Abstract
Electrostatic Dust Collectors (EDCs) are in use for passive sampling of bioaerosols, but particular aspects of their performance have not yet been evaluated. This study investigated the effect of mailing EDCs on endotoxin loading and the effect of EDC deployment in front of and away from heated ventilation on endotoxin sampling. Endotoxin sampling efficiency of heated and unheated EDC cloths was also evaluated. Cross-country express mailing of dust-spiked EDCs yielded no significant changes in endotoxin concentrations compared to dust-only samples for both high spiked-EDCs (p=0.30) and low spiked-EDCs (p=0.36). EDCs were also deployed in 20 identical apartments with one EDC placed in front of the univent heater in each apartment and contemporaneous EDC placed on the built-in bookshelf in each apartment. The endotoxin concentrations were significantly different (p=0.049) indicating that the placement of EDC does impact endotoxin sampling. Heated and unheated EDCs were deployed for 7 days in pairs in farm homes. There was a significant difference between endotoxin concentrations (p=0.027) indicating that heating EDCs may diminish their electrostatic capabilities and impact endotoxin sampling. The last study investigated the electrostatic charge of 12 heated and 12 unheated EDC cloths. There was a significant difference in charge (p=0.009) which suggests that heating EDC cloths may make them less effective for sampling. In conclusion, EDCs can be mailed to and from deployment sites, EDC placement in relationship to ventilation is crucial, and heating EDCs reduces their electrostatic charge which may diminish their endotoxin sampling capabilities.
Keywords: endotoxin, exposure assessment, house dust, passive sampling
INTRODUCTION
The Electrostatic Dust Collector (EDC) is an easily-used passive sampling device consisting of a polypropylene folder holding 2 or 4 electrostatic cloths. EDCs employ electret fibers which have been shown to enhance allergen particle retention (1-3). The advantage of the EDC method over other methods is its ability to be mailed to and from sites, eliminating the need to deploy field staff to study sites. However, it has not been determined whether mailing samples interferes with the endotoxin loading during shipment. EDC cloths are also easily inserted and removed from the polypropylene folders for EDC preparation and extraction, giving EDCs an advantage over other passive sampling devices such as the petri dish method and pizza box-aluminum foil sampling device; also known as a dust fall collector (DFC) (4-5). Similar to active sampling methods, both of these methods require field workers for deployment and recovering settled dust samples for endotoxin analysis is difficult (4, 6-7).
Endotoxin concentrations sampled with EDCs have been compared to other sampling methods. A PM10 Harvard impactor, reservoir dust from vacuum sampling and EDCs were deployed and compared in farm (n=9) and nonfarm homes (n=7) in the Netherlands (7). EDCs correlated well to the Harvard impactor (r=0.70) and with reservoir floor dust (r=0.65). Although floor dust correlated poorly with the Harvard impactor (r=30)(7). Other studies have also reported poor correlation between floor dust and active sampling(8-9). Another study compared EDCs to inhalable dust PAS-6 samplers deployed in an animal companion hospital and found that they were moderately correlated (r=0.70) (9). Button aerosol samplers and EDCs deployed in farm homes had moderately correlated (r=0.70) endotoxin concentrations (10). DFCs and EDCs deployed in Danish homes had a moderate correlation between endotoxin concentrations (r=0.58) but the DFCs released significantly more particles compared to EDCs(5). The moderate associations between EDCs and active samplers/floor dust may indicate that EDCs actually captures intermediate particles that are larger than PM10 and smaller than dust particles captured during vacuum sampling(7).EDCs have been used for sampling low endotoxin home environments, including apartments and farm homes, and for sampling high endotoxin occupational environments, including a companion hospital and a social room at a composting plant (7, 9, 11-12). EDCs are easily deployed on flat surfaces to sample settling, airborne dust over a specified time period, typically two weeks. Because EDCs are deployed on an elevated surface, only airborne dust settles on the electrostatic cloth surface. A previous study has indicated that bookshelf deployment may restrict airflow for sampling (13). Furthermore, it is unknown whether or not EDCs should be placed away from ventilation diffusers. The ventilation diffuser outlets might alter air currents increasing or decreasing the amount of dust settling on the EDCs. It is unknown whether there is a relationship between placement in front of or away from ventilation.
Commercial electrostatic cloths are manufactured with no intent of keeping the cloths free of endotoxin or other microbial agents. A previous study found that contact of electrostatic cloths with packaging resulted in higher endotoxin concentrations compared to cloths not in contact with packaging (14). As a result, it was proposed that EDC cloth preparations include heating cloths for overnight at 200°C to degrade endotoxin on the cloths prior to assembly (7). However, it is unknown if heating affects the cloths’ electrostatic charge and/or its ability to sample endotoxin. EDCs have also been deployed for varying time periods including 7, 14, and 28 days, and even 8 weeks (9, 11, 15-16). It has not been investigated whether the cloths maintain their electrostatic charge over longer sampling periods or if the charge dissipates over time which would affect the sampling capabilities.
The goal of this study was to investigate four factors that could potentially influence the ability of EDCs to be a reliable exposure assessment device. The effect of mailing EDCs was evaluated by spiking EDCs with a known mass of house dust, mailing the EDCs, and then determining whether mailing the EDCs resulted in a loss of sampled endotoxin. Multiple EDCs were placed in apartments to determine the relationship between EDC deployment location and ventilation diffusers on the measured endotoxin levels. Third, the effect of pre-heating EDC cloths on their sampling capabilities was examined. A side study was conducted to investigate the effect of heat and deployment time on EDC cloth charge because electrostatic charge may be an important factor for effectively sampling endotoxin.
METHODS
EDC Assembly
EDCs were 2-sided polypropylene folders that each held 2 electrostatic cloths. Heated EDC cloths were heated for 6 h at 160°C instead of overnight at 200°C because the latter protocol resulted in deterioration of the EDC cloths. Cloths are heated to degrade preexisting endotoxin present from manufacturing and packaging. EDC cloths designated as unheated did not receive any heat treatment (for the Heated/Unheated Cloth Study and the Electrostatic Charge Study). The cloths were securely fastened into polypropylene folders that had been cleaned with 70% alcohol solution under endotoxin-free conditions.
Mailing Study
Extra high and low endotoxin quality control (QC) dust prepared for the National Health and Nutrition Examination Survey (NHANES) study (17) was used to spike two-cloth EDCs. High QC dust was blended to contain an endotoxin concentration of 82 EU/mg while the low QC dust had an endotoxin concentration of 20 EU/mg. Three EDCs each were spiked with either 5 mg or 10 mg of high QC test dust. Similarly, three EDCs each were spiked with 5 mg or 10 mg of low QC dust. Both EDC cloths within the same EDC folder were spiked with aliquots of the same dust weight. Since EDCs cannot be weighed with sufficient accuracy in a microbalance, dust aliquot vials used for spiking were pre-weighed and weighed post-spiking to determine the exact dust weight used for EDC cloth spiking.
Four aliquots of high QC dust and 4 aliquots of low QC dust were analyzed for endotoxin to compare to the mailed dust spiked-EDCs. Two blank EDCs remained closed and were mailed with the 18 dust-spiked EDCs. Each EDC was closed with two clasps and mailed in its own Ziploc bag. EDCs were placed into one of two express mail boxes. The EDCs were express mailed during the fall from University of Iowa to North Carolina, and were returned using FedEx. One of the 4 blanks was below the limit of detection (LOD) and a value of zero was assigned. The average endotoxin concentration of the blank EDCs was low (0.442 EU/sample) and spiked-EDCs were not blank corrected. Endotoxin values for mailed EDCs were calculated to be expressed in EU/mg to normalize the endotoxin concentrations to the weight of the spiked dust. The endotoxin concentrations of the two cloths within each EDC were averaged.
Apartment Study
This study was conducted during the winter in 20 flats in one apartment building, all with individual adjustable univent heaters and with the same apartment layout. Forty EDCs were deployed with one EDC deployed on a bookshelf and the other on a music stand (Hamilton KB95E Encore Symphonic). The music stand was set approximately 25 cm from a univent ventilator located near a window. Each EDC rested on the shelf portion of a music stand adjusted to a horizontal position at a height of 135 cm. An additional EDC was placed on top of a shelf within the built-in book shelves present in each apartment. Participants were instructed not to touch EDCs and to avoid disturbing them. After the 14-day sampling period, all EDCs were collected and stored at −20°C to await endotoxin analysis. Endotoxin concentrations were compared as EU/m2. All values in the Apartment Study were blank corrected using the average endotoxin concentration of ten blank EDC cloths (0.069 EU/sample).
Heated/Unheated Cloth Study
Nine heated and 9 unheated EDCs, each with 2 electrostatic cloths, were assembled as described above. EDCs were deployed for 7 summer days in 9 farm homes located within a 25 km radius of Maquoketa, IA. These farm households consisted of an individual or a family farming livestock (usually cattle) or row crops (corn and soybeans). Home occupancy ranged from one to six individuals. Two EDCs were deployed as previously described on music stands in the main living area of each home where residents stated they spent the most time. Two heated and two unheated EDCs were deployed in four different homes to serve as blanks. The blank EDCs were removed from packaging, opened for 1 min and returned to their Ziploc® bags. After collection, the samples were stored at −20°C until endotoxin analysis. The averages of these heated and unheated blank EDC cloths were 1.23 and 1.71 EU/sample, respectively, and these values were used to blank correct heated and unheated cloths.
Endotoxin Extraction
Glassware was rendered endotoxin-free by heating overnight at 200°C prior to use. All EDC cloths were extracted in 10 ml sterile, endotoxin-free water (Lonza, Inc) as previously described(11). Cloths were shaken for 1 h at 22°C. The extracts were transferred and the recovery volume recorded. The extracts were centrifuged for 20 min at 600 xG at 4°C and the pellet was discarded.
For the EDC Mailing Study, all samples and dust aliquots were extracted in 10 ml of endotoxin-free water (Lonza, Inc). Dust, spiked EDCs, and blank EDC extracts were diluted using 4-fold serial dilutions in endotoxin-free water. Samples from the Apartment Study were diluted using 3-fold serial dilutions and samples from the Heated/Unheated Cloth Study were diluted into 4-fold serial dilutions.
Kinetic chromogenic Limulus Amebocyte Assay (LAL) assay
EDC eluates were analyzed using the kinetic chromogenic Limulus Amebocyte Lysate (LAL) assay (Kinetic-QCL; Lonza, Inc., Walkersville, MD), as previously described (18) but without the addition of 0.05% Tween 20. The reagents used for each study were from the same lot (lot HL0476). All samples and standard dilutions were prepared in endotoxin-free borosilicate glass tubes heated overnight. A 12-point standard curve was generated using 2-fold serial dilutions of endotoxin standard (Escherichia coli E50-643; Lonza, Inc.; 13 EU/ng). Dilutions were assayed in endotoxin-free microtiter plates (Costar no. 3596; Corning, Inc.) and analyzed using a microplate reader (SpectraMax 340, Molecular Devices, Inc.) with photometric measurements taken at 37°C every 30 s for 90 min at 405 nm. The same microplate reader was used for all samples of the same study. SoftMaxPro software (Ver 5.4 and 4.7.1, Molecular Devices, Inc.) was used for data analysis. The minimum acceptable r2 value was 0.995 for the standard curve.
Electrostatic Charge Study
A grounded electrometer (Pasco, Inc.) was attached to a faraday “ice pail” (Pasco, Inc.) to measure the voltage of EDC cloths to determine charge. Twelve heated and twelve unheated cloths were attached with tape to a cardstock paper tube with a wooden dowel handle to expose the entire surface area of the cloth to the faraday pail for accurate voltage measurements. After zeroing the electrometer, the EDC cloth was inserted into the inner ice pail and the voltage recorded. Ten measurements were taken for each cloth and all measurements were conducted in the same day to minimize the effects of variation in temperature and humidity, which can alter measurement of electrostatic charge. For comparing electrostatic charges, voltage measurements (V) were converted to picocoulombs (Q) using the equation Q=CV, with the given internal capacitance being 150 pf (C). The 10 charge measurements for each cloth were then averaged.
Another small group of samples was deployed to investigate whether field deployment time may affect the electrostatic charge of the EDC cloths. To determine the effect of deployment time on charge, three EDC with heated cloths were deployed on three side-by-side music stands in the main living area of a farm home. The shelves of each music stand were adjusted to a horizontal position, extended to a height of 135 cm, and assigned one of three deployment periods: 7, 14 or 28 days. A blank EDC remained closed for the 28 days. Following each designated deployment period, EDC folders were closed and placed into a Ziploc® bag. Following 28 days of sampling, voltage measurements were taken as described above using the same cardstock tube for measuring each cloth. For 7, 14, and 28 days of sampling, the average charge in picocoulombs (pC) for the EDC at each time point were compared. This was done to determine if the cloth charge changed over the deployment period.
Scanning Electron Microscopy
An EDC cloth heated for 6 h at 160°C and an unheated EDC cloth were deployed for 28 days in a farm home and were imaged using SEM. An unheated EDC cloth and a heated cloth used as a wipe were also imaged. All cloths were mounted and sputter coated with 60/40 gold-palladium using a K550 Emitech sputter coater. The cloths were imaged using a Hitachi S-4800 SEM.
Statistical Analysis
All endotoxin concentrations were log-normally distributed. Endotoxin values from samples below the limit of detection (LOD) were assigned the value of LOD/√2. For the Mailing Study, a one-way between-subject Analysis of Variance (ANOVA) was conducted on high and low QC dust to compare the effect of mailing 5 and 10 mg dust-spiked EDCs to dust-only endotoxin concentrations. A paired t-test was performed on endotoxin concentrations in the Apartment Study and the Heated/Unheated Cloth Study. A Pearson correlation analysis and unpaired, equal variances t-test was performed between log-transformed electrostatic charges of heated and unheated cloths from the Electrostatic Charge Study.
For all statistical analyses, P-values below 0.05 were considered significant and values below 0.01 were considered highly significant. Analyses were performed using SigmaPlot version 11.0 (Systat Software, Inc.; Santa Clara, CA).
RESULTS
Table I displays the descriptive statistics for the Mailing Study, the Heated/Unheated Cloth Study, the Apartment Study and the Electrostatic Charge Study. In the Mailing Study, the geometric mean (GM) for the dust-only samples (82 EU/mg) were 28% higher than the high QC dust spiked EDCs (59 EU/mg). For the low QC dust spiked EDCs and low QC dust-only, the GM was 35% higher for the dust-only samples (20 EU/mg) compared to the dust spiked EDCs (13 EU/mg). In the Heated/Unheated Cloth Study, unheated EDC cloths had a 30% higher GM compared to heated EDC cloths (935 EU/m2vs. 659 EU/m2, respectively). The Apartment Study had a 36% higher GM for EDCs deployed in front of the univent (211 EU/m2) compared to EDC deployed away from the univent (134 EU/m2). In the Electrostatic Charge Study, the unheated EDC cloths (1737 pC) had a 1.7-fold higher GM compared to heated EDC cloths (998 pC). Coefficients of variation were 15% for unheated cloths and 30% for heated cloths.
TABLE I.
Descriptive statistics of the endotoxin loads (EU/m2) in the Mailing Study, the Heated/Unheated Cloth Study that sampled in 9 farm homes, and the Apartment Study that sampled in 17 apartments. Summary data for the charge (pC) are shown for the Electrostatic Charge Study.
| N | GM | GSD | Median | Interquartile Range | |
|---|---|---|---|---|---|
| Mailing study (EU/mg) | |||||
| High QC Dust spike | 12 | 59 | 1.6 | 57 | 42-86 |
| High QC Dust-only | 4 | 82 | 1.2 | 79 | 75-89 |
| Low QC Dust Spike | 12 | 13 | 1.7 | 12 | 10-15 |
| Low QC Dust-only | 4 | 20 | 1.3 | 19 | 16-24 |
| Apartment Study (EU/m2) | |||||
| In front of Univent | 17 | 211 | 2.1 | 210 | 135-316 |
| Away from Univent | 17 | 134 | 2.1 | 165 | 90-203 |
| Heated/Unheated Cloth Study (EU/m2) | |||||
| Heated Cloths | 18 | 730 | 1.9 | 605 | 519-859 |
| Unheated Cloths | 18 | 1059 | 2.8 | 655 | 500-2399 |
| Electrostatic Charge Study (pC) | |||||
| Heated Cloths | 12 | 998 | 1.8 | 1114 | 611-1380 |
| Unheated Cloths | 12 | 1737 | 1.4 | 1799 | 1276-2265 |
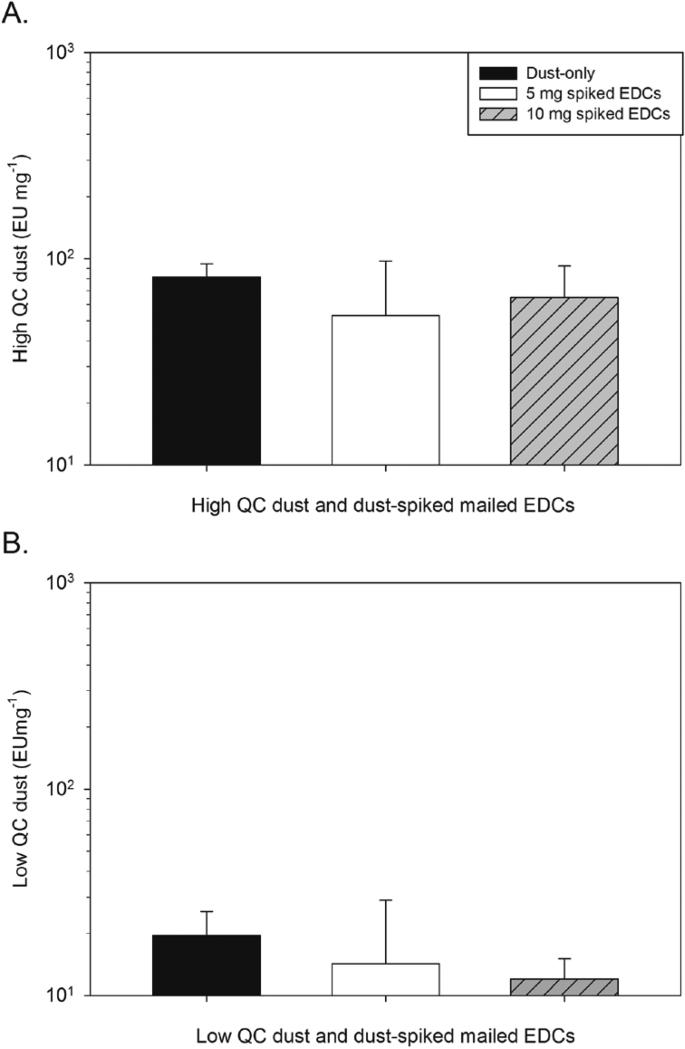
Figure 1a displays the GM in EU/mg of each mailed high QC spiked EDC pair and the solid black bar is the GM of the endotoxin content of the dust used to spike the EDC samples. Figure 1b displays the same information for low QC dust-spiked EDC cloths. The values are normalized to the endotoxin per mg of spiked-dust and as a result the endotoxin concentrations should be similar for all EDCs. A one-way between-subject ANOVA indicated no significant difference (p=0.30) between any pairwise comparisons of 5 and 10 mg of high QC spiked EDCs and high QC dust-only endotoxin concentrations. An ANOVA model also determined no significant difference between any pairwise comparisons of 5 and 10 mg of low QC spiked EDCs and low QC dust-only endotoxin values (p=0.36). Thus, mailing EDCs did not significantly affect the measured endotoxin concentrations.
FIGURE 1.
Geometric mean (GM) and geometric standard deviation (GSD) of endotoxin concentrations from dust-spiked and mailed EDCs normalized to EU/mg for EDCs spiked with 5 mg (open bar) and 10 mg (hashed bar) of (a) high endotoxin QC dust and (b) low endotoxin QC dust compared to the endotoxin content of the dust itself (solid bar).
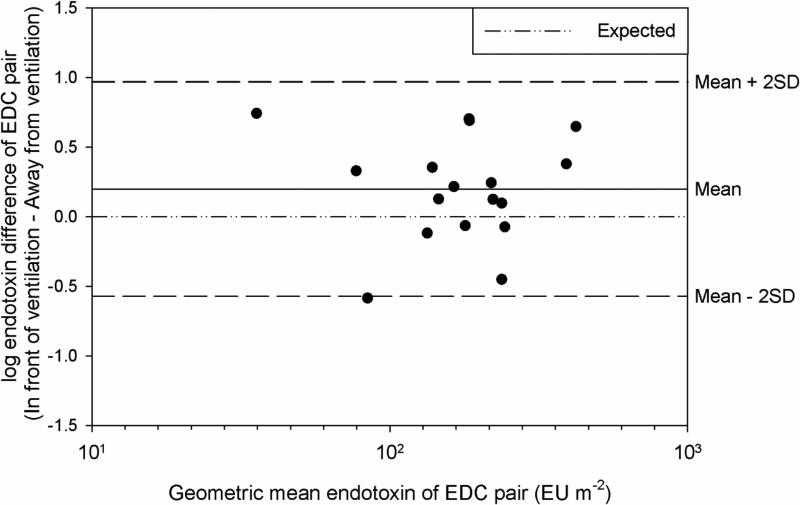
EDCs exposed to direct, heated univent ventilation in the Apartment Study were compared to EDCs deployed on bookshelves, away from direct ventilation. The average outdoor temperature over the sampling 14-day sampling period was −5.6°C. Heated ventilation has been suspected to interfere with endotoxin loading of the EDCs and deployment instructions from several prior studies specified avoiding such locations. The Bland-Altman plot (Figure 2) shows surprisingly poor agreement between EDCs deployed in front of compared to away from ventilation in the 17 apartments. EDCs deployed in the same apartment would be expected to have comparable endotoxin concentrations. However, a paired t-test indicated a significant difference between EDC endotoxin concentrations in front of and away from the univent (p=0.049). This difference in endotoxin concentrations between EDCs deployed in different locations in the same apartment suggests that EDC deployment location requires careful attention and needs to be controlled. In most apartments, EDCs deployed in front of the ventilation had higher endotoxin concentrations than the EDC deployed on the bookshelf. This suggests that heating units expose the EDCs to more house dust when they are in use or particle loading was limited by obstruction from the bookshelf. Since the samples were taken during the winter months, the heating unit was in use during sampling.
FIGURE 2.
Bland-Altman comparison of endotoxin concentrations in front of and away from heated ventilation in 17 apartments. The expected line represents a mean difference of zero between the two groups.
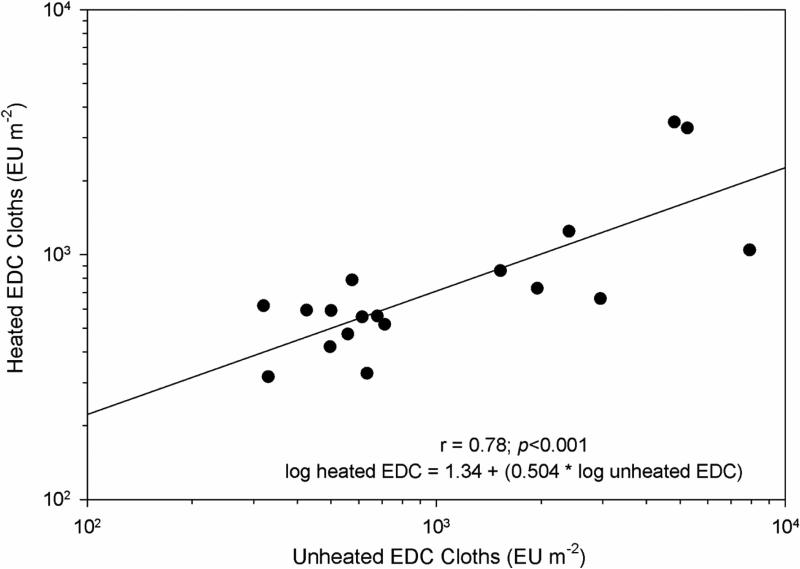
Comparisons of side-by-side EDC sampling in farm homes using heated and unheated EDC cloths is displayed in Figure 3. Over the range of endotoxin values, the unheated EDC cloth had higher endotoxin concentrations than the heated EDC cloths. The correlation between heated and unheated cloths was highly significant (r=0.78; p<0.001). A paired t-test indicated a significant difference between endotoxin concentrations of heated and unheated EDC cloths endotoxin concentrations (p=0.027).
FIGURE 3.
Comparison of 18 heated and 18 unheated EDC cloths and heated EDC cloth endotoxin concentrations sampled side-by-side for 7 days in 9 farm homes.
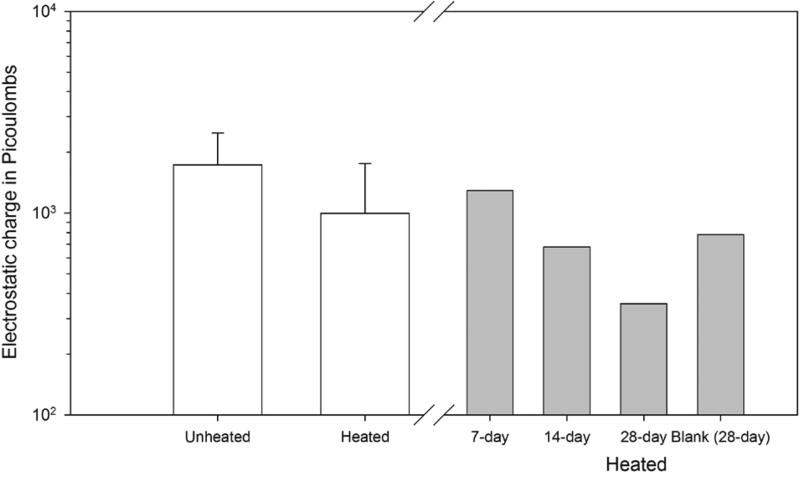
Figure 4 (open bars) displays the GM and geometric standard deviations (GSD) of the electrostatic charges detected in the12 EDC cloths measured per treatment. The electrostatic charge of the unheated EDC cloths had a higher GM compared to the heated EDC cloths. A t-test of the charges of heated and unheated cloth were significantly different (p=0.009). Figure 4 (filled bars) illustrates mean charge of 4 heated EDCs deployed in a farm home for 7, 14, or 28 days. EDC cloth charge decreased over time indicating that dust loading quenched the surface charge or that there is charge dissipation in the cloths over time. These data suggest that unheated cloths may sample and retain settled dust more effectively than heated cloths. However, since EDC cloths are not manufactured endotoxin free, unheated cloths may have inconsistent contamination that cannot be readily corrected using blanks.
FIGURE 4.
Charge comparisons between undeployed heated and unheated cloths (open bars) and EDCs deployed over 7, 14, and 28 days of sampling (shaded bars) showing progressive loss of charge over deployment time.
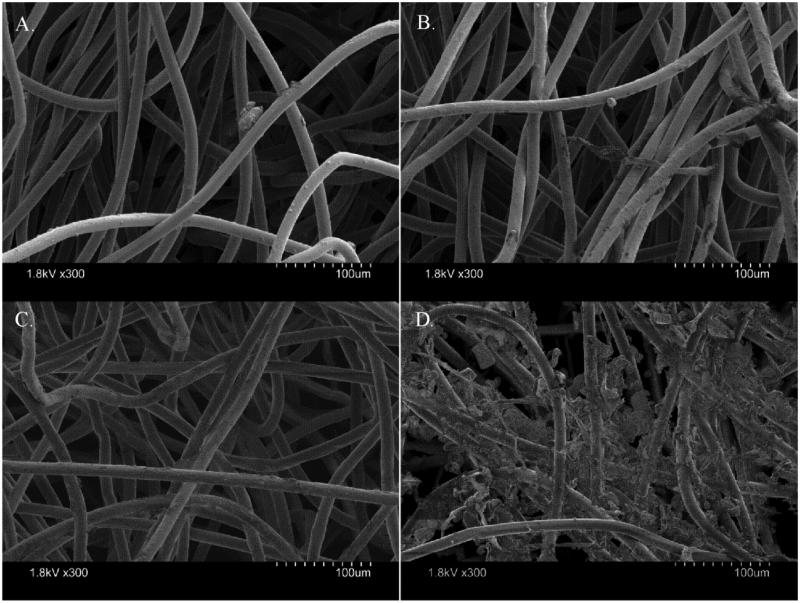
Figure 5 contains four SEM images of a heated EDC cloth deployed for 28 days (Figure 5a) and of an unheated EDC cloth deployed for 28 days (Figure 5b) on a scale of 100 μm. In each image, particulate matter of approximately 10 μm diameter is visible. In Figure 5b, a fiber approximately 110 μm long and 10 μm wide is shown. Figure 5c is an unheated blank EDC cloth and Figure 5d is a heated EDC cloth used to wipe a bookshelf (shown for comparison). Figure 5d is saturated with particulate matter. The pores in the EDC cloths appear to be up to 150 μm in size with the strands about 10 to 15 μm in diameter. There were no visual differences between particulate matter retention between the heated and unheated EDC cloths. The unheated EDC cloths (Figure 5b) did appear to have more fibers from sampling present on the cloths, which may be due to the unheated cloths having a stronger electrostatic charge. Comparing Figure 5a to Figure 5d, it is obvious that the EDC cloths are not becoming saturated during 28 days of sampling.
FIGURE 5.
SEM images of a heated EDC cloth deployed for 28 days (A), an unheated EDC cloth deployed for 28 days (B), an unheated blank EDC cloth (C), and an EDC cloth used as a wipe on a bookshelf (D) all displayed at the same magnification (see 100 μm scale).
DISCUSSION
In large cohort studies, the ability to mail EDCs to and from locations would be advantageous to lower study cost, increase sample size, and to obtain more realistic measurements of airborne endotoxin compared to vacuum sampling of reservoir floor dust. This study suggests that endotoxin concentrations are not significantly altered during mailing. This finding is reassuring because some studies already have mailed EDCs without knowing the effect mailing may have on endotoxin samples (7, 15).
In the Apartment Study, the EDCs deployed in front of ventilation compared to away from ventilation were significantly different. There was a significant difference in endotoxin loading between the two deployment areas, with bookshelves resulting in an under-sampling of endotoxin. An effort was made to avoid a full bookshelf with restrictive air movement; however, even an empty bookshelf may impede endotoxin from settling onto the EDCs. The ventilation units may be the source of increased endotoxin; particularly because the univents were older and may not have had their filters replaced or have been cleaned regularly. Madsen et al. (13) conducted a study in the Copenhagen area at the same time the Apartment Study was performed. Madsen et al. (13)deployed 6 EDCs on bookshelves and compared endotoxin concentrations to 6 EDCs deployed in an open space and found a significant difference between the two locations. This may partially explain EDCs deployed on bookshelves, away from ventilation had lower endotoxin concentrations compared to EDC deployed in front of ventilation in the Apartment Study. The combination of results from the Apartment Study and Madsen et al.(13) indicate that EDCs should be deployed away from heated ventilation and that constricted spaces such as bookcases should be avoided. The use of music stands with a horizontal platform at a height of 135 cm is an innovative approach, which was used to deploy EDCs in the Heated/Unheated Cloth Study to avoid the restrictive air circulation of a bookshelf.
The Apartment Study interquartile ranges for endotoxin concentrations were lower compared to EDCs used to sample 7 student homes in the Netherlands, where the lowest values were between 400 and 500 EU/m2 (19). Another study sampled 27 flats in the greater Copenhagen area situated close to trafficked roads and occupied by couples (n=21) or single occupants (n=6) above 50 years of age (13). The occupants were non-smokers and the endotoxin range with results for winter and spring combined was 145 to 12,919 EU/m2 with a median of 1560 EU/m2 (13). The range for that study was much higher than the range of the Apartment Study (interquartile range: 90 to 316 EU/m2). The differences in endotoxin concentrations between the two studies may partially be explained by the individuals being retired, and being at home for longer periods of time compared to the college students in the Apartment Study. Madsen et al.(13) also concluded that endotoxin was 40-times higher when occupants were home than when they were absent. Other factors that influence endotoxin concentrations such as home hygiene practices, pet ownership, or even socioeconomic status may explain some of the differences between the studies (8, 13, 20). In particularly, students in the Apartment Study were not allowed to house a pet according to University rules.
EDCs have previously been heated according to establish protocols to degrade preexisting endotoxin present from cloth packaging but the effect heating has on electrostatic charge and endotoxin sampling is unknown (7, 14). The presumption was that the impact of heating cloths would minimally affect collection efficiency and would not significantly impact the EDC cloths’ performance. In the Heated/Unheated Cloth Study, EDC cloths deployed in farm homes had significantly different endotoxin concentrations with unheated having higher endotoxin concentrations. The difference could not be explained by the endotoxin content of unsampled cloths. In the Electrostatic Charge Study, heated EDC cloths were found to have a significantly decreased electrostatic charge compared to unheated cloths. The reduction of charge due to heating could decrease the effectiveness of the electrostatic cloths to attract dust particles. The Heated/Unheated Cloth Study and the Electrostatic Charge Study both suggest that sampling efficiency may be affected by heat-treatment of EDC cloths. An alternative strategy could be to use unheated cloths and correct for background endotoxin loads by assaying a set of field blanks. Another option would be to accept the diminished the electrostatic charges of the cloths through heating and then use some method to restore the electrostatic charge on the cloths. However, it may also be possible to adjust for the difference of endotoxin concentrations between heated and unheated cloths because there was a highly significant correlation between their endotoxin concentrations.
The loss of electrostatic charge on the cloths through heat-treatment may impact the endotoxin sampling efficiency. The higher electrostatic charge of unheated EDC cloths may enhance their ability to attract and hold endotoxin and larger particles compared to the lower electrostatic charges found in heated cloths. This may explain the significant difference in endotoxin because large particles have been shown to contain larger amounts of endotoxin than smaller particles (21-22). Furthermore, heating the cloths appeared to structurally change the matrix by melting the strands together.
In a small follow up study, the electrostatic charge of heated EDC cloths deployed in a farm home over 7, 14, and 28 days was evaluated. Charge on the cloths dissipated over 7, 14, and 28 days of sampling. The dissipation of charge indicates that longer sampling periods, such as 28 days, may affect the ability of EDCs to uniformly sample endotoxin over time. As a result, 7 or 14 days of sampling may be more effective for sampling endotoxin than 28 days. Noss et al.(19) reported an anecdotal finding that EDC endotoxin concentrations only increased by 5% from 2 to 4 weeks, respectively. Assuming uniform distribution rate over the time period, the values would be expected to double from 2 to 4 weeks. Noss et al. (19)offered the explanation that EDC cloths were becoming saturated when deployment times were extended to 4 weeks. However, this study suggests that the failure of endotoxin concentrations to double from 2 to 4 weeks may partially be due to the dissipation of charge on the cloths. Kilburg-Basnyat et al.(11) support this finding as there was not a doubling in endotoxin concentrations between 14 and 28 days. However, the SEM images indicated that the cloths are capable of adhering to particles of varying sizes and that the EDC cloths fail to become saturated over a 28 day sampling period. This may further indicate that EDC cloth saturation for longer deployment times is not an issue but rather the electrostatic charge dissipating over time may be a limiting factor.
CONCLUSION
EDCs can be mailed to and from deployment sites without loss of endotoxin loading. EDCs should be deployed to avoid heated ventilation, air ducts and obstruction for more consistent sampling. Heating EDC cloths to inactivate endotoxin prior to sampling reduces their electrostatic charge and significantly reduces their endotoxin sampling efficiency. Over time, the electrostatic charge dissipates even more, indicating sampling periods beyond 14 days are ill advised.
ACKNOWLEDGEMENTS
The authors thank the occupants of the studied farm homes and the apartments for their cooperation and hospitality. Nick Verna provided assistance by express mailing the EDCs back to the University of Iowa from North Carolina. The authors would like to acknowledge use of the University of Iowa Central Microscopy Research Facility. The authors would also like to thank the University of Iowa Physics Department for providing the apparatus for electrostatic charge measurements and Sarah S. Perry for reviewing the statistical analyses.
FUNDING
This work was supported by the University of Iowa, Environmental Health Sciences Research Center [NIH P30 ES005605]. The Hitachi S-4800 SEM was acquired by the University of Iowa Central Microscopy Research Facility through an NIH Shared Instrumentation Grant [1 S10 RR022498-01].
REFERENCES
- 1.Hughes J, Gaunt L, Gaynor P. Electrostatic targeting for allergen removal and pest control applications. Journal of electrostatics. 2002;55(3):237–245. [Google Scholar]
- 2.Gaynor P, Hughes J. Dust anchoring characteristics of electret fibres with respect to Der p 1 allergen carrying particles. Medical and Biological Engineering and Computing. 1998;36(5):615–620. doi: 10.1007/BF02524433. [DOI] [PubMed] [Google Scholar]
- 3.Hughes J. Electro-Technology. 1996. Electrostatic Allergen Control. pp. 13–16. [Google Scholar]
- 4.Würtz H, Sigsgaard T, Valbjørn O, Doekes G, Meyer HW. The dustfall collector–a simple passive tool for long-term collection of airborne dust: a project under the Danish Mould in Buildings program (DAMIB). Indoor Air. 2005;15(s9):33–40. doi: 10.1111/j.1600-0668.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 5.Frankel M, Timm M, Hansen EW, Madsen AM. Comparison of sampling methods for the assessment of indoor microbial exposure. Indoor Air. 2012;22(5):405–414. doi: 10.1111/j.1600-0668.2012.00770.x. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson AS, Hedren M, Almqvist C, Larsson K, Renström A. Evaluation of Petri dish sampling for assessment of cat allergen in airborne dust. Allergy. 2002;57(2):164–168. doi: 10.1034/j.1398-9995.2002.1s3297.x. [DOI] [PubMed] [Google Scholar]
- 7.Noss I, Wouters IM, Visser M, Heederik DJJ, Thorne PS, Brunekreef B, et al. Evaluation of a Low-Cost Electrostatic Dust Fall Collector for Indoor Air Endotoxin Exposure Assessment. Applied and Environmental Microbiology. 2008;74(18):5621–5627. doi: 10.1128/AEM.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J-H, Spiegelman DL, Gold DR, Burge HA, Milton DK. Predictors of airborne endotoxin in the home. Environmental Health Perspectives. 2001;109(8):859. doi: 10.1289/ehp.01109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samadi S, Heederik DJ, Krop EJ, Jamshidifard A-R, Willemse T, Wouters IM. Allergen and endotoxin exposure in a companion animal hospital. Occupational and Environmental Medicine. 2010;67(7):486–492. doi: 10.1136/oem.2009.051342. [DOI] [PubMed] [Google Scholar]
- 10.Kilburg-Basnyat B, Peters TM, Perry SS, Thorne PS. Electrostatic Dust Collectors Compared to Inhalable Samplers for Measuring Endotoxin Concentrations in Farm Homes. 2015 doi: 10.1111/ina.12243. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilburg-Basnyat B, Metwali N, Thorne PS. Effect of Deployment Time on Endotoxin and Allergen Exposure Assessment Using Electrostatic Dust Collectors. Annals of Occupational Hygiene. 2015;59(1):104–115. doi: 10.1093/annhyg/meu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liebers V, van Kampen V, Bünger J, Düser M, Stubel H, Brüning T, et al. Assessment of airborne exposure to endotoxin and pyrogenic active dust using electrostatic dustfall collectors (EDCs). Journal of Toxicology and Environmental Health, Part A. 2012;75(8-10):501–507. doi: 10.1080/15287394.2012.674919. [DOI] [PubMed] [Google Scholar]
- 13.Madsen AM, Matthiesen CB, Frederiksen MW, Frederiksen M, Frankel M, Spilak M, et al. Sampling, extraction and measurement of bacteria, endotoxin, fungi and inflammatory potential of settling indoor dust. Journal of Environmental Monitoring. 2012;14(12):3230–3239. doi: 10.1039/c2em30699a. [DOI] [PubMed] [Google Scholar]
- 14.Thorne PS, Metwali N, Avol E, McConnell RS. Surface sampling for endotoxin assessment using electrostatic wiping cloths. Annals of Occupational Hygiene. 2005;49(5):401–406. doi: 10.1093/annhyg/mei002. [DOI] [PubMed] [Google Scholar]
- 15.Vredegoor DW, Willemse T, Chapman MD, Heederik DJ, Krop EJ. Can f 1 levels in hair and homes of different dog breeds: lack of evidence to describe any dog breed as hypoallergenic. Journal of Allergy and Clinical Immunology. 2012;130(4):904–909. e907. doi: 10.1016/j.jaci.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs JH, Krop EJ, de Wind S, Spithoven J, Heederik DJ. Endotoxin levels in homes and classrooms of Dutch school children and respiratory health. European Respiratory Journal. 2013;42(2):314–322. doi: 10.1183/09031936.00084612. [DOI] [PubMed] [Google Scholar]
- 17.Thorne PS, Metwali N, Mendy A, Salo P, Co C, Jaramillo R, et al. Endotoxin exposure:predictors and associated respiratory health outcomes prevalent in the U.S. In preparation. 2015 doi: 10.1164/rccm.201502-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorne PS. Inhalation toxicology models of endotoxin- and bioaerosol-induced inflammation. Toxicology. 2000;152(1–3):13–23. doi: 10.1016/s0300-483x(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 19.Noss I, Wouters IM, Bezemer G, Metwali N, Sander I, Raulf-Heimsoth M, et al. β-(1,3)-Glucan Exposure Assessment by Passive Airborne Dust Sampling and New Sensitive Immunoassays. Applied and Environmental Microbiology. 2010;76(4):1158–1167. doi: 10.1128/AEM.01486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne PS, Cohn RD, Mav D, Arbes SJ, Jr, Zeldin DC. Predictors of Endotoxin Levels in US Housing. Environmental Health Perspectives. 2009;117(5):763–771. doi: 10.1289/ehp.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soukup JM, Becker S. Human Alveolar Macrophage Responses to Air Pollution Particulates Are Associated with Insoluble Components of Coarse Material, Including Particulate Endotoxin. Toxicology and Applied Pharmacology. 2001;171(1):20–26. doi: 10.1006/taap.2000.9096. [DOI] [PubMed] [Google Scholar]
- 22.Schins RPF, Lightbody JH, Borm PJA, Shi T, Donaldson K, Stone V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicology and Applied Pharmacology. 2004;195(1):1–11. doi: 10.1016/j.taap.2003.10.002. [DOI] [PubMed] [Google Scholar]