Abstract
The craniofacial skeleton is derived from both neural crest cells and mesodermal cells; however, the majority of the bone, cartilage, and connective tissue is derived from the neural crest. Dentin sialophosphoprotein (DSPP) is a precursor protein that is expressed by the connective tissues of the craniofacial skeleton, namely, bone and dentin with high expression levels in the dentin matrix. Gene ablation studies have shown severe dental defects in DSPP-null mutant mice. Therefore, to elucidate the role of DSPP on the developing dental-craniofacial complex, we evaluated phenotypic changes in the structure of intramembranous bone and dentin mineralization using 3 different age groups of DSPP-null and wild-type mice. Results from micro–computed tomographic, radiographic, and optical microscopic analyses showed defective dentin, alveolar and calvarial bones, and sutures during development. The impaired mineralization of the cranial bone correlated well with low expression levels of Runx2, Col1, and OPN identified using calvarial cells from DSPP-null and wild-type mice in an in vitro culture system. However, the upregulation of MMP9, MMP2, FN, and BSP was observed. Interestingly, the null mice also displayed low serum phosphate levels, while calcium levels remained unchanged. Alizarin red and von Kossa staining confirmed the dysfunction in the terminal differentiation of osteoblasts obtained from the developing calvaria of DSPP-null mice. Immunohistochemical analysis of the developing molars showed changes in Runx2, Gli1, Numb, and Notch expression in the dental pulp cells and odontoblasts of DSPP-null mice when compared with wild-type mice. Overall, these observations provide insight into the role of DSPP in the normal development of the calvaria, alveolar bone, and dentin-pulp complex.
Keywords: dentin sialophosphoprotein, mineralization, calvaria, mandible, odontoblasts, dental pulp
Introduction
The bones of the calvaria are formed by an intramembranous ossification process, where mesenchymal precursor cells derived from both the neural crest and mesoderm convert directly into osteoblasts without a cartilage intermediate (Jiang et al. 2002). Intramembranous ossification begins with centers of condensing mesenchymal cells in which osteoblasts subsequently differentiate. These centers then expand, and adjacent bones form sutures that consist of mesenchymal cells, providing a reservoir of stem cells for further bone growth (Opperman 2000). Dentin, on the other hand, is synthesized by odontoblasts, which are neural crest–derived ectomesenchymal precursor cells (Ruch 1985; Ruch et al. 1995; Sharpe 2001). The development of calvarial and mandibular bones and teeth is a complex process, and the dynamic extracellular matrix (ECM) provides the cues for mineralization (Ravindran and George 2014). Importantly, the interplay between the self-assembled type I collagen matrix and the noncollagenous proteins in the ECM serves as a template for mineral nucleation and growth (Veis 2005; Goldberg et al. 2011). One such noncollagenous protein initially identified in the dentin matrix is dentin sialophosphoprotein (DSPP) (MacDougall et al. 1997; George et al. 1998; George et al. 1999; Sreenath et al. 1999). Subsequently, DSPP was identified in intramembranous endochondral bone and in several nonmineralized tissues (Qin et al. 2002; Fisher and Fedarko 2003; Qin et al. 2003; Ogbureke and Fisher 2004). Mutations in the DSPP gene were responsible for human hereditary disorders such as dentinogenesis imperfecta and dentin dysplasia (MacDougall 1998; Hart and Hart 2007; Kim and Simmer 2007). DSPP is a precursor protein that codes for 2 major noncollagenous proteins in the dentin matrix, namely, dentin sialoprotein (DSP) and dentin phosphoprotein (DPP) (Yamakoshi 2008; Suzuki et al. 2009). Both these proteins are developmentally regulated and have been implicated to play specific roles in dentin formation. Recently, we have shown that DSPP contains an internal ribosomal entry site element responsible for the expression of the carboxyl domain of DSPP (Zhang et al. 2013).
To study the role of the DSPP gene in the formation of mineralized tissues of the dental-craniofacial complex, we used micro–computed tomography (µCT) and X-ray imaging to examine the cranium, alveolar bone, and molars of DSPP-null mice during early development. Using the calvarial cell culture system, we further show that in the absence of DSPP, the functional differentiation of osteoblasts from neural crest–derived osteoblast progenitor cells was impaired.
Materials and Methods
Generation of DSPP-null Mice
The detailed procedure for the generation of DSPP knockout mice has been described by Sreenath et al. (2003). One-, 2-, and 3-mo-old DSPP-null mice and age-matched wild-type (WT) mice were generated. All mice were generated and maintained according to the University of Illinois at Chicago (UIC) Animal Care Committee. All studies were performed per the UIC protocol (Animal Assurance Number 13-210).
Radiographic Analysis
The dissected calvaria and hemimandibles were imaged by high-resolution digital radiography using a digital radiography machine (model MX-20; Faxitron Corp., Lincolnshire, IL, USA). Specimens subject to comparison were exposed using the same settings on the digital sensor and the radiographic densities of the craniofacial region compared between the WT and DSPP-null mice.
µCT Analyses
One-, 2-, and 3-mo-old mandibles from WT and DSPP-null mice were fixed in 10% neutral buffered formalin and scanned using a µCT40 desktop cone-beam scanner with accompanying software (Scanco Medical AG, Bassersdorf, Switzerland). The detailed protocol is described in the Appendix.
Biochemical Assays to Determine Serum Calcium and Phosphate Levels
Serum was obtained from 1-mo-old animals, and serum calcium and inorganic phosphorus concentrations were determined by a calcium detection kit (Abcam, Cambridge, UK) and a phosphorus Liqui-UV kit (Stanbio Laboratory, Boerne, TX, USA) per the manufacturers’ protocols.
Quantitative Real-time Polymerase Chain Reaction (PCR)
RNA was extracted from cultured cells using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s manual. One microgram of total RNA was reverse-transcribed with Superscript III (Invitrogen) using an oligodT primer (Invitrogen, Carlsbad, CA, USA) for 60 min at 50 °C. Quantitative real-time PCR was carried out using the ABI StepOnePlus instrument (Applied Biosystems, Foster City, CA, USA). The relative gene expression level was estimated by the 2−ΔΔCT method, with the GAPDH gene expression level used as an internal control.
Alizarin Red and von Kossa Staining for Mineralization Assays or Tissue Sections
The detailed protocol is provided in the Appendix.
Immunohistochemistry and Immunocytochemistry
Postnatal 7-d mouse heads were fixed in 10% neutral buffered formalin for 3 d at 4 °C and then processed for paraffin embedding as published before (Hao et al. 2004; Hao et al. 2009). The antibodies used are presented in the Appendix.
Statistical Analyses
Data are presented as the mean ± standard deviation of at least 3 independent experiments or 5 animals per group, respectively. Probability values were calculated using the Student’s t test. P < 0.05 was considered significant.
Results
DSPP-null Mice Exhibit Impaired Cranial Bone Development
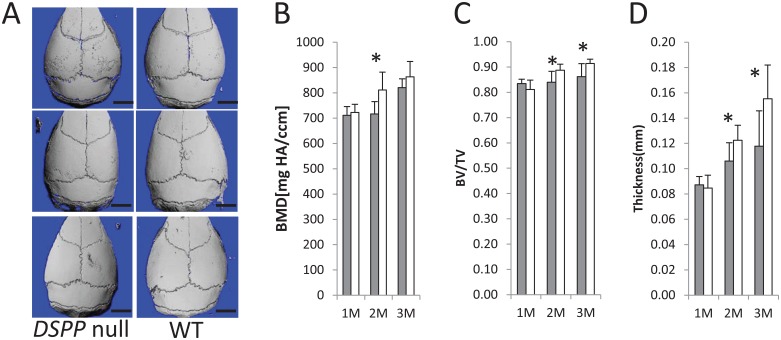
In order to determine whether the loss of DSPP expression produces a calvarial phenotype, we investigated, by µCT, the calvarial bone structure of 3 different age groups, namely, 1-, 2-, and 3-mo-old WT and DSPP-null mice. µCT scans showed the mineralization defects of the frontal and parietal bones (Fig. 1A). Interestingly, the bone volume/total volume (BV/TV) and thickness of the bone were significantly lower in the null mice at 2 and 3 mo (Fig. 1C, D), and the bone mineral density was significantly lower at 2 mo (Fig. 1B).
Figure 1.
Characterization of the calvarial bones in DSPP-null and gender-matched wild-type (WT) mice at 1, 2, and 3 mo by micro–computed tomography (µCT). (A) Three-dimensional µCT scans of calvaria from 1-, 2-, and 3-mo-old DSPP-null and WT mice (n = 5). Note that the parietal frontal bones of the DSPP-null mice are hypomineralized. Bar = 2.5 mm. (B–D) Comparison of the WT and DSPP-null mice for bone mineral density (B). Bone volume/total volume (C) and bone thickness (D) at 1, 2, and 3 mo. Gray bar = DSPP null; white bar = WT. Statistically significant difference: *P < 0.05. Comparisons were performed using the Student’s t test.
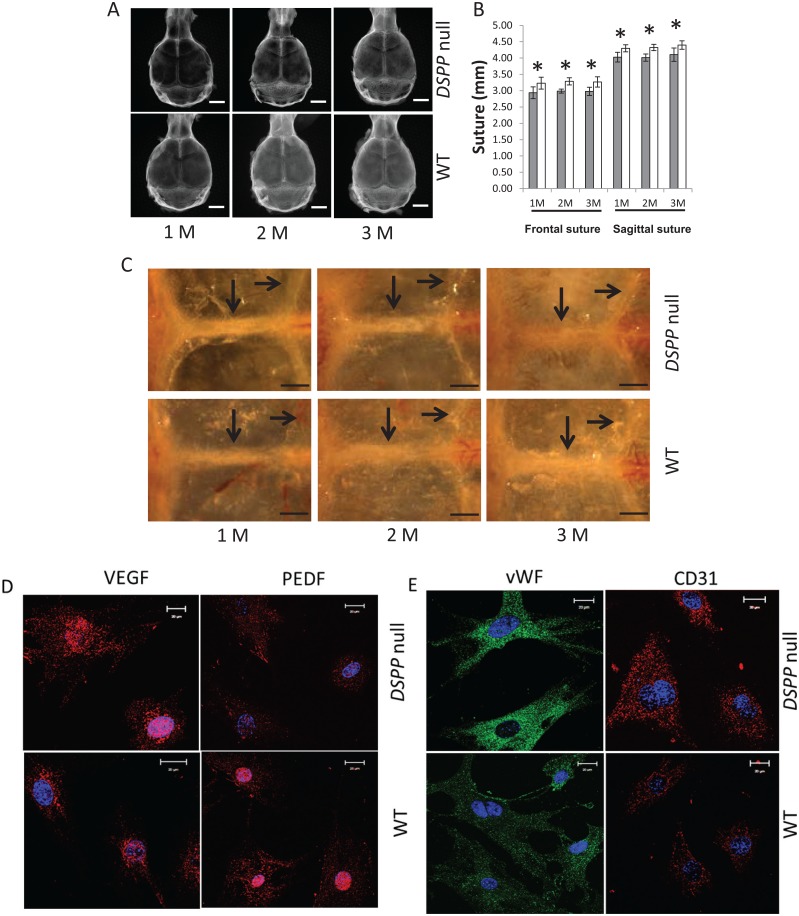
Radiography confirmed that the cranial bones were poorly mineralized in the DSPP-null mice as evidenced by their low radio-opacity (Fig. 2A). Specifically, in the DSPP-null mice, the phenotype showed reduced ossification of the frontal and parietal bones as early as 1 mo and remained poorly calcified until 3 mo. The length of the frontal and sagittal sutures was significantly lower in the DSPP-null mice (Fig. 2B).
Figure 2.
Characterization of the calvarial bones in DSPP-null and gender-matched wild-type (WT) mice at 1, 2, and 3 mo by radiography and optical microscopy. (A) Representative radiographs reveal reduced mineralization of the calvarial bones. For radiographic imaging, the inferior side was facing the X-ray, and axial scans were obtained (n = 5). (B) Length of the frontal suture (FS) and sagittal suture (SS) from radiographic analysis. The mean ± standard deviation of measurements of the FS and SS from 5 samples for each group (*P < 0.05). Gray bar = DSPP null; white bar = WT. (C) Visualization through a dissection microscope of the coronal and sagittal sutures of the dissected skulls of 1-, 2-, and 3-mo-old WT and DSPP-null mice. Arrows point to the sutures. Note the increased vascularity of the parietal bones in the null mice. Bar = 1 mm. Arrows indicate frontal (long arrow) and lambdoid (short arrow) sutures. (D) Expression of angiogenic markers in the calvarial osteoblasts isolated from 7-d WT and DSPP-null mice. Anti-VEGF and anti-PEDF fluorescence immunocytochemistry performed on calvarial cells isolated from 7-d WT and DSPP-null mice. (E) Expression of endothelial markers in the calvarial osteoblasts isolated from 7-d WT and DSPP-null mice. Anti–von Willebrand factor and anti-CD31 fluorescence immunocytochemistry performed on calvarial cells isolated from 7-d WT and DSPP-null mice.
Optical image analysis showed an invasion of blood vessels around the parietal bones in 2- and 3-mo-old DSPP-null mice (Fig. 2C). Angiogenesis was confirmed by the increased presence of vascular endothelial growth factor (VEGF) in the calvarial osteoblasts of DSPP-null mice when compared with the controls (Fig. 2D). Pigment epithelium-derived factor (PEDF) is one of the most effective inhibitors of angiogenesis, and this was correspondingly lower in the null mice when compared with the WT mice (Fig. 2D). Immunocytochemistry showed that the calvarial osteoblasts were derived from endothelial cells, and the expression of the endothelial markers von Willebrand factor and CD31 was higher in DSPP-null osteoblasts.
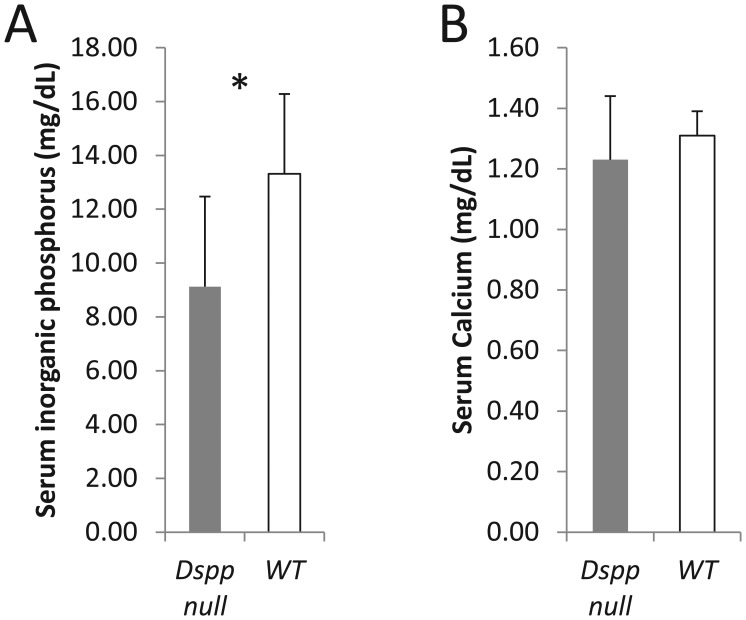
Loss of DSPP Affects Serum Phosphate Levels
The serum biochemical profile of the WT and DSPP-null mice showed no difference in Ca2+ levels; however, significantly reduced Pi levels were observed in the null mice (Fig. 3).
Figure 3.
Serum biochemistry profiles in DSPP-null and wild-type (WT) mice. (A) Serum phosphate levels were significantly lower in DSPP-null mice when compared with the WT mice (P < 0.05), while serum calcium levels (B) were statistically unchanged in null and WT mice (P = 0.31) (n = 4 in each group). Experiments performed in triplicate for each sample. Comparisons were performed using the Student’s t test.
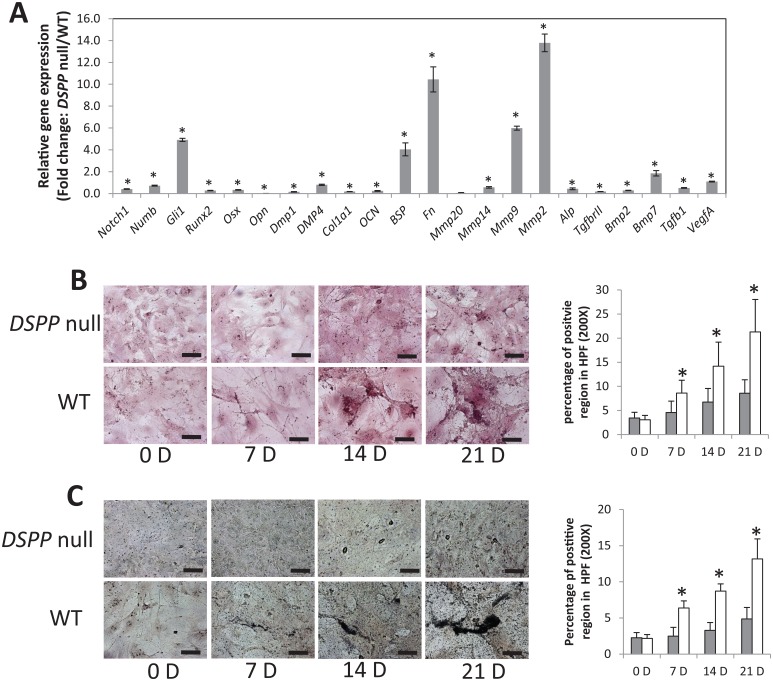
Loss of DSPP Alters the Expression of Key Markers of Osteoblast Differentiation and Mineralized Matrix Formation
Several osteogenic markers were selected, and their expression levels were quantified by quantitative PCR using mRNA isolated from primary calvarial cells (Fig. 4A). Interestingly, the expression levels of Runt-related transcription factor 2 (Runx2), the osteoblast-specific master transcription factor, were attenuated when compared with the WT mice. Osterix, a transcription factor that is regulated by Runx2 and essential for osteoblast differentiation, was also reduced in the calvarial cells of null mice. Type I collagen, essential for matrix mineralization, and osteocalcin, a marker of mature mineralized tissue, were also downregulated. Matrix metalloproteinase 14 (MMP14), shown to be essential for cartilage removal in rodent craniofacial development, was lower in the DSPP-null mice. However, the MMP2 and MMP9 expression levels were upregulated in the DSPP-null mice when compared with the WT mice. Interestingly, the expression levels of bone sialoprotein (BSP) and fibronectin (FN) were upregulated in the DSPP-null mice (Fig. 4A).
Figure 4.
The loss of DSPP alters the expression of osteoblast differentiation genes in 5-d calvarial tissues and the formation of a calcified matrix. (A) Total RNA was isolated from primary calvarial cells generated from DSPP-null and wild-type (WT) mice (n = 20) and subjected to quantitative real-time polymerase chain reaction. Analyses were carried out for the mRNA levels of Notch 1, Numb, Gli1, Runx2, Osx, OPN, DMP1, DMP4, ColI, OCN, FN, MMP20, MMP14, MMP9, MMP2, ALP, TGFβRII, BMP2, BMP7, TGFβ1, and VEGFA. The results were normalized to mGAPDH. The gene expression fold change was relative to that of WT mice, which was normalized as 1. Values are the mean ± standard deviation of 3 independent samples. Note the downregulation of most of the osteogenic differentiation markers. A statistically significant difference was observed: *P < 0.05 vs WT. Comparisons were performed using the Student’s t test. (B, C) Cultured osteoblast cells of 5-d WT and DSPP-null mice were fixed at 0, 7, 14, and 21 d after the initiation of differentiation. Histological staining for Alizarin red (B) and von Kossa (C) was performed as described in Materials and Methods. Statistically significant amounts of phosphate and calcium were observed in the WT mice when compared with the DSPP-null mice. *P < 0.05. Comparisons were performed using the Student’s t test.
Alizarin red and von Kossa staining performed on calvarial cultures showed poor results for calcium and phosphate deposits in the DSPP-null mice when compared with WT mice (Fig. 4B, C). Significantly higher amounts of phosphate and calcium deposits were observed in the WT mice when compared with the null mice. These findings corroborate well with the impaired differentiation of mesenchymal cells to preosteoblasts and to mature functional osteoblasts when DSPP expression is attenuated.
Disruption of DSPP Leads to Defects in the Alveolar Bone and Dentin
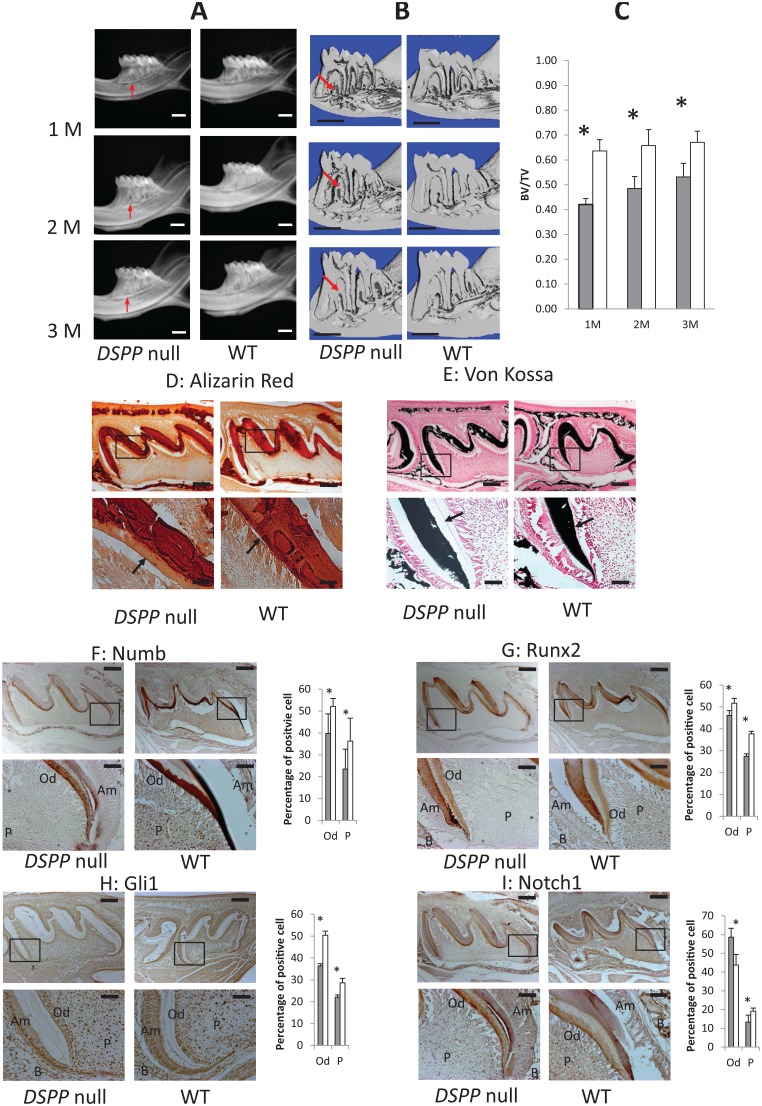
µCT of 1-, 2-, and 3-mo-old DSPP-null and WT mice was performed to evaluate the mineralization of dentin and alveolar bone. The hypomineralization of dentin was observed by both µCT and radiographic imaging (Fig. 5A, B). Remarkable alveolar bone loss was observed in the DSPP-null mice. The roots of the DSPP-null mice were irregular in shape with resorption pit–like structures. Quantitative µCT analysis of the alveolar bone in the furcation region of the first molar showed a significant decrease in the BV/TV in DSPP-null mice, indicating abnormalities in alveolar bone formation and mineralization (Fig. 5C). The hypomineralized alveolar bone phenotype was observed in all 3 different age groups.
Figure 5.
Characterization of the mandibular bone and dentin by radiographic and micro–computed tomographic (µCT) analyses. (A) Representative radiographs show hypomineralized dentin at 1, 2, and 3 mo of age, and the alveolar bone is highly porous when compared with the wild-type (WT) mice (n = 5). (B) The µCT scans show longitudinal first molar sections displaying qualitative alveolar bone defects (red arrows) (n = 5). Bar = 1 mm. (C) Quantitative 3-dimensional µCT parameters were measured, and the represented bone volume/total volume values measured show a progressive decrease in bone volume in the DSPP-null mice when compared with the WT mice. Data were obtained for 5 per group, and the Student’s t test was used for statistical analysis. *P < 0.05. Gray bar = DSPP null; white bar = WT. (D) Alizarin red staining shows calcium deposits in the alveolar bone and dentin matrix of WT and DSPP-null mice. Arrows indicate broad predentin in the DSPP-null mice and narrow physiological predentin in the WT mice. (E) von Kossa staining shows phosphate deposits in the alveolar bone of WT and DSPP-null mice. Arrows indicate broad predentin in the null mice and narrow physiological predentin in the WT mice. (F–I) Immunohistochemical analysis to identify the differential expression pattern of Numb, Runx2, Gli1, and Notch in the developing 7-d molars of WT and DSPP-null mice. (F) A lower expression of Numb in DSPP-null odontoblasts and dental pulp cells is observed when compared with the WT counterparts. (G) Higher expression of Runx2 in WT odontoblasts and pulp cells when compared with the DSPP-null counterparts. (H) Higher expression of Gli1 in WT odontoblasts and pulp cells when compared with the DSPP-null counterparts. (I) Higher expression of Notch 1 in DSPP-null odontoblasts when compared with the WT odontoblasts. Scale bar: upper row = 200 µm; lower row = 50 µm. Gray bar = DSPP null; white bar = WT. Am, ameloblasts; B, bone; Od, odontoblasts; P, dental pulp cells. n = 5 sections per antibody and representative serial sections were imaged.
Disruption of DSPP Affects the Expression of Numb, Runx2, and Glioma-associated Oncogene 1 (Gli1) and the Odontoblasts and Dental Pulp of 7-d Mice
We then analyzed the expression levels of key proteins responsible for the differentiation of dental pulp stem cells into odontoblasts by immunohistochemical analysis. Numb expression was higher in the odontoblasts and pulp cells of WT mice when compared with DSPP-null mice (Fig. 5F). Runx2 expression was higher in both the pulp and odontoblasts of the WT mice (Fig. 5G). The expression of the transcription factor Gli1 was significantly lower in the DSPP-null mice when compared with the WT mice (Fig. 5H), while Notch 1 showed higher expression in the odontoblasts and lower expression in the pulp cells of DSPP-null mice (Fig. 5I). Alizarin red and von Kossa staining of the sections showed no apparent changes in the amount of extracellular calcium and phosphate levels (Fig. 5D, E). However, wider unmineralized predentin in the null mice was readily apparent as published previously (Sreenath et al. 2003).
Discussion
The calvarial bones (frontal, parietal, and interparietal) are formed through intramembranous ossification in which mesenchymal precursor cells derived from both the neural crest and mesoderm convert directly into osteoblasts without the precondition of a cartilage intermediate (Jiang et al. 2002). During teeth formation, neural crest–derived ectomesenchymal cells differentiate to functional odontoblasts, which are responsible for the synthesis and secretion of the dentin matrix. The dental pulp cells are of ectomesenchymal origin and contain pulp stromal fibroblasts; mesenchymal stem cells; and neuronal, vascular, and immune system cells.
DSPP is expressed by several cell types of the dental-craniofacial tissue. It is most abundant in the dentin matrix and is the terminal differentiation marker of odontoblasts. Immediately after its synthesis, DSPP is cleaved into DSP and DPP. BMP1, MMP2, and MMP20 have been implicated in the proteolytic process (Yamakoshi et al. 2006; von Marschall and Fisher 2010). In this study, we demonstrate that the DSPP gene is essential for the formation of mineralized tissues of the dental-craniofacial complex.
Radiographic imaging showed that both the frontal and parietal calvarial bones were poorly mineralized during early development. The frontal bone–derived osteoblasts are from the cranial neural crest, and the parietal bones arise from osteoprogenitors that differentiate from the paraxial mesoderm. The reduced mineralization of the frontal and parietal bones suggests that DSPP is required for the osteoblast differentiation of embryonic precursor–derived cells from both the mesoderm and cranial neural crest. Consistent with this finding, gene expression analysis by quantitative PCR confirmed that silencing DSPP expression altered the mRNA levels of key master regulators of osteogenesis, namely, Runx2 and Osterix. Runx2 is a transcription factor that regulates osteoblast cell differentiation. It binds to the promoters of several genes that regulate osteoblast differentiation. Interestingly, dentin matrix phosphoprotein 1 (DMP1), alkaline phosphatase, and osteocalcin are Runx2 target genes, and their mRNA levels are reduced in the absence of DSPP. BSP, an essential component of the bone ECM, was upregulated. As BSP functions as a hydroxyapatite nucleator, it is possible that the bony spicules seen in the frontal and parietal bones might be formed as a result of BSP being upregulated. Osteopontin is a negative regulator of mineralization, and the mRNA levels were highly attenuated in the null mice. The upregulation of MMP2 and MMP9 in the null mice was particularly interesting, and this might indicate that the turnover of the ECM might be responsible for the poorly mineralized phenotype of the calvaria. FN was also upregulated in the DSPP-null mice, and this could contribute to the formation of highly vascularized parietal bones. Recently, it was shown that FN remodeling is essential for normal vasculogenic sprout formation, and perturbed FN assembly alters endothelial cell motility (Zhou et al. 2008). It is thus possible to envisage the role of MMPs in remodeling the FN matrix to promote vascularization in DSPP-null mice. Alizarin red and von Kossa staining confirmed the impaired terminal differentiation of osteoblasts in the DSPP-null mice.
There are several molecular determinants of mineralization in which regulators of mineral metabolism such as phosphate can affect mineralized tissues. Serum phosphate levels were perturbed in the DSPP-null mice. Low serum phosphate levels were observed, while calcium levels showed no difference between the WT and null mice. Published reports showed that DMP1-null mice exhibited low serum phosphate levels, leading to defective osteocytes and resulting in osteomalacia (Feng et al. 2006). In the DSPP-null mice, it is unclear if the hypophosphatemic phenotype, and consequently the mineralization defects, is a direct effect of DSPP on serum phosphate levels or an indirect effect due to lower DMP1 levels observed in null mice.
The development of sutures was particularly interesting in the DSPP-null mice. The sagittal suture and frontal suture were shorter. Tissue architecture analysis further showed that at 1 mo, the null mice had wider sutural gaps perhaps due to the delayed recruitment of mesenchymal cells to the osteogenic cell lineage. However, this observation needs to be explored further. Interestingly, the parietal bone surrounding the sutures was highly vascularized in the DSPP-null mice. Angiogenesis is controlled by factors that promote angiogenesis and those that inhibit the process. Interestingly, VEGF, which promotes vessel growth, was higher in the DSPP-null mice, and PEDF, which blocks angiogenesis, was lower. The undifferentiated state of calvarial osteoblasts was further confirmed with antibodies specific for endothelial cells, namely, CD31 and von Willebrand factor. Calvarial cells from both the WT and DSPP-null mice express these markers; however, higher expression levels were seen in the null mice. This suggests that the osteoblasts were derived from endothelial-derived mesenchymal stem-like cells, and in the absence of DSPP, their differentiation potential was suppressed. This finding provides new insights into the potential role of endothelial-to-mesenchymal transition in the presence and absence of DSPP. Similar endothelial cell plasticity and their conversion into multipotent stem-like cells have been reported (Medici et al. 2010).
In vertebrates, the cranial neural crest–derived dental mesenchyme consists of the dental papilla and dental follicle. The dental follicle gives rise to the periodontium, including the osteoblasts that contribute to the alveolar process (Zhang et al. 2003). Results from 3-dimensional µCT analysis showed a greater reduction of the mandibular alveolar bone in DSPP-null mice when compared with the WT mice. Results also showed a loss of bone in the furcation region of the first mandibular molar at 1 mo, and this continued until 3 mo. The alveolar bone volume fractions (BV/TV) at 1, 2, and 3 mo were lower than the WT mice. A recent publication by Gibson et al. (2014) has shown by histological analyses that the osteocyte lacunae in the alveolar bone of the DSPP-null mice appeared irregular with disorganized and fewer canaliculi. Thus, DSPP is required for mandibular mesenchymal cells to differentiate into functional osteoblasts and form a mineralized matrix.
In the developing vertebrate tooth, the dental papilla gives rise to odontoblasts and the dental pulp. We had previously shown that DSPP mRNA is highly expressed in odontoblasts and pulp cells (Hao et al. 2009). DSPP-null mice showed a hypomineralized dentin phenotype with enlarged pulp chambers (Sreenath et al. 2003). µCT studies using 3 different age groups showed poor mineralized dentin matrix formation. Impaired odontogenic differentiation was further confirmed by the decreased expression of Runx2, Numb, and Gli1, while Notch 1 was higher in the odontoblasts of null mice and lower in the pulp cells. It is well established that Runx2 is necessary for the differentiation of dental pulp cells to odontoblasts and also controls their maturation (Yang et al. 2014). The expression of Runx2 is developmentally regulated. It is highly expressed in preodontoblasts and downregulated in functional odontoblasts. Runx2 transcriptionally regulates DSPP and DMP1 by directly binding to their promoters in dental pulp stem cells (Chen et al. 2002; Feng et al. 2002). Observations in this study suggest that in the absence of DSPP, both DMP1 and Runx2 expression levels are affected, leading to impaired odontoblast differentiation. Numb is a multifunctional protein involved in asymmetric cell differentiation, proliferation, and maintenance. Higher Numb levels in WT odontoblasts suggest a differentiated state, while lower levels in the null mice suggest impaired asymmetric cell division, and the cells are in a self-renewal state. The inhibition of Numb signaling reduces stem cell progenitors with dentinogenic potential (Li et al. 2013). Notch 1, a transmembrane protein, has been shown to be important in specifying the dental cell type identity. The inhibition of Notch signaling leads to impaired growth of the mouse incisor cervical loop with reduced proliferation and increased apoptosis. Overexpression of the constitutively activated Notch 1 intracellular domain inhibited the odontoblast differentiation of dental pulp stem cells (Zhang et al. 2008). In the DSPP-null mice, Notch expression was lower in the dental pulp cells. Numb can inhibit Notch signaling through interaction with the Notch intracellular domain and promotes its ubiquitination and degradation (McGill and McGlade 2003). Thus, Notch and Numb signaling appear to play a critical role in the maturation and differentiation of odontoblasts and the regulation of dental pulp stem cell differentiation. Gli genes encode the transcription effectors of sonic hedgehog signaling. Gli1 is now known to be regulated by several protein mediators besides components of the hedgehog signaling pathway and is now known to be a marker for mesenchymal stem cells in the dental pulp (Zhao et al. 2014). In bone, Gli1 was shown to promote osteoblast differentiation but also repressed osteoblast maturation. Activated Gli1 translocates into the nucleus to induce the expression of various context-specific genes. Higher expression levels of Gli1 in the odontoblasts of null mice suggest their undifferentiated state. The differential regulation of these signaling molecules in the absence of DSPP suggests the regulatory role played by these proteins to orchestrate the process of dentin formation by directing mesenchymal cell commitment toward the odontoblast differentiation and maturation process.
Overall, DSPP-null mice have developmental defects in the dental-craniofacial complex that persist through postnatal growth. These mice have altered mandibular bone volume as observed by µCT imaging, reduced ossification of the calvarial bones, and decreased expression of key osteogenic markers such as Runx2 and several ECM proteins. The mechanisms that control the range of dental-craniofacial mineralization phenotypes observed might be multifactorial and complex, resulting from the absence of the functional activity of DSPP. Thus, DSPP directly or indirectly regulates a number of transcription factors, matrix molecules, and serum phosphate levels. Further studies are warranted to identify the signaling mechanisms stimulated by DSPP during matrix mineralization of the craniofacial complex.
Author Contributions
Y. Chen, contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Y. Zhang, contributed to design and data acquisition, drafted the manuscript; A. Ramachandran, contributed to design and data analysis, drafted the manuscript; A. George, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
Thanks to Dr. Ashok Kulkarni, NIDCR, for generating the initial DSPP-null mice.
Footnotes
This work was supported by the National Institute of Dental and Craniofacial Research, National Institutes of Health, US Department of Health and Human Services (R01 DE 19633), and by the Brodie Endowment Fund and research funding provided by Dr. Carla Evans, chair of the Department of Orthodontics.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Chen S, Gu TT, Sreenath T, Kulkarni AB, Karsenty G, MacDougall M. 2002. Spatial expression of Cbfa1/Runx2 isoforms in teeth and characterization of binding sites in the DSPP gene. Connect Tissue Res. 43(2–3):338–344. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, et al. 2006. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 38(11):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, Harris SE, MacDougall M. 2002. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique marker gene. J Bone Miner Res. 17(10):1822–1831. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Fedarko NS. 2003. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 44(Suppl 1):33–40. [PubMed] [Google Scholar]
- George A, Srinivasan R, Thotakura S, Liu K, Veis A. 1999. Rat dentin matrix protein 3 is a compound protein of rat dentin sialoprotein and phosphophoryn. Connect Tissue Res. 40(1):49–57. [DOI] [PubMed] [Google Scholar]
- George A, Srinivasan R, Thotakura S, Veis A. 1998. The phosphophoryn gene family: identical domain structures at the carboxyl end. Eur J Oral Sci. 106(Suppl 1):221–226. [DOI] [PubMed] [Google Scholar]
- Gibson MP, Jani P, Wang X, Lu Y, Qin C. 2014. Overexpressing the NH-terminal fragment of dentin sialophosphoprotein (DSPP) aggravates the periodontal defects in knockout mice. J Oral Biosci. 56(4):143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M, Kulkarni AB, Young M, Boskey A. 2011. Dentin: structure, composition and mineralization. Front Biosci. 3:711–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Ramachandran A, George A. 2009. Temporal and spatial localization of the dentin matrix proteins during dentin biomineralization. J Histochem Cytochem. 57(3):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Zou B, Narayanan K, George A. 2004. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone. 34(6):921–932. [DOI] [PubMed] [Google Scholar]
- Hart PS, Hart TC. 2007. Disorders of human dentin. Cells Tissues Organs. 186(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. 2002. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 241(1):106–116. [DOI] [PubMed] [Google Scholar]
- Kim JW, Simmer JP. 2007. Hereditary dentin defects. J Dent Res. 86(5):392–399. [DOI] [PubMed] [Google Scholar]
- Li H, Ramachandran A, Gao Q, Ravindran S, Song Y, Evans C, George A. 2013. Expression and function of NUMB in odontogenesis. Biomed Res Int. 2013:182965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall M. 1998. Refined mapping of the human dentin sialophosphoprotein (DSPP) gene within the critical dentinogenesis imperfecta type II and dentin dysplasia type II loci. Eur J Oral Sci. 106(Suppl 1):227–233. [DOI] [PubMed] [Google Scholar]
- MacDougall M, Simmons D, Luan X, Gu TT, DuPont BR. 1997. Assignment of dentin sialophosphoprotein (DSPP) to the critical DGI2 locus on human chromosome 4 band q21.3 by in situ hybridization. Cytogenet Cell Genet. 79(1–2):121–122. [DOI] [PubMed] [Google Scholar]
- McGill MA, McGlade CJ. 2003. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem. 278(25):23196–23203. [DOI] [PubMed] [Google Scholar]
- Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. 2010. Conversion of vascular endothelial cells into multipotent stem–like cells. Nat Med. 16(12):1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbureke KU, Fisher LW. 2004. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 83(9):664–670. [DOI] [PubMed] [Google Scholar]
- Opperman LA. 2000. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 219(4):472–485. [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Butler WT. 2003. Dentin sialoprotein in bone and dentin sialophosphoprotein gene expressed by osteoblasts. Connect Tissue Res. 44(Suppl 1):179–183. [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, Nagai N, Butler WT. 2002. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 81(6):392–394. [DOI] [PubMed] [Google Scholar]
- Ravindran S, George A. 2014. Multifunctional ECM proteins in bone and teeth. Exp Cell Res. 325(2):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch JV. 1985. Odontoblast differentiation and the formation of the odontoblast layer. J Dent Res. 64(Spec No):489–498. [DOI] [PubMed] [Google Scholar]
- Ruch JV, Lesot H, Bègue-Kirn C. 1995. Odontoblast differentiation. Int J Dev Biol. 39(1):51–68. [PubMed] [Google Scholar]
- Sharpe PT. 2001. Neural crest and tooth morphogenesis. Adv Dent Res. 15:4–7. [DOI] [PubMed] [Google Scholar]
- Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. 2003. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 278(27):24874–24880. [DOI] [PubMed] [Google Scholar]
- Sreenath TL, Cho A, MacDougall M, Kulkarni AB. 1999. Spatial and temporal activity of the dentin sialophosphoprotein gene promoter: differential regulation in odontoblasts and ameloblasts. Int J Dev Biol. 43(6):509–516. [PubMed] [Google Scholar]
- Suzuki S, Sreenath T, Haruyama N, Honeycutt C, Terse A, Cho A, Kohler T, Muller R, Goldberg M, Kulkarni AB. 2009. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol. 28(4):221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis A. 2005. Materials science: a window on biomineralization. Science. 307(5714):1419–1420. [DOI] [PubMed] [Google Scholar]
- von Marschall Z, Fisher LW. 2010. Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix products by three isoforms of bone morphogenetic protein-1 (BMP1). Matrix Biol. 29(4):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakoshi Y. 2008. Dentin sialophophoprotein (DSPP) and dentin. J Oral Biosci. 50(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakoshi Y, Hu JC, Iwata T, Kobayashi K, Fukae M, Simmer JP. 2006. Dentin sialophosphoprotein is processed by MMP-2 and MMP-20 in vitro and in vivo. J Biol Chem. 281(50):38235–38243. [DOI] [PubMed] [Google Scholar]
- Yang F, Xu N, Li D, Guan L, He Y, Zhang Y, Lu Q, Zhang X. 2014. A feedback loop between RUNX2 and the E3 ligase SMURF1 in regulation of differentiation of human dental pulp stem cells. J Endod. 40(10):1579–1586. [DOI] [PubMed] [Google Scholar]
- Zhang C, Chang J, Sonoyama W, Shi S, Wang CY. 2008. Inhibition of human dental pulp stem cell differentiation by Notch signaling. J Dent Res. 87(3):250–255. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Song Y, Ravindran S, Gao Q, Huang CC, Ramachandran A, George A. 2013. DSPP contains an IRES element responsible for the translation of dentin phosphophoryn. J Dent Res. 93(2):155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhang X, Tang J, Chen J, Chen Y. 2003. Msx1/Bmp4 genetic pathway regulates mammalian alveolar bone formation via induction of Dlx5 and Cbfa1. Mech Dev. 120(12):1469–1479. [DOI] [PubMed] [Google Scholar]
- Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, Chai Y. 2014. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 14(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Rowe RG, Hiraoka N, George JP, Wirtz D, Mosher DF, Virtanen I, Chernousov MA, Weiss SJ. 2008. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Genes Dev. 22(9):1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.