in the developing epiphysis: early events on the pathway to equine osteochondrosis? Equine Vet J. 2008;40(5):442–454. doi: 10.2746/042516408X297453. [DOI] [PubMed] [Google Scholar]
- 11.McCoy AM, Toth F, Dolvik NI, et al. Articular osteochondrosis: a comparison of naturally-occurring human and animal disease. Osteoarthritis Cartilage. 2013;21(11):1638–1647. doi: 10.1016/j.joca.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nissi MJ, Toth F, Carlson CS, Ellermann J. Improved visualization of cartilage canals using semi-quantitative susceptibility mapping. Paper presented at: Intl Soc Mag Reson Med; 2014; Milan, Italy. [Google Scholar]
- 13.Nissi MJ, Toth F, Zhang J, et al. Susceptibility weighted imaging of cartilage canals in porcine epiphyseal growth cartilage ex vivo and in vivo. Magn Reson Med. 2014;71(6):2197–2205. doi: 10.1002/mrm.24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olstad K, Hendrickson EH, Carlson CS, Ekman S, Dolvik NI. Transection of vessels in epiphyseal cartilage canals leads to osteochondrosis and osteochondrosis dissecans in the femoro-patellar joint of foals; a potential model of juvenile osteochondritis dissecans. Osteoarthritis Cartilage. 2013;21(5):730–738. doi: 10.1016/j.joca.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Early lesions of osteochondrosis in the distal tibia of foals. J Orthop Res. 2007;25(8):1094–1105. doi: 10.1002/jor.20375. [DOI] [PubMed] [Google Scholar]
- 16.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Epiphyseal cartilage canal blood supply to the distal femur of foals. Equine Vet J. 2008;40(5):433–439. doi: 10.2746/042516408X300269. [DOI] [PubMed] [Google Scholar]
- 17.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Epiphyseal cartilage canal blood supply to the tarsus of foals and relationship to osteochondrosis. Equine Vet J. 2008;40(1):30–39. doi: 10.2746/042516407X239836. [DOI] [PubMed] [Google Scholar]
- 18.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Early lesions of articular osteochondrosis in the distal femur of foals. Vet Pathol. 2011;48(6):1165–1175. doi: 10.1177/0300985811398250. [DOI] [PubMed] [Google Scholar]
- 19.Toth F, Nissi MJ, Wang L, Ellermann JM, Carlson CS. Surgical induction, histological evaluation, and MRI identification of cartilage necrosis in the distal femur in goats to model early lesions of osteochondrosis. Osteoarthritis Cartilage. 2015;23(2):300–307. doi: 10.1016/j.joca.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toth F, Nissi MJ, Zhang J, et al. Histological confirmation and biological significance of cartilage canals demonstrated using high field MRI in swine at predilection sites of osteochondrosis. J Orthop Res. 2013;31(12):2006–2012. doi: 10.1002/jor.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visco DM, Hill MA, Van Sickle DC, Kincaid SA. The development of centres of ossification of bones forming elbow joints in young swine. J Anat. 1990;171:25–39. [PMC free article] [PubMed] [Google Scholar]
- 22.Wilsman NJ, Van Sickle DC. Cartilage canals, their morphology and distribution. Anat Rec. 1972;173(1):79–93. doi: 10.1002/ar.1091730107. [DOI] [PubMed] [Google Scholar]
- 23.Ytrehus B, Andreas Haga H, Mellum CN, et al. Experimental ischemia of porcine growth cartilage produces lesions of osteochondrosis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2004;22(6):1201–1209. doi: 10.1016/j.orthres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Ytrehus B, Carlson CS, Ekman S. Etiology and pathogenesis of osteochondrosis. Vet Pathol. 2007;44(4):429–448. doi: 10.1354/vp.44-4-429. [DOI] [PubMed] [Google Scholar]
- 25.Ytrehus B, Carlson CS, Lundeheim N, et al. Vascularisation and osteochondrosis of the epiphyseal growth cartilage of the distal femur in pigs--development with age, growth rate, weight and joint shape. Bone. 2004;34(3):454–465. doi: 10.1016/j.bone.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Ytrehus B, Ekman S, Carlson CS, Teige J, Reinholt FP. Focal changes in blood supply during normal epiphyseal growth are central in the pathogenesis of osteochondrosis in pigs. Bone. 2004;35(6):1294–1306. doi: 10.1016/j.bone.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Ytrehus B, Grindflek E, Teige J, et al. The effect of parentage on the prevalence, severity and location of lesions of osteochondrosis in swine. J Vet Med A Physiol Pathol Clin Med. 2004;51(4):188–195. doi: 10.1111/j.1439-0442.2004.00621.x. [DOI] [PubMed] [Google Scholar]