Abstract
The balance between bone resorption and bone formation is vital for maintenance and regeneration of alveolar bone and supporting structures around teeth and dental implants. Tissue regeneration in the oral cavity is regulated by multiple cell types, signaling mechanisms, and matrix interactions. A goal for periodontal tissue engineering/regenerative medicine is to restore oral soft and hard tissues through cell, scaffold, and/or signaling approaches to functional and aesthetic oral tissues. Bony defects in the oral cavity can vary significantly, ranging from smaller intrabony lesions resulting from periodontal or peri-implant diseases to large osseous defects that extend through the jaws as a result of trauma, tumor resection, or congenital defects. The disparity in size and location of these alveolar defects is compounded further by patient-specific and environmental factors that contribute to the challenges in periodontal regeneration, peri-implant tissue regeneration, and alveolar ridge reconstruction. Efforts have been made over the last few decades to produce reliable and predictable methods to stimulate bone regeneration in alveolar bone defects. Tissue engineering/regenerative medicine provide new avenues to enhance tissue regeneration by introducing bioactive models or constructing patient-specific substitutes. This review presents an overview of therapies (e.g., protein, gene, and cell based) and biomaterials (e.g., resorbable, nonresorbable, and 3-dimensionally printed) used for alveolar bone engineering around teeth and implants and for implant site development, with emphasis on most recent findings and future directions.
Keywords: tissue engineering, alveolar bone, gene therapy, 3D printing, growth factors, regeneration
Pathogenesis of Defects Associated with Periodontal and Peri-implant Diseases
Alveolar bone lining the socket containing teeth is remodeled continuously. This fine-tuned balance between bone resorption and bone formation is maintained by multiple cell types and signaling mechanisms (Nanci and Bosshardt 2006; Fig. 1). In susceptible individuals, the inflammatory response to bacteria can initiate the destructive process of periodontitis, leading to loss of connective tissue and bone, as well as apical migration of the junctional epithelium (Seymour et al. 2015). While disruption of subgingival microbial biofilm and resolution of periodontal inflammation can be achieved by nonsurgical therapy, restitution ad integrum cannot normally be expected, leaving a reduced periodontium and potentially residual alveolar bone defects. In more advanced cases of periodontitis, the tooth loses support and is exfoliated or extracted. To ensure masticatory function and aesthetics, replacement of missing teeth is often considered necessary. However, due to extensive previous bone resorption, alveolar bone defects may prevent correct positioning of dental implants, requiring augmentation. Following successful osseointegration, dental implants can also undergo a process of microbially driven chronic inflammation leading to bone resorption (peri-implantitis) and potentially implant loss (Carcuac and Berglundh 2014). Therefore, the clinical need for alveolar bone regeneration arises to improve long-term prognosis of teeth with periodontitis and implants affected by peri-implantitis and for the development of alveolar bone sites for implant placement. In addition to bone loss due to chronic inflammation, bone regeneration may be needed to correct defects of other origins, including trauma, tumor resection, or congenital and developmental conditions (Giannobile 2014). Efforts have been made over recent decades to predictably stimulate bone regeneration for alveolar bone defects around teeth as well as more recently in edentulous areas and around implants affected by peri-implantitis. This review highlights therapies and biomaterials used for alveolar bone engineering, with emphasis on the most recent findings and future avenues.
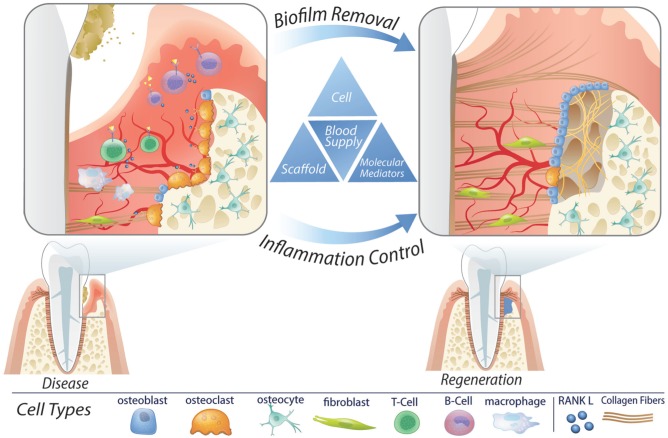
Figure 1.
Influencing factors for periodontal regenerative medicine. Periodontal diseases can result in significant damage to the periodontal structures. Following resolution of etiologic factors and controlling host response/inflammation, periodontal regeneration is necessary to restore health. Periodontal regeneration can be achieved clinically through the use of cell therapy, biologics, and biomaterial scaffolds in the context of an adequate blood supply.
Tissue Engineering/Regenerative Medicine
Mason and Dunnill (2008) described regenerative medicine as a therapy replacing or regenerating human cells, tissues, or organs to restore or establish their normal function. This is now an established research field called tissue engineering/regenerative medicine (TE/RM), its purpose being “to stimulate regeneration of tissues and organs by either implanting biomaterials for in vivo regeneration or by constructing substitutes in vitro” (Brouwer et al. 2015). TE/RM is a translational research area including a broad range of disciplines, such as stem cell biology, material sciences, medicine, chemistry, and manufacturing (Webber et al. 2015). Recently, nanotechnology was introduced as a new area in TE/RM and in periodontal tissue engineering, with emerging studies demonstrating significant influence of nanoscaled topography and geometry on cell differentiation, behavior, and enhanced 3-dimensional (3D) regeneration (Rios et al. 2011; Bartold et al. 2016). For example, nanofibrous scaffolds designed to mimic the natural architecture of dentin have been shown to increase dental pulp stem cell proliferation and differentiation in conjunction with magnesium ion release (Qu et al. 2014). There is also an emergence of nanotechnology-based applications as therapeutic strategies for treatment of periodontitis-induced bone loss. A recent study showed that nano-proresolving medicines designed specifically for treatment of inflammation-induced bone loss resulted in increased bone formation in a large animal model (Van Dyke et al. 2015). Ongoing research into the importance of nanoscale features for regeneration of periodontal complex tissues will further elucidate the required scaffold design parameters and therapeutic capabilities of nanotechnology-based applications.
There is a significant public health need for effective strategies for tissue engineering that can easily translate into everyday use in clinical dental practice. However, TE/RM technologies present a challenge for the regulatory agency evaluation system (e.g., Food and Drug Administration [FDA] or European Medicines Agency), since these approaches may be regarded as tissues, biological products, drugs, and/or medical devices, thereby requiring evaluation in all of the applicable pathways (Webber et al. 2015). In 2014, 28 TE/RM products were approved for clinical use and made commercially available for various applications by the FDA (Bertram et al. 2015).
Bone deficiencies in the oral cavity vary from localized bone loss commonly resulting from periodontal disease to extensive bone defects associated with facial trauma or tumor resection (Pagni et al. 2012). Creating complete regeneration of craniofacial defects is a challenge: not only must the engineered tissue have sufficient strength to sustain mechanical forces and architectural properties, but it also requires a porous internal network and a surface optimized for attachment, migration, proliferation, and differentiation of cells, while concomitantly providing a therapy that is simple and cost-efficient for patient-specific fabrication (Obregon et al. 2015; Webber et al. 2015; Bartold et al. 2016). New advances in cell and gene therapy present ways to improve the outcome of current techniques and introduce approaches for regulating inflammatory host responses.
Current Therapies
The goal of periodontal regenerative techniques is to restore the periodontal tissues and their function. Recent advances in TE/RM are based on delivery of cells, proteins, and genes in biodegradable scaffolds (Fig. 2). Early on, periodontal regeneration used the concept of guided tissue regeneration, allowing selection of the cell population to colonize the periodontal wound following surgical exposure. The use of bone substitutes in conjunction with barriers aims to prevent epithelial migration into the regenerating area. This allows the slower migrating cells of the periodontal ligament (PDL) to repopulate the protected area, providing positive clinical outcomes in selected clinical cases (Gottlow et al. 1984). Decades of research have expanded on this initial experience, resulting in a number of different biomaterials that are available to the clinician and researcher for alveolar bone regeneration. The Table shows biomaterials used for alveolar bone regeneration broadly divided in subgroups according to their origins and mechanisms of action. It should be noted that treatment components (biomaterials, growth factors, recombinant proteins, etc.) are often not used alone but in combinations. Biomaterials can be broadly divided into 1) materials that replace the missing portion of alveolar bone (“bone grafts” or “bone substitutes”); 2) materials that cover the alveolar bone loss area, protecting it from epithelial downgrowth (“barriers”); and 3) materials with biological activity that can be administered directly to the defect (“biologics” or “cell therapy”). Some examples of studies where these biomaterials have been used clinically or preclinically over the past 5 y are provided in the Table. It is suggested that these original reports and reviews be examined for more comprehensive listing of studies.
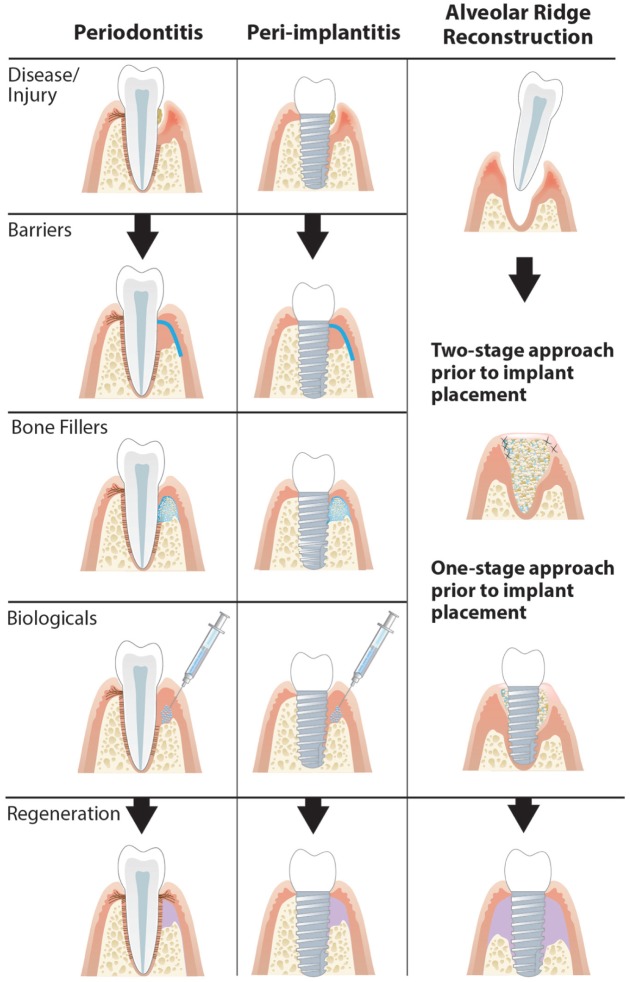
Figure 2.
Approaches for regenerating periodontal and peri-implant supporting tissues. Guided tissue regeneration involving bone substitutes and barrier membrane and bone fill can be used for tissue regeneration in periodontitis defects and peri-implantitis defects. Development of implant sites after tooth extraction can be obtained in either a 1- or 2-stage bone augmentation process.
Table.
Periodontal, Peri-implant, and Alveolar Ridge Regenerative Approaches.
| Therapy | |||
|---|---|---|---|
| Type: Clinical Example |
Periodontal Application |
Implant-based Application |
Implant Site Development / Alveolar Ridge Reconstruction |
| Bone Replacement Grafts | |||
| Autogenous: intraoral, extraoral bone blocks | Intrabony defects (Hassan et al. 2015; Mathur et al. 2015) Furcation defects (Lafzi et al. 2013) |
Regenerative treatment of peri-implantitis (Aghazadeh et al. 2012) | Nonmolar extraction socket for ridge preservation (Wood and Mealey 2012) Restoration of severe segmental or crestal bone defects in maxilla (Restoy-Lozano et al. 2015) |
| Allogenic: fresh-frozen bone, freeze-dried bone, demineralized freeze-dried allografts | Intrabony defects (Ogihara and Tarnow 2014; Agarwal et al. 2016) | Regenerative treatment of peri-implantitis (Froum et al. 2012) | Lateral ridge augmentation prior to implant placement (Urban et al. 2013) Vertical ridge augmentation (Chan et al. 2015) |
| Xenogenic: deproteinized bovine bone, equine bone graft | Deep noncontained intrabony defects (Iorio-Siciliano et al. 2014) Mandibular grade II furcation defect (Kannan et al. 2014) |
Regenerative treatment of peri-implantitis (Roccuzzo et al. 2011; Aghazadeh et al. 2012; Matarasso et al. 2015) | Socket preservation (Cardaropoli et al. 2012) Horizontal ridge preservation (Di Stefano et al. 2015) Extraction site defects (Nevins, Reynolds, et al. 2013) |
| Alloplastic: ceramics (i.e., biphasic calcium phosphate, beta-tricalcium phosphate, hydroxyapatite), bioactive glass, titanium granules | Furcation defects (Wohlfahrt et al. 2012) Periodontal intrabony defects (Hoffmann et al. 2015) |
Regenerative treatment of peri-implantitis (Wohlfahrt et al. 2012) | Deficient alveolar ridges (Nevins, Kao, et al. 2013) Alveolar ridge reconstruction (Santana and Santana 2015) |
| Synthetic: polymeric scaffolds | Large periodontal osseous defect (Rasperini et al. 2015) | Regenerative treatment of peri-implantitis (Diniz et al. 2015) | Alveolar ridge reconstruction (Goh et al. 2015) |
| Barriers | |||
| Autogenous: periosteal pedicle graft, buccal fat pad | Two-wall intrabony defect (Singhal et al. 2013) Furcation defect (Deliberador et al. 2012) |
Implant placement (Boora et al. 2015) | Alveolar ridge augmentation (Troedhan et al. 2015) |
| Allogenic: amnion membrane, chorionic membrane freeze-dried dura mater | Furcation defects (Patel et al. 2012) | Implant placement (Velez et al. 2010) | Guided bone regeneration (Holtzclaw 2015) |
| Xenogenic: bovine-derived, porcine membrane | Treament of severe chronic periodontitis (Gamal and Iacono 2013) | Treatment of peri-implantitis (Agazadeh et al. 2012; Matarasso et al. 2015) | Socket preservation (Cardaropoli et al. 2012) |
| Alloplastic: Gore-Tex, resorbable copolymers, Ti-reinforced PTFE, titanium mesh | Treatment of chronic periodontitis (Wadhawan et al. 2012) | Peri-implantitis (Roos-Jansaker et al. 2014) | Vertical ridge augmentation (Urban et al. 2013) Horizontal bone augmentation (Di Stefano et al. 2015) |
| Biologics | |||
| Bioactive factors: EMD, BMPs, PDGF, FGF2, teriparatide, GDF-5 | Intrabony defects (Döri et al. 2013; Oortgeisen et al. 2014) Periodontal osseous defects (Bashutski et al 2010; Windisch et al. 2012; Nevins, Kao, et al. 2013) Two- or 3-walled vertical bone defects (Kitamura et al. 2011) |
Peri-implantitis-affected implants (Froum et al. 2012; Jung et al. 2015) Implant osseointegration (Kuchler et al. 2011) |
Extraction site defects (Nevins, Reynolds, et al. 2013) Alveolar ridge and maxillary sinus augmentation (Koch et al. 2010; Freitas et al. 2015) Autogenous bone block grafting (Santana and Santana 2015) Ridge augmentation in saddle-type bone defects (Hoshi et al. 2016) |
| Stem cells: bone mesenchymal stem cells, periodontal ligament, gingival margin–derived, bone marrow concentrate | Periodontal defects (Du et al. 2014) | Treatment of peri-implantitis (Park et al. 2015) | Maxillary sinus floor elevation (Kim et al. 2015; Kaigler et al 2015) Alveolar ridge augmentation (Kaigler et al. 2013) |
| Gene therapy: osteoprotegerin, BMP2, BMP7, LMP3 | Treatment of periodontitis (Tang et al. 2015) | Treatment of peri-implantitis (Park et al. 2015) | |
Sample references (i.e., noncomprehensive listing) of recent studies published for each therapy (human studies in bold). Darker gray cells indicate fields where human randomized controlled trials have been conducted; paler gray cells indicate fields where human studies (non–randomized controlled trials) have been conducted; and very pale gray cells indicate preclinical studies.
BMP, bone morphogenetic protein; EMD, enamel matrix derivative; FGF2, fibroblast growth factor 2; GDF-5, growth and differentiation factor 5; PDGF, platelet-derived growth factor; PTFE, polytetrafluoroethylene.
Bone Grafts
Periodontal defects are often considered amenable to bone regeneration when residual bony walls are still preserved and can provide blood supply as well as mechanical support for the placement of a bone-filling material in the resorption site (Cortellini and Tonetti 2015). In addition to scaffolding function, space provision, and blood clot stabilization, bone grafts themselves may possess osteoconductive and/or osteoinductive characteristics.
To generate a scaffold for a particular function, knowledge of the tissue structure and behavior is needed. Four fundamental requirements for a scaffold include form, function, formation, and fixation, where formation is enhancement of tissue regeneration and fixation is the attachment of a scaffold to the surrounding tissue (Hollister 2009). Several important aspects of scaffold assembly need to be fulfilled: macrostructure, degradation time, release mechanisms, as well as exposure and duration of bioactive molecules (Hollister 2009). The material used must not only provide mechanical support but also promote cell adherence, migration, and proliferation on the surface to stimulate extracellular matrix (ECM) production. Furthermore, the composition of ECM needs to mimic the native ECM and, in periodontal regeneration, provide a space for PDL cellular growth while excluding epithelial cell downgrowth (Webber et al. 2015; Bartold et al. 2016).
Based on their origin, bone grafts have been classically divided into autogenous, allogenic, xenogenic, and synthetic or alloplastic (Pilipchuk et al. 2015). Several biomaterials belonging to the 4 classes above have been and are still used for alveolar bone regeneration, usually in combination with barriers and/or biologics. The clinical efficacy of bone substitutes for the treatment of intrabony defects and for lateral alveolar bone reconstruction has recently been systematically reviewed (Matarasso et al. 2015; Sanz-Sánchez et al. 2015).
Barriers
Upon surgical exposure and debridement of a bone defect, different types of cells can recolonize the wound, including epithelial cells, connective tissue cells, bone cells, and PDL cells. Physical barriers are used for guided tissue regeneration or guided bone regeneration to exclude downgrowth of epithelial cells and long junctional epithelium formation from the bone defect, providing space for cells capable of tissue regeneration. Membranes have been used in conjunction with bone grafts, which provide added physical support. More recent, “third generation” membranes act not only as barriers but also as delivery devices for specific agents, such as antibiotics and growth factors (Sam and Pillai 2014). Barrier membranes are traditionally divided into resorbable and nonresorbable (needing a second surgical procedure for removal) categories. Based on their origins, barriers can also be divided into autogenous, allogenic, xenogenic, and alloplastic categories. More traditional derivations of barrier membranes are xenogenic (e.g., bovine or porcine) and synthetic (Pilipchuk et al. 2015). The clinical efficacy of different membranes for guided tissue regeneration has recently been reviewed (Kao et al. 2015; Sculean et al. 2015).
Biologics
Biological mediators might be considered the most recent and fast-developing generation of agents used for alveolar bone engineering. They could be broadly categorized into growth factors, stem cells, and gene therapy agents. Growth factors used in periodontal tissue engineering include enamel matrix derivative (EMD), platelet-derived growth factor (PDGF), bone morphogenetic proteins (BMPs), vascular endothelial growth factor, growth and differentiation factor 5, and transforming growth factor β. Several studies have reported on their impact on tissue regeneration in both preclinical and clinical settings, reviewed by Pilipchuk and coworkers (2015). BMPs are members of the transforming growth factor β superfamily and are important factors in bone formation. Recombinant human BMP-2 and BMP-7 have been approved by the FDA for clinical use, including alveolar ridge augmentation and sinus elevation procedures (McKay et al. 2007). Growth and differentiation factor 5 has been evaluated in human clinical trials for periodontal and sinus floor augmentation applications (Koch et al 2010; Windisch et al. 2012). Several studies on treatment of sinus augmentation reported on the consistent improvement in bone gain with BMP-2 and the ability to preserve alveolar ridge heights as reported in a recent systematic review (Freitas et al. 2015).
PDGF plays an essential role in periodontal tissue repair by promoting PDL, fibroblast, and cementum proliferation and has been extensively used (Khoshkam et al. 2015). PDGF-BB is approved for periodontal regeneration and is commercially available, and it has been tested for treatment of intrabony defects and alveolar bone regeneration. Long-term evaluation showed promising results and improved treatment outcomes in periodontal regeneration (Lin et al. 2015). A long-term stable clinical and radiographic improvement of localized periodontal defects was shown in a randomized controlled clinical trial using recombinant human PDGF-BB protein in combination with a scaffold (Nevins, Kao, et al. 2013). Fibroblast growth factor 2 (FGF-2) is an important factor in wound-healing and angiogenic processes. FGF-2 inhibits osteogenic differentiation of PDL cells, maintaining their differentiation potential and increasing cellular proliferation (Pilipchuk et al. 2015). As shown in a recent systematic review, this factor has shown to improve bone fill in periodontal regeneration in phase II studies (Lin et al. 2015). In a clinical trial treatment of intrabony defects, periodontal regeneration and bone fill were significantly improved with FGF-2 therapy (Kitamura et al. 2011). EMD was introduced for the treatment of intrabony defects in human studies nearly 20 y ago and applied clinically in intrabony defects (Heijl 1997) and, more recently, in suprabony defects (Di Tullio et al. 2013) and implant site development. Beneficial effects of EMD have not been demonstrated in peri-implantitis defects, and at present EMD has been clinically applied in only a case series (Froum et al. 2012). The clinical efficacy of EMD for periodontal regeneration of intrabony defects has recently been reviewed (Kao et al. 2015; Sculean et al. 2015).
Another factor showing promising results on periodontal tissue regeneration is brain-derived neurotrophic factor. This factor is known to influence bone remodeling by increasing synthesis of osteopontin, BMP-2, and collagen in PDL cells. A recent study on nonhuman primates reported a promising effect on treatment of periodontal furcation defects (Jimbo et al. 2014).
Teriparatide (PTH 1-34) is a parathyroid hormone–derived biologic used predominantly for treatment of osteoporosis but with substantial evidence of increasing bone formation in extraction sockets and around dental implants (Kuchler et al. 2011; Vasconcelos et al. 2014). It is suggested that patients with metabolic bone diseases would most benefit from a teriparatide-based treatment (Rios et al. 2015), given its ability to reduce osteoblast apoptosis while increasing preosteoblast proliferation. A clinical trial evaluating the effect of teriparatide on patients following periodontal surgery found significantly increased osseous defect resolution with administration of teriparatide, with improved clinical outcomes as compared with standard of care (Bashutski et al. 2010).
Emerging Technologies
Stem Cell Therapies
Cell therapy can be defined as treatment of disease by introducing new cells into a tissue (Rios et al. 2011). For cell-based techniques in tissue engineering, somatic cells and stem cells can both be used (Pagni et al. 2012). Somatic cells can be harvested, cultured, and delivered to the site of tissue destruction. PDL fibroblasts, cementoblasts, and dental follicle cells all show the ability to mineralize in vitro and promote periodontal regeneration (Pagni et al. 2012). However, somatic cells lack the self-renewal capacity and differentiation potential that stem cells have. Stem cells can be harvested from a number of locations, including bone marrow, adipose tissue, dental pulp, and PDL (Seo et al. 2004; Kaukua et al. 2014).
Induced pluripotent stem cells (iPSCs) have become of particular interest in TE/RM (Takahashi and Yamanaka 2006). Utilization of iPSC-based therapy requires the harvest of somatic cells and subsequent gene therapy to reprogram cells into a multipotent or pluripotent state (iPSCs), followed by differentiation of iPSCs into a homogenous population of patient-specific terminally differentiated cells. There are, however, some concerns regarding the use of iPSCs, including possible integration of viral genes into the host, immunogenic responses, and a potential induction of tumorigenesis (Hong et al. 2014).
Bone repair cells, also termed ixmyelocel-T, are derived from enriched CD90+ and CD14+ bone marrow–derived cell populations. With a bioreactor, these MSCs can be expanded from a small sample of autologous bone marrow and then delivered back to the patient. This method provides a technique to produce patient-specific cells and has been shown in phase I/II clinical trials to increase bone formation and accelerate early osteogenesis (Kaigler et al. 2013; Kaigler et al. 2015). Ixmyelocel-T also contains a population of macrophages with a unique expression of markers for tissue repair and regeneration, as well as a decreased secretion of proinflammatory markers in response to inflammatory stimuli, indicating a potential role in tissue repair and regeneration (Ledford et al. 2013).
Recently, another cell type, periosteum-derived cells, has been suggested as an alternative cell source for bone regeneration, since harvesting these cells is less invasive for patients and an easier procedure for the dentists. Isolated cells involved in periodontal regeneration are grown on temperature-sensitive sheets. When a layer of cells (PDL cell sheet) is formed, changes in temperature are used to detach the sheet, which can then be implanted into a defect (Bartold et al. 2016). Preclinical studies have shown improved cementogenesis and PDL fibers with cell sheets (Lin et al. 2015). Mesenchymal stem cells have a number of immunomodulatory properties that can support a regenerative microenvironment, making them strong candidates for tissue engineering applications. By targeting these signaling pathways, oral tissue regenerative outcomes may be improved. For clinical application, the many challenges of handling autologous or allogeneic cells due to regulatory requirements need to be better managed to make such approaches more clinically feasible.
Gene Therapy
To circumvent the limitations of using recombinant proteins in TE/RM, such as the short half-life of growth factors in vivo and the limited control over distribution of protein, gene therapy presents a promising option. Gene therapy uses genetically modified cells to deliver specific doses of a bioactive protein for a sustained period. There are currently several different methods for transfecting the gene of interest into cells or interfering with cellular expression of a particular gene (Fig. 3).
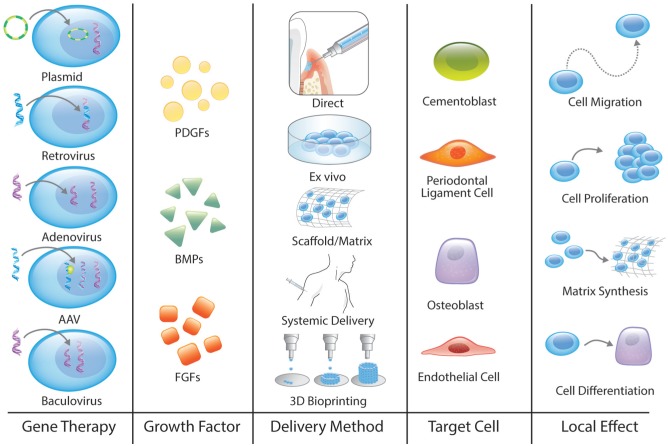
Figure 3.
Gene therapeutics for tissue engineering/regenerative medicine. Gene therapy includes several steps: consider the gene therapy vector, a tissue growth factor, the method of delivery, the target cells, and finally, the local effect. 3D, 3-dimensional; AAV, adeno-associated virus; BMP, bone morphogenetic protein; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor.
Nonviral vectors are plasmids—that is, small circular DNA structures that can replicate in the cell independently of chromosomes. These molecules are considered safer for the host than viral vectors, since they are not incorporated into the chromosomes. Even though they have been regarded as less effective, they do provide a method for transient expression of a protein, and with different carriers, they have been shown to improve bone formation (Lu et al. 2013). New, nonintegrating lentivirus vectors are being developed and have recently been used for reprogramming fibroblasts into iPSCs (Driscoll et al. 2015). In contrast, adenoviruses do not incorporate the gene into host chromosomes (Lu et al. 2013). An in vivo delivery approach with Ad-BMP7 in collagen matrix around implants showed an enhanced bone regeneration and osseointegration (Dunn et al. 2005). Recently, a new, localized adenovirus-mediated system was designed to immobilize Ad-BMP7 on titanium discs, resulting in attachment and differentiation of osteoblasts (Chen et al. 2013). Gene therapy vectors for delivery of PDGF have been used in several studies showing a sustained and biologically active expression as well as improved alveolar bone and cementum regeneration (Jin et al. 2004). In addition, AdPDGF-B used with a collagen matrix in periodontal osseous defects was considered safe for possible use in human clinical studies (Chang et al. 2009). Locally delivered osteoprotegerin vector (Ad-hOPG) added to femoral defects prior to implant placement resulted in accelerated bone deposition and enhanced osseointegration of titanium implants (Yin et al. 2015). A combinatory gene therapy using AdBMP7 alone or with adenovirus with LIM domain mineralization protein 3 (LMP3) gene promoted in vivo bone formation via PDL progenitor cells (Jin et al. 2003; Lin et al. 2013).
Adeno-associated virus (AAV) has a single-stranded DNA genome and therefore requires the host DNA polymerase to make a complementary strand (McCarty et al. 2001). The long-term transgene expression and efficient gene transfer with AAV have made it a very promising approach for gene therapy. The use of AAVs in periodontitis has been reported; treatment of Porphyromonas gingivalis–induced periodontal disease using an AAV vector with tumor necrosis factor receptor–immunoglobulin Fc inhibited disease progression and prevented alveolar bone loss (Cirelli et al. 2009). Baculoviruses—which do not replicate, are not toxic to mammalian cells, and degrade in host cells over time—have also been used. Several animal studies have used baculovirus with BMP2 and vascular endothelial growth factor transgenes for promoting bone repair (Lu et al. 2013).
Gene therapy can also be performed with different techniques, such as direct delivery, systemic delivery, local delivery, and recently, microRNAs (miRNAs; Lu et al. 2013). miRNAs are small noncoding RNAs that regulate expression of a target gene (Hobert 2008). Numerous miRNAs have been reported to influence osteogenesis by down- and/or upregulation of genes directly involved in bone formation, as reviewed in Fang et al. (2015). For example, miR-2861, miR/3960, and miR/378 promote osteoblastic differentiation, while miR-204, miR-204, miR-211, and miR-133 inhibit osteoblastic differentiation. miRNA has been suggested to contribute to the pathogenesis of rheumatoid arthritis, a disease characterized by osteoclastic bone destruction (Jing et al. 2015). Currently, research on miRNAs in bone development is originating from in vitro cell culture experiments to a large extent. However, there are some published in vivo studies showing promising results using miRNAs to improve bone repair (Fang et al. 2015). Interestingly, Wu et al. (2013) coated titanium discs with miRNA lipoplexes. In this in vitro study, the combination of antimiR-138 and miR-29b resulted in upregulation of expression of osteogenic genes, enhanced alkaline phosphatase and collagen production, and increased ECM mineralization.
Three-dimensional Printing
Three-dimensionally printed biomedical devices can be used to precisely restore defects or potentially even to reconstruct entire organs with complex microstructure. Three-dimensional printing (3DP) uses inkjet printing to apply a liquid binder solution onto a powder bed and can simultaneously arrange multiple cell types, deposit ECM, and provide fined-tuned control over bioactive molecules deposition. To date, peptides, proteins, DNA plasmids, and living cells have been printed (Chia and Wu 2015); 3DP has also been used to produce a 3D cell culture model for generating ECM on scaffolds (Pati et al. 2015). The 3DP method can further be used for indirect printing, which refers to the printing of a mold that is then cast with the final polymer. With this technique, a computed tomography scan of the patient’s defect can act as a template for making a 3D mold. This mold is then used for making a scaffold for gene therapy and a growth factor delivery system. Park and coworkers (Park et al. 2012; Park et al. 2014) designed a 3D wax mold to produce a fiber-guiding scaffold to improve integration of PDL fibers into mineralized tissues. In a randomized controlled clinical trial, the use of prefabricated 3D polycaprolactone (PCL) scaffolds in postextraction sockets resulted in normal bone healing and better maintenance of the alveolar ridge as compared with extraction sockets without scaffolds (Goh et al. 2015).
The fused deposition modeling technique for 3DP of thermoplastic material, such as PCL and poly(lactic-co-glycolic acid) (PLGA), can create scaffolds with mechanical strength, high porosity, and controlled morphology. However, it does not allow for incorporation of living cells or temperature-sensitive biological molecules (Chia and Wu 2015). Additionally, 3D plotting is a technique to make soft tissue scaffolds, such as hydrogels, with direct incorporation of cells while retaining their normal activity (Chia and Wu 2015). A potential limitation of the hydrogel as a scaffold includes inhibition of cell-to-cell interactions, which may influence cell signaling. In contrast, 3D printing of living cells, either in cell aggregates or seeded onto 3DP scaffolds, may enhance cell signaling and promote tissue formation (Obregon et al. 2015). Organ printing, defined as layer-by-layer additive biomanufacturing, is a technique with the potential to remove the need for a scaffold. In the so-called mini-tissue-based approach, tissue spheroids are used as building blocks that fuse to form a tissue. For example, self-assembled vascular spheroids can form a branched vascular system within a 3D construct, thereby providing blood supply to all parts of newly forming tissue (Mironov et al. 2009). Interestingly, recent studies report on the use of 3DP to build complex tissues, such as constructing periodontium-like tissue (Lee et al. 2014). A 3DP bioresorbable scaffold has been used for periodontal repair (Rasperini et al. 2015; Fig. 4); 3DP also has the potential to make complex, patient-specific constructs such as temporomandibular joints (Chia and Wu 2015).
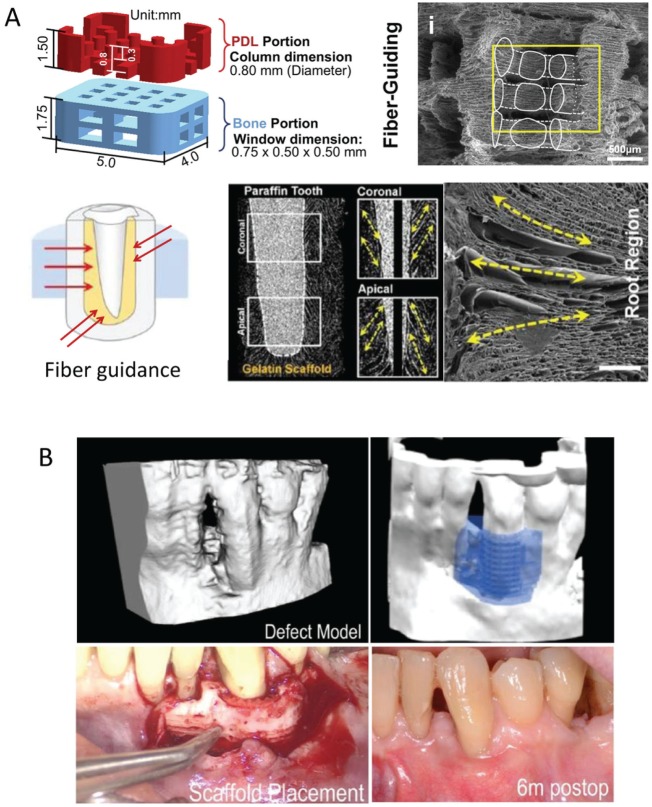
Figure 4.
Design of a customized scaffold using 3-dimensional (3D) printing. (A) 3D printing has been used to design a scaffold consisting of a periodontal ligament (PDL) portion and a bone portion. This was further modified to improve the fiber-guiding potential as well as the direction of the PDL to mimic the topography of the different kinds of fibers in the PDL (first row). Digitalized cross-sectional view of a 3D-reconstructed image. Longitudinal cross-section image showed pore morphologies at coronal and apical portions. Scanning electron microscopy image providing longitudinal pores produced by a freeze-casting method. Adapted from Park et al. (2010), Park et al. (2012), and Park et al. (2014) with permission. (B) 3D printing using polycaprolactone was made to fit the periosseous defect based on the patient’s cone beam computed tomography scan. Adapted from Rasperini et al. (2015) with permission.
Biohybrid Interfaces
Modern dental implants lack the force dissipation, proprioception, and specialized cell types present in the PDL. Furthermore, Carcuac et al. (2013) reported an increased rate of disease progression, inflammatory infiltrate, and tissue destruction in peri-implant diseases as compared with corresponding periodontal lesions in natural teeth. Due to a lack of a periodontal ligamentous attachment, osseointegrated dental implants are not ideally placed in growing patients with adjacent natural dentition or orthodontically relocated. Thus, a PDL-attached implant, or “ligaplant,” could provide a promising solution to many clinical situations and a mechanism allowing orthodontically mediated implant movement (Giannobile 2010). In addition, perpendicular ligament attachment may contribute to encapsulation of the inflammatory infiltrate and thereby slow bone loss and gingival recession (Carcuac et al. 2013; Carcuac and Berglundh 2014). Recently, a functional murine ligaplant model was developed by Oshima et al. (2014), using hydroxyapatite-coated dental implants surrounded with embryonic dental follicle tissues. Results demonstrated regenerated cementum, PDL, and alveolar bone with physiologic function. Gault et al. (2010) reported preclinical and human clinical studies conducted using the ligaplant model. The findings from these studies showed a layer of cementum and cementocytes present on the implant surface, as well as PDL tissues, lamina dura, and mechanical properties similar to those of natural teeth, without adverse tissue reactions or bone loss. Limitations include clinical unpredictability as well as fundamental technical challenges of labor, high cost associated with autologous cell culture, and increased time of processing patient-specific specimens. Although still at a very early stage, the use of bioinspired implants combined with biomaterials and cellular platforms will allow for individualized therapeutic options that optimize the benefits of complete restoration of human periodontium or hybrid interfaces against implants.
Summary and Future Directions
Challenges remain in TE/RM for restoring all periodontal components (including bone, ligament, cementum, and surrounding connective tissues) to the same degree in structure and function. For both peri-implant and alveolar ridge regeneration, bone regeneration is required; however, complete 3D reconstruction is difficult to achieve in a predictable fashion within the bony envelope for functional implant stability. Future approaches to develop reproducible methods for periodontal and peri-implant regeneration will need to consider the following key elements: 1) occlusal load/biomechanical influences of the newly regenerated tissues; 2) effects of microbial load and contamination of wounds due to the microbiome in the local environment; 3) wound stability to maintain the 3D conformation of the wound site to reconstitute the original periodontal topography; and 4) appropriate cellular signals to recruit and direct cell populations to recapitulate the tissue-regenerative response in the proper conformation at the tooth or implant interface. Many exciting advances in materials science, engineering, and cell biology begin to address the existing obstacles in the field for predictive reconstructive modalities. These new technologies work toward a common goal: to make available cost-effective, patient-specific treatment options that provide maximal function and esthetics.
Author Contributions
L. Larsson, A.M. Decker, W.V. Giannobile, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; S.P. Pilipchuk, L. Nibali, T. Berglundh, contributed to data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
Special acknowledgment for illustrations and graphic design contributions are given to Stephanie O’Neil and Ken Rieger at the University of Michigan School of Dentistry graphic design department. W.V.G. has consulted for Sunstar, Straumann, Geistlich Pharma, Biomimetic Therapeutics, and Medtronic.
Footnotes
This work was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research DE13397 and DE025570 to W.V.G. and by Wilhelm och Martina Lundgrens stipendiefond to L.L. A.M.D. was supported by the University of Michigan Tissue Engineering and Regeneration Training Program (NIH T32 DE007057). L.N. was supported by the British Society of Periodontology Clinical Fellowship Award. S.P.P. acknowledges support from the National Science Foundation (NSF GRFP 1256260).
The remaining authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Agarwal A, Gupta ND, Jain A. 2016. Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: a randomized split mouth clinical trail. Acta Odontol Scand. 74(1):36–43. [DOI] [PubMed] [Google Scholar]
- Aghazadeh A, Rutger Persson G, Renvert S. 2012. A single-centre randomized controlled clinical trial on the adjunct treatment of intra-bony defects with autogenous bone or a xenograft: results after 12 months. J Clin Periodontol. 39(7):666–673. [DOI] [PubMed] [Google Scholar]
- Bartold PM, Gronthos S, Ivanovski S, Fisher A, Hutmacher DW. 2016. Tissue engineered periodontal products. J Periodontal Res. 51(1):1–15. [DOI] [PubMed] [Google Scholar]
- Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, Giannobile WV, McCauley LK. 2010. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 363(25):2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram TA, Johnson PC, Tawil BJ, Van Dyke M, Hellman KB. 2015. Enhancing tissue engineering/regenerative medicine product commercialization: the role of science in regulatory decision making for TE/RM product development. Tissue Eng Part A. 21(19–20):2476–2479. [DOI] [PubMed] [Google Scholar]
- Boora P, Rathee M, Bhoria M. 2015. Effect of platelet rich fibrin (PRF) on peri-implant soft tissue and crestal bone in one-stage implant placement: a randomized controlled trial. J Clin Diagn Res. 9(4):ZC18–ZC21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer KM, Lundvig DM, Middelkoop E, Wagener FA, Von den Hoff JW. 2015. Mechanical cues in orofacial tissue engineering and regenerative medicine. Wound Repair Regen. 23(3):302–311. [DOI] [PubMed] [Google Scholar]
- Carcuac O, Abrahamsson I, Albouy JP, Linder E, Larsson L, Berglundh T. 2013. Experimental periodontitis and peri-implantitis in dogs. Clin Oral Implants Res. 24(4):363–371. [DOI] [PubMed] [Google Scholar]
- Carcuac O, Berglundh T. 2014. Composition of human peri-implantitis and periodontitis lesions. J Dent Res. 93(11):1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardaropoli D, Tamagnone L, Roffredo A, Gaveglio L, Cardaropoli G. 2012. Socket preservation using bovine bone mineral and collagen membrane: a randomized controlled clinical trial with histologic analysis. Int J Periodontics Restorative Dent. 32(4):421–430. [PubMed] [Google Scholar]
- Chan HL, Benavides E, Tsai CY, Wang HL. 2015. A titanium mesh and particulate allograft for vertical ridge augmentation in the posterior mandible: a pilot study. Int J Periodontics Restorative Dent. 35(4):515–522. [DOI] [PubMed] [Google Scholar]
- Chang PC, Cirelli JA, Jin Q, Seol YJ, Sugai JV, D’Silva NJ, Danciu TE, Chandler LA, Sosnowski BA, Giannobile WV. 2009. Adenovirus encoding human platelet-derived growth factor-B delivered to alveolar bone defects exhibits safety and biodistribution profiles favorable for clinical use. Hum Gene Ther. 20(5):486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yang J, Wang H, Chao Y, Zhang C, Shen J, Zhang P. 2013. Adenovirus encoding BMP-7 immobilized on titanium surface exhibits local delivery ability and regulates osteoblast differentiation in vitro. Arch Oral Biol. 58(9):1225–1231. [DOI] [PubMed] [Google Scholar]
- Chia HN, Wu BM. 2015. Recent advances in 3D printing of biomaterials. J Biol Eng. 9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli JA, Park CH, MacKool K, Taba M, Jr, Lustig KH, Burstein H, Giannobile WV. 2009. AAV2/1–TNFR:Fc gene delivery prevents periodontal disease progression. Gene Ther. 16(3):426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortellini P, Tonetti MS. 2015. Clinical concepts for regenerative therapy in intrabony defects. Periodontol 2000. 68(1):282–307. [DOI] [PubMed] [Google Scholar]
- D’Amato S, Tartaro G, Itro A, Nastri L, Santagata M. 2015. Block versus particulate/titanium mesh for ridge augmentation for mandibular lateral incisor defects: clinical and histologic analysis. Int J Periodontics Restorative Dent. 35(1):e1–e8. [DOI] [PubMed] [Google Scholar]
- Deliberador TM, Mendes RT, Storrer CL, Giovanini AF, Zielak JC, Lopes TR. 2012. Autogenous bone graft combined with buccal fat pad as barrier in treatment of class II furcation defect: a case report. Bull Tokyo Dent Col. 53(3):127–132. [DOI] [PubMed] [Google Scholar]
- Diniz IM, Chen C, Ansari S, Zadeh HH, Moshaverinia M, Chee D, Marques MM, Shi S, Moshaverinia A. 2015. Gingival mesenchymal stem cell delivery system based on RGD-coupled alginate hydrogel with antimicrobial properties: a novel treatment modality for peri-implantitis. J Prosthodont [epub ahead of print 27 July 2015] in press. doi: 10.1111/jopr.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano DA, Greco GB, Cinci L, Pieri L. 2015. Horizontal-guided bone regeneration using a titanium mesh and an equine bone graft. J Contemp Dent Pract. 16(2):154–162. [DOI] [PubMed] [Google Scholar]
- Di Tullio M, Femminella B, Pilloni A, Romano L, D’Arcangelo C, De Ninis P, Paolantonio M. 2013. Treatment of supra-alveolar-type defects by a simplified papilla preservation technique for access flap surgery with or without enamel matrix proteins. J Periodontol. 84(8):1100–1110. [DOI] [PubMed] [Google Scholar]
- Döri F, Arweiler NB, Szántó E, Agics A, Gera I, Sculean A. 2013. Ten-year results following treatment of intrabony defects with an enamel matrix protein derivative combined with either a natural bone mineral or a β-tricalcium phosphate. J Periodontol. 84(6):749–757. [DOI] [PubMed] [Google Scholar]
- Driscoll CB, Tonne JM, El Khatib M, Cattaneo R, Ikeda Y, Devaux P. 2015. Nuclear reprogramming with a non-integrating human RNA virus. Stem Cell Res Ther. 6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Shan Z, Ma P, Wang S, Fan Z. 2014. Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration. J Dent Res. 93(2):183–188. [DOI] [PubMed] [Google Scholar]
- Dunn CA, Jin Q, Taba M, Jr, Franceschi RT, Bruce Rutherford R, Giannobile WV. 2005. BMP gene delivery for alveolar bone engineering at dental implant defects. Mol Ther. 11(2):294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Deng Y, Gu P, Fan X. 2015. MicroRNAs regulate bone development and regeneration. Int J Mol Sci. 16(4):8227–8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas RM, Spin-Neto R, Marcantonio Junior E, Pereira LA, Wikesjo UM, Susin C. 2015. Alveolar ridge and maxillary sinus augmentation using rhBMP-2: a systematic review. Clin Implant Dent Relat Res. 17 Suppl 1:e192–e201. [DOI] [PubMed] [Google Scholar]
- Froum SJ, Froum SH, Rosen PS. 2012. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 32(1):11–20. [PubMed] [Google Scholar]
- Gamal AY, Iacono VJ. 2013. Enhancing guided tissue regeneration of periodontal defects by using a novel perforated barrier membrane. J Periodontol. 84(7):905–913. [DOI] [PubMed] [Google Scholar]
- Gault P, Black A, Romette JL, Schroeder K, Thillou F, Brune T, Berdal A, Wurtz T. 2010. Tissue-engineered ligament: implant constructs for tooth replacement. J Clin Periodontol. 37(8):750–758. [DOI] [PubMed] [Google Scholar]
- Giannobile WV. 2010. Getting to the root of dental implant tissue engineering. J Clin Periodontol. 37(8):747–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannobile WV. 2014. Commentary: treatment of periodontitis. Destroyed periodontal tissues can be regenerated under certain conditions. J Periodontol. 85(9):1151–1154. [DOI] [PubMed] [Google Scholar]
- Goh BT, Teh LY, Tan DB, Zhang Z, Teoh SH. 2015. Novel 3D polycaprolactone scaffold for ridge preservation: a pilot randomised controlled clinical trial. Clin Oral Implants Res. 26(3):271–277. [DOI] [PubMed] [Google Scholar]
- Gottlow J, Nyman S, Karring T, Lindhe J. 1984. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 11(8):494–503. [DOI] [PubMed] [Google Scholar]
- Hassan KS, Alagl AS. 2015. A comparative study of bovine bone used alone and in combination with transforming growth factor-beta for the treatment of periodontal osseous defects in humans. Saudi Journal of Medicine and Medical Sciences. 3(1) 33–39. [Google Scholar]
- Heijl L. 1997. Periodontal regeneration with enamel matrix derivative in one human experimental defect: a case report. J Clin Periodontol. 24(9 Pt 2):693–696. [DOI] [PubMed] [Google Scholar]
- Hobert O. 2008. Gene regulation by transcription factors and microRNAs. Science. 319(5871):1785–1786. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Al-Machot E, Meyle J, Jervøe-Storm PM, Jepsen S. 2015. Three-year results following regenerative periodontal surgery of advanced intrabony defects with enamel matrix derivative alone or combined with a synthetic bone graft. Clin Oral Investig. 50(3):347–355. [DOI] [PubMed] [Google Scholar]
- Hollister SJ. 2009. Scaffold design and manufacturing: from concept to clinic. Adv Mater. 21(32–33):3330–3342. [DOI] [PubMed] [Google Scholar]
- Holtzclaw D. 2015. Maxillary sinus membrane repair with amnion-chorion barriers: a retrospective case series. J Periodontol. 86(8):936–940. [DOI] [PubMed] [Google Scholar]
- Hong SG, Winkler T, Wu C, Guo V, Pittaluga S, Nicolae A, Donahue RE, Metzger ME, Price SD, Uchida N, et al. 2014. Path to the clinic: assessment of iPSC-based cell therapies in vivo in a nonhuman primate model. Cell Rep. 7(4):1298-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi S, Akizuki T, Matsuura T, Ikawa T, Kinoshita A, Oda S, Tabata Y, Matsui M, Izumi Y. 2016. Ridge augmentation using recombinant human fibroblast growth factor-2 with biodegradable gelatin sponges incorporating β-tricalcium phosphate: a preclinical study in dogs. J Periodontal Res. 51(1):77–85. [DOI] [PubMed] [Google Scholar]
- Iorio-Siciliano V, Andreuccetti G, Blasi A, Matarasso M, Sculean A, Salvi GE. 2014. Clinical outcomes following regenerative therapy of non-contained intrabony defects using a deproteinized bovine bone mineral combined with either enamel matrix derivative or collagen membrane. J Periodontol. 85(10):1342–1350. [DOI] [PubMed] [Google Scholar]
- Jimbo R, Tovar N, Janal MN, Mousa R, Marin C, Yoo D, Teixeira HS, Anchieta RB, Bonfante EA, Konishi A, et al. 2014. The effect of brain-derived neurotrophic factor on periodontal furcation defects. PloS one. 9(1):e84845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. 2004. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 9(4):519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. 2003. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol. 74(2):202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing D, Hao J, Shen Y, Tang G, Li ML, Huang SH, Zhao ZH. 2015. The role of microRNAs in bone remodeling. Int J Oral Sci. 7:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung UW, Lee IK, Park JY, Thoma DS, Hämmerle CH, Jung RE. 2015. The efficacy of BMP-2 preloaded on bone substitute or hydrogel for bone regeneration at peri-implant defects in dogs. Clin Oral Implants Res. 26(12):1456–1465. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Avila-Ortiz G, Travan S, Taut AD, Padial-Molina M, Rudek I, Wang F, Lanis A, Giannobile WV. 2015. Bone engineering of maxillary sinus bone deficiencies using enriched CD90+ stem cell therapy: a randomized clinical trial. J Bone Miner Res. 30(7):1206–1216. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Pagni G, Park CH, Braun TM, Holman LA, Yi E, Tarle SA, Bartel RL, Giannobile WV. 2013. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell Transplant. 22(5):767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan AL, Bose BB, Muthu J, Perumalsamy R, Pushparajan S, Namasivayam A. 2014. Efficacy of combination therapy using anorganic bovine bone graft with resorbable GTR membrane vs. open flap debridement alone in the management of grade II furcation defects in mandibular molars: a comparative study. J Int Soc Prev Community Dent. 4 Suppl 1:S38–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao RT, Nares S, Reynolds MA. 2015. Periodontal regeneration–intrabony defects: a systematic review from the AAP regeneration workshop. J Periodontol. 86(2):S77–S104. [DOI] [PubMed] [Google Scholar]
- Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, et al. 2014. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 513(7519):551–554. [DOI] [PubMed] [Google Scholar]
- Khoshkam V, Chan HL, Lin GH, Mailoa J, Giannobile WV, Wang HL, Oh TJ. 2015. Outcomes of regenerative treatment with rhPDGF-BB and rhFGF-2 for periodontal intrabony defects: a systematic review and meta-analysis. J Clin Periodontol. 42(3):272–280. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Chung JH, Shin SY, Shin SI, Kye SB, Kim NK, Kwon TG, Paeng JY, Kim JW, Oh OH, et al. 2015. Efficacy of rhBMP-2/hydroxyapatite on sinus floor augmentation: a multicenter, randomized controlled clinical trial. J Dent Res. 94(9):158S–165S. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Akamatsu M, Machigashira M, Hara Y, Sakagami R, Hirofuji T, Hamachi T, Maeda K, Yokota M, Kido J, et al. 2011. FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial. J Dent Res. 90(1):35–40. [DOI] [PubMed] [Google Scholar]
- Koch FP, Becker J, Terheyden H, Capsius B, Wagner W. 2010. A prospective, randomized pilot study on the safety and efficacy of recombinant human growth and differentiation factor-5 coated onto β-tricalcium phosphate for sinus lift augmentation. Clin Oral Implants Res. 21(11):1301–1308. [DOI] [PubMed] [Google Scholar]
- Kuchler U, Luvizuto ER, Tangl S, Watzek G, Gruber R. 2011. Short-term teriparatide delivery and osseointegration: a clinical feasibility study. J Dent Res. 90(8):1001–1006. [DOI] [PubMed] [Google Scholar]
- Lafzi A, Shirmohammadi A, Faramarzi M, Jabali S, Shayan A. 2013. Clinical comparison of autogenous bone graft with and without plasma rich in growth factors in the treatment of grade II furcation involvement of mandibular molars. J Dent Res Dent Clin Dent Prospects. 7(1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford KJ, Zeigler F, Bartel RL. 2013. Ixmyelocel-T, an expanded multicellular therapy, contains a unique population of M2-like macrophages. Stem Cell Res Ther. 4(6):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Hajibandeh J, Suzuki T, Fan A, Shang P, Mao JJ. 2014. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A. 20(7–8):1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Rios HF, Cochran DL. 2015. Emerging regenerative approaches for periodontal reconstruction: a systematic review from the AAP Regeneration Workshop. J Periodontol. 86(2):S134–S152. [DOI] [PubMed] [Google Scholar]
- Lin Z, Rios HF, Park CH, Taut AD, Jin Q, Sugai JV, Robbins PD, Giannobile WV. 2013. LIM domain protein-3 (LMP3) cooperates with BMP7 to promote tissue regeneration by ligament progenitor cells. Gene Ther. 20(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CH, Chang YH, Lin SY, Li KC, Hu YC. 2013. Recent progresses in gene delivery-based bone tissue engineering. Biotechnol Adv. 31(8):1695–1706. [DOI] [PubMed] [Google Scholar]
- Mason C, Dunnill P. 2008. A brief definition of regenerative medicine. Regen Med. 3(1):1–5. [DOI] [PubMed] [Google Scholar]
- Matarasso M, Iorio-Siciliano V, Blasi A, Ramaglia L, Salvi G, Sculean A. 2015. Enamel matrix derivative and bone grafts for periodontal regeneration of intrabony defects: a systematic review and meta-analysis. Clin Oral Investig. 19(7):1581–1593. [DOI] [PubMed] [Google Scholar]
- Mathur A, Bains VK, Gupta V, Jhingran R, Singh GP. 2015. Evaluation of intrabony defects treated with platelet-rich fibrin or autogenous bone graft: a comparative analysis. Eur J Dent. 9(1):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Monahan PE, Samulski RJ. 2001. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 8(16):1248–1254. [DOI] [PubMed] [Google Scholar]
- McKay WF, Peckham SM, Badura JM. 2007. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop. 31(6):729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. 2009. Organ printing: tissue spheroids as building blocks. Biomaterials. 30(12):2164–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanci A, Bosshardt DD. 2006. Structure of periodontal tissues in health and disease. Periodontol 2000. 40:11–28. [DOI] [PubMed] [Google Scholar]
- Nevins M, Kao RT, McGuire MK, McClain PK, Hinrichs JE, McAllister BS, Reddy MS, Nevins ML, Genco RJ, Lynch SE, et al. 2013. Platelet-derived growth factor promotes periodontal regeneration in localized osseous defects: 36-month extension results from a randomized, controlled, double-masked clinical trial. J Periodontol. 84(4):456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins ML, Reynolds MA, Camelo M, Schupbach P, Kim DM, Nevins M. 2013. Recombinant human platelet-derived growth factor BB for reconstruction of human large extraction site defects. Int J Periodontics Restorative Dent. 34(2):157–163. [DOI] [PubMed] [Google Scholar]
- Obregon F, Vaquette C, Ivanovski S, Hutmacher DW, Bertassoni LE. 2015. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J Dent Res. 94(9):143S–152S. [DOI] [PubMed] [Google Scholar]
- Ogihara S, Tarnow DP. 2014. Efficacy of enamel matrix derivative with freeze-dried bone allograft or demineralized freeze-dried bone allograft in intrabony defects: a randomized trial. J Periodontol. 85(10):1351–1360. [DOI] [PubMed] [Google Scholar]
- Oortgiesen DA, Walboomers XF, Bronckers AL, Meijer GJ, Jansen JA. 2014. Periodontal regeneration using an injectable bone cement combined with BMP-2 or FGF-2. J Tissue Eng Regen Med. 8(3):202–209. [DOI] [PubMed] [Google Scholar]
- Oshima M, Inoue K, Nakajima K, Tachikawa T, Yamazaki H, Isobe T, Sugawara A, Ogawa M, Tanaka C, Saito M, et al. 2014. Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci Rep. 4:6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagni G, Kaigler D, Rasperini G, Avila-Ortiz G, Bartel R, Giannobile WV. 2012. Bone repair cells for craniofacial regeneration. Adv Drug Deliv Rev. 64(12):1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Rios HF, Jin Q, Bland ME, Flanagan CL, Hollister SJ, Giannobile WV. 2010. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 31(23):5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Rios HF, Jin Q, Sugai JV, Padial-Molina M, Taut AD, Flanagan CL, Hollister SJ, Giannobile WV. 2012. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials. 33(1):137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Rios HF, Taut AD, Padial-Molina M, Flanagan CL, Pilipchuk SP, Hollister SJ, Giannobile WV. 2014. Image-based, fiber guiding scaffolds: a platform for regenerating tissue interfaces. Tissue Eng Part C Methods. 20(7):533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kim KH, Gwak EH, Rhee SH, Lee JC, Shin SY, Koo KT, Lee YM, Seol YJ. 2015. Ex vivo bone morphogenetic protein 2 gene delivery using periodontal ligament stem cells for enhanced re-osseointegration in the regenerative treatment of peri-implantitis. J Biomed Mater Res A. 103(1):38–47. [DOI] [PubMed] [Google Scholar]
- Patel S, Kubavat A, Ruparelia B, Agarwal A, Panda A. 2012. Comparative evaluation of guided tissue regeneration with use of collagen-based barrier freeze-dried dura mater allograft for mandibular class 2 furcation defects (a comparative controlled clinical study). J Contemp Dent Pract. 13(1):11–15. [DOI] [PubMed] [Google Scholar]
- Pati F, Song TH, Rijal G, Jang J, Kim SW, Cho DW. 2015. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials. 37:230–241. [DOI] [PubMed] [Google Scholar]
- Pilipchuk SP, Plonka AB, Monje A, Taut AD, Lanis A, Kang B, Giannobile WV. 2015. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent Mater. 31(4):317–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu T, Jing J, Jiang Y, Taylor RJ, Feng JQ, Geiger B, Liu X. 2014. Magnesium-containing nanostructured hybrid scaffolds for enhanced dentin regeneration. Tissue Eng Part A. 20(17–18):2422–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasperini G, Pilipchuk S, Flanagan C, Park C, Pagni G, Hollister S, Giannobile W. 2015. 3D-printed bioresorbable scaffold for periodontal repair. J Dent Res. 94(9):153S–157S. [DOI] [PubMed] [Google Scholar]
- Restoy-Lozano A, Dominguez-Mompell JL, Infante-Cossio P, Lara-Chao J, Lopez-Pizarro V. 2015. Calvarial bone grafting for three-dimensional reconstruction for severe maxillary defects: a case series. Int J Oral Maxillofac Implants. 30(4):880–990. [DOI] [PubMed] [Google Scholar]
- Rios HF, Bashutski JD, McAllister BS, Murakami S, Cobb CM, Chun YP, Lin Z, Mandelaris GA, Cochran DL. 2015. Emerging regenerative approaches for periodontal reconstruction: practical applications from the AAP Regeneration Workshop. Clinic Adv Periodontics. 5(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios HF, Lin Z, Oh B, Park CH, Giannobile WV. 2011. Cell- and gene-based therapeutic strategies for periodontal regenerative medicine. J Periodontol. 82(9):1223–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccuzzo M, Bonino F, Bonino L, Dalmasso P. 2011. Surgical therapy of peri-implantitis lesions by means of a bovine-derived xenograft: comparative results of a prospective study on two different implant surfaces. J Clin Periodontol. 38(8):738–745. [DOI] [PubMed] [Google Scholar]
- Roos-Jansaker AM, Persson GR, Lindahl C, Renvert S. 2014. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: a 5-year follow-up. J Clin Periodontol. 41(11):1108–1114. [DOI] [PubMed] [Google Scholar]
- Sam G, Pillai BR. 2014. Evolution of barrier membranes in periodontal regeneration: “Are the third generation membranes really here?” J Clin Diagn Res. 8(12):ZE14–ZE17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana RB, Santana CM. 2015. A clinical comparison of guided bone regeneration with platelet-derived growth factor-enhanced bone ceramic versus autogenous bone block grafting. Int J Oral Maxillofac Implants. 30(3):700–706. [DOI] [PubMed] [Google Scholar]
- Sanz-Sánchez I, Ortiz-Vigón A, Sanz-Martín I, Figuero E, Sanz M. 2015. Effectiveness of lateral bone augmentation on the alveolar crest dimension: a systematic review and meta-analysis. J Dent Res. 94(9):128S–142S. [DOI] [PubMed] [Google Scholar]
- Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple IL, Stavropoulos A. 2015. Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontol 2000. 68(1):182–216. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. 2004. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 364(9429):149–155. [DOI] [PubMed] [Google Scholar]
- Seymour G, Berglundh T, Trombelli L. 2015. Pathogenesis of periodontitis. In: Lindhe J, Lang NP, editors. Clinical periodontology and implant dentistry. London (UK): Wiley-Blackwell. [Google Scholar]
- Singhal R, Nandlal Kumar A, Rastogi P. 2013. Role of space provision in regeneration of localized two-wall intrabony defects using periosteal pedicle graft as an autogenous guided tissue membrane. J Periodontol. 84(3):316–324. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126(4):663–676. [DOI] [PubMed] [Google Scholar]
- Tang H, Mattheos N, Yao Y, Jia Y, Ma L, Gong P. 2015. In vivo osteoprotegerin gene therapy preventing bone loss induced by periodontitis. J Periodontal Res. 50(4):434–43. [DOI] [PubMed] [Google Scholar]
- Troedhan A, Wainwright M, Kurrek A, Schlichting I. 2015. Biomechanical stability of dental implants in augmented maxillary sites: results of a randomized clinical study with four different biomaterials and PRF and a biological view on guided bone regeneration. Biomed Res Int. http://dx.doi.org/10.1155/2015/850340 [DOI] [PMC free article] [PubMed]
- Urban IA, Nagursky H, Lozada JL, Nagy K. 2013. Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: a prospective case series in 25 patients. Int J Periodontics Restorative Dent. 33(3):299–307. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, Serhan CN. 2015. Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res. 94(1):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos DF, Marques MR, Benatti BB, Barros SP, Nociti FH, Jr, Novaes PD. 2014. Intermittent parathyroid hormone administration improves periodontal healing in rats. J Periodontol. 85(5):721–728. [DOI] [PubMed] [Google Scholar]
- Velez I, Parker WB, Siegel MA, Hernandez M. 2010. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: a pilot study. J Periodontol. 81(12):1797–1804. [DOI] [PubMed] [Google Scholar]
- Wadhawan A, Gowda TM, Mehta DS. 2012. Gore-tex® versus resolut adapt® GTR membranes with perioglas® in periodontal regeneration. Contemp Clin Dent. 3(4):406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber MJ, Khan OF, Sydlik SA, Tang BC, Langer R. 2015. A perspective on the clinical translation of scaffolds for tissue engineering. Ann Biomed Eng. 43(3):641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch P, Stavropoulos A, Molnár B, Szendröi-Kiss D, Szilágyi E, Rosta P, Horváth A, Capsius B, Wikesjö UM, Sculean A. 2012. A phase IIa randomized controlled pilot study evaluating the safety and clinical outcomes following the use of rhGDF-5/β-TCP in regenerative periodontal therapy. Clin Oral Investig. 16(4):1181–1189. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt JC, Lyngstadaas SP, Heijl L, Aass AM. 2012. Porous titanium granules in the treatment of mandibular class II furcation defects: a consecutive case series. J Periodontol. 83(1):61–69. [DOI] [PubMed] [Google Scholar]
- Wood RA, Mealey BL. 2012. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J Periodontol. 83(3):329–336. [DOI] [PubMed] [Google Scholar]
- Wu K, Song W, Zhao L, Liu M, Yan J, Andersen MØ, Kjems J, Gao S, Zhang Y. 2013. MicroRNA functionalized microporous titanium oxide surface by lyophilization with enhanced osteogenic activity. ACS Appl Mater Interfaces. 5(7):2733–2744. [DOI] [PubMed] [Google Scholar]
- Yin G, Chen J, Wei S, Wang H, Chen Q, Lin Y, Hu J, Luo E. 2015. Adenoviral vector-mediated overexpression of osteoprotegerin accelerates osteointegration of titanium implants in ovariectomized rats. Gene Ther. 22(8):636–644. [DOI] [PubMed] [Google Scholar]