Abstract
Recovery of hand function following lesions in the primary motor cortex (M1) is associated with a reorganization of premotor areas in the ipsilesional hemisphere, and this reorganization depends on the size of the lesion. It is not clear how lesion size affects motor representations in the contralesional hemisphere and how the effects in the 2 hemispheres compare. Our goal was to study how lesion size affects motor representations in the ipsilesional and contralesional hemispheres. In rats, we induced lesions of different sizes in the caudal forelimb area (CFA), the equivalent of M1. The effective lesion volume in each animal was quantified histologically. Behavioral recovery was evaluated with the Montoya Staircase task for 28 days after the lesion. Then, the organization of the CFA and the rostral forelimb area (RFA)—the putative premotor area in rats—in the 2 cerebral hemispheres was studied with intracortical microstimulation mapping techniques. The distal forelimb representation in the RFA of both the ipsilesional and contralesional hemispheres was positively correlated with the size of the lesion. In contrast, lesion size had no effect on the contralesional CFA, and there was no relationship between movement representations in the 2 hemispheres. Finally, only the contralesional RFA was negatively correlated with chronic motor deficits of the paretic forelimb. Our data show that lesion size has comparable effects on motor representations in premotor areas of both hemispheres and suggest that the contralesional premotor cortex may play a greater role in the recovery of the paretic forelimb following large lesions.
Keywords: contralesional, cortex, hand, intact hemisphere, ipsilesional, lesion size, motor representations, plasticity, recovery, stroke
Introduction
Cortical lesions, such as those that occur following stroke, trigger plasticity in distant regions of the brain.1,2 A key factor that appears to affect motor function and plasticity is the size of the lesion. The relationship between lesion size and the reorganization of motor representations in the ipsilesional cortex was well established in a series of experiments conducted in monkeys.3,4 In each animal, lesions were made to selectively target different proportions of cortical gray matter in the hand area of M1. After recovery, there was a reorganization of the hand representation in the ventral premotor cortex (PMv), and the cortical area evoking movements of the paretic hand in the PMv was proportional to the size of injury.
Several studies also support the idea that there is a relationship between lesion size and contralesional plasticity. For example, in the days following middle cerebral artery occlusions (MCAos) in rats, there is shift of activation toward the contralesional hemisphere and atypically high hemodynamic activity in M1 and the primary somatosensory cortex.5 The increase in contralesional activity is greater in animals with larger lesions. Many neuroanatomical changes are also observed in the contralesional hemisphere after brain injuries, and they too are greater in animals with larger lesions.6,7 The size of lesion may also affect the role of the contralesional hemisphere in the recovery of the paretic limb. Reversible inhibition of the contralesional hemisphere induces more profound motor impairments with the paretic forelimb in rats that recovered from large lesions than in those that recovered from small lesions.8 Along the same lines in humans, a treatment based on contralesional inhibition that improves the control of the paretic arm for mildly impaired patients with relatively small damage can have detrimental effects for patients with greater deficits and more extensive damage.9
Whereas there is extensive support for contralesional plasticity, it is not quite clear in which area of the motor network these changes are more pronounced. Human stroke studies show atypical patterns of hemodynamic activity in the contralesional M1 but also in premotor areas, with much disparity about the site with the greatest changes across studies.10-12 We currently have a limited understanding of how hemodynamic changes are related to the organization of movement representations in the contralesional hemisphere. It would, thus, be interesting to understand better the effect of lesion size on the organization of motor representations in the contralesional cortex and how changes in the contralesional hemisphere relate to changes in the ipsilesional hemisphere.
To provide insights into these questions, we used a rat model of cortical injury. One strength of this model, in comparison to clinical populations that typically have lesions of various sizes and locations, is that it enabled us to specifically target the cortical territory involved in the forelimb movements in M1 (the caudal forelimb area [CFA]) and to vary the lesion size in different animals. Following spontaneous recovery, we studied how lesion size affected motor representations in the CFA and the rostral forelimb area (RFA)—the putative premotor area in rats13-16—of the ipsilesional and contralesional hemispheres.
Methods
Our experimental protocol followed the guidelines of the Canadian Council on Animal Care and was approved by the Comité de Déontologie de l’Expérimentation sur les Animaux of the Université de Montréal.
A total of 25 Sprague-Dawley rats of approximately 3 months of age were used (250-300 g; Charles River Laboratories, QC, Canada). All animals were housed singly. They were randomly assigned to a control group (n = 5) or to 2 groups with cortical lesions. To study lesions of variable sizes, we constituted 1 group with lesions induced with 6 (n = 10) and 1 group with 12 (n = 10) cortical injections of the vasoconstrictor endothelin-1 (ET-1; see below).
The motor performance of animals with ischemic lesions was quantified with the Montoya Staircase task.17 The performance score is based on the number of food pellets animals can successfully retrieve from wells on a staircase (Figure 1A). Details of our familiarization and behavioral data collection protocols have been previously published.18 Briefly, animals were familiarized for 10 days with the task and food pellets. A baseline performance score was established at the end of this period (Figure 1C). Following the lesion, motor performance was evaluated twice in the first week and then once per week for the 3 following weeks. On any given testing day, the performance score of each arm was the average number of eaten pellets of the best 3 trials out of 4. For each trial, rats had 3 minutes to retrieve up to 7 pellets (1 per well). During the familiarization period, to prevent inducing a bias for the use of 1 arm, we alternated which forelimb we tested first. Following the lesion, we tested the paretic hand first to minimize the effect of motivation on the scores obtained with this arm.
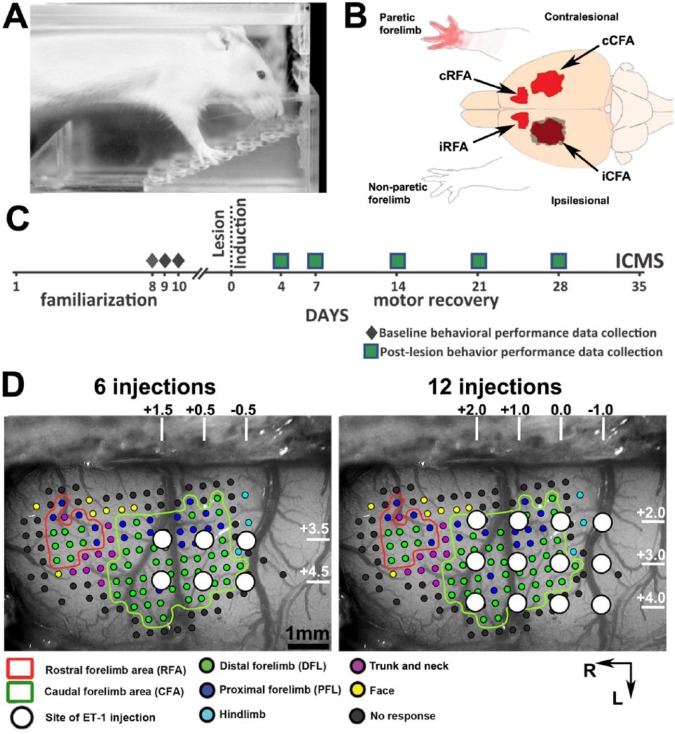
Figure 1.
Experimental design: A. Side view of a rat performing the behavioral task in the Montoya staircase box. For a trial, a single food pellet is placed on each step, and the rat has 3 minutes to retrieve the pellets. Its performance score is based on the number of pellets left at the end of the 3-minute period. B. Cartoon showing the location of the different motor representations in the 2 hemispheres and the nonparetic and paretic forelimbs in relation to the lesion (gray shadow). The ischemic lesion was induced in the cortex contralateral to the arm with the best performance score. C. Timeline of experimental procedures for animals with cortical lesions. Baseline behavioral performance data were collected prior to the lesion and averaged to create a baseline score. After the lesion, recovery was monitored for 4 weeks. Intracortical microstimulation techniques (ICMS) were used to study the organization of motor areas in a terminal experiment on the 35th day after the lesion. D. To create a range of cortical lesion sizes, we used 2 lesion protocols. Each panel shows ICMS data from a control animal where CFA and RFA are outlined. Small dots on the panels show sites of electrode penetration and ICMS stimulation. The color of the dots indicates the category of the movements evoked at the site. Based on stereotaxic coordinates (numbers and white lines on top and right of each panel), the locations of the 6 (left panel) and 12 (right panel) ET-1 injections used in the 2 lesion protocols are overlaid onto the ICMS data. The protocols were designed so that they both spare the RFA, but lesions induced with 12 ET-1 injections should destroy a greater portion of the CFA. Scale bar = 1 mm.
Note: Color version of the figure is present with the online March 2016 issue at www.nnr.sagepub.com.
Abbreviations: ET-1, endothelin-1; iRFA, ipsilesional RFA; iCFA, ipsilesional CFA; cRFA, contralesional RFA; cCFA, contralesional CFA; L, lateral; R, rostral.
The final recovery score of each animal was calculated using the following formula:
Accordingly, a recovery score of 0 indicates full recovery, whereas a negative score reveals persistent deficits at the end of the spontaneous recovery period.
Electrophysiological data were collected in a terminal experiment on the 35th day after the lesion. In control animals, electrophysiological data were collected after 5 weeks of being single housed in our facility. Controls did not undergo the familiarization period as this was shown to have no effect on motor maps.19
Lesion Induction Surgery
To create cortical gray matter lesions of different sizes, we made different numbers of relatively small cortical ET-1 injections in the CFA in different animals (either 6 or 12 injections). Lesion surgeries were done aseptically. Anesthesia was induced with ketamine hydrochloride (80 mg/kg, intraperitoneal) and sustained with ~2% isoflurane. Lesions targeted the CFA based on stereotaxic coordinates (Figures 1B and 1D). For the animals with 6 ET-1 injections, 6 holes 0.7 mm in diameter were drilled through the skull (stereotaxic coordinates = +1.5, +0.5, −0.5 mm anteroposterior, +2.5, +3.5 mm mediolateral to bregma). In each hole, a syringe (Hamilton Company, NV) was lowered to a depth of −1.5 mm from the surface of the cortex to inject 330 nL of ET-1 (0.3 µg/µL in saline) at a rate of 3 nL/s with a microinjector. For animals with 12 injections, ET-1 was injected in a similar manner in 12 holes (+2.0, +1.0, 0.0, −1.0 mm anteroposterior, +2.0, +3.0, +4.0 mediolateral to bregma). After injections, the holes were sealed with bone wax and the skin sutured. At the end of the procedure all animals received dexamethasone (Vetoquinol; 1 mg/kg), enrofloxacin (Baytril; 10 mg/kg), carprofen (Rimadyl; 10 mg/kg), and buprenorphine (Temgesic; 0.005 mg/kg). The recovery of every animal was closely monitored, and the antibiotic and analgesic medication was continued for 2 days following the lesion induction procedures.
Electrophysiological Mapping Surgery
The electrophysiological data collection was done 35 days following the lesion, which corresponds to the time when behavioral recovery reaches a plateau in our model.18 Thus, the electrophysiological data should reflect the organization of the motor cortex after most of the spontaneous recovery has occurred.
In a terminal experiment, ICMS techniques were used to derive cortical motor maps of forelimb movements in the CFA and RFA of both hemispheres (interpenetration distance ≈333 µm). A first craniotomy exposed the contralesional hemisphere under isoflurane anesthesia. Anesthesia was switched to ketamine hydrochloride for data collection (~3-5 mg/kg/10 min; intraperitoneal). A glass insulated tungsten microelectrode (~1.0 MΩ) was lowered into the cortex to a depth of 1550 to 1600 µm to target layer V. Cortical maps were derived using standard ICMS trains.18,20 A stimulation train consisted of 13 monophasic square pulses (0.2 ms duration; 3.3 ms interpulse interval), and trains were delivered at 1 Hz. At each penetration site, the movement evoked at threshold current intensity (the current at which movements were evoked by 50% of the stimulation trains) was identified and used for subsequent analyses. If no movement was evoked at a maximum current intensity of 100 µA, the site was defined as unresponsive. Following completion of the contralesional motor maps, a second craniotomy exposed the ipsilesional cortex for motor mapping. In some cases, because of complications during the experiment, mapping in the RFA and/or CFA was incomplete. In these cases, the forearm representation that was considered incomplete—that is, that was not entirely surrounded by nonresponsive sites or cortical sites from other representations—was not included in subsequent analyses. Thus, the number of data points for each movement representation is not identical and is specified in the text and figures (see Results).
We analyzed forelimb movements in 2 categories: distal forelimb representation (DFL) and proximal forelimb representation (PFL). Movements of digits, wrist, and forearm (pronation/supination) were included in the DFL, and movements of the elbow and shoulder were included in the PFL.3,13,21 Movements of the neck, trunk, vibrissae, hindlimb, and nonresponsive sites were used to define the borders of the CFA and RFA. The surface area for each movement category was calculated with a custom-made program in Matlab (MathWorks, MA). The algorithm expanded each colored dot on the map to neighboring pixels using nearest-neighbor interpolation until all pixels were assigned a color. The total number of pixels with the same color was scaled to real cortical surface area according to a calibration ruler that was placed on the brain for the digital picture of the cortex.
Histology
On completion of the electrophysiological data collection, animals were killed with a lethal dose of sodium pentobarbital and transcardially perfused. The brain was fixed, cryoprotected,22 and cut coronally (40 µm thickness). One out of 6 sections was Nissl stained and used to determine the lesion size. For each animal, 19 sections (total span from 3.48 mm rostral to 0.6 mm caudal to bregma), extending beyond the lesion both caudally and rostrally, were reconstructed using Neurolucida (MicroBrightField, VT). To limit the potential impact of different factors such as tissue shrinkage caused by the histological processing or by the lesion scar, the lesion size was calculated according to the following formula18:
Statistical Analyses
Values are reported as mean ± standard deviation. Statistical analyses were carried out with SPSS Statistics 19. The effect of the lesion on the behavioral performance was tested with a 1-way repeated-measures ANOVA using time as a factor, with differences being considered significant at P < .05. Post hoc comparisons were conducted with LSD (significant at P < .05) and tested if the average behavioral performance on a given postlesion day was different from the prelesion baseline value. Correlation analyses were conducted with Pearson’s correlations. A t statistic was used to establish if the correlation was statistically significant (P < .05).
Results
Effective Lesion Volume
Visual inspection of the histological sections confirmed that the ischemic injury destroyed all cortical layers of the sensorimotor cortex (Figure 2A). In most animals, the lesion was limited to the cortical gray matter, but 2 rats had clear striatal lesions and were, therefore, excluded from the study. Our lesion protocols resulted in a wide range of lesion volumes (from 3.07 to 22.96 mm3, corresponding to 1.98% to 14.55% of the reconstructed hemisphere). Figure 2B shows the size of lesions from histological reconstructions for each animal. The effective lesion size values were distributed as a continuum across cases. We concluded from this analysis that animals with ischemic lesions should be considered as a single experimental group that recovered from lesions of various sizes.
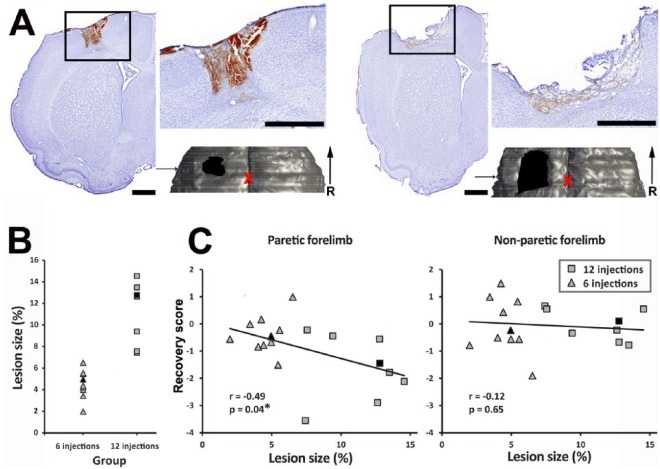
Figure 2.
Effective lesion size and behavioral recovery: A. Example of a Nissl stained section of the ipsilesional hemisphere in an animal that recovered from a small (left panel) and a large lesion (right panel). The box shows the location of the high-resolution photograph of the lesion in the inset. Lesions were essentially limited to the cortex and affected all layers of the cortical gray matter. For each animal, we made a histological reconstruction of the brain to quantify the lesion size. The 3D reconstruction of the tissue bloc is shown below the inset. The bloc is viewed from above. The location and extent of the lesion is outlined in black, and the arrow indicates the location of the Nissl-stained section in the reconstructed bloc. The red X shows the location of Bregma. Scale bars = 1 mm. B. Values of effective lesion size for each animal included in the study (n = 18). Lesion sizes are reported as a percentage of the reconstructed contralesional hemisphere. Our lesion protocols resulted in a wide range of cortical lesion sizes. Filled black symbols show animals from 2A. C. Relationship between the size of the lesion and the recovery score of the paretic (left panel) and nonparetic (right panel) forelimbs for individual animals. Only the recovery score of the paretic forelimb was significantly related to the lesion size (*). Filled black symbols show animals from 2A.
Note: Color version of the figure is present with the online March 2016 issue at www.nnr.sagepub.com.
Abbreviation: R, rostral.
Behavioral Recovery and Lesion Size
The injury significantly affected the behavioral performance with the nonparetic forelimb (F = 9.9; P < .001). The deficits were significant on the fourth day after the lesion (P < .001), but performance recovered to baseline by the seventh day. As expected, the lesions also caused profound behavioral impairments with the paretic forelimb (F = 23.7; P < .001). The deficits were significant on the fourth day after the lesion (P < .001). There was improvement with time, but the behavioral performance was still lower than baseline at the end of the recovery period (28th day; P = .02). When looking at individual animals, there was a wide range of final recovery scores for both the nonparetic and paretic forelimbs. However, only the recovery score of the paretic forelimb was correlated with the lesion size (r = −0.49; P = .04; Figure 2C). Animals with larger cortical lesions had greater chronic deficits with the paretic forelimb.
Motor Representations in Control Animals
Figures 3A and 4A show examples of ICMS data and the generated map of forelimb motor representations in control animals. As described by others, CFA and RFA were typically separated by cortical territory evoking movements of the trunk and vibrissae.13-15 CFA was larger than RFA (5.76 ± 0.82 mm2 and 1.23 ± 0.19 mm2, respectively; n = 5). In the CFA, the area of DFL was 3.83 ± 1.07 mm2 and occupied 66.3% ± 15.5% of the total surface area of the CFA. The average area of PFL was 1.92 ± 0.79 mm2. In RFA, the area of DFL was 0.87 ± 0.14 mm2, which represented 71.1% ± 7.9% of the total surface area of the RFA. The average area of PFL was 0.36 ± 0.13 mm2. We used the average area and the 95% confidence interval of the standard deviation for each motor representation to compare the motor representations in animals that recovered from cortical lesions with that of controls (see below).
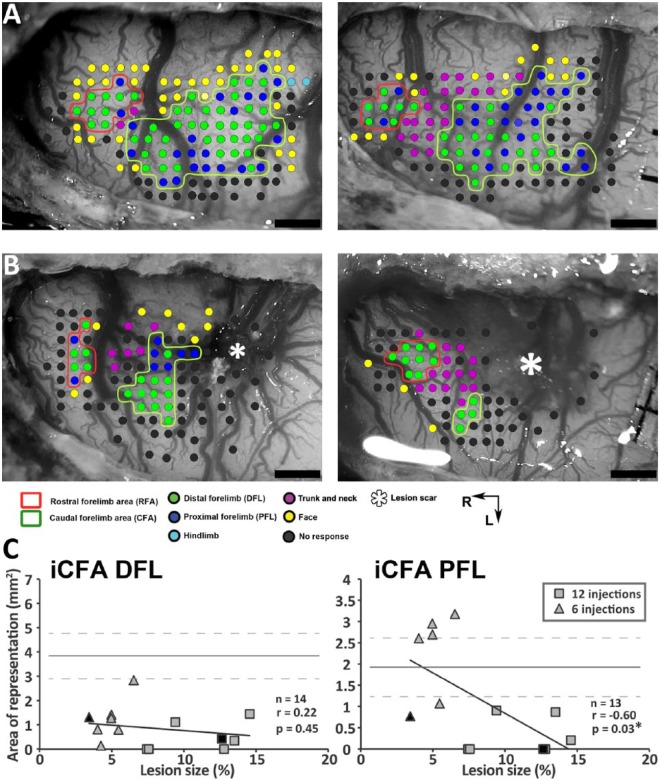
Figure 3.
Impact of the lesions on the ipsilesional CFA (iCFA): A. Examples of ICMS data in 2 control rats. Each dot shows a stimulation site and the evoked movement (color coded). The CFA and RFA are outlined. B. Examples of ipsilesional ICMS data in 2 rats that recovered from a small (left panel) and large lesion (right panel). In all animals from which we collected ipsilesional data, we found sites that evoked trunk, neck, or face movements between the lesion and RFA, supporting that iRFA was spared by the lesion. The lesion scar is shown with an asterisk. Scale bar = 1 mm. C. Correlation between the size of the lesion and the cortical surface area of DFL (left panel) and PFL (right panel) in the iCFA. The mean surface area value of the control group for each representation is shown with a solid line, and the 95% confidence interval with dashed gray lines. There was a significant negative correlation between the size of the lesion and the PFL in the iCFA (*). Filled black symbols show the 2 animals from 3B.
Note: Color version of the figure is present with the online March 2016 issue at www.nnr.sagepub.com.
Abbreviations: ICMS, intracortical microstimulation techniques; L, lateral; R, rostral.
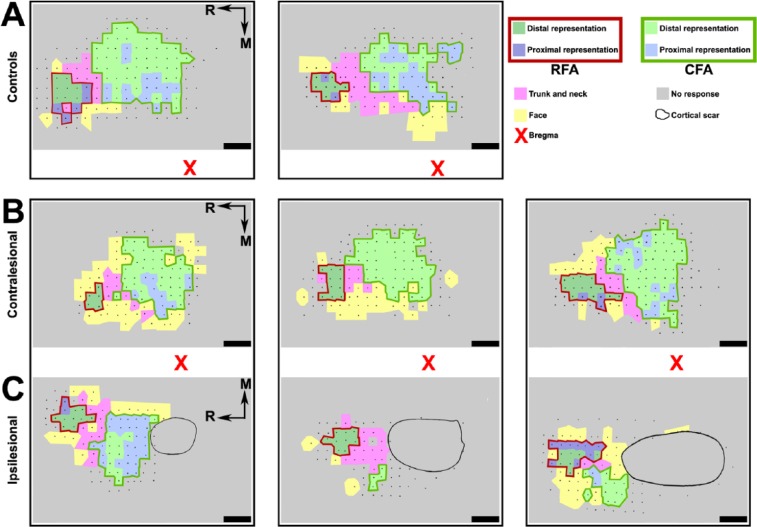
Figure 4.
Examples of analyzed motor maps: A. Examples of motor maps generated from the ICMS data of 2 control animals. Each small black dot shows the location of an electrode penetration and cortical stimulation. Evoked movements at threshold current intensity are color coded, and CFA and RFA are outlined. Contralesional (B) and ipsilesional (C) motor maps of 3 animals that recovered from cortical lesions of various sizes (5.0%, 12.6%, and 14.6%, from left to right). The approximate location of the lesion scar is shown, but lesion size was determined with histological reconstructions (see Figure 2). The red X shows the location of bregma. Scale bars = 1 mm.
Note: Color version of the figure is present with the online March 2016 issue at www.nnr.sagepub.com.
Abbreviations: ICMS, intracortical microstimulation techniques; CFA, caudal forelimb area; RFA, rostral forelimb area; M, medial; R, rostral.
Impact of Lesions on the Ipsilesional CFA (iCFA)
We studied the organization of motor areas with ICMS mapping techniques 5 weeks following the ischemic injury. We collected data to calculate the DFL and PFL areas of the iCFA in 14 and 13 rats, respectively. Figure 3B shows examples of ipsilesional ICMS data in 2 rats. In most cases, we found some sites in the iCFA that evoked movements of the forelimb, and in all cases, we evoked trunk or vibrissa movements at the rostral border of the lesion scar, supporting the idea that the ipsilesional RFA (iRFA) was spared by the injury. The relationships between movement representations in the iCFA and the size of the lesion are shown in Figure 3C. The area of DFL was smaller in animals that recovered from cortical injuries than in controls (<95% confidence interval on the mean), but there was no relationship between lesion size and DFL. The area of PFL was more variable across animals, and the relationship between lesion size and PFL was significant (r = −0.60; P = .03). Animals with large lesions had smaller PFL than controls. In animals with small lesions, PFL tended to be bigger than in animals with large lesions, and in some cases, PFL was even bigger than in controls.
Effect of Lesion Size on Spared Motor Areas of the Ipsilesional and Contralesional Hemispheres
For DFL, we collected ICMS data in the iRFA from 14 rats and in the contralesional RFA (cRFA) and CFA (cCFA) from 18 rats. For PFL, we obtained data in the iRFA from 12 rats, in the cRFA from 17 rats, and in the cCFA from 13 rats. Figure 4 shows 3 examples of rats in which we were able to complete ICMS data collection for all forelimb representations in both hemispheres.
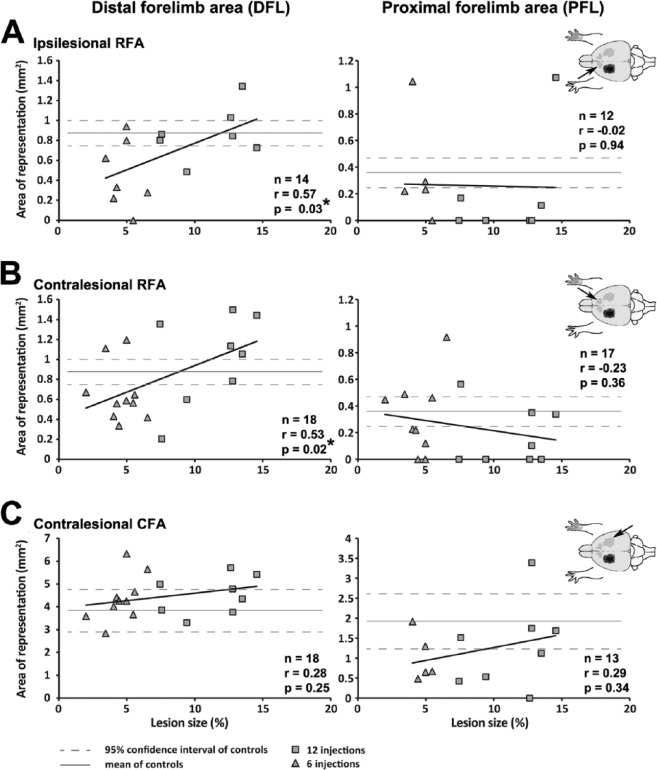
We conducted correlation analyses to evaluate the relation between the lesion size and forelimb representations in the iRFA, cRFA, and cCFA (Figure 5). For iRFA, animals with small lesions tended to have smaller DFL than animals with large lesions and controls. The relationship between lesion size and the area of DFL in the iRFA was significant (r = 0.57; P = .03). In contrast, PFL in the iRFA was smaller than controls in most animals and was not related to the size of the lesion. A similar pattern was found for cRFA. Most animals with small lesions had smaller DFL than animals with large lesions and controls. Many animals with large lesions had bigger DFL than controls. The relationship between lesion size and the area of DFL in the cRFA was also significant (r = 0.53; P = .02). We found no relation between lesion size and the area of PFL in the cRFA. Finally, we found no significant relationship between lesion size and motor representations in the cCFA.
Figure 5.
The effect of lesion size on motor representations. Plots of motor representations in relation to the size of lesion for individual animals. For each row, a cartoon on the right shows the location of the motor representation in the brain. The area of the distal forelimb representation (DFL) and the proximal forelimb representation (PFL) are plotted in the left and right columns, respectively. A. In the ipsilesional RFA (iRFA), there was a significant correlation between the size of the lesion and the DFL. Lesion size did not affect the PFL in the iRFA. B. Similarly, in the contralesional RFA, animals with larger lesions had bigger DFL, but there was no relationship between lesion size and the PFL. C, In the contralesional CFA, DFL and PFL were not affected by the size of the lesion.
Relationship Between Motor Representations Across Hemispheres
Apart from the size of the lesion, another factor that could be driving changes of motor representations was the residual forelimb area in the perilesional cortex. We, therefore, conducted correlations to evaluate if the area of DFL or PFL representations in the iCFA was related to the motor representations in the iRFA, cRFA, and cCFA. We found no significant correlations. For example, the area of PFL in the iCFA was not related to the area of DFL in the iRFA (r = −0.35; P = .24) or in the cRFA (r = −0.39; P = .21).
Yet another possibility is that with smaller lesions, reorganization in the motor network relies on ipsilesional plasticity and that recovery from increasingly larger lesions would require reorganization of the contralesional hemisphere. If so, there should be a relation between motor representations in the iRFA (ipsilesional plasticity) and in the contralesional cortex. We, therefore, tested if the area of DFL in the iRFA was correlated to motor representations in the contralesional hemisphere. Once again, we found no significant correlations. For example, the area of DFL in the iRFA was not related to the area of DFL in the cRFA (r = 0.47; P = .10).
It, thus, appears that motor representations in the 2 hemispheres were poorly related to one another. However, there was a similar relation between the size of the lesion and the area of DFL in both the iRFA and cRFA, the premotor areas in the 2 hemispheres.
Interaction Between Motor Representations and Final Recovery
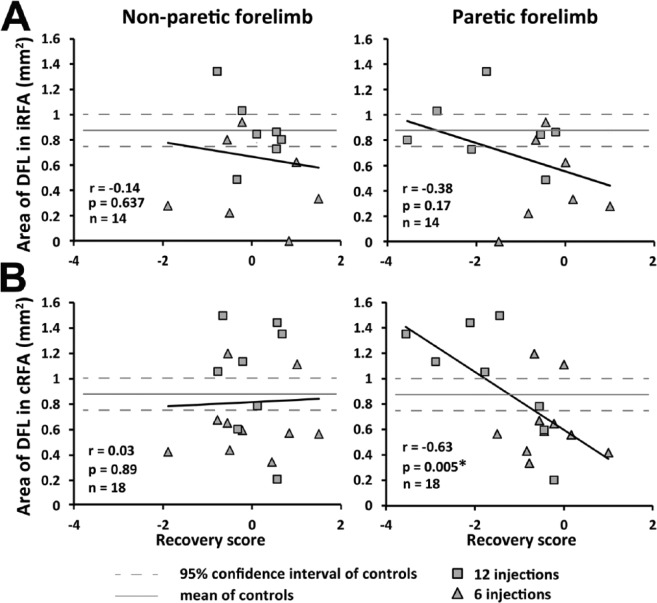
Finally, we evaluated the relationship between the recovery score and the area of DFL in the iRFA and cRFA, the 2 representations affected by the size of the lesion. For the nonparetic forelimb, there was no significant correlation between the recovery score and the area of DFL in the iRFA or cRFA (Figure 6). For the paretic forelimb, the recovery score was not correlated to the area of DFL in the iRFA. However, there was a significant inverse correlation between the recovery score and the area of DFL in the cRFA (r = −0.63; P = .005).
Figure 6.
Relation between the distal forelimb representation (DFL) in the ipsilesional and contralesional rostral forelimb areas (iRFA and cRFA) and the final recovery. A. Plot showing the relationship between the size of DFL in the iRFA and the recovery score of the nonparetic forelimb (left panel) and the paretic forelimb (right panel) for each rat (n = 14). There was no relationship between the size of the DFL area in the iRFA and the functional recovery of the 2 forelimbs. B. Plot showing the relationship between the DFL area in the cRFA and the recovery score of the 2 forelimbs (n = 18). No relationship was found between the cRFA and the recovery of the nonparetic forelimb (left panel). However, there was a significant relationship between the DFL area in the cRFA and the recovery score of the paretic forelimb (right panel). Rats with greater chronic deficits with the paretic forelimb (lower recovery scores) had bigger DFL in the cRFA.
Discussion
Our objective was to study the effect of lesion size on the organization of motor representations in the ipsilesional and contralesional hemispheres. We took advantage of a rat model in which we specifically targeted the cortex in the CFA—the primary motor cortex in rats—and induced a wide range of lesions sizes confirmed in postmortem histological reconstructions. At the chronic state of recovery, we used invasive cortical mapping techniques to study in detail the organization of the CFA and RFA (the putative premotor area) in both the ipsilesional and contralesional hemispheres. We found that larger lesions induced greater deficits of the paretic forelimb. Lesion size was correlated with the area of DFL in both the iRFA and cRFA, but only DFL in the cRFA was correlated to recovery of the paretic forelimb. Our data support the idea that lesion size has comparable effects on motor representations in the 2 hemispheres and suggest that the contralesional premotor cortex may play a greater role in the recovery of the paretic forelimb following large lesions.
The Effect of Lesion Size on the Reorganization of Ipsilesional Motor Maps
In the perilesional cortex (iCFA), the area of DFL was smaller than in controls, and animals with small lesions tended to have a bigger PFL area than controls. These results are comparable to the ones described in squirrel monkeys after small cortical lesions in the hand representation of M1.23 Following spontaneous recovery, there is a reduction of the digit representation and an increase of the proximal representation in the perilesional cortex. In macaque monkeys, after recovery from lesions in the hand representation of M1, many cortical sites in the perilesional cortex evoking digit movements prior to the lesion become nonresponsive or are replaced by more proximal muscle territories after recovery.24 It is possible that the lack of reemergence of hand movement in the perilesional cortex reflects suboptimal plasticity in spontaneously recovering animals that can be reversed by rehabilitative therapy.25
In the iRFA, we found that the area of DFL correlated with the size of the lesion. Animals with small lesions tended to have smaller DFL than controls and animals with large lesions. Once again, these results are much in line with previous studies in monkeys.3,4 Following recovery from small lesions in M1, there is a decrease of the hand representation in the ipsilesional PMv, and large M1 lesions result in an increase of the hand representation in the PMv. However, in contrast to rats, monkeys have several premotor areas26 that may be undergoing changes simultaneously after a lesion to contribute to motor recovery. Lesions in M1 also trigger cortical reorganization of the supplementary motor area,27 and physiological reorganization in premotor areas of monkeys is associated with cortical rewiring28 and changes of their corticospinal projections.29
In monkeys that spontaneously recover from M1 lesions, reversible inactivation of premotor areas induces motor deficits that are much more profound that what is observed in intact animals.30,31 Similarly, in humans, disrupting the activity in the ipsilesional dorsal premotor cortex with transcranial magnetic stimulation induces greater deficits with the paretic limb in well-recovered chronic stroke patients than in controls.32 These data support the idea that plasticity in the ipsilesional premotor areas allows these areas to vicariously take over functions that were lost as a result of the lesion. Based on studies such as these, novel treatments are being developed to specifically engage the premotor cortex after stroke, and they have been shown to improve function.33
The results from the present study show that cortical lesions in rats induce changes of ipsilesional motor representations that have many similarities to monkeys and humans. Spontaneously recovering rats have small hand representations in the iCFA, and much like in a premotor area, the hand representation in the iRFA increases as a function of lesion size. Thus, whereas rats have a less complex motor network than primates, motor recovery appears to be supported by comparable reorganization of the ipsilesional motor network in the 2 species.
The Effect of Lesion Size on the Reorganization of Contralesional Motor Maps
We found a significant relationship between lesion size and the area of DFL in the cRFA. However, there was no relationship between motor representations in the 2 hemispheres. It, thus, seems that lesion size, but not organization of ipsilesional motor areas, drives plasticity of motor representation in the contralesional hemisphere.
To date, the few studies that looked at the organization of cortical motor maps in the contralesional hemisphere have found no effect of lesion.19,34,35 The failure to identify changes in the cRFA in these studies may be explained by the restricted range of lesion sizes or by lesion location.
For example, following recovery from lesions induced with 8 microinjections of ET-1, ICMS mapping revealed no difference between the cRFA of recovered animals and controls.19 The relationship that we found between lesion size and motor representations in the cRFA somewhat predicts this result (Figure 5). The size of lesions resulting from 8 ET-1 injections should be in the middle of the range we obtained with 6 and 12 ET-1 injections. Following these midsize lesions, the DFL area in the cRFA is expected to fall within the 95% confidence interval of controls.
In another study, motor mapping was conducted in the contralesional hemisphere following recovery from MCAo.34 Lesions resulting from MCAos in that study were likely larger than the ones we obtained with 12 ET-1 injections. However, MCAo lesions in rodents typically spare the motor cortex.36 Thus, the difference in lesion location in animals with MCAos could explain the absence of reorganization of motor areas in the contralesional hemisphere in this study.
The reorganization of the cRFA in rats can be associated with the atypical activation of the contralesional premotor cortex following stroke.11,12,37 Abnormal contralesional premotor activity after stroke correlates with decreased corticospinal tract integrity, suggesting that patients with more affected corticospinal outputs are more likely to recruit the contralesional premotor cortex to perform more demanding tasks.38 In light of our results, it appears that the organization of the cRFA, the putative premotor cortex in rats16 is more sensitive to lesions in the opposite hemisphere than the cCFA. It is tempting to propose that following larger lesions, the premotor cortex may be more involved in recovery of the paretic limb, positively or negatively.
The Relation Between Motor Representations in the Contralesional Hemisphere and Recovery
The relation between reorganization in the contralesional hemisphere and recovery has been and still is a topic of debate. There is evidence in the literature that reorganization of the contralesional hemisphere can interfere with recovery of the paretic limb, support it, or favor motor learning with the nonparetic limb.11,39,40
In the present study, rats with poorer recovery had larger DFL in the cRFA. Similarly, in humans, atypical activity in the contralesional hemisphere is more frequent in patients with poor recovery.41,42 Studies in humans showing that inhibition of this hemisphere after stroke can favor recovery of the paretic limb support the idea that at least part of the contralesional activity can have a negative effect.43-45 In rats, we also found that pharmacological inactivation of the cCFA can improve recovery of the paretic forearm.18 Interestingly, none of the studies using contralesional inhibition as a treatment to improve recovery in animals or humans has specifically targeted the premotor cortex. It is possible that contralesional inactivation of the cRFA would be more effective to improve recovery than inactivation of the cCFA. Likewise in humans, inhibition of the contralesional premotor cortex may have better outcomes than inhibition of M1.
Studies in which the activity in contralesional premotor areas is disturbed to evaluate their contribution to movements of the paretic arm show that they can both contribute37,38 or not be involved in the recovery of the paretic forelimb.31,32 To date, none of these studies has systematically investigated the role of contralesional motor areas in function of the size of lesions restricted to M1. One possibility is that lesions of various sizes may lead to different changes in contralesional premotor areas, allowing them to contribute differently to recovery. Supporting this hypothesis, in rats that recovered from large MCAo lesions, reversible inhibition of the contralesional cortex induced greater deficits in the paretic limb than in control rats or animals that recovered from small lesions.8 In humans, inhibition of the contralesional cortex can also be detrimental to the paretic arm function following large lesions.9 In the present study, the relation we found between motor representations in the cRFA and lesion size could suggest that cRFA only contributes to recovery of the paretic forelimb following large lesions.
Finally, rats that suffered a cortical lesion are better at learning novel tasks with the nonparetic limb.46 Even though we found no correlation between cRFA and the function of the nonparetic forelimb, our data do not exclude the possibility that changes in the cRFA support learning of compensatory behavior of the nonparetic forelimb. However, in intact rats, motor training affects the organization of the CFA but not the RFA.13 Thus, if changes in motor maps of the contralesional hemisphere strictly support learning of the nonimpaired forelimb, our results suggest that after a lesion, motor learning is achieved through very different mechanisms that preferentially involve the RFA over the CFA.
Acknowledgments
We thank Trevor Drew and Kelsey N. Dancause for insightful comments, suggestions, and editing.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Heart and Stroke Foundation Canadian Partnership for Stroke Recovery National Expansion grant to ND. ND is supported by a Canadian Institutes of Health Research (CIHR) New Investigator salary award.
References
- 1. Dancause N. Vicarious function of remote cortex following stroke: recent evidence from human and animal studies. Neuroscientist. 2006;12:489-499. [DOI] [PubMed] [Google Scholar]
- 2. Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol. 2006;16:638-644. [DOI] [PubMed] [Google Scholar]
- 3. Dancause N, Barbay S, Frost SB, et al. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol. 2006;96:3506-3511. [DOI] [PubMed] [Google Scholar]
- 4. Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205-3214. [DOI] [PubMed] [Google Scholar]
- 5. Dijkhuizen RM, Singhal AB, Mandeville JB, et al. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003;23:510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp Neurol. 2006;201:479-494. [DOI] [PubMed] [Google Scholar]
- 7. Kim SY, Jones TA. Lesion size-dependent synaptic and astrocytic responses in cortex contralateral to infarcts in middle-aged rats. Synapse. 2010;64:659-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21:989-999. [DOI] [PubMed] [Google Scholar]
- 9. Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. 2012;22:2662-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cramer SC, Nelles G, Benson RR, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518-2527. [DOI] [PubMed] [Google Scholar]
- 11. Gerloff C, Bushara K, Sailer A, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129(pt 3):791-808. [DOI] [PubMed] [Google Scholar]
- 12. Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55:1081-1088. [DOI] [PubMed] [Google Scholar]
- 13. Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321-3325. [DOI] [PubMed] [Google Scholar]
- 14. Neafsey EJ, Bold EL, Haas G, et al. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77-96. [DOI] [PubMed] [Google Scholar]
- 15. Neafsey EJ, Sievert C. A second forelimb motor area exists in rat frontal cortex. Brain Res. 1982;232:151-156. [DOI] [PubMed] [Google Scholar]
- 16. Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10:269-289. [DOI] [PubMed] [Google Scholar]
- 17. Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36:219-228. [DOI] [PubMed] [Google Scholar]
- 18. Mansoori BK, Jean-Charles L, Touvykine B, Liu A, Quessy S, Dancause N. Acute inactivation of the contralesional hemisphere for longer durations improves recovery after cortical injury. Exp Neurol. 2014;254:18-28. [DOI] [PubMed] [Google Scholar]
- 19. Barbay S, Guggenmos DJ, Nishibe M, Nudo RJ. Motor representations in the intact hemisphere of the rat are reduced after repetitive training of the impaired forelimb. Neurorehabil Neural Repair. 2013;27:381-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stowe AM, Plautz EJ, Eisner-Janowicz I, et al. VEGF protein associates to neurons in remote regions following cortical infarct. J Cereb Blood Flow Metab. 2007;27:76-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dancause N, Barbay S, Frost SB, et al. Ipsilateral connections of the ventral premotor cortex in a new world primate. J Comp Neurol. 2006;495:374-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144-2149. [DOI] [PubMed] [Google Scholar]
- 24. Wyss AF, Hamadjida A, Savidan J, et al. Long-term motor cortical map changes following unilateral lesion of the hand representation in the motor cortex in macaque monkeys showing functional recovery of hand functions. Restor Neurol Neurosci. 2013;31:733-760. [DOI] [PubMed] [Google Scholar]
- 25. Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791-1794. [DOI] [PubMed] [Google Scholar]
- 26. Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav. 2002;77:677-682. [DOI] [PubMed] [Google Scholar]
- 27. Eisner-Janowicz I, Barbay S, Hoover E, et al. Early and late changes in the distal forelimb representation of the supplementary motor area after injury to frontal motor areas in the squirrel monkey. J Neurophysiol. 2008;100:1498-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dancause N, Barbay S, Frost SB, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167-10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McNeal DW, Darling WG, Ge J, et al. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518:586-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoogewoud F, Hamadjida A, Wyss AF, et al. Comparison of functional recovery of manual dexterity after unilateral spinal cord lesion or motor cortex lesion in adult macaque monkeys. Front Neurol. 2013;4:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res. 1999;128:149-159. [DOI] [PubMed] [Google Scholar]
- 32. Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127(pt 4):747-758. [DOI] [PubMed] [Google Scholar]
- 33. Dodakian L, Sharp KG, See J, et al. Targeted engagement of a dorsal premotor circuit in the treatment of post-stroke paresis. NeuroRehabilitation. 2013;33:13-24. [DOI] [PubMed] [Google Scholar]
- 34. Gonzalez CL, Gharbawie OA, Williams PT, Kleim JA, Kolb B, Whishaw IQ. Evidence for bilateral control of skilled movements: ipsilateral skilled forelimb reaching deficits and functional recovery in rats follow motor cortex and lateral frontal cortex lesions. Eur J Neurosci. 2004;20:3442-3452. [DOI] [PubMed] [Google Scholar]
- 35. Maggiolini E, Viaro R, Franchi G. Suppression of activity in the forelimb motor cortex temporarily enlarges forelimb representation in the homotopic cortex in adult rats. Eur J Neurosci. 2008;27:2733-2746. [DOI] [PubMed] [Google Scholar]
- 36. Gharbawie OA, Gonzalez CL, Williams PT, Kleim JA, Whishaw IQ. Middle cerebral artery (MCA) stroke produces dysfunction in adjacent motor cortex as detected by intracortical microstimulation in rats. Neuroscience. 2005;130:601-610. [DOI] [PubMed] [Google Scholar]
- 37. Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518-14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lotze M, Beutling W, Loibl M, et al. Contralesional motor cortex activation depends on ipsilesional corticospinal tract integrity in well-recovered subcortical stroke patients. Neurorehabil Neural Repair. 2012;26:594-603. [DOI] [PubMed] [Google Scholar]
- 39. Jones TA, Jefferson SC. Reflections of experience-expectant development in repair of the adult damaged brain. Dev Psychobiol. 2011;53:466-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641-656. [DOI] [PubMed] [Google Scholar]
- 41. Calautti C, Naccarato M, Jones PS, et al. The relationship between motor deficit and hemisphere activation balance after stroke: a 3T fMRI study. Neuroimage. 2007;34:322-331. [DOI] [PubMed] [Google Scholar]
- 42. Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126(pt 6):1430-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fregni F, Boggio PS, Mansur CG, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551-1555. [DOI] [PubMed] [Google Scholar]
- 44. Nowak DA, Grefkes C, Dafotakis M, et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008;65:741-747. [DOI] [PubMed] [Google Scholar]
- 45. Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681-2686. [DOI] [PubMed] [Google Scholar]
- 46. Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597-8606. [DOI] [PMC free article] [PubMed] [Google Scholar]