Abstract
Background: Little is known about the acute or immediate effects of walking exercise and yoga on mood in people with multiple sclerosis (MS). Such an examination is important for identifying an exercise modality for inclusion in exercise-training interventions that yields mood benefits in MS. We examined the effects of single bouts of treadmill walking and yoga compared with a quiet, seated-rest control condition on acute mood symptoms in MS.
Methods: Twenty-four participants with MS completed 20 minutes of treadmill walking, yoga, or quiet rest in a randomized, counterbalanced order with 1 week between sessions. Participants completed the Profile of Mood States questionnaire before and immediately after each condition. Total mood disturbance (TMD) and the six subscales of the Profile of Mood States were analyzed using repeated-measures analysis of variance and paired-samples t tests.
Results: There was a significant condition × time interaction on TMD scores (ηp2 = 0.13). Walking and yoga conditions yielded comparable reductions in TMD scores. There was a significant condition × time interaction on vigor (ηp2 = 0.23) whereby walking but not yoga yielded an improvement in vigor. There was a significant main effect of time on anger, confusion, depression, and tension (P < .05) but not on fatigue.
Conclusions: Walking and yoga yielded similar improvements in overall acute mood symptoms, and walking improved feelings of vigor. These effects should be further investigated in long-term exercise-training studies.
Multiple sclerosis (MS) is an immune-mediated, demyelinating disease of the central nervous system with an estimated prevalence of more than 2 million adults worldwide.1 This disease results in a wide range of psychological symptoms, including moods of tension/anxiety, depression, fatigue, and anger.2,3 These moods are associated with negative consequences for people with MS, including sleep disturbances, poor quality of life,4 and mobility disability.5 This underscores the importance of identifying approaches for managing the mood disturbances associated with MS.
Exercise training, defined as a planned, structured regimen of regular physical activity deliberately performed to improve one or more components of physical fitness (ie, aerobic capacity or muscle strength and endurance),6 represents a promising option for managing mood disturbances in MS.7 For example, a recent meta-analysis indicated a small but statistically significant mood-enhancing effect of exercise training on depressive mood states in people with MS.8 Such a small effect of exercise training on depressive and possibly other moods in MS might be associated with a lack of focal inquiry regarding the exercise modality for yielding mood benefits. That is, some modes of exercise might yield larger changes in mood than others.
One approach that might help clarify the exercise modality necessary for maximizing mood benefits in MS involves examining the effects of single bouts of exercise on acute mood symptoms. The assumption of this approach is that the mood benefits associated with a single bout of exercise might accumulate over time with repetitive, or long-term, exercise training. This would seemingly translate into larger benefits of exercise training on mood states in MS—if we identify and include a stimulus that is effective for short-term benefits. Such an approach is consistent with the framework for studying the physiological effects of single bouts of exercise that then accumulate as long-term, physiological adaptations with exercise training.9 Collectively, this represents a promising starting point for systematically developing exercise-training interventions for improving mood states in people with MS.
To date, there has been limited research examining the effect of single bouts of exercise on acute mood symptoms in people with MS, although there is a large body of research examining single bouts of exercise (eg, cycling, walking, resistance training, or aqua aerobics)10–13 on acute mood symptoms in the general population.14 We are aware of one study15 that examined changes in total mood disturbance (TMD; higher scores indicate worse mood) on the Profile of Mood States (POMS) questionnaire after a single session of cycling exercise in women with MS. That study reported significant reductions in TMD scores 20 and 60 minutes after the single session of cycling exercise. The magnitude of the improvement was moderate based on Cohen's d values of 0.59 and 0.54 for TMD scores 20 and 60 minutes after exercise, respectively. The primary limitation of that study included the absence of a control condition that accounted for the passage of time, repeated test administration, and interactions with researchers. An additional limitation is the lack of reporting regarding specific mood changes on the POMS subscales. Another limitation included the focus on a single modality of exercise. Indeed, there has been limited comparison of other forms of exercise (ie, walking) with yoga, as an example, for improving acute mood symptoms in people with MS, and such a comparison is important for recommending yoga as an intervention tool for improving mood in people with MS.16
Yoga is a relatively less frequently studied exercise stimulus compared with other, more traditional modes of exercise (eg, walking and cycling) in MS, and reviews comparing the beneficial effects of yoga with other forms of exercise modalities indicate potential benefits of a regular yoga practice.17 A qualitative review of yoga as a therapeutic approach for a multitude of disease conditions suggested that regular yoga practice can enhance well-being and mental health, including symptoms of depression, tension, and fatigue.18 However, there are minimal data on the immediate effects of such stimuli on acute mood symptoms. One study comparing the effects of single sessions of aerobic dance and hatha yoga on psychological well-being in young adults (ie, college-aged individuals) reported that both conditions reduced perceived stress and negative affect comparably; however, only hatha yoga resulted in an improvement in the physiological correlates (ie, cortisol response) that might be underlying the stress and negative affect.19 These results further emphasize the importance of comparing different exercise modalities for improving acute mood symptoms.
The present study compared the effects of acute bouts of treadmill walking and guided yoga exercise with a quiet rest control condition on acute mood symptoms in people with MS using a within-subjects design. The hypotheses were based on the bipolar activation theory of Thayer.20 This theory states that there are two general mood systems that exist on opposite ends of a single continuum of activation dimensions, namely, energetic activation/arousal (ranging from feeling sleepy to feeling awake) and tense activation/arousal (ranging from feeling calm to feeling nervous).21 This theory further suggests that there is a dynamic balance between tense arousal and energetic arousal that is evident in exercise; for example, a tired person (such as after exercising) will likely experience little anxiety.22 Accordingly, we hypothesized that walking and yoga would be associated with reductions in TMD scores compared with the control condition but that there would be differential patterns of change for the vigor subscale in response to treadmill walking versus guided yoga. We hypothesized that walking would result in increased scores on the vigor subscale based on heightened activation levels and valence compared with the yoga condition. This hypothesis is further in line with the possible physiological basis of the acute mood symptoms of yoga as cited in previous studies.17–19 We further hypothesized that the POMS subscales of anger, confusion, tension, depression, and fatigue would respond with greater improvements (ie, score reductions) after guided yoga and walking compared with after quiet rest.
Methods
Participants
Prospective participants were identified from a database of individuals with MS who were previously involved in studies conducted in our laboratory at the University of Illinois at Urbana-Champaign. The study protocol was described to prospective participants via telephone. If the individual was interested, screening for the following inclusion criteria was conducted: 1) definite diagnosis of MS, 2) relapse-free for the past 30 days, 3) ability to read a 14-point font, 4) ability to walk independently or with minimal assistance (ie, a cane or crutch but not a walker/bilateral support), 5) age 18 to 54 years, 6) willingness and ability to walk on a motor-driven treadmill and complete serial mood assessments, and 7) low risk of contraindications of maximal exercise testing. The low risk of contraindications was based on no more than a single “yes” response on the Physical Activity Readiness Questionnaire,23 along with a physician's approval. We did not screen for mood disorders. Of 84 individuals contacted via telephone, 36 were interested and underwent screening. Eleven participants who qualified declined to participate in the study, citing lack of time; one participant did not qualify based on the age criterion. This resulted in a final sample of 24 people with MS.
The POMS
This study included the 30-item abbreviated POMS. This version of the POMS was designed to measure transient or fluctuating affective states in psychiatric and normal samples.24 The abbreviated version consists of 30 adjectives that are rated using the “Right Now” response set on a 5-point scale with anchors of 0 (not at all) and 4 (extremely). The “Right Now” response set allows for measuring the changes in mood from immediately before to after exercise, thereby isolating the factors that can influence mood during the exercise bouts. The primary outcome, TMD, was calculated by adding all the scores from the subscales of Anxiety-Tension, Anger-Hostility, Depression-Dejection, Fatigue-Inertia, and Confusion-Bewilderment and then subtracting the score on the Vigor-Activity subscale. The POMS has strong evidence for its validity and reliability across diverse samples,25,26 and it is the most common measure of mood states for studies of single sessions of exercise.14
Aerobic Capacity
We measured aerobic capacity for describing the fitness level of the sample and precise prescription of the intensity of the treadmill walking exercise condition. Briefly, aerobic capacity was measured as peak oxygen consumption (V̇o2peak) using an incremental exercise test to exhaustion on an electronically braked, computer-driven cycle ergometer (Lode BV; Groningen, the Netherlands) and a calibrated open-circuit spirometry system (TrueOne; Parvo Medics, Sandy, UT) for analyzing expired gases. Participants initially rested on the cycle ergometer for measurement of resting heart rate (HR). The incremental exercise test was preceded by a 3-minute warm-up at 0 W. The initial work rate for the incremental exercise test was 0 W, and the work rate continuously increased at a rate of 15 W/min until the participant reached volitional fatigue (ie, the inability to continue exercising). This protocol has been validated in people with MS for measuring peak aerobic capacity.27 The V̇o2, respiratory exchange ratio, and work rate were measured continuously by the open-circuit spirometry system and are expressed as 20-second averages. The HR was displayed using a Polar HR monitor (Polar Electro Oy, Kempele, Finland), and HR and rating of perceived exertion (RPE)28 were recorded every minute. The V̇o2peak was expressed in mL/kg/min based on the highest recorded 20-second V̇o2 value when two of three criteria were satisfied: 1) respiratory exchange ratio of at least 1.10, 2) peak HR within 10 bpm of the age-predicted maximum (ie, ~1 SD), and 3) peak RPE of at least 17.
To prescribe the intensity of the treadmill walking exercise condition, we calculated 60% HR reserve (HRR; ie, moderate intensity) using the Karvonen equation ([(peak HR − resting HR) × 0.6] + resting HR) using resting and peak HR values obtained during the incremental exercise test.9 This exercise intensity is consistent with previous research on the effects of acute exercise on mood outcomes.15
Disability Status
All the participants underwent a neurologic examination by a Neurostatus-certified examiner (IE or BMS) who generated Expanded Disability Status Scale (EDSS)29 scores. The EDSS scores were generated for describing the sample.
Procedure and Experimental Sessions
The procedures were approved by the University of Illinois institutional review board, and all the participants provided written informed consent. The study procedures consisted of four testing sessions conducted in our laboratory, and the sessions were separated by 1 week. The first testing session was a baseline session, and the other sessions (ie, sessions 2–4) consisted of the experimental conditions (ie, treadmill walking, guided yoga, and quiet rest).
During the baseline session, participants initially completed a short demographics questionnaire. The Neurostatus-certified assessor then administered a neurologic examination to generate an EDSS score. Participants then underwent the incremental exercise test to exhaustion on a cycle ergometer for measurement of aerobic capacity and V̇o2 as a function of HR for subsequent and precise prescription of the exercise intensity for the submaximal treadmill walking session.
The subsequent experimental conditions (ie, sessions 2–4) occurred in a within-subjects design, and the order of the sessions was counterbalanced and randomized across participants. Participants initially completed the POMS questionnaire while seated in a comfortable chair (ie, pretest). Participants then performed the assigned condition (ie, treadmill walking, guided yoga, or quiet rest) and completed the POMS again within 5 minutes after the condition (ie, posttest). All the participants were asked to void their bladder before beginning any exercise protocol to avoid interruptions later on and were allowed to use the restroom if needed during exercise. However, none chose to do so.
Treadmill Walking
The treadmill walking condition began with a 5-minute warm-up, proceeding to 20 minutes of exercise at an intensity that was associated with 60% HRR (ie, moderate intensity),9 followed by a 5-minute cooldown. The treadmill speed and grade work rate were initially selected using American College of Sports Medicine equations based on energy expenditure,9 and the actual intensity during each acute session was monitored and documented by HR (using the Polar heart rate monitor [Polar Electro Oy]) and RPE. The walking protocol was conducted individually on a one-on-one basis with the participant. The experimenter (BMS), who has experience and training in supervising and monitoring of exercise protocols in individuals with MS, was accompanied by a research assistant, who was trained in exercise supervision of individuals with MS, for help if/when necessary. There was minimal interaction between researchers and participants, except when recording the RPE.
Yoga Exercise
The yoga session began with 5 minutes of centering, continued with 20 minutes of hatha yoga modified for people with MS, and concluded with 5 minutes of meditation/deep breathing (ie, savasana or corpse pose). The hatha yoga consisted of nine postures (ie, seated cat and cow, seated twist, child's pose, downward-facing dog, supported forward bend, sun salutation, seated warrior-2, supported triangle pose, and seated forward bend) that involved isometric muscle contraction and relaxation of different muscle groups in conjunction with regulated breathing. Participants mirrored an exercise leader who performed the postures alongside the participants and spotted them when necessary. The intensity during each acute yoga session was monitored and documented by HR and RPE. The yoga protocol was developed by a licensed yoga teacher who works as a yoga instructor at local yoga studios. The session was delivered to the participants on a one-on-one basis by either the yoga instructor or the experimenter (BMS), who was trained by the yoga instructor on the delivery process. This is the same experimenter who supervised the walking session and who has experience and training in exercise supervision of individuals with MS. A research assistant trained in the protocol and experienced in exercise training with individuals with MS was present as a spotter if/when necessary.
Control Condition
The control condition involved sitting quietly without doing any other tasks for 30 minutes in a comfortable chair located in the research laboratory in the same area as the experimenter, who was, therefore, present to monitor the participant during this period. The participant did not read, check his or her mobile phone, or sleep during this condition. The participant did not engage with anyone or get up from the chair, and the experimenter was able to monitor the participant to make sure that he or she was following the protocol. The experimenter checked on the participant with the same regularity as the interactions during the walking exercise condition. This is an optimal control condition for studies of acute exercise and mood because it accounts for the passage of time and its potential transient effect on mood.30 Neither HR nor RPE was monitored during the resting condition, but we attempted to control for the degree of interaction between the experimenter and the participant by having the experimenter check on the participant at regular intervals and having the participant sit in the same area as the experimenter during the 30-minute period.
Data Analysis
All data analyses were conducted using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY). Descriptive statistics for demographic and clinical characteristics are given as mean (SD) throughout the text and Table 1. Separate repeated-measures analyses of variance (ANOVAs) were conducted for identifying the interactions and main effects of time (pre-post) and condition (walking, yoga, and control) on TMD scores, vigor subscale scores, and scores from each of the five remaining subscales. We present effect sizes (ESs) as Hedges' g, which is computed by subtracting the post-intervention POMS score from the pre-intervention POMS score and dividing this change by the pooled standard deviation of change per condition. Hedges' g was chosen to estimate ES because this statistic corrects for the smaller sample size and, therefore, provides a better estimate of the variance.31 The magnitude of the ES was interpreted using the cutoff points for small (0.2), moderate (0.5), and large (0.8) changes.32 The alpha value a priori was set at 0.05.
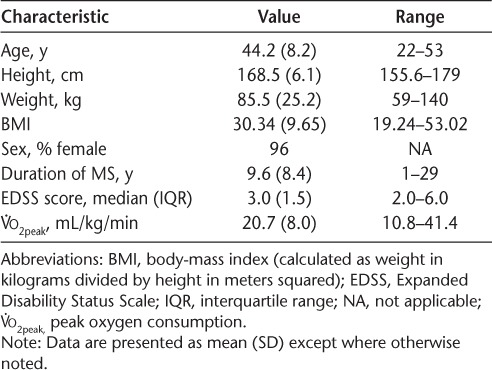
Table 1.
Sample baseline characteristics of the 24 study participants
Results
Sample Characteristics
Demographic and clinical characteristics of the sample are provided in Table 1. The sample included 24 individuals (23 women [96%]; mean age, 44.2 years) with relapsing-remitting MS. The mean disease duration was 9.6 years, and the median EDSS score was 3.0, which reflects that, on average, the sample was fully ambulatory, with mild-to-moderate disability.29 The sample demonstrated a mean V̇o2peak of 20.7 mL/kg/min. This value is similar to other samples of individuals with mild-to-moderate MS disability.27,33
Experimental Conditions
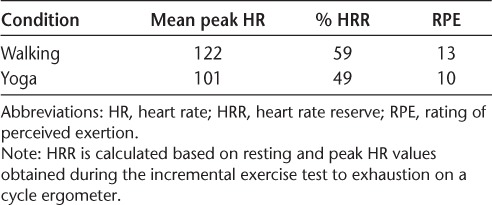
Descriptive statistics for exercise intensity (ie, HR and RPE) for treadmill walking exercise and yoga are provided in Table 2. Based on these data, participants were working approximately at the target moderate intensity (ie, 60% HRR) for the treadmill walking exercise and slightly below moderate intensity (ie, 49%) for the yoga session (Table 2). Mean peak RPE values reported in each condition were 13 in the walking condition and 10 in the yoga condition. Collectively, this indicates that the treadmill walking exercise and yoga conditions were performed at relatively moderate intensities (ie, HRR 40%–60% based on the American College of Sports Medicine physical activity guidelines).9
Table 2.
Peak HR and RPE and % HRR during treadmill walking exercise and guided yoga
TMD Scores
The pre-post condition mean (SD) scores for TMD are provided in Table 3. The repeated-measures ANOVA indicated a statistically significant condition × time interaction on TMD (F2,46 = 3.48, P < .05, ηp2 = 0.13) (Figure 1). The walking and yoga conditions resulted in comparable pre-post condition reductions of TMD scores (Δ = 3.67 and 3.70 points, respectively), and the changes were moderate in magnitude, as determined by the ESs per condition compared with the control condition (ie, walking vs. quiet rest, yoga vs. quiet rest) (Hedges' g = 0.30 and 0.27 for walking and yoga, respectively). There was no significant pre-post change in TMD for the control condition (P < .05). Paired-samples t tests indicated a significant pre-post change in TMD scores for the walking and yoga conditions (T2-3 = 2.47 and 2.65, respectively, P < .05).
Table 3.
Mean (SD) POMS subscores pre-post condition (N = 24)
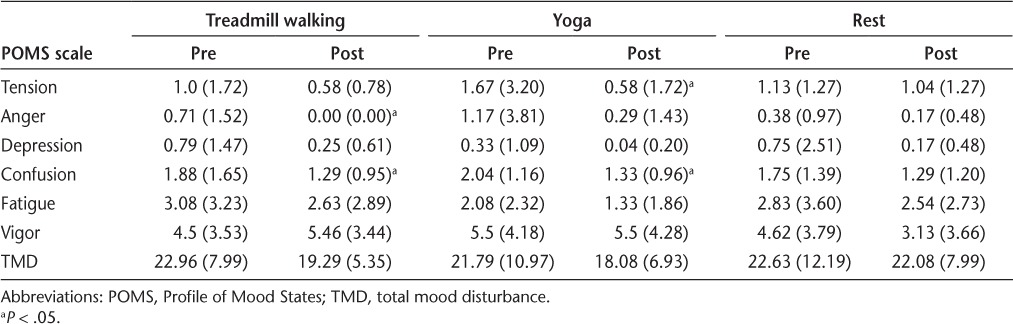
Figure 1.
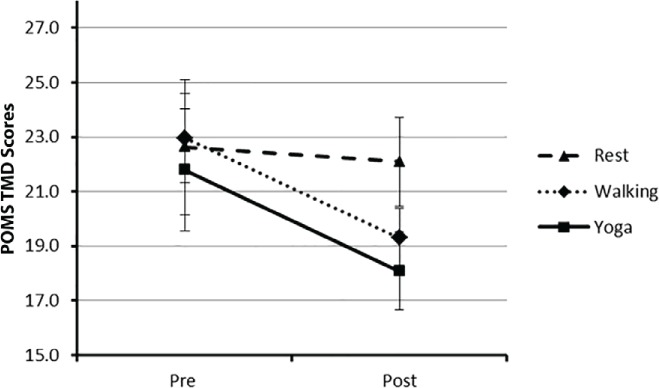
Mean total mood disturbance (TMD) scores for all three conditions pre-post condition
Error bars represent standard errors. POMS, Profile of Mood States.
Vigor
The pre-post condition mean (SD) scores for the vigor subscale are provided in Table 3. The ANOVA indicated a significant condition × time interaction on vigor scores (F2,46 = 6.70, P < .05, ηp2 = 0.23). Vigor scores increased immediately after walking but remained the same immediately after yoga and decreased after the control condition (Figure 2). The magnitude of change was moderate based on the a priori cutoff points for ES estimation.32 These patterns of change were further confirmed by paired-samples t tests (T2-3 = −1.82, 0.00, and 3.27 for walking, yoga, and quiet rest, respectively, P < .05).
Figure 2.
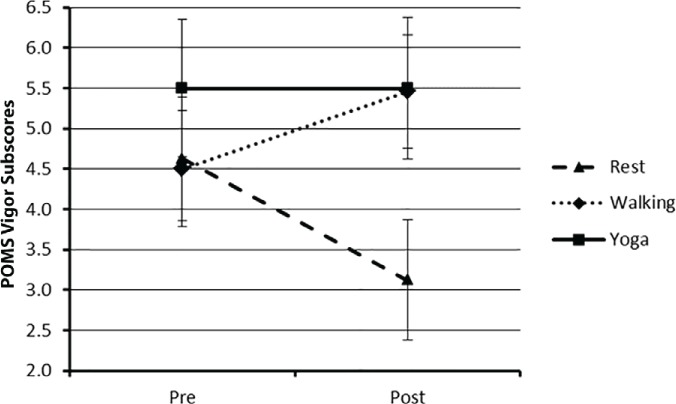
Mean vigor subscores for all three conditions pre-post condition
Error bars represent standard errors. POMS, Profile of Mood States.
Tension, Depression, Anger, Confusion, and Fatigue
The pre-post condition mean (SD) scores for the subscales for all three conditions are provided in Table 3. There were no significant interactions on fatigue or the tension, depression, anger, and confusion subscales (P > .05). There were significant time main effects for the tension (F1,23 = 4.91, ηp2 = 0.18), depression (F1,23 = 4.23, ηp2 = 0.16), anger (F1,23 = 5.15, ηp2 = 0.18), and confusion (F1,23 = 12.57, ηp2 = 0.35) subscales but not for fatigue (F1,23 = 2.54, ηp2 = 0.10). Paired-samples t tests further confirmed improved scores on anger and confusion after walking (T2-3 = 2.29, P < .05 and T2-3 = 2.51, P < .05, respectively) and improved scores on tension and confusion after yoga (T2-3 = 2.52, P < .05 and T2-3 = 3.33, P < .05, respectively) (Table 3).
Discussion
To our knowledge, this is the first study to investigate the differential effects of single sessions of walking and guided yoga on acute mood symptoms in people with MS using a within-subjects design. The TMD improved by a moderate magnitude after both treadmill walking and yoga but remained unchanged for the control condition; the effects of walking and yoga on TMD were not statistically significantly different. We further observed an increase in vigor after walking but not after the yoga session (ηp2 = 0.23). There were no interactions for fatigue or the tension, depression, anger, and confusion subscales, but there were main effects of time on the tension, depression, anger, and confusion subscales. This indicated that the three conditions were associated with nonspecific reductions in those acute mood symptoms. Collectively, the present results might have informational value for the design of future studies of exercise training for managing acute mood symptoms in MS.
The present findings extend those from Petruzzello et al.15 by suggesting that acute mood symptoms can be improved within minutes of a single bout of exercise using two new modalities other than cycling (ie, treadmill walking and yoga). These results may have several implications. Treadmill walking and yoga improved mood after only a single bout of exercise, which suggests that those modalities of exercise can be an effective tool for managing acute mood symptoms for individuals with MS. This is important considering that the prevalence of poor mood symptoms in adults with MS is approximately three times that of the general population.34 This emphasizes the importance of engaging in moderate-intensity exercise for people with MS to potentially manage and perhaps prevent this cluster of negative mood symptoms. The ability of these exercise stimuli to induce positive effects less than 5 minutes after the termination of exercise suggests that exercise is particularly useful when dealing with acute mood symptoms. Single bouts of various forms of exercise seem to be well tolerated by individuals with MS who have low levels of neurologic disability and may provide an immediate improvement in well-being (ie, improvement in acute mood symptoms).
Another noteworthy finding from the present study is that vigor increased after treadmill walking exercise but not after yoga relative to quiet rest. This was consistent with the secondary hypothesis and indicates that walking was more energizing than yoga for individuals with MS. Of note, similar results were obtained in a previous study conducted with 113 psychiatric inpatients who completed the POMS before and after participation in a session of yoga.35 The authors of that study noted improvements in the five negative mood states (ie, depression, tension, anger, confusion, and fatigue), but there was no change in vigor scores.35 This might be related to feelings of postexercise activation (ie, increased energetic arousal) based on Thayer's multidimensional model of energetic activation.20 Participants might have felt more “activated” or “energized” immediately after moderate-intensity treadmill walking than immediately after yoga, which would seemingly explain the differential ratings of vigor across both conditions. Although both walking and yoga were performed at relatively moderate intensities, the yoga protocol ended with 5 minutes of meditation/deep breathing (ie, corpse pose or savasana). Based on Thayer's activation model,20 it is possible that those final 5 minutes of savasana at the end may have resulted in lowered activation in the energy-vigor dimension, which may have led to a de-energized state after the yoga stimulus. Finally, fatigue did not change significantly after guided yoga or walking. This suggests that acute bouts of guided yoga and treadmill walking might not induce fatigue in individuals with MS. This is important given that fatigue is a highly prevalent symptom of MS.36
This study is not without limitations. First, the sample was primarily composed of women (ie, 96%), and, therefore, the results might not be representative of the acute mood changes that occur in men. Similarly, based on the V̇o2peak levels measured during the incremental exercise test, the sample had relatively lower levels of fitness compared with healthy samples without MS, and, therefore, individuals with higher fitness levels might react differently to the same stimulus. Similarly, the sample had a relatively short duration of MS (ie, ~10 years) and relatively mild MS disability based on EDSS scores; and previous studies indicate changes in mood symptoms with increasing age and EDSS scores.37 Therefore, the present results might not be applicable to those with MS for longer durations or with a higher disability status. Another limitation is the possibility of a supervision effect; that is, there was more social interaction and engagement in the walking and guided yoga conditions than in the quiet rest condition. Note that, as described previously in the “Methods” section, there was some engagement between the participants and the experimenter during the quiet rest condition to match the other two experimental conditions; however, this might not have been comparable. Finally, we do not have data on POMS scores at later time points (eg, 1 hour after the condition) or on the possible prevalence of mood disorders in the participants at baseline; these should be addressed in future studies.
In conclusion, the present results indicate that a single bout of exercise might be beneficial in inducing mood-improving effects in people with MS and that the modality of the exercise might not matter if the goal is to improve overall acute mood symptoms (eg, TMD). However, different modalities of exercise might be more effective for improving vigor. Future studies should account for trait anxiety, clinical mood disturbances, and habitual physical activity levels in the sample to be able to account for the possible influence of these two important factors on the acute mood symptom effects of acute exercise. The mental health benefits of exercise for individuals with MS need to be replicated in other research endeavors to increase our understanding of the specific factors that might be influencing the individual responses to different modalities and intensities of exercise. For example, one important issue involves examining the acute mood-enhancing benefits of treadmill walking at different intensities to determine whether different intensities of acute walking are more beneficial for improving acute mood symptoms in individuals with MS. Similarly, comparing different types of yoga (eg, hatha, vinyasa, yin, etc.) to identify the optimal style when considering varying levels of disability can be particularly helpful for individuals with MS. Finally, identifying the immediate effects of different exercise modalities has further benefits for the individual because this gives more choices for exercises, meaning that they can choose what they enjoy the most, which increases the likelihood of adherence to a regular exercise regimen in the longer term.
PracticePoints.
Acute bouts (ie, ~20 minutes) of moderate-intensity exercise can be sufficient to improve acute mood symptoms in people with MS.
Different types of exercise, such as walking and yoga, can be equally effective in improving acute mood symptoms in people with MS.
Future studies should compare the acute mood-enhancing effects of different types and intensities of exercise in people with MS.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Wade BJ. Spatial analysis of global prevalence of multiple sclerosis suggests need for an updated prevalence scale. Mult Scler Int. 2014;2014:124578. doi: 10.1155/2014/124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joffe RT, Lippert GP, Gray TA, Sawa G, Horvath Z. Mood disorder and multiple sclerosis. Arch Neurol. 1987;44:376–378. doi: 10.1001/archneur.1987.00520160018007. [DOI] [PubMed] [Google Scholar]
- 3.Jones KH, Ford DV, Jones PA et al. A large-scale study of anxiety and depression in people with multiple sclerosis: a survey via the web portal of the UK MS Register. PLoS One. 2012;7:e41910. doi: 10.1371/journal.pone.0041910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobentanz IS, Asenbaum S, Vass K et al. Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neurol Scand. 2004;110:6–13. doi: 10.1111/j.1600-0404.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 5.Marrie RA, Rudick R, Horwitz R et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74:1041–1047. doi: 10.1212/WNL.0b013e3181d6b125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 7.Feinstein A, Rector N, Motl R. Exercising away the blues: can it help multiple sclerosis–related depression? Mult Scler. 2013;19:1815–1819. doi: 10.1177/1352458513508837. [DOI] [PubMed] [Google Scholar]
- 8.Ensari I, Motl RW, Pilutti LA. Exercise training improves depressive symptoms in people with multiple sclerosis: results of a meta-analysis. J Psychosom Res. 2014;76:465–471. doi: 10.1016/j.jpsychores.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 9.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. Baltimore, MD: Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 10.Focht BC. Pre-exercise anxiety and the anxiolytic responses to acute bouts of self-selected and prescribed intensity resistance exercise. J Sports Med Phys Fitness. 2002;42:217–223. [PubMed] [Google Scholar]
- 11.Ströhle A, Feller C, Onken M, Godemann F, Heinz A, Dimeo F. The acute antipanic activity of aerobic exercise. Am J Psychiatry. 2005;162:2376–2378. doi: 10.1176/appi.ajp.162.12.2376. [DOI] [PubMed] [Google Scholar]
- 12.Wininger SR. The anxiolytic effect of aqua aerobics in elderly women. Percept Mot Skills. 2002;94:338–340. doi: 10.2466/pms.2002.94.1.338. [DOI] [PubMed] [Google Scholar]
- 13.Smith JC. Effects of emotional exposure on state anxiety after acute exercise. Med Sci Sports Exerc. 2013;45:372–378. doi: 10.1249/MSS.0b013e31826d5ce5. [DOI] [PubMed] [Google Scholar]
- 14.Berger BG, Motl RW. Exercise and mood: a selective review and synthesis of research employing the profile of mood states. J Appl Sport Psychol. 2000;12:69–92. [Google Scholar]
- 15.Petruzzello SJ, Snook EM, Gliottoni RC, Motl RW. Anxiety and mood changes associated with acute cycling in persons with multiple sclerosis. Anxiety Stress Coping. 2009;22:297–307. doi: 10.1080/10615800802441245. [DOI] [PubMed] [Google Scholar]
- 16.Cramer H, Lauche R, Azizi H, Dobos G, Langhorst J. Yoga for multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2014;9:e112414. doi: 10.1371/journal.pone.0112414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross A, Thomas S. The health benefits of yoga and exercise: a review of comparison studies. J Altern Complement Med. 2010;16:3–12. doi: 10.1089/acm.2009.0044. [DOI] [PubMed] [Google Scholar]
- 18.Woodyard C. Exploring the therapeutic effects of yoga and its ability to increase quality of life. Int J Yoga. 2011;4:49–54. doi: 10.4103/0973-6131.85485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West J, Otte C, Geher K, Johnson J, Mohr DC. Effects of Hatha yoga and African dance on perceived stress, affect, and salivary cortisol. Ann Behav Med. 2004;28:114–118. doi: 10.1207/s15324796abm2802_6. [DOI] [PubMed] [Google Scholar]
- 20.Thayer R. Toward a psychological theory of multidimensional activation (arousal) Motiv Emot. 1978;2:1–34. [Google Scholar]
- 21.Schimmack U, Rainer R. Experiencing activation: energetic arousal and tense arousal are not mixtures of valence and activation. Emotion. 2002;2:412–417. doi: 10.1037/1528-3542.2.4.412. [DOI] [PubMed] [Google Scholar]
- 22.Thayer RE. The Biopsychology of Mood and Arousal. New York, NY: Oxford University Press; 1989. [Google Scholar]
- 23.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Can J Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- 24.McNair D, Lorr M, Droppleman L. Profile of Mood States (POMS) Manual. San Diego, CA: Education and Industrial Testing Service; 1971. [Google Scholar]
- 25.Reddon JR, Marceau R, Holden RR. A confirmatory evaluation of the Profile of Mood States: convergent and discriminant item validity. J Psychopathol Behav Assess. 1985;7:243–259. [Google Scholar]
- 26.McNair D, Lorr M, Droppleman L. Profile of Mood States (POMS)–Revised Manual. 2nd ed. San Diego, CA: Education and Industrial Testing Service; 1992. [Google Scholar]
- 27.Motl RW, Fernhall B. Accurate prediction of cardiorespiratory fitness using cycle ergometry in minimally disabled persons with relapsing-remitting multiple sclerosis. Arch Phys Med Rehabil. 2012;93:490–495. doi: 10.1016/j.apmr.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 29.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 30.Breus MJ, O'Connor PJ. Exercise-induced anxiolysis: a test of the “time out” hypothesis in high anxious females. Med Sci Sports Exerc. 1998;30:1107–1112. doi: 10.1097/00005768-199807000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Turner HM, Bernard RM. Calculating and synthesizing effect sizes. Contemp Issues Commun Sci Disord. 2006;33:42–55. [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 33.Sandroff BM, Motl RW. Fitness and cognitive processing speed in persons with multiple sclerosis: a cross-sectional investigation. J Clin Exp Neuropsychol. 2012;34:1041–1052. doi: 10.1080/13803395.2012.715144. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Foley FW, Picone MA, Halper J, Zemon V. Depression levels and interferon treatment in people with multiple sclerosis. Int J MS Care. 2012;14:10–16. doi: 10.7224/1537-2073-14.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavey R, Sherman T, Mueser KT, Osborne DD, Currier M, Wolfe R. The effects of yoga on mood in psychiatric inpatients. Psychiatr Rehabil J. 2005;28:399–402. doi: 10.2975/28.2005.399.402. [DOI] [PubMed] [Google Scholar]
- 36.Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45:435–437. doi: 10.1001/archneur.1988.00520280085020. [DOI] [PubMed] [Google Scholar]
- 37.Wood B, van der Mei I, Ponsonby AL et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2012;19:217–224. doi: 10.1177/1352458512450351. [DOI] [PubMed] [Google Scholar]


