Abstract
Heterotopic cartilage develops in certain pathologic conditions, including those affecting the human temporomandibular joint (TMJ), but the underlying molecular mechanisms remain obscure. This is in part due to the fact that a reliable animal model of such TMJ diseases is not available. Here, we show that aberrant chondrocyte differentiation and ectopic cartilage formation occur spontaneously in proteoglycan 4 (Prg4) mutant TMJ discs without further invasive procedure. By 2 mo of age, mutant disc cells displayed chondrocyte transdifferentiation, accompanied by strong expression of cartilage master gene Sox9 and matrix genes aggrecan and type II collagen. By 6 mo, heterotopic cartilage had formed in the discs and expressed cartilage hypertrophic markers Runx2 and ColX. The ectopic tissue grew in size over time and exhibited regional mineralization by 12 mo. Bone morphogenetic protein (BMP) signaling was activated with the ectopic chondrogenic cells and chondrocytes, as indicated by phosphorylated Smad 1/5/8 nuclear staining and by elevated expression of Bmp2, Bmpr1b, Bmpr2, and BMP signaling target genes. Likewise, we found that upon treatment with recombinant human BMP 2 in high-density micromass culture, mutant disc cells differentiated into chondrocytes and synthesized cartilage matrix more robustly than control cells. Importantly, a specific kinase inhibitor of BMP receptors drastically attenuated chondrogenesis in recombinant human BMP 2–treated mutant disc cultures. Unexpectedly, we found that Prg4 was expressed at joint-associated sites, including disc/muscle insertion and muscle/bone interface, and all these structures were abnormal in Prg4 mutants. Our data indicate that Prg4 is needed for TMJ disc integrity and function and that its absence leads to ectopic chondrogenesis and cartilage formation in conjunction with abnormal BMP signaling. Our findings imply that the BMP signaling pathway could be a potential therapeutic target for prevention or inhibition of ectopic cartilage formation in TMJ disease.
Keywords: articular disc, temporomandibular joint, TMJ disorder, lubricin, ectopic cartilage, joint lubrication
Introduction
The articular disc is 1) a fibrocartilagenous tissue located between the mandibular condyle and glenoid fossa in the temporal bone, 2) a major component of the temporomandibular joint (TMJ), and 3) essential for joint movement. The disc has important roles in shock absorbance, load distribution, protection of articular cartilage and joint lubrication, and overall TMJ function (Tanaka et al. 2008). Given the multifaceted demands imposed onto the TMJ during mastication, the disc is constantly exposed to considerable biomechanical stress and load. Indeed, anatomic and structural changes in the disc, including disc displacement, perforation, or malformations, significantly increase the risk and incidence of TMJ disorders (Scrivani et al. 2008).
The disc is composed of fibroblasts and chondrocyte-like cells (fibrochondrocytes) and is commonly distinguished into 3 regions along the anteroposterior axis: anterior band, intermediate zone, and posterior band (Molinari et al. 2007). Because fibroblasts predominate, their characteristic type I collagen is more abundant than other collagens, and type II collagen is present in the pericellular matrix of chondrocyte-like cells. Within the disc regions, there is spatial variability in cellular and matrix composition, and changes in these parameters are observed with age and in TMJ pathologies (Molinari et al. 2007; Lu et al. 2009). For example, calcium content and/or calcification of TMJ discs increases progressively with aging, suggesting that TMJ disc degenerative changes take place as a result of deranged mechanical stress (Jibiki et al. 1999; Takano et al. 1999). In other pathologic conditions, patients with juvenile idiopathic arthritis or chronic TMJ disorders and those undergoing TMJ surgery can display ectopic chondrocyte differentiation and cartilage formation within the TMJ’s synovial space or soft tissue (Lindqvist et al. 1992; Luz et al. 2003; Ringold et al. 2011). In such conditions, patients have limited TMJ mobility, and their facial development can be compromised (Shibuya et al. 2003; Jensen et al. 2010). Therefore, it is important to define the cellular and molecular mechanisms underlying these conditions and, in particular, heterotopic cartilage formation that could in turn provide insights into prevention or treatment.
Proteoglycan 4 (Prg4) encodes superficial zone protein/lubricin, megakaryocyte-stimulating factor, and other secreted products (Hui et al. 2012). Lubricin is synthesized by synoviocytes and articular cartilage surface cells and is secreted into the joint space (Flannery et al. 1999; Schmidt et al. 2007; Coles et al. 2010). It contains an extensive mucin-like region rich in O-linked oligosaccharides and is thought to be responsible for boundary lubrication by itself or together with other lubricants during joint movement (Swann et al. 1985; Seror et al. 2015). PRG4 loss-of-function mutations in humans cause camptodactyly-arthropathy-coxa-vara-pericarditis syndrome (CACP; Bahabri et al. 1998; Marcelino et al. 1999). The limb joints in CACP patients are characterized by noninflammatory hyperplasia and fibrosis of the synovial membrane (Bahabri et al. 1998). Importantly, altered lubricin levels have been associated with osteoarthritis and rheumatoid arthritis (Elsaid et al. 2008; Ungethuem et al. 2010). We and others recently reported that mice lacking Prg4 exhibit 1) hyperplasia of articular cartilage of glenoid fossa and condyle, 2) disappearance of superficial cells, 3) abnormal protein deposition, and 4) articular cartilage degradation (Hill et al. 2014; Koyama et al. 2014). Prg4-null TMJ discs were also severely deranged, lost their characteristic concave shape, and importantly, exhibited increased chondrocytic cell differentiation by as early as 3 mo (Koyama et al. 2014).
Bone morphogenetic protein (BMP) signaling has well-established roles in endochondral bone formation during skeletal development and growth, and its abnormal activation causes heterotopic cartilage and bone formation (Billings et al. 2008; Huegel et al. 2015; Long and Ornitz 2015). Thus, we hypothesized that BMP signaling could be involved in heterotopic chondrocyte differentiation and cartilage formation in the discs of Prg4-null mice. Here we do show that BMP signaling was excessive in heterotopic cartilage forming in Prg4-null TMJ discs and that inhibition of BMP signaling blocked the increased responsiveness of mutant disc cells to exogenous recombinant human BMP 2 (rhBMP-2).
Materials and Methods
Mice
Prg4-/- mutant mice were kindly provided by Dr. Matthew Warman (Boston Children’s Hospital, Boston, MA, USA; Rhee et al. 2005). Animals were maintained in accordance with the National Institutes of Health’s Guide for Care and Use of Laboratory Animals, and protocols were approved by the Institutional Animal Care and Use Committee of The Children’s Hospital of Philadelphia.
Histologic, Histochemical, Immunohistochemical, Immunocytochemical, and In Situ Hybridization Analyses
Age-matched Prg4 mutants and wild-type littermates were evaluated at postnatal day 14 (2 wk, n = 6), 2 mo (n = 12), 4 mo (n = 6), 6 mo (n = 12), 12 mo (n = 6), and 15 mo (n = 4) of age. Mice were fixed with 4% paraformaldehyde overnight, decalcified for 2 wk in 10% ethylenediaminetetraacetic acid (EDTA) / 2% paraformaldehyde, dehydrated, and embedded in paraffin. Immunostaining for phosphorylated Smad 1/5/8 (pSmad1/5/8) was carried out with paraffin sections. After deparaffinization, antigen retrieval was performed in citrate buffer (pH 6.0) by boiling for 10 min and then incubating in citrate butter at room temperature for 10 min. Endogenous peroxidase was blocked by incubation with 3% H2O2 for 10 min. Sections were treated with Tween-20 in phosphate-buffered saline 1% bovine serum albumin for blocking and were incubated with a rabbit pSmad1/5/8 antibody (1:100; Cell Signaling, Beverly, MA, USA) overnight at 4 °C. Following rinsing, sections were then incubated with biotinylated goat anti-rabbit secondary antibody, and the signal was visualized with a horseradish peroxidase / DAB detection immunohistochemistry kit according to the manufacturer’s instructions (Abcam Plc., Cambridge, UK). Companion serial sections were treated together, except for inclusion of primary antibodies, and served as negative controls.
For in situ hybridization, sections were hybridized with antisense or sense 35S-labeled probes as described in detail previously (Koyama et al. 2007).
Micromass Cell Culture
Micromass cultures were prepared from the disc cells of 2-mo-old wild-type and Prg4 mutant mice described previously (Asai et al. 2014) with some modification. Isolated articular discs were incubated with 2.5 U/mL of Dispase (MP Biomedicals, LLC, Solon, OH, USA) and 600 U/mL (3 mg/mL) of type I collagenase (Worthington Biochemical Corporation, Lakewood, NJ, USA) in Hank’s balanced salt solution with Ca2+ and Mg2+ for 30 min at 37 °C with agitation. The dissociated cells were grown on collagen 1 substrate–coated 6-well culture plates in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum and antibiotics. The cultures were passaged 3 to 5 times prior to use in experiments.
Micromass cultures were initiated by spotting 20 µL of the cell suspensions (4 × 105 cells) onto collagen 1 substrate in 24-well tissue culture plates. The cultures were grown for 10 d in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum and supplemented with rhBMP-2 (R&D Systems, Minneapolis, MN, USA) at a concentration of 100 to 200 ng/mL and in absence or presence of the BMP signaling inhibitor LDN-193189, which selectively blocks the transcriptional activity of the BMP type 1 receptor kinases (Selleckchem, Houston TX, USA; Cuny et al. 2008) at a concentration of 0.2 or 0.4 µM. Day 10 cultures were fixed and stained with alcian blue (pH 1.0), and the integrated density of alcian blue–stained cultures was semiquantified with ImageJ.
Results
Prg4 Expression in Discs and Heterotopic Chondrogenic Differentiation in Prg4-null Discs
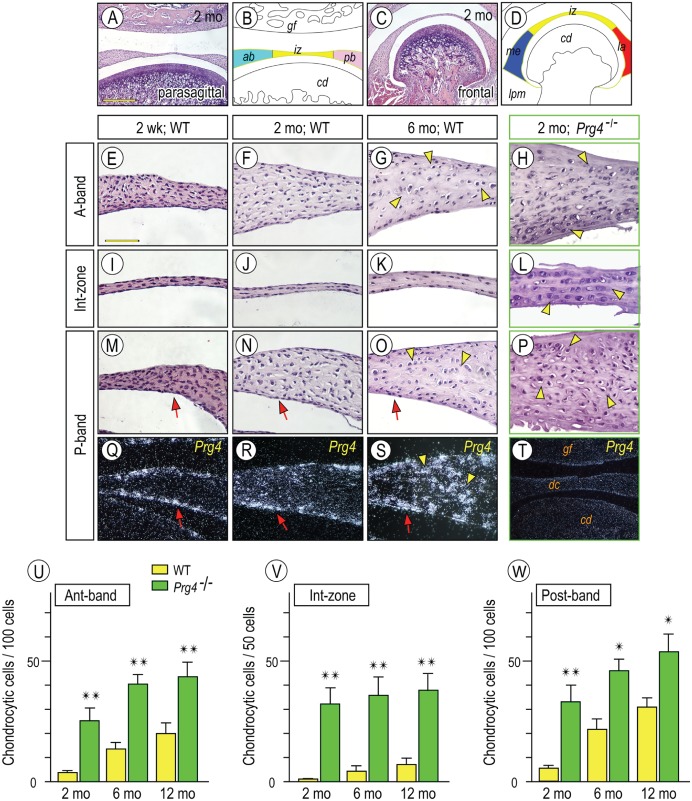
Little is known about possible links between chondrogenic transdifferentiation and Prg4 expression in TMJ discs, and such information could provide important insights into heterotopic cartilage formation in certain TMJ diseases. To this end, we further examined the expression patterns of Prg4 expression and the structural and phenotypic changes occurring in Prg4-null discs over time. Parasagittal (Fig. 1A) and frontal (Fig. 1C) disc sections were prepared, and the histologically identified disc regions are schematically depicted in Figure 1B and D. Chondrocyte-like cells were assessed by a round shape and type II collagen (Col2α1) gene expression. In 2-wk- and 2-mo-old discs, flat and compacted fibroblasts predominated in all regions, while few if any chondrocyte-like cells were present in anterior (Fig. 1E, F), posterior (Fig. 1M, N), and intermediate zones (Fig. 1I, J). By 6 mo of age, the chondrocyte-like cells became appreciable in the central portion of anterior/posterior bands (Fig. 1G, O, arrowheads), while fibroblasts lined the disc surfaces (hereafter referred to as disc-lining cells). Prg4 transcripts were present in disc-lining cells as early as at 2 wk of age and continued to be present over time (Fig. 1Q–S, arrow). Interestingly, Prg4 expression became detectable in chondrocyte-like cells at 6 mo (Fig. 1S, arrowhead). In Prg4-null discs, however, chondrocyte-like cells were already appreciable at 2 mo, were present throughout (Fig. 1H, L, P), and increased in number with age (Fig. 1U–W). Importantly, there were almost no detectable disc-lining cells in 2-mo-old mutants (Figs. 1H, L, P), and some unknown material coated the tissue surface. In situ hybridization for Prg4 with Prg4-null sections produced no signals, indicating specificity of signal (Fig. 1T).
Figure 1.
Temporospatial Prg4 expression in wild-type discs and aberrant differentiation of chondrocyte-like cells in Prg4-/- discs. Diagram (B, D) illustrating respective disc portions from parasagittal (A) and frontal sections (C) of temporomandibular joints (TMJs). TMJs from mice were analyzed by histology: 2-wk-old (E, I, M, Q), 2-mo-old (F, J, N, R), and 6-mo-old (G, K, O, S) control and 2-mo-old Prg4-/- (H, L, P, T). In situ hybridization with isotope-labeled riboprobes for Prg4 expression (Q–T). Note that Prg4 is expressed in disc-lining cells (arrows) and chondrocyte-like cells (arrowheads). Chondrocyte-like cells were counted in anterior (Ant) band, intermediate (Int) zone, and posterior (Post) band in distinct TMJ sections from control and Prg4-null mice at ages of 2, 6, and 12 mo (U–W; cell number is present per approximately 100 cells in anterior and posterior bands and approximately 50 cells in intermediate zone). Areas were randomly selected from 5 to 8 sections per sample (n = 3 for mouse/each group; *P < 0.05, **P < 0.01) and presented as average ± SD. Scale bars: 250 µm in A, also for C, T; 65 µm in E, also for F–S. ab, anterior band; cd, condyle; dc, disc; gf, glenoid fossa; iz, intermediate zone; la, lateral part; lpm, lateral pterygoid muscle; me, medial part; pb, posterior band; WT, wild type.
Molecular Evaluation of Heterotopic Cartilage Formation in Prg4-null Discs
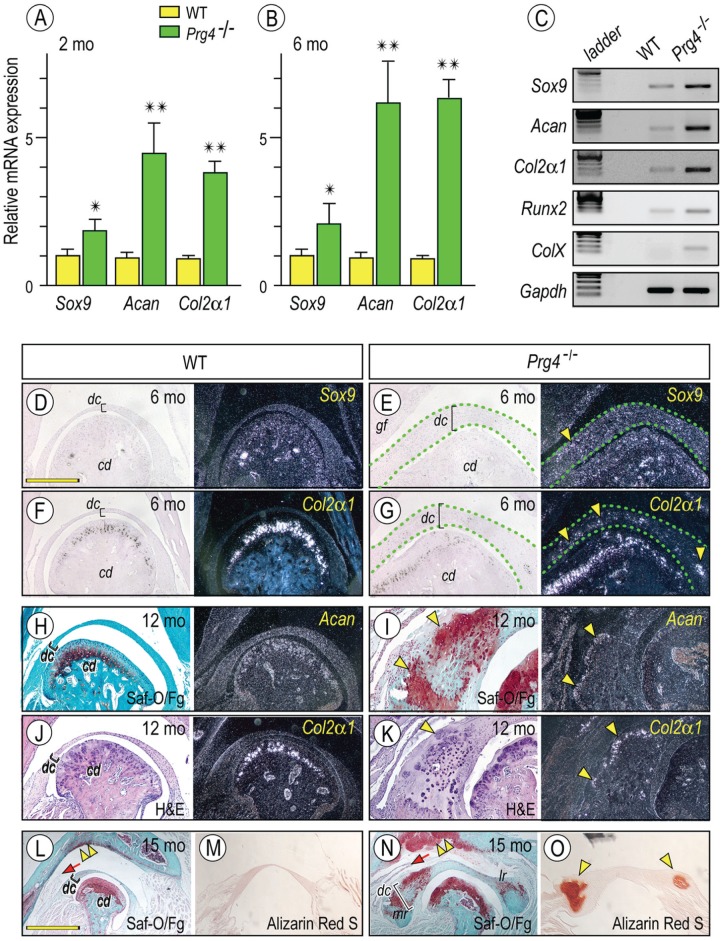
Next, we characterized in greater detail the process of ectopic chondrogenesis in mutant discs. RNAs isolated from 2-mo-old control and Prg4-null discs were processed for quantitative polymerase chain reaction (PCR; Fig. 2A, B). Sox 9, a key transcription factor regulating early chondrocyte differentiation, was upregulated in mutant versus wild-type discs. as were the cartilage characteristic genes aggrecan and Col2α1 (Fig. 2A, B). Similar trends were seen with semiquantitative PCR (Fig. 2C). Importantly, Runx2, a key transcription factor for chondrocyte hypertrophy and endochondral ossification, was slightly increased in mutants and accompanied the increased expression of type X collagen (ColX), a marker for chondrocyte hypertrophy. To verify data, we performed in situ hybridization to assess the topographic expression of Sox-9, Col2α1, and aggrecan (Fig. 2E, G, I, K). Safranin O/fast green staining clearly revealed that the medial region of mutant discs contained ectopic cartilage (Fig. 2I [arrowheads], N) composed of chondrocytes expressing Sox9, aggrecan, Col2α1, and ColX (Fig. 2I, K; Appendix Fig. 2). Heterotopic cartilage also developed in the glenoid fossa, and the medial synovial membrane appeared elongated (Fig. 2N, double arrowhead and arrow, respectively). Alizarin red staining revealed regional mineralization in the ectopic cartilage (Fig. 2N, O, arrowheads).
Figure 2.
Increased expression of chondrocyte markers in Prg4-/- discs. Histograms depicting the relative expression of Sox9, aggrecan (Acan), and Col2α1 in Prg4-/- and control discs at 2 mo (A) and 6 mo (B) of age and presented as average ± SD (n = 3 for mouse/each group, *P < 0.05, **P < 0.01). Semiquantitative reverse transcription polymerase chain reaction reveals increased expression of early (Sox9, Acan, and Col2α1) and maturing chondrocyte (Runx2 and ColX ) markers in Prg4-/- discs as compared with control littermates at age of 6 mo (C). Frontal sections prepared from TMJs and discs from 6-mo-old (D–G), 12-mo-old (H–K), and 15-mo-old (L–O) control (D, F, H, J, L, M) and Prg4-/- (E, G, I, K, N, O) mice were processed for in situ hybridization with isotope-labeled riboprobes for Sox9 (D, E, arrowhead), Col2α1 (F, G, J, K, arrowhead), and Acan (H, I). Mutant discs were delineated by green dashed lines (E, G). Sections were also stained with safranin O (Saf-O) / fast green (Fg; H, I, L, N) and alizarin red S (M, O) and evaluate proteoglycans and/or mineralization in discs, respectively. Safranin O–stained cartilage (I, bright field, arrowheads) was composed of Col2α1- and Acan-expressing chondrocytes (I, K, dark field, arrowheads). Alizarin red S–stained mineralized tissues were detected in the mesial and lateral portions of the mutant discs (O, arrowheads). Note also the massive safranin O–stained cartilage developed in the mutant glenoid fossa as compared with control (L, N, double arrowhead, respectively) and the elongated medial synovial membrane in mutants (L, N, arrow). Scale bars: 1.2 mm in D, also for E–K, M, O; 2.5 mm in L, also for N. cd, condyle; dc, disc; gf, glenoid fossa; H&E, hematoxylin and eosin; WT, wild type.
Aberrant Canonical BMP Signaling Is Associated with Heterotopic Cartilage Formation
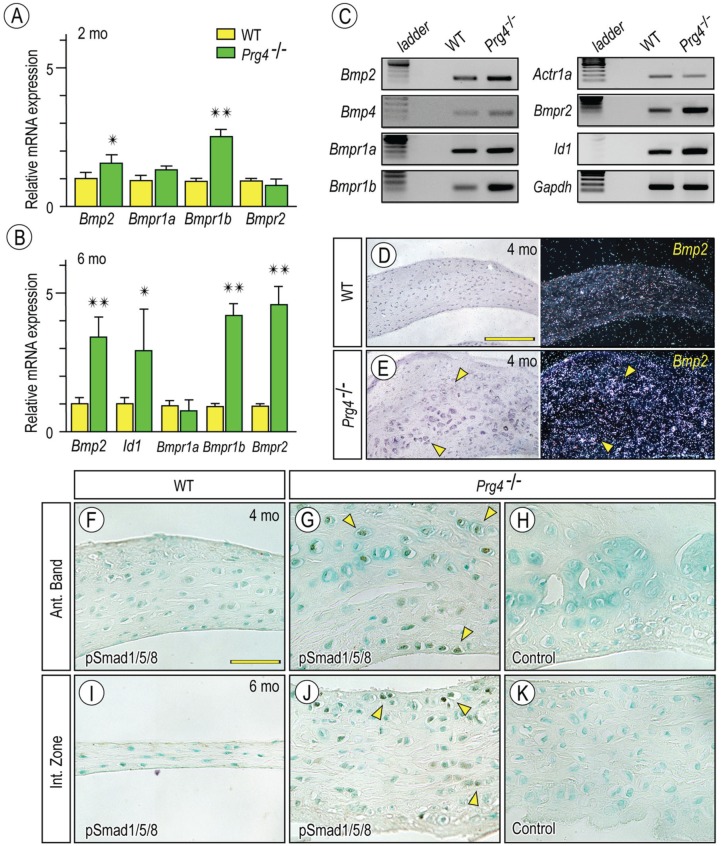
We next sought to clarify mechanisms underlying ectopic cartilage formation in mutant discs. BMP signaling is well known for its positive and active roles in chondrogenesis and endochondral ossification in normal and pathologic conditions (Billings et al. 2008; Long and Ornitz 2015). To determine whether ectopic BMP signaling was involved in ectopic cartilage formation in Prg4-null discs, we examined expression of BMP and their receptors in 2-mo- and 6-mo-old control and mutant discs (Fig. 3A, B). PCR showed that Bmp2, Bmpr1b, and Bmpr2 were all upregulated in 6-mo-old mutants relative to respective controls (Fig. 3B, C). The BMP signaling downstream mediator inhibitor of differentiation 1 (ld-1) was also upregulated in mutants, confirming increased Bmp signaling (Fig. 3B, C). In situ hybridization showed that Bmp2 transcripts were particularly clear in chondrocyte-like cells surrounding the presumptive cartilaginous nodules (Fig. 3E). pSmad1/5/8 immunostaining was quantified as fraction of nuclear-stained chondrocyte-like cells in anterior and intermediate zones, and significant differences were observed between control and Prg4-null discs (Fig. 3F, G, I, J; Appendix Fig. 3A). Increased activation of BMP signaling in mutant discs was also evident by Western blotting (Appendix Fig. 3B). Sections in which primary pSmad1/5/8 antibody was omitted displayed no signal, validating specificity of immunostaining data (Fig. 3H, K).
Figure 3.
Increased bone morphogenetic protein (BMP) signaling in Prg4-/- discs. Histograms depicting the relative expression of Bmp2, Bmpr1b, and Bmpr2 in Prg4-/- and control discs at age of 2 mo (A) and 6 mo (B) and presented as average ± SD (n = 3 for mouse/each group, *P < 0.05, **P < 0.01). Semiquantitative reverse transcription polymerase chain reaction showing that increased expression of Bmp2, Bmp type I and type II receptors, and Id-1 in discs from Prg4-/- mice as compared with wild type (WT) at age of 6 mo (C). TMJ discs from 4-mo-old control (D, F, I) and Prg4-/- (E, G, H, J, K) mice were analyzed by in situ hybridization with isotope-labeled riboprobes for Bmp2 (D, E) or by immunohistochemistry with phosphorylated Smad 1/5/8 (pSmad1/5/8) antibodies. Sections not treated with primary antibodies served as control (H, K). Note increased Bmp2 expression (E; arrowheads) and pSmad1/5/8-nulear translocation (G, J; arrowheads) are detected in chondrocyte-like cells in the anterior band (Ant. Band) and the intermediate zone (Int. Zone) in Prg4-/- discs. Scale bars: 90 µm in D, also for E; 55 µm in F, also for G–K.
Inhibition of BMP Signaling Blocks Chondrogenesis in Micromass Culture of Prg4-null Disc Cells
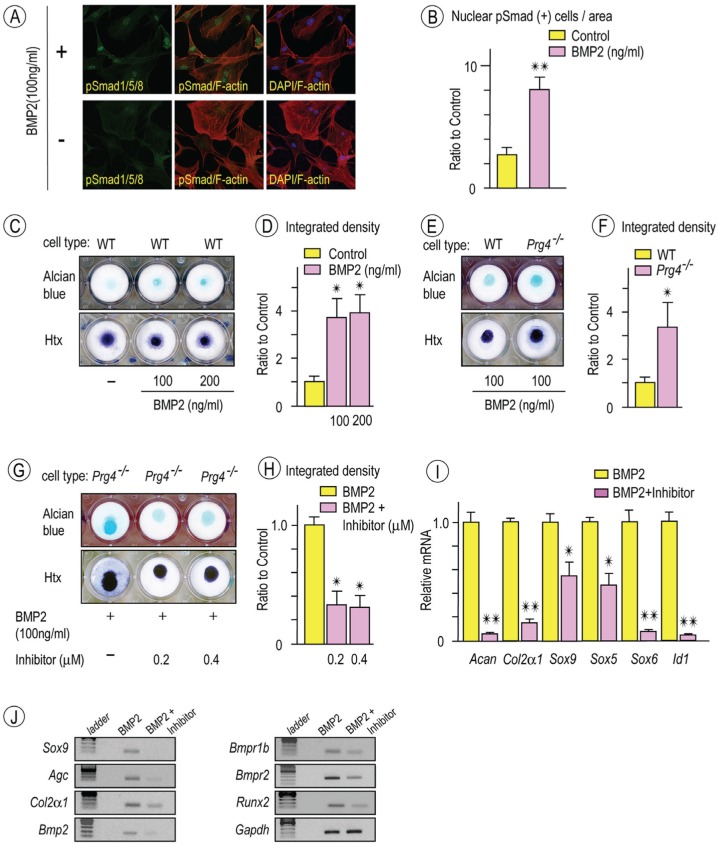
The data above indicate that increased BMP signaling is associated with heterotopic chondrogenesis and cartilage formation in mutant discs. Thus, we asked how mutant disc cells would behave in culture with regard to chondrogenic differentiation and whether they would respond to a BMP antagonist. Disc cells were first isolated from control 2-mo-old mice, reared in culture, treated with rhBMP2, and monitored for pSmad1/5/8 nuclear translocation for 10, 30, and 60 min. Immunocytochemistry showed a significant and rapid increase of pSmad1/5/8 nuclear translocation in rhBMP-2-treated as compared with untreated cells (Fig. 4A, B). To analyze chondrogenesis, the cells were seeded in high-density micromass culture (20 × 106 cells/mL) and treated with rhBMP2 (0 to 200 ng/mL). Staining with alcian blue on day 10 showed that exogenous rhBMP2 had induced chondrogenesis in wild-type cell cultures (Fig. 4C, D). As expected, disc cells from Prg4 mutant mice in companion micromass cultures appeared to undergo more exuberant chondrogenesis in response to exogenous rhBMP-2 (Fig. 4E, F). Note that chondrogenesis in control and mutant cell cultures was low in the absence of rhBMP2. To determine whether chondrogenesis in control and mutant cultures could be counteracted with a BMP signaling inhibitor, cultures were treated continuously with 0.2μM and 0.4μM LDN-193189. Alcian blue staining (Fig. 4G, H) and expression analysis of cartilage transcription factors and chondrogenic markers showed that the antagonist did in fact reduce chondrogenesis (Fig. 4I). The data were further affirmed by semiquantitative PCR, revealing that Bmp2, Bmpr1b, Bmpr2, and Runx2 were all decreased (Fig. 4J).
Figure 4.
Chondrogenic potential of Prg4-/- disc cells stimulated by recombinant human bone morphogenetic protein 2 (rhBMP-2) and attenuated by bone morphogenetic protein (BMP) inhibitor. Disc cells from 2-mo-old wild-type (WT) mice were plated on coverslips and grown in serum-starved Dulbecco’s modified Eagle’s medium for 12 h. Cultures were then stimulated with rhBMP2 for 30 min, fixed, and incubated with a rabbit phosphorylated Smad 1/5/8 (pSmad1/5/8) primary antibody followed by anti-rabbit Alexa Fluor 488 (green), with rhodamine phalloidin for staining the actin cytoskeleton in cells (red; A, upper panel). Culture without rhBMP2 treatment were served as controls (A, lower panel). DAPI stains were used to detect nuclei (blue). Images of pSmad1/5/8 (green) or DAPI (blue) were merged with rhodamine phalloidin-stained cytoskeletal images (red). Disc cells with pSmad1/5/8 in the nucleus were counted in randomly selected 6 to 8 areas (approximately 15 to 20 cells/area; n = 5, **P < 0.01) and presented as average ± SD (B). Day 10 micromass cultures of WT disc cells stimulated with rhBMP-2 (100 or 200 ng/mL) exhibited increased alcian blue staining compared with control cultures (C, upper panel; n = 4). Micromass cultures were also counterstained with hematoxylin (Htx) to reveal the presence of cells in cultures (C, lower panel). Integrated density of alcian blue–stained cultures was measured (D; n = 4, *P < 0.05). Day 10 micromass cultures of Prg4-/- disc cells stimulated with rhBMP-2 (100 ng/mL; E, upper panel) revealed increased alcian blue staining compared to WT disc cells (E, upper panel). Htx counterstaining (E; lower panel). Integrated density of alcian blue–stained cultures were measured (F; n = 4, *P < 0.05). Micromass cultures of Prg4-/- disc cells were treated with rhBMP-2 (100 ng/mL) alone or rhBMP-2 (100 ng/mL) / LDN-193189 (BMP inhibitor) at indicated concentration (G). Alcian blue staining (G, upper panel) and Htx counterstaining (G; lower panel). Integrated density of alcian blue–stained cultures was measured (H; n = 4, *P < 0.05). Histograms depicting relative expression of chondrocyte makers, Sox5, Sox6, Sox9, and BMP target were decreased in BMP inhibitor-treated cultures as compared with controls (I; n = 3, *P < 0.05, **P < 0.01). Semiquantitative reverse transcription polymerase chain reaction revealed decreased expression of chondrocyte markers, Bmp2, Bmp receptors, and Runx2 in Prg4-/- discs as compared with controls (J). Graphs depict mean ± SD. Control data were set as 1 (D, F, H, I).
Defective Structures at Nonarticulation Sites in Prg4-null TMJs
We further investigated the relationship between Prg4 expression and abnormalities of TMJ soft tissues in mutant mice, with particular attention to the tissue-tissue interface where Prg4 action may be required. We found that Prg4 transcripts were also detected in TMJ-associated tissues, including disc-pterygoid muscle interface (Fig. 5A) and pterygoid muscle/tendon-condylar neck insertion site (Fig. 5B). In good correlation, Prg4-null TMJs showed characteristic defective structures in those sites; in particular, the disc fibers lost a smooth insertion into the muscles (Figs. 5D, arrowheads), and ectopic cartilage had formed in the muscle/tendon insertion site into the condyle (Fig. 5F, arrowheads).
Figure 5.
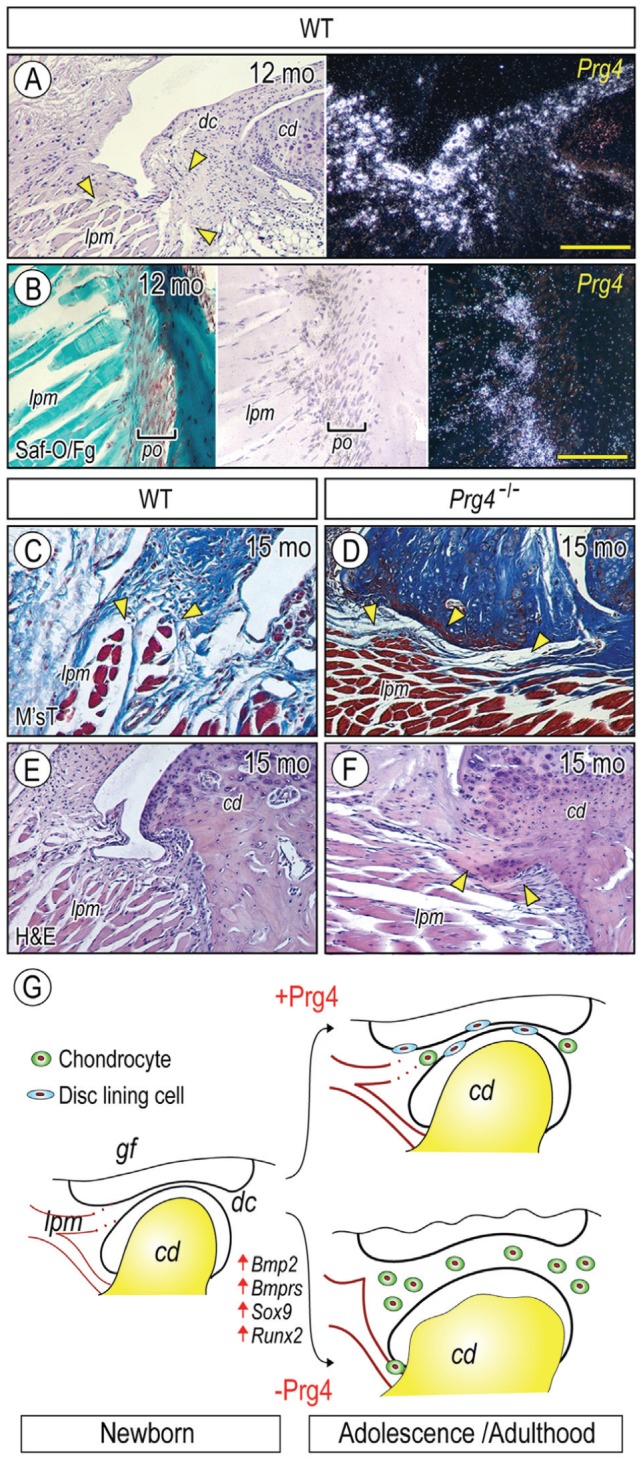
Prg4 expression at nonarticulation sites and tissue derangement in Prg4-/- mice. Parasagittal sections of TMJs from 12-mo (A, B) and 15-mo (C–F) control (A, B, C, E) and Prg4-/- (D, F) mice were processed for histology and in situ hybridization with isotope-labeled riboprobes for Prg4 expression (A, B). Sections were stained with safranin O/fast green (Saf-O/Fg; B), Masson’s trichrome (M’sT; C, D), and hematoxylin and eosin (H&E; E, F). Note that Prg4 is expressed at disc/lateral pterygoid muscle junction (A, arrowheads) and muscle/tendon insertion site into the mandibular condyle (B). Note also the disrupted muscle insertion into the articular disc in mutant (D, arrowheads) as compared with control (C, arrowheads) and ectopic cartilage formation at muscle/tendon insertion site into the mandibular condyle in mutant (F, arrowheads). (G) Model of heterotopic ossification in TMJs caused by absence of Prg4 action and involvement of abnormal BMP signaling. (G) Model of increased chondrogenesis and subsequent cartilage formation in Prg4 mutant discs. TMJ appears to develop normally, and the disc separates the joint space into the upper and lower joint cavities. With further development, the disc displays a concaved shape; the disc-lining cells cover the surface of the disc; and a few chondrogenic cells and chondrocytes differentiate in the anterior and posterior bands in wild-type disc (with Prg4 action). In sharp contrast, in Prg4 mutant mice (without Prg4 action), the disc becomes much thicker, and this is accompanied by increased chondrocyte number and ectopic cartilage formation throughout the disc. In addition, the lateral pterigoid muscle loses its smooth integration into the disc. The increased chondrogenesis and subsequent cartilage formation throughout the disc are accompanied by activation of Bmp signaling and increased expression of Sox9 and Runx2. Scale bars: 120 µm in A, also for E, F; 60 µm in B, also for C, D. cd, condyle; dc, disc; lpm, lateral pterygoid muscle; po, periosteum.
Discussion
Here, we report that heterotopic cartilage develops spontaneously in Prg4-null discs without additional insult or invasive surgical intervention. The expression levels of Bmp2, BMP type I and type II receptors, and ld-1 were all significantly and concurrently increased in mutant discs, indicative of abnormal activation of BMP signaling. Consistent with this notion, mutant discs displayed an increased number of chondrocytic cells and chondrocytes concomitant with pSmad1/5/8-nuclear staining. In good correlation, we found that mutant disc cells in micromass cultures were more responsive to rhBMP2 treatment than control cells were, as indicated by cartilage matrix accumulation. Importantly, a BMP signaling inhibitor attenuated chondrogenesis in control and mutant cultures. Taken together, the data indicate that the mutant disc cell population is more prone than control cells to undergo chondrogenic cell differentiation in conjunction with enhanced BMP signaling. The data also suggest that the Prg4-null mice are a useful model for the study of TMJ heterotopic ossification and that Prg4 is normally needed for postnatal TMJ disc integrity and function.
Our findings clearly show that wild-type TMJ discs dynamically undergo remodeling with age, as observed in discs in humans and other animals (Minarelli and Liberti 1997; Berkovitz and Pacy 2000). Chondrocyte-like cells preferentially differentiate in anterior and posterior bands, likely representing an adaptive response of disc cells to the demands of TMJ function. The results correlate well with analysis of biomechanical properties that were found to change significantly in terms of compressive modulus in those sites with age (Wang et al. 2014). Interestingly, we find that these chondrocyte-like cells/chondrocytes in discs of aged mice start to express Prg4, indicating that the interface between these cells may need lubricating action for proper TMJ function. Prior studies have shown an increased coefficient of friction in loaded joints from Prg4-null mice (Jay et al. 2007; Drewniak et al. 2012). Therefore, abnormal friction could be one of major culprits for aberrant chondrocyte differentiation and subsequent cartilaginous tissue formation in discs as well as glenoid fossae and condyles. Given our finding from this and previous work (Shibukawa et al. 2007; Koyama et al. 2014), we propose the following abnormal modeling process in the TMJs of Prg4-mutants (Fig. 5G). The disc primordium first becomes apparent once the superior and inferior articular cavities are creased by early postnatal life. Soon after, the mutant disc cells show increased cell proliferation throughout the disc, leading to disc hypertrophy. In conjunction with the deformation of articular cartilage of glenoid fossa and condyle, the disc could be subjected to abnormal mechanical stimuli, making the cells more prone to differentiate into chondrocytes. In such processes, aberrant BMP signaling could be activated and would induce heterotopic cartilage and bone formation.
We utilized high-density micromass cultures to analyze the chondrogenic potentials of cells isolated from the fibrocartilaginous disc of TMJs (Hayes and Ralphs 2011). We show here that cells isolated from wild-type discs were able to undergo chondrogenesis but that cells isolated from Prg4-mutant discs displayed higher chondrogenic potential. Interestingly, proteoglycan accumulation in wild-type cultures was relatively low but increased substantially after rhBMP-2 treatment (Fig. 4C, D). The data indicate that disc cells are able to retain their chondrogenic potentials during culture expansion and can undergo chondrogenesis in response to BMP signaling. The more exuberant chondrogenesis that we observe in mutant cell cultures in response to exogenous rhBMP-2 (Fig. 4E, F) may reflect the possibility that mutant cultures contain a more numerous population of chondroprogenitor and/or chondrocytic cells.
Our data clearly indicate that a characteristic BMP ligand-receptor signaling axis operates in the Prg4-mutant discs. We find in mutant TMJ discs at 6 mo of age that expression levels of Bmp2, Bmpr1b, and Bmpr2 are about 3-fold higher than controls. Previous studies have shown that BMP type I receptors possess distinct biological functions during skeletal development. Intriguingly, expression of dominant-negative Bmpr1b displayed more severe skeletal defects when compared with Bmpr1a, implying that Bmpr1b-mediated BMP signaling is essential for early chondrogenesis (Chen et al. 1998). In agreement with previous studies, our results show that blocking BMP signaling can markedly decrease the expression of Bmpr1b and Bmp2, resulting in decreased proteoglycan synthesis and accumulation and decreased chondrocyte differentiation. Our findings imply that the BMP signaling pathway could be a potential therapeutic target for prevention and/or treatment for ectopic chondrocyte differentiation and/or cartilage formation in affected TMJ discs.
Our histomorphometric observations and gene expression analyses clearly indicate that mutant discs in juvenile mice contain more chondrogenic cells than those in age-matched controls. In our mouse model, however, ectopic cartilage formation appears to be slower than that seen in discs subjected to surgical intervention (Embree et al. 2015). Therefore, our model may represent a chronic pathologic process of heterotopic ossification in discs. Note that there are no apparent inflammatory signs in synovial joints in CACP patients (Rhee et al. 2005). It is therefore intriguing to ask if there are any links between the acceleration of heterotopic cartilage formation and inflammatory response in synovium and other TMJ sites accompanied by surgical intervention or joint injuries.
Finally, we show that Prg4 is expressed in nonarticulation sites of TMJ associated tissues: disc/lateral pterygoid muscle (LPM) interface and condylar neck/LPM insertion site. The LPM is a muscle with 2 separate heads, attaches to the anteromedial surface of the TMJ disc and the condylar neck, and coordinates the movement of TMJ components (Juniper 1981). Clinical studies have pointed out relationships among muscle hypertrophy/atrophy, damaged discs and TMJ internal derangement (Dergin et al. 2012; Imanimoghaddam et al. 2013). Our data indicate that loss of Prg4 could alter the associations of LPM/TMJ disc and LPM/condyle and could compromise TMJ function. Ectopic cartilage also develops in the muscle/bone insertion site. Taken together, our data lead to the intriguing possibility that Prg4 has previously unsuspected roles in such joint-associated tissues and sites. Further investigations will be necessary to clarify this and other possibilities.
Author Contributions
T.E. Bechtold, C. Saunders, C. Mundy, H. Um, R.S. Decker, I. Salhab, N. Kurio, P.C. Billings, contributed to data analysis, drafted the manuscript; M. Pacifici, H.D. Nah, E. Koyama, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank Dr. Matthew Warman for kindly proving the Prg4-null mice.
Footnotes
This study was supported by National Institutes of Health grants (National Institute of Dental and Craniofacial Research grant RO1DE023841 and National Institute of Arthritis and Musculoskeletal and Skin Diseases grant RO1AR061758). R.S.D. is the recipient of a postdoctoral training grant (1F32AR064071) from the National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Asai S, Otsuru S, Candela ME, Cantley L, Uchibe K, Hofmann TJ, Zhang K, Wapner KL, Soslowsky LJ, Horwitz EM, et al. 2014. Tendon progenitor cells in injured tendons have strong chondrogenic potential: the CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells. 32(12):3266–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahabri SA, Suwairi WM, Laxer RM, Polinkovsky A, Dalaan AA, Warman ML. 1998. The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum. 41(4):730–735. [DOI] [PubMed] [Google Scholar]
- Berkovitz BK, Pacy J. 2000. Age changes in the cells of the intra-articular disc of the temporomandibular joints of rats and marmosets. Arch Oral Biol. 45(11):987–995. [DOI] [PubMed] [Google Scholar]
- Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. 2008. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res. 23(3):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. 1998. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 142(1):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JM, Zhang L, Blum JJ, Warman ML, Jay GD, Guilak F, Zauscher S. 2010. Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis Rheum. 62(6):1666–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. 2008. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 18(15):4388–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergin G, Kilic C, Gozneli R, Yildirim D, Garip H, Moroglu S. 2012. Evaluating the correlation between the lateral pterygoid muscle attachment type and internal derangement of the temporomandibular joint with an emphasis on MR imaging findings. J Craniomaxillofac Surg. 40(5):459–463. [DOI] [PubMed] [Google Scholar]
- Drewniak EI, Jay GD, Fleming BC, Zhang L, Warman ML, Crisco JJ. 2012. Cyclic loading increases friction and changes cartilage surface integrity in lubricin-mutant mouse knees. Arthritis Rheum. 64(2):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, Shalvoy R, Jay GD. 2008. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 58(6):1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree MC, Iwaoka GM, Kong D, Martin BN, Patel RK, Lee AH, Nathan JM, Eisig SB, Safarov A, Koslovsky DA, et al. 2015. Soft tissue ossification and condylar cartilage degeneration following TMJ disc perforation in a rabbit pilot study. Osteoarthritis Cartilage. 23(4):629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, Caterson B. 1999. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 254(3):535–541. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Ralphs JR. 2011. The response of foetal annulus fibrosus cells to growth factors: modulation of matrix synthesis by TGF-beta1 and IGF-1. Histochem Cell Biol. 136(2):163–175. [DOI] [PubMed] [Google Scholar]
- Hill A, Duran J, Purcell P. 2014. Lubricin protects the temporomandibular joint surfaces from degeneration. PLoS One. 9(9):e106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huegel J, Enomoto-Iwamoto M, Sgariglia F, Koyama E, Pacifici M. 2015. Heparanase stimulates chondrogenesis and is up-regulated in human ectopic cartilage: a mechanism possibly involved in hereditary multiple exostoses. Am J Pathol. 185(6):1676–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL. 2012. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med. 4(1):15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanimoghaddam M, Madani AS, Hashemi EM. 2013. The evaluation of lateral pterygoid muscle pathologic changes and insertion patterns in temporomandibular joints with or without disc displacement using magnetic resonance imaging. Int J Oral Maxillofac Surg. 42(9):1116–1120. [DOI] [PubMed] [Google Scholar]
- Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, Elsaid K, Kim KS, Cui Y, Warman ML. 2007. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 56(11):3662–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AW, Viozzi CF, Foote RL. 2010. Long-term results of radiation prophylaxis for heterotopic ossification in the temporomandibular joint. J Oral Maxillofac Surg. 68(5):1100–1105. [DOI] [PubMed] [Google Scholar]
- Jibiki M, Shimoda S, Nakagawa Y, Kawasaki K, Asada K, Ishibashi K. 1999. Calcifications of the disc of the temporomandibular joint. J Oral Pathol Med. 28(9):413–419. [DOI] [PubMed] [Google Scholar]
- Juniper RP. 1981. The superior pterygoid muscle? Br J Oral Surg. 19(2):121–128. [DOI] [PubMed] [Google Scholar]
- Koyama E, Saunders C, Salhab I, Decker RS, Chen I, Um H, Pacifici M, Nah HD. 2014. Lubricin is required for the structural integrity and post-natal maintenance of TMJ. J Dent Res. 93(7):663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, Maeda Y, Lanske B, Song B, Serra R, et al. 2007. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 134(11):2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist C, Söderholm AL, Hallikainen D, Sjövall L. 1992. Erosion and heterotopic bone formation after alloplastic temporomandibular joint reconstruction. J Oral Maxillofac Surg. 50(9):942–949. [DOI] [PubMed] [Google Scholar]
- Long F, Ornitz DM. 2015. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 5(1):a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XL, Mow VC, Guo XE. 2009. Proteoglycans and mechanical behavior of condylar cartilage. J Dent Res. 88(3):244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz JG, Rodrigues L, Chilvarquer I, Soler JM. 2003. Mineralization of stylohyoid ligament complex in patients with temporomandibular disorders and asymptomatic individuals: a comparative study. J Oral Rehabil. 30(9):909–13. [DOI] [PubMed] [Google Scholar]
- Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, Sood R, Makalowska I, Baxevanis A, Johnstone B, et al. 1999. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 23(3):319–322. [DOI] [PubMed] [Google Scholar]
- Minarelli AM, Liberti EA. 1997. A microscopic survey of the human temporomandibular joint disc. J Oral Rehabil. 24(11):835–840. [DOI] [PubMed] [Google Scholar]
- Molinari F, Manicone PF, Raffaelli L, Raffaelli R, Pirronti T, Bonomo L. 2007. Temporomandibular joint soft-tissue pathology: I. Disc abnormalities. Semin Ultrasound CT MR. 28(3):192–204. [DOI] [PubMed] [Google Scholar]
- Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, Jay GD, Stewart M, Wang H, Warman ML, et al. 2005. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 115(3):622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold S, Thapa M, Shaw EA, Wallace CA. 2011. Heterotopic ossification of the temporomandibular joint in juvenile idiopathic arthritis. J Rheumatol. 38(7):1423–1428. [DOI] [PubMed] [Google Scholar]
- Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. 2007. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 56(3):882–891. [DOI] [PubMed] [Google Scholar]
- Scrivani SJ, Keith DA, Kaban LB. 2008. Temporomandibular disorders. N Engl J Med. 359(25):2693–2705. [DOI] [PubMed] [Google Scholar]
- Seror J, Zhu L, Goldberg R, Day AJ, Klein J. 2015. Supramolecular synergy in the boundary lubrication of synovial joints. Nat Commun. 6:6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibukawa Y, Young B, Wu C, Yamada S, Long F, Pacifici M, Koyama E. 2007. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev Dyn. 236(2):426–434. [DOI] [PubMed] [Google Scholar]
- Shibuya T, Kino K, Kitamura Y, Takahashi T. 2003. Synovial osteochondromatosis accompanying an ossified articular disk in the temporomandibular joint: a case report. J Oral Pathol Med. 32(7):441–442. [DOI] [PubMed] [Google Scholar]
- Swann DA, Silver FH, Slayter HS, Stafford W, Shore E. 1985. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J. 225(1):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano Y, Moriwake Y, Tohno Y, Minami T, Tohno S, Utsumi M, Yamada M, Okazaki Y, Yamamoto K. 1999. Age-related changes of elements in the human articular disk of the temporomandibular joint. Biol Trace Elem Res. 67(3):269–276. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Detamore MS, Mercuri LG. 2008. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 87(4):296–307. [DOI] [PubMed] [Google Scholar]
- Ungethuem U, Haeupl T, Witt H, Koczan D, Krenn V, Huber H, von Helversen TM, Drungowski M, Seyfert C, Zacher J, et al. 2010. Molecular signatures and new candidates to target the pathogenesis of rheumatoid arthritis. Physiol Genomics. 42A(4):267–282. [DOI] [PubMed] [Google Scholar]
- Wang XD, Cui SJ, Liu Y, Luo Q, Du RJ, Kou XX, Zhang JN, Zhou YH, Gan YH. 2014. Deterioration of mechanical properties of discs in chronically inflamed TMJ. J Dent Res. 93(11):1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.