Abstract
The regenerative potential of c‐kit+ cardiac stem cells (CSCs) is severely limited by the poor survival of cells after transplantation in the infarcted heart. We have previously demonstrated that preconditioning human CSCs (hCSCs) with the heme oxygenase‐1 inducer, cobalt protoporphyrin (CoPP), has significant cytoprotective effects in vitro. Here, we examined whether preconditioning hCSCs with CoPP enhances CSC survival and improves cardiac function after transplantation in a model of myocardial infarction induced by a 45‐minute coronary occlusion and 35‐day reperfusion in immunodeficient mice. At 30 minutes of reperfusion, CoPP‐preconditioned hCSCsGFP+, hCSCsGFP+, or medium were injected into the border zone. Quantitative analysis with real‐time qPCR for the expression of the human‐specific gene HLA revealed that the number of survived hCSCs was significantly greater in the preconditioned‐hCSC group at 24 hours and 7 and 35 days compared with the hCSC group. Coimmunostaining of tissue sections for both green fluorescent protein (GFP) and human nuclear antigen further confirmed greater hCSC numbers at 35 days in the preconditioned‐hCSC group. At 35 days, compared with the hCSC group, the preconditioned‐hCSC group exhibited increased positive and negative left ventricular (LV) dP/dt, end‐systolic elastance, and anterior wall/apical strain rate (although ejection fraction was similar), reduced LV remodeling, and increased proliferation of transplanted cells and of cells apparently committed to cardiac lineage. In conclusion, CoPP‐preconditioning of hCSCs enhances their survival and/or proliferation, promotes greater proliferation of cells expressing cardiac markers, and results in greater improvement in LV remodeling and in indices of cardiac function after infarction. Stem Cells 2015;33:3596–3607
Keywords: Heart failure, Myocardial infarction, Human cardiac stem cell, Cell survival, Preconditioning, HO‐1 inducer, Cobalt protoporphyrin
Significance Statement.
The results of the multiple clinical trials are very exciting and suggest that human cardiac stem cell (hCSC) therapies may revolutionize the treatment of heart failure in patients with ischaemic cardiomyopathy. However, due to the very low level of donor cell survival after transplantation, it is crucial to find strategies that enhance cardiac stem cell survival. We previously showed that in vitro preconditioning of hCSCs with a small molecule, cobalt protoporphyrin (CoPP), a heme oxygenase‐1 inducer, enhances resistance to apoptosis through activation of the survival signaling pathway and release of various cytokines. Here, we examine the in vivo relevance of these observations. We demonstrate that preconditioning hCSCs with CoPP leads to a significant increase in the in vivo cell survival and/or proliferation after transplantation in the infarcted heart, a greater attenuation of ventricular remodeling, and improvement of cardiac function, suggesting that preconditioning cells with CoPP may enhance the effectiveness of cell therapy for heart disease. Our results indicate that pretreatment of hCSCs with CoPP may be a simple, safe, and effective intervention to augment the utility of CSC therapy.
Introduction
Stem cell‐based therapies have considerable potential for repairing cardiac damage due to ischemia/reperfusion injury 1, 2. Among the many types of cells being investigated, c‐kit+ cardiac stem cells (CSCs) appear to be particularly promising because they normally reside in the adult myocardium and have been reported to be capable of differentiating into all three major cardiac cell types (cardiomyocytes, smooth muscle cells, and endothelial cells) and improving cardiac function after myocardial infarction (MI) in an animal model 3. In the past decade, results from several independent laboratories have clearly demonstrated the ability of human and rodent CSCs to promote cardiac regeneration and attenuate MI‐induced left ventricular (LV) dysfunction and remodeling in various animal models 4, 5, 6, 7, 8, 9, 10, 11. These encouraging results led to the first clinical trial of c‐kit+ CSCs, cardiac stem cell infusion in patients with ischemic cardiomyopathy (SCIPIO) 12, 13, whose initial results were encouraging. However, many challenges remain before CSC‐based therapies become a clinical reality. One of the main problems is the very poor survival of donor cells. In mice with MI, it has been shown that >90% of transplanted CSCs die within a week and >95% by 5 weeks 14, 15; it is self‐evident that this massive loss of cells limits their effectiveness as a therapy. Strategies that enhance CSC survival after adoptive transfer would have significant therapeutic implications for patients with ischemic heart disease and post‐MI heart failure.
One of the strategies to increase cell survival is to “precondition” (or prime) the cells with a variety of techniques, including heat shock of the cells prior to transplantation, forced expression of survival factors in the donor cells, and exposure of cells to soluble pro‐survival factors 6, 16, 17, 18. In this study, we have explored the utility of a new approach—the induction of heme oxygenase‐1 (HO‐1). HO‐1 is a stress‐inducible protein that degrades heme, producing equimolar quantities of biliverdin (which is converted to bilirubin by biliverdin reductase), iron, and carbon monoxide (CO) 19. HO‐1 induces cellular protection in diverse conditions, such as injury, inflammation, and oxidative stress 20, 21, 22. Mounting evidence indicates that HO‐1 exerts important protective functions in the cardiovascular system, which is ascribed to its end products, biliverdin and CO 22, 23. One of the most potent inducer of HO‐1 is cobalt protoporphyrin (CoPP) 24. Previous studies have shown that CoPP enhances the survival of cardiomyocytes and restores contractility to unvascularized three‐dimensional adult cardiomyocyte grafts implanted in vivo 25 as well as protects the heart from ischemic damage in both normal and diabetic rats 26. We have previously reported that preconditioning hCSCs with CoPP promotes cell survival and resistance to oxidative stress in vitro 27. The mechanism appears to involve activation of survival signaling pathways such as the ERK/NRF2 pathway as well as paracrine effects via increased release of cytokines 27. In this study, we investigated whether preconditioning hCSCs with CoPP enhances in vivo hCSC survival and improves cardiac function and structure after transplantation into the infarcted heart of severe combined immunodeficiency (SCID) mice.
Materials and Methods
Detailed methodology is provided in the online‐only Data Supporting Information. The protocol for this study is shown in Figure 1E. Briefly, 7 days before MI, parameters of LV function and dimensions were measured by echocardiography in SCID immunodeficient mice. MI was induced by a 45‐minute coronary occlusion followed by reperfusion. At 30 minutes of reperfusion, mice were randomly allocated to three groups to receive intramyocardial injections of (a) F12 medium only (vehicle control group); (b) nonpreconditioned hCSCs (hCSC group); and (c) hCSCs preconditioned with CoPP (PC‐hCSC group). Four intramyocardial injections were performed in the border zone as indicated in Supporting Information Figure S3A. In a subset of mice, the heart was harvested to assess hCSC survival at different time points (5 minutes, 24 hours, and 7 and 35 days) following cell transplantation. The rest of the mice were used to evaluate cardiac function and structure by echocardiography and hemodynamic studies as well as by histological studies after 35 days. To quantify the number of c‐kit+ hCSCs that survived in the mouse heart following transplantation, we constructed a standard curve based on the human‐specific gene HLA‐A, a unique endogenous marker that we used as a probe for human cells 14, 15, 28 (Fig. 1C). The standard curve (Fig. 1D) was established with a known ratio of hCSCs and mouse heart genomic DNA, and the cycle threshold (C t) value derived from real‐time qPCR. This assay is a modification of our previous method 14 and is based on the assumption that each diploid mouse cell contains ∼7.2 pg genomic DNA and each diploid human cell contains ∼7.7 pg genomic DNA.
Figure 1.
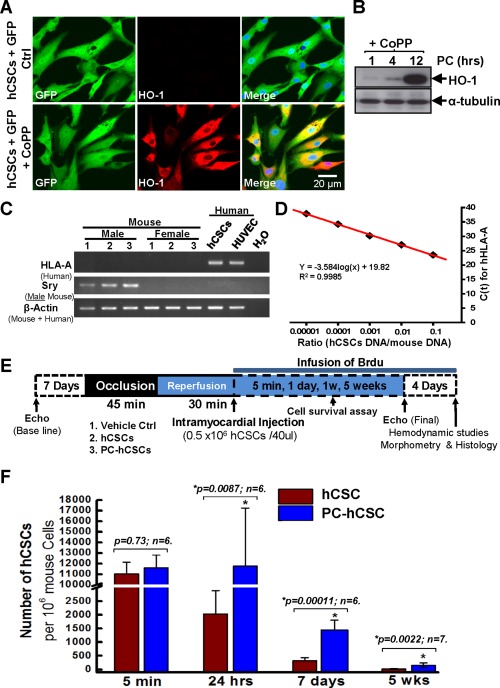
Increased in vivo survival of hCSCs after preconditioning with CoPP. (A): Confocal images show that 100% of hCSCs are GFP‐positive cells (green) after infection with a lentivirus expressing GFP, while CoPP‐preconditioned hCSCs show positive staining for HO‐1 (red). (B): Western blot shows significant upregulation of HO‐1 expression after 12‐hour preconditioning with CoPP. (C): Gel images of PCR products from mouse or human genomic DNA show the unique gene expression of HLA‐A only in human cells, including hCSCs and HUVEC. (D): To determine the number of hCSCs that survived in the SCID mouse hearts, a standard curve was set up with the C t values (cycle threshold) from qPCR reactions for a series of known ratios of hCSC and SCID mouse heart genomic DNA. (E): Protocol for in vivo studies of transplantation of preconditioned hCSCs into SCID mice following myocardial infarction. (F): A significant increase in the number of surviving hCSCs was observed at 24 hours, 1 and 5 weeks after hCSC transplantation of CoPP‐preconditioned cells compared with control cells. Data are means ± SD. Abbreviations: CoPP, cobalt protoporphyrin; GFP, green fluorescent protein; hCSCs, human cardiac stem cells; HO‐1, heme oxygenase‐1; HUVEC, human umbilical vein endothelial cells.
Results
Preconditioning hCSCs with CoPP Enhances In Vivo Cell Survival and/or Proliferation
hCSCs were isolated from right atrial appendages obtained from patients undergoing coronary artery bypass surgery, as described previously 12, 29. Our previous in vitro study has shown that preconditioning hCSCs with CoPP, an HO‐1 inducer, significantly increases resistance of cells to oxidative stress via the ERK/NRF2 signal pathway 27. Thus, we hypothesized that preconditioning hCSCs with CoPP may enhance in vivo cell survival and therapeutic efficacy after MI. Prior to transplanting cells into the mouse hearts, purified c‐kit+/Lin− hCSCs were first labeled with eGFP by transduction with a lentivirus carrying the eGFP gene. As shown in Figure 1A, more than 98% of the cells were eGFP positive, which was also confirmed by fluorescence‐activated cell sorting analysis (data not shown). Immunostaining with an HO‐1 antibody demonstrated a significant increase in HO‐1 expression following a 12‐hour preconditioning with CoPP; this was confirmed by Western blot (Fig. 1B). The cells maintained >95% viability for up to 60 minutes in basal medium, which was the vehicle used for the transplanted cells used in the following sections (Supporting Information Fig. S1). Furthermore, the cells maintained a high c‐kit positivity (up to 87%) in vitro for 19 passages (Supporting Information Fig. S2), similar to our previous results 29. The cells used in the following studies were expanded for less than 15 passages.
In order to examine the number of cells that remain in vivo, we used the standard curve shown in Figure 1D. Genomic DNA was isolated from tissue harvested from three different regions (left ventricle, right ventricle, and atria) at 5 minutes, 24 hours, and 7 and 35 days after cell transplantation. After real‐time qPCR with HLA‐A primers for individual genomic DNA samples from different groups at various time points, the total number of hCSCs in the heart was calculated and compared based on the standard curve (Fig. 1D) and the Ct value. As shown in Figure 1F and Supporting Information Figure S3B, compared with the hCSC group, in vivo survival of preconditioned hCSCs showed no difference at 5 minutes following transplantation. However, a more than fivefold increase in the number of hCSCs was observed in the CoPP‐preconditioned group at 24 hours. Although the number of hCSCs decreased dramatically after the first few days, the PC‐hCSC group exhibited an approximately four‐ and eightfold greater abundance of CSCs in the heart than the hCSC group at 1 and 5 weeks after transplantation, respectively. Importantly, in the hCSC group (Fig. 1F and Supporting Information Fig. S3B, red columns), 81.5% of the hCSCs present in the LV at 5 minutes were lost in the ensuing 24 hours, and only 3.2% of the CSCs present at 5 minutes could still be found in the LV at 7 days after transplantation. By 35 days, only 0.18% of the cells present at 5 minutes were detected in the heart. In the preconditioned hCSC group (Fig. 1F and Supporting Information Fig. S3B, blue columns), 101.8% of the hCSCs present in the LV at 5 minutes remained in the heart at 24 hours after transplantation, indicating that the rate of cell proliferation exceeded the rate of cell loss. Furthermore, 12.6% of the CSCs present at 5 minutes could still be found in the LV at 7 days after transplantation, which is significantly greater than the hCSC group (12.6% vs. 3.2%, p < .001). After 35 days, 1.35% of the cells present at 5 minutes were detected in the heart, which was still more than the hCSC group (1.35% vs. 0.18%, p < .005). No hCSCs could be detected in the right ventricle or atria at any time points (data not shown). Taken together, these data suggest that preconditioning hCSCs with CoPP significantly enhanced in vivo hCSC survival and/or proliferation.
To independently verify these conclusions, the number of hCSCs was assessed by staining LV sections harvested at 39 days with antibodies specific for green fluorescent protein (GFP) or human nuclear antigen (HNA). The number of cells positive for either GFP or human HNA was counted and quantified with confocal imaging (Fig. 2). A few GFP+ cells did not show positive staining for human HNA (Supporting Information Fig. S4, yellow arrow) at 5 minutes following intramyocardial injection, which may be due to the focal plane, and an occasional HNA+ cell did not show positive staining for GFP (Supporting Information Fig. S4, white arrow). Quantitative analysis showed that the percentage of cells that were GFP+ and HNA+ was similar (Supporting Information Fig. S4B). At 39 days after intramyocardial injection, the number of either GFP+ cells or HNA+ cells in the risk region was significantly greater in the PC‐hCSC group compared with the hCSC group (Fig. 2), further confirming that CoPP preconditioning promoted in vivo hCSC survival and/or proliferation after transplantation.
Figure 2.
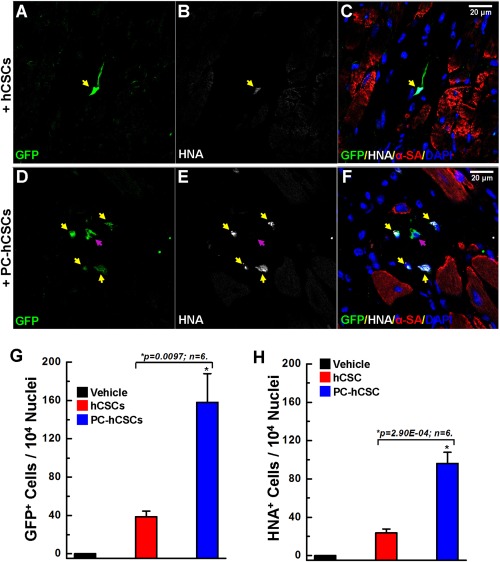
Confocal images showing increased survival of cobalt protoporphyrin (CoPP)‐preconditioned hCSCs in the infarcted mouse heart. Following immunostaining with antibodies against GFP (green), HNA (white), and α‐SA (red), as well as counterstaining nuclei with DAPI, representative confocal images show that in the CoPP‐preconditioned hCSC group (D, E, F) more cells are positive for both GFP and HNA compared to the control cell group (A, B, C). Yellow arrows indicate cells positive for both GFP and HNA; purple arrows indicate cells positive only for GFP. Quantitative analysis showed a significantly greater number of surviving hCSCs in the preconditioned group compared with the nonpreconditioned cell group, as indicated by the more GFP+ cells (G) and HNA+ cells (H). Data are means ± SD. Abbreviations: GFP, green fluorescent protein; hCSCs, human cardiac stem cells; HNA, human nuclear antigen; α‐SA, α‐sarcomeric actin.
Intramyocardial Injection of PC‐hCSCs Alleviates LV Dysfunction and Remodeling
Echocardiographic Assessment
Body weight and heart rate were similar in all groups at baseline and at 5 and 35 days after cell injection (Supporting Information Fig. S5). By experimental design, rectal temperature remained within a narrow, physiologic range (36.8–37.2°C) in all groups.
Echocardiography showed that the impairment in LV contractile performance at 5 days after reperfusion was comparable in all groups, indicating that the injury inflicted by ischemia/reperfusion was similar (Fig. 3). As expected, in the vehicle group between 5 and 35 days after reperfusion, the parameters of regional myocardial function, including anterior and apical wall longitudinal strain rate, and those of global LV performance (LV ejection fraction [EF]), exhibited either no change or deterioration (Fig. 3). In contrast, in the hCSC‐treated groups (both the group that received hCSC and that which received CoPP‐preconditioned hCSCs), LV EF was significantly greater than in the vehicle group at 35 days (Fig. 3A); in addition, both hCSC‐treated groups exhibited a significantly smaller LV end‐systolic volume (Fig. 3C) and greater LV anterior wall thickness (Fig. 3E) compared with the vehicle group. The attenuation of LV remodeling in the PC‐hCSC group was significantly greater than that in both the hCSC group and the vehicle group, as evidenced by the fact that left ventricular end diastolic volume (LVEDV) (Fig. 3B) was significantly reduced, and LV posterior wall thickness (Fig. 3F) was significantly increased in the PC‐hCSC group.
Figure 3.
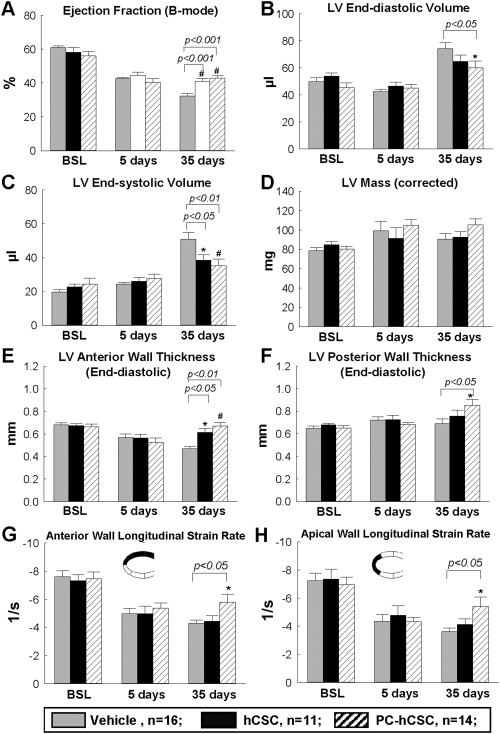
Echocardiographic analysis. Serial echocardiographic studies were performed at BSL (4 days prior to coronary occlusion/reperfusion), 5 days after CSC treatment, and 35 days after CSC transplantation in the vehicle control group (n = 16), hCSC group (n = 11), and PC‐hCSC group (n = 14). Data are means ± SEM. Abbreviations: BSL, baseline; hCSCs, human cardiac stem cells; LV, left ventricular.
The overall longitudinal strain rate was significantly increased in the PC‐hCSC group compared with both the vehicle and hCSC control groups at 35 days (Supporting Information Fig. S7). In a more detailed assessment, both the anterior and the apical wall longitudinal strain rates were significantly higher in the PC‐hCSC group compared with either the hCSC group or the vehicle group (Fig. 3G, 3H), whereas the longitudinal strain rates for the posterior wall, basal wall, and middle wall showed no difference (Supporting Information Fig. S8). Taken together, the echocardiographic data demonstrate that, compared with hCSCs or vehicle, PC‐hCSCs produced a greater improvement in LV remodeling and in regional wall motion in the infarcted region.
Hemodynamic Measurements
To independently assess the effect of intramyocardial injection of hCSCs on cardiac function, vehicle‐ and hCSC‐injected mice were subjected to hemodynamic studies using a Millar catheter. To prevent any after‐effects of the anesthesia used during the echocardiographic assessment 35 days after CSC transplantation, a 4‐day interval was allowed between echocardiographic and hemodynamic measurements; thus, the hemodynamic studies were performed just before euthanasia, 39 days after hCSC or vehicle administration.
As shown in Figure 4A, compared with the vehicle group, both the hCSC and PC‐hCSC groups exhibited a significantly greater LV EF, and there was no difference between these two groups. However, unlike the hCSC group, the PC‐hCSC group exhibited a significant increase in end‐systolic elastance (a load‐independent parameter of LV performance) and stroke work (Fig. 4B, 4C), LV dP/dtmax and dP/dtmin (Fig. 4D, 4E), and cardiac output and stroke volume (Fig. 4F, 4G) compared with the vehicle group. Thus, although EF was not increased, load‐independent measures of LV performance were improved by PC‐hCSCs but not by hCSCs.
Figure 4.
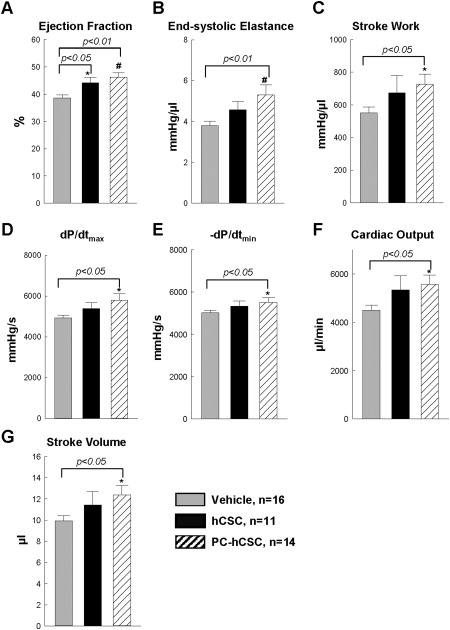
Hemodynamic analysis. At 39 days after cell transplantation, hemodynamic measurements were performed using a Millar catheter in the vehicle control group (n = 16), hCSC group (n = 11), and PC‐hCSC group (n = 14). Data are means ± SEM. *, p < .05; #, p < .01 versus vehicle group. Abbreviation: hCSCs, human cardiac stem cells.
Morphometric Analysis
The effects of hCSC transplantation on LV remodeling were evaluated by Masson's trichrome staining as described previously 11. As illustrated in Figure 5 and Supporting Information Figure S9, morphometric analysis demonstrated that there were no appreciable differences among the three groups with respect to the size of the region at risk (expressed as a percentage of the left ventricle), indicating that the magnitude of the ischemic insult was comparable in all groups (Supporting Information Fig. S9). The administration of PC‐hCSCs was associated with a reduction in scar area: in mice that received PC‐hCSCs, the scar area (34.4% ± 6.1% of the region at risk) was smaller than that in the corresponding vehicle‐treated group (57.1% ± 3.9%; p < .01; Fig. 5D); the scar area in mice treated with hCSCs (45.8% ± 3.5%) was also smaller than that in the vehicle‐treated group (57.1% ± 3.9%; p < .05; Fig. 5D). Both the hCSC group and PC‐hCSC group showed an increase in the amount of viable myocardium in the region at risk (Fig. 5E). However, the thickness of the LV anterior (infarcted) wall was increased (Fig. 5F) and the LV expansion index was decreased only in the PC‐hCSC group (Fig. 5G). In summary, both hCSCs and PC‐hCSCs reduced scar tissue and increased viable tissue, but the salubrious effects of PC‐hCSCs on LV remodeling were greater than those of hCSCs.
Figure 5.
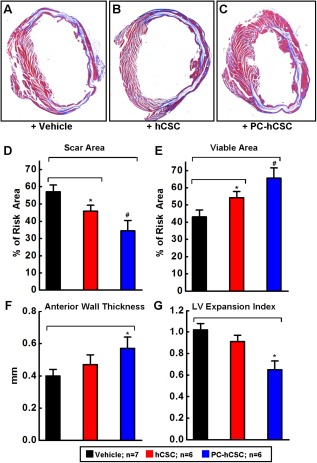
Morphometric analysis of LV remodeling after hCSC transplantation. (A–C): Representative Masson's trichrome‐stained myocardial sections from mice injected with vehicle (A), hCSCs (B), or cobalt protoporphyrin‐preconditioned hCSCs (C). Scar tissue and viable myocardium are identified in blue and red, respectively. (D–G): Quantitative analysis of LV morphometric parameters as indicated. LV expansion index = (LV cavity area/LV total area)/(noninfarcted region wall thickness/risk region wall thickness). Values are means ± SEM. *, p < .05; #, p < .01 versus vehicle group. Abbreviations: hCSCs, human cardiac stem cells; LV, left ventricular.
PC‐hCSCs Promote Endogenous Cardiac Regeneration
In order to identify newly formed cells, BrdU was infused continuously for 39 days with miniosmotic pumps beginning at the time of vehicle or hCSC infusion and continuing until euthanasia, as described previously 11. Evidence for formation of new myocytes was provided by the colocalization of α‐sarcomeric actin (α‐SA) and BrdU. Representative images of LV sections stained with antibodies for both α‐SA and BrdU are shown in Figure 6A–6E. Quantitative analysis revealed that the total number of newly formed cells (indicated by the incorporation of BrdU) in the risk region was significantly greater in PC‐hCSC‐treated hearts than in either vehicle‐treated hearts or hCSC‐treated hearts (Fig. 6F). There were no statistical differences in the total numbers of newly formed cells positive for BrdU or newly formed cells expressing cardiac markers (α‐SA/BrdU double positive cells) in the remote region (Supporting Information Fig. S10). However, the total content of newly formed cells expressing cardiac markers (α‐SA+/BrdU+ cells) in the risk region was significantly higher in the PC‐hCSC group than in either the vehicle or control hCSC group (Fig. 6G).
Figure 6.
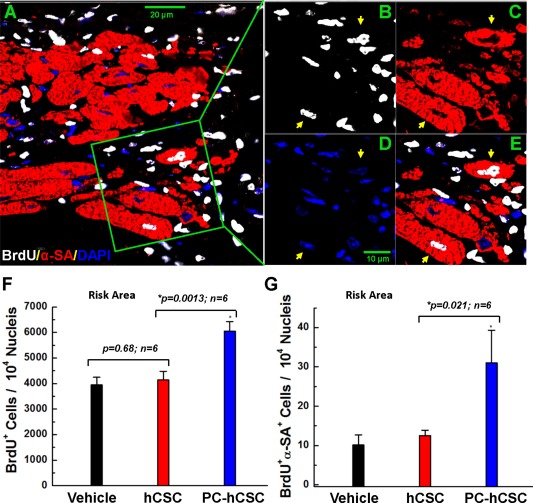
Proliferation of endogenous cells following hCSC transplantation. Mice received BrdU beginning at the time of cell transplantation and continuing until euthanasia (for 39 days). Positivity for BrdU (white) in representative confocal microscopic images obtained from the infarcted region identifies newly formed cells (A–E): α‐SA is red. Nuclei were stained with DAPI (blue). Yellow arrows indicate cells positive for BrdU, α‐SA, and DAPI. (F): Quantitative analysis of the number of BrdU‐positive cells (total newly formed cells) at 39 days after vehicle or cell transplantation. (G): Quantitative analysis of the number of BrdU/α‐SA double positive cells (newly formed cells with apparent commitment to myocytic differentiation) at 39 days after vehicle or cell transplantation. Values are means ± SD. The risk region comprises both the border zones and the scar region. Abbreviations: hCSCs, human cardiac stem cells; LV, left ventricular; α‐SA, α‐sarcomeric actin.
PC‐hCSCs Exhibit Enhanced Proliferation and Differentiation After Transplantation
The human origin of cells in myocardial sections was evidenced by immunolabeling for eGFP, and evidence for proliferation of hCSCs was provided by the colocalization of eGFP and BrdU (Supporting Information Fig. S11). The total content of newly formed human cells (eGFP/BrdU double positive cells) in the risk region was significantly higher in the PC‐hCSC group than in the hCSC group (60.2 ± 16.2 vs. 22.1 ± 5.3 per 104 nuclei, p < .05 Supporting Information Fig. S11E). Differentiation of transplanted hCSCs into a cardiogenic lineage was evaluated by the presence of eGFP and HNA double positive cells that expressed alpha‐sarcomeric actin (GFP+HNA+α‐SA+). As shown in Figure 2 and Supporting Information Figure S11, at 39 days after cell transplantation, the majority of surviving hCSCs were not differentiated into a cardiogenic lineage; they were negative for alpha‐sarcomeric actin (no sarcomeres were evident) and the cell size was quite small. However, a few cells exhibited positive staining for all three markers (GFP+HNA+α‐SA+), which is indicative of cardiomyocytic commitment (Fig. 7A–7D). These cells, derived from human CSCs, were also positive for the cardiomyogenesis marker, GATA‐4, as evidenced by triple staining for GATA‐4/HNA/α‐SA (Supporting Information Fig. S12A–S12D, yellow arrow). Quantitative analysis showed that the total number of GFP+HNA+α‐SA+ triple positive cells in the risk region was significantly greater in the PC‐hCSC group than in the hCSC control group (Fig. 7E). However, the absolute numbers were very small (<10 cells per 105 nuclei), indicating that these cells were exceedingly rare (Fig. 7E). Similarly, the total number of hCSC‐derived cells positive for GATA‐4/HNA/α‐SA staining was significantly higher in the PC‐hCSC group than in the hCSC group (Supporting Information Fig. S12E), but, again, the numbers were exceedingly small. These cells were found exclusively in the risk area; no hCSC‐derived cells expressing cardiac markers were seen in the remote area (data not shown). Collectively, these results suggest that PC‐hCSCs have an enhanced ability to proliferate and to form cells that express cardiac markers; however, these cells do not acquire the phenotype of mature myocytes and their numbers are extremely small.
Figure 7.
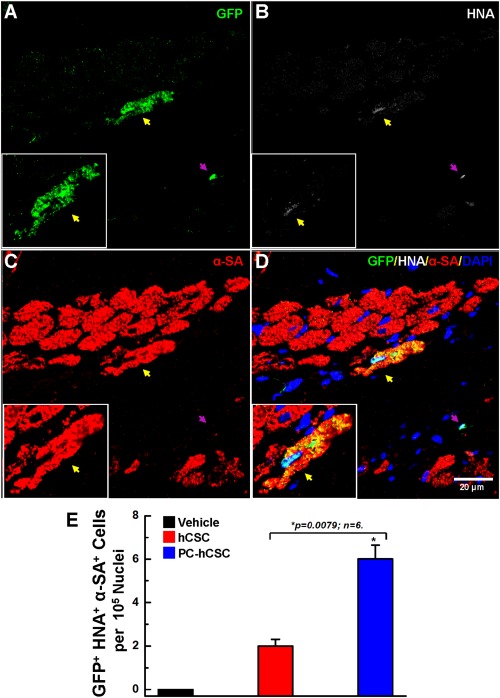
Differentiation of hCSCs at 39 days after transplantation. (A–D): Representative confocal microscopic images show the differentiation of transplanted hCSCs after 39 days of transplantation. Shown is the immunofluorescent detection of eGFP (green) (A), HNA (white) (B), α‐sarcomeric actin (α‐SA, red) (C), and nuclear DAPI (blue) in the risk region of hearts that received PC‐hCSCs. The yellow arrow indicates a cardiomyocyte that is positive for eGFP, HNA, and α‐SA. The purple arrow indicates a nondifferentiated human cell negative for α‐SA, but positive for both GFP and HNA. Insets are enlarged cell images indicating newly formed cardiomyocytes derived from hCSCs. (E): Quantitative analysis of the number of GFP/HNA/α‐SA triple positive cells. Data are means ± SD. The risk region comprises both the border zones and the scar region. Abbreviations: GFP, green fluorescent protein; hCSCs, human cardiac stem cells; HNA, human nuclear antigen; α‐SA, α‐sarcomeric actin.
Analysis of Proteins Related to Myocardial Apoptosis, Angiogenesis, and Calcium Handling Capacity in Formalin‐Fixed and Paraffin‐Embedded Heart Tissue
In order to further investigate the mechanism of the therapeutic benefits afforded by CoPP‐preconditioned hCSCs, we performed Western blot analysis of tissue lysates extracted from formalin‐fixed and paraffin‐embedded (FFPE) heart tissue samples obtained from the border zone. Based on previously described protocols 30 and our pilot studies, tissue lysates extracted from FFPE blocks should allow recovery of ∼70% of antigens for Western blot. We examined the protein expression of BCL2, MCL1, BAX, and BAD (related to apoptosis), angiopoietin‐1 (related to angiogenesis), and SERCA2 (related to calcium handling) in 14 samples (four for the vehicle group and five for both the hCSC and PC‐hCSC groups). As shown in Supporting Information Figure S13, there were no significant differences among the three groups in the expression of any of the proteins examined.
Discussion
Although preconditioning hCSCs with the HO‐1 inducer, CoPP, enhances resistance to cell death in vitro 27, it is unknown whether transplantation of CoPP‐preconditioned hCSCs in infarcted myocardium results in a significant increase in hCSC numbers and greater LV functional and structural improvement. This study was undertaken to answer this question.
Our results can be summarized as follows: (a) in this immunodeficient mouse model of acute MI, intramyocardial injection of 5 × 105 CoPP‐preconditioned hCSCs resulted in significantly greater numbers of cells remaining in the recipient heart at 24 hours, 7 days, and 35 days after injection compared with mice given nonpreconditioned hCSCs; (b) compared with nonpreconditioned cells, administration of CoPP‐preconditioned hCSCs resulted in greater improvement in LV remodeling (greater reductions in anterior LV wall thinning, LV expansion index, end‐diastolic LV volume, and posterior LV wall thinning) as well as in greater improvement in regional function in the infarcted region and in various parameters of global LV function; (c) these salubrious effects were associated with greater cell proliferation, greater formation of new cells that expressed cardiac‐specific proteins, and greater differentiation of transplanted cells into myocytes. Taken together, these results indicate that CoPP‐preconditioned hCSCs are more efficacious than unmodified hCSCs in the treatment of post‐MI LV dysfunction.
One of the major challenges associated with stem cell therapy is the poor survival of transplanted stem cells 14, 15. Various approaches have been used to overcome this problem, including preconditioning stem cells by genetic or pharmacologic manipulations or by exposing them to in vitro conditions that mimic the harsh environment of an ischemic heart. Preconditioning can be induced by a variety of techniques including (but not limited to) in vitro hypoxia 31, exposure to SDF‐1 32, and over‐expression of HO‐1 33. In general, all these methods have been found to enhance the post‐transplantation survival and therapeutic potential of the cells. Quantitative comparison of the effectiveness of the various methods is difficult because of variable models and protocols in various laboratories. In this study, we pretreated hCSCs with CoPP, an HO‐1 inducer. The rationale for selecting this small molecule was its known cytoprotective actions 25 as well as its ability to protect the heart from ischemic damage in both normal and diabetic rats 26. Our results indicate that pretreatment of hCSCs with CoPP is a simple, safe, and effective intervention to augment the utility of CSC therapy.
Traditional methods to assess cell retention, including counting immunostained cells on tissue sections, are imprecise and laborious. Here, we used a highly sensitive and accurate method, based on real‐time PCR detection of a human‐specific gene 14, 28, to determine the number of exogenous hCSCs that remain in the heart after transplantation. Our results show that in the hCSC group, there was a rapid disappearance of transplanted nonpreconditioned hCSCs, with more than 95% of them being lost after 7 days (Fig. 1F and Supporting Information Fig. S3B). These results are consistent with our previous studies of mouse and rat CSCs 9, 14, 15 as well as with those obtained by others with other stem cells, all of which have shown low initial retention and almost complete disappearance after a few weeks 33, 34, 35. However, following preconditioning of hCSCs with CoPP, greater numbers of hCSCs were observed at all the time points, with an approximately fivefold increase at 24 hours, an approximately fourfold increase at 7 days, and an approximately eightfold increase at 35 days following cell transplantation compared with the nonpreconditioned cell group (Fig. 1F and Supporting Information Fig. S3B). Similar data were obtained with the traditional method of immunostaining for either GFP or HNA (Fig. 2).
In a previous study 14 in which we monitored the retention and engraftment of mouse CSCs in the murine heart following intramyocardial injection, we found that that >75% of the CSCs present at 5 minutes were lost in the ensuing 24 hours; only 7.6% ± 2.1% of the CSCs present at 5 minutes could still be found at 7 days after injection, and only 2.8% ± 0.5% at 35 days. Thus, even after direct intramyocardial injection, the number of CSCs that remained in the murine heart was minimal. This study was performed to determine whether preconditioning human CSCs significantly increases cell retention. Using nonpreconditioned human CSCs, we observed that >80% of the hCSCs present at 5 minutes were lost in the 24 hours after intramyocardial injection, which is comparable to the loss of mouse CSCs observed previously 14. In contrast, for the human CSCs preconditioned with CoPP, we found that the number of cells that remained in the mouse heart at 24 hours after intramyocardial injection was ∼102% of those present at 5 minutes. Approximately 13% of the hCSCs present at 5 minutes could still be found at 7 days after injection, which is significantly more than nonpreconditioned hCSC group at the same time, and 1.35% ± 0.72% of the CSCs present at 5 minutes could still be found at 35 days (Fig. 1F and Supporting Information Fig. S3B), which is also significantly more than the nonpreconditioned hCSCs group. Taken together, these results show that the number of CoPP‐preconditioned hCSCs that remain in the donor heart is greater than that of nonpreconditioned hCSCs (although, in absolute terms, both numbers are minuscule), indicating greater survival and/or proliferation.
Extensive preclinical studies have been conducted which provide evidence for the capacity of c‐kit+ CSCs to improve cardiac function after MI 4, 6, 9, 11, 15, 18, 36, 37. The results of SCIPIO, the first study of these cells in humans, are also encouraging 12, 13. The mechanism by which exogenous c‐kit+ CSCs (and stem/progenitor cells in general) improve cardiac function, however, remains unclear. This data indicate that most hCSCs transplanted into the mouse heart do not survive long‐term irrespective of whether they are preconditioned or nonpreconditioned, although retention was better for CoPP‐preconditioned hCSCs (Fig. 1). In addition, only few cells positive for GFP or HNA were found to express cardiomyocyte markers (α‐sarcomeric actin) (Fig. 7 and Supporting Information Fig. S12), making it difficult to argue that cardiomyogenic differentiation of transplanted hCSCs occurs in the recipient heart in a functionally meaningful manner. Despite their poor engraftment and differentiation, however, both CoPP‐preconditioned and nonpreconditioned hCSCs significantly improved LV EF compared with the vehicle control (Figs. 3A, 4A). The clear dissociation between cell number and functional improvement observed here provides cogent evidence to support the concept that the major mechanism whereby the transplanted CoPP‐preconditioned hCSCs improve LV function must involve paracrine actions that modulate the function of adjacent cells. Numerous studies have demonstrated that stem cells contribute to tissue repair and regeneration by releasing growth factors, including cytokines and chemokines, in a dynamic spatial‐temporal manner that can promote donor cell survival, angiogenesis, tissue repair, and remodeling 17, 38, 39, 40, 41, 42, 43. Emerging evidence suggests that transplanted c‐kit+ CSCs also function in a paracrine manner 2, 9, 15, 44, 45. Our previous in vitro study has shown that CoPP‐preconditioning of hCSCs induces a global increase in cytokine release 27. The increased number of BrdU‐positive cells as well as BrdU‐positive cells expressing cardiac proteins in the risk region of hearts receiving PC‐hCSCs (Fig. 6F, 6G) may reflect augmentation of endogenous repair mechanisms by the transplanted PC‐hCSCs 2, 7.
Although we did not find any significant difference in myocardial levels of proteins related to apoptosis, angiogenesis, and calcium handling at 35 days (Supporting Information Fig. S13), these data must be interpreted with caution because of several reasons, including the nonuniformity of the border zone samples (which may underlie the large variance observed), the limited number of samples (caused by the fact that most hearts were used for pathological analysis), and the timing of sample harvest (35 days after transplantation, when changes in protein expression may have already subsided). However, even if we had found a significant change in one or more proteins, we could not conclude that that is the mechanism of the benefits afforded by PC‐hCSCs; the change in protein level(s) may be simply an epiphenomenon that is not causally related to the beneficial effects of cell therapy. The number of potential paracrine mechanisms is very large and includes secretion of growth factors, cytokines, miRs, exosomes, and so forth, all of which may modulate many different functions of the host myocardium. Identification of the specific molecular mechanisms whereby CoPP‐preconditioned hCSCs improve LV function is a formidable challenge that will require an extensive investigative effort.
Despite the increased cell retention in the heart and the greater improvement in LV remodeling associated with PC‐hCSCs, preconditioning of hCSCs did not improve all measures of LV function. Compared with mice treated with nonpreconditioned cells, LV EF was not increased by preconditioning, although regional function and load‐independent parameters of contractility were improved. The lack of a more striking difference between PC‐hCSCs and hCSCs may reflect an inherently small increase in effectiveness, the overwhelming extent of cell loss after transplantation (which may have precluded beneficial effects of preconditioning from becoming manifest), or the relatively short follow‐up of 35 days. Longer observation periods will be necessary to determine the effectiveness of CoPP preconditioning of hCSCs. It is important to note that EF is a load‐dependent variable and that load‐independent variables, which are more accurate parameters to assess cardiac contractility, were improved more by preconditioned cells than by nonpreconditioned cells (Fig. 4). Thus, although CoPP preconditioning did not increase EF versus nonpreconditioned CSCs, end‐systolic elastance was significantly improved only by PC‐hCSCs. Moreover, hemodynamic studies showed that other load‐dependent variables (stroke work, LV dP/dtmax, LV dP/dtmin, cardiac output, and stroke volume) were significantly improved by PC‐hCSCs but not by hCSCs. Thus, the totality of evidence supports the conclusion that PC‐hCSCs are more efficacious than hCSCs in alleviating post‐MI LV dysfunction.
Conclusions
In conclusion, this study demonstrates that preconditioning hCSCs with a small molecule, CoPP, leads to a significant increase in cell survival and/or proliferation after transplantation in the infarcted heart, a greater attenuation of LV remodeling, and an improvement of many indices of cardiac function, suggesting that exposing cells to CoPP prior to transplantation may enhance the effectiveness of cell therapy for heart disease. The mechanism(s) responsible for the improved outcome with CoPP‐preconditioned CSCs remain to be defined. HO‐1 is a multifaceted protein that exerts a plethora of beneficial actions 46. Given the poor survival of transplanted cells, and our in vitro data indicating that preconditioning hCSCs induces a global increase in cytokine release 27, it is likely that paracrine actions involving multiple growth factors and trophic factors acting in synergy are involved in the reparative actions of PC‐hCSCs.
Author Contributions
C.C.: conception and design, manuscript writing and revision, hCSCs preparation, cell survival, and histological data collection, analysis, and interpretation; Y.G.: conception and design, manuscript revision, MI surgery, echocardiographic, and hemodynamic data collection, analysis, and interpretation; R.B.: conception and design, and manuscript revision; L.T., M.T., and X.W.: hCSCs preparation, cell survival, and histological data collection, analysis, and interpretation; J.D., W.W., M.B., Y.N., X.Z., and W.X.: MI surgery, echocardiographic, and hemodynamic data collection, analysis, and interpretation; Q.L.: provision of study material (BrdU pumps); K.U.H.: provision of study material (HLA primers). C.C. and Y.G. contributed equally to this work.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Acknowledgments
This study was supported by NIH Grants R01HL114951 (to C.C.), 5UM1HL113530 and P01HL78825 (to R.B.) from the National Institutes of Health, and Research Grant 12BGIA9090005 (to C.C.) from the American Heart Association.
References
- 1. Hong KU, Bolli R. Cardiac stem cell therapy for cardiac repair. Curr Treat Options Cardiovasc Med 2014;16:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanganalmath SK, Bolli R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 2013;113:810–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bearzi C, Rota M, Hosoda T et al. Human cardiac stem cells. Proc Natl Acad Sci USA 2007;104:14068–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolli R, Tang XL, Sanganalmath SK et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 2013;128:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linke A, Muller P, Nurzynska D et al. Stem cells in the dog heart are self‐renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA 2005;102:8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fischer KM, Cottage CT, Wu W et al. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim‐1 kinase. Circulation 2009;120:2077–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Angert D, Berretta RM, Kubo H et al. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circ Res 2011;108:1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dawn B, Stein AB, Urbanek K et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA 2005;102:3766–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang XL, Rokosh G, Sanganalmath SK et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30‐day‐old infarction. Circulation 2010;121:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taghavi S, Sharp TE, 3rd , Duran JM et al. Autologous c‐Kit+ mesenchymal stem cell injections provide superior therapeutic benefit as compared to c‐Kit+ cardiac‐derived stem cells in a feline model of isoproterenol‐induced cardiomyopathy. Clin Transl Sci 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Q, Guo Y, Ou Q et al. Intracoronary administration of cardiac stem cells in mice: A new, improved technique for cell therapy in murine models. Basic Res Cardiol 2011;106:849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolli R, Chugh AR, D'Amario D et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2011;378:1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Chugh AR, Beache GM, Loughran JH et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The SCIPIO trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 2012;126:S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hong KU, Li QH, Guo Y et al. A highly sensitive and accurate method to quantify absolute numbers of c‐kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol 2013;108:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hong KU, Guo Y, Li QH et al. c‐kit+ Cardiac stem cells alleviate post‐myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One 2014;9:e96725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laflamme MA, Chen KY, Naumova AV et al. Cardiomyocytes derived from human embryonic stem cells in pro‐survival factors enhance function of infarcted rat hearts. Nat Biotechnol 2007;25:1015–1024. [DOI] [PubMed] [Google Scholar]
- 17. Haider H, Ashraf M. Preconditioning and stem cell survival. J Cardiovasc Transl Res 2010;3:89–102. [DOI] [PubMed] [Google Scholar]
- 18. Mohsin S, Khan M, Toko H et al. Human cardiac progenitor cells engineered with Pim‐I kinase enhance myocardial repair. J Am Coll Cardiol 2012;60:1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryter SW, Alam J, Choi AM. Heme oxygenase‐1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev 2006;86:583–650. [DOI] [PubMed] [Google Scholar]
- 20. Tsoyi K, Kim HJ, Shin JS et al. HO‐1 and JAK‐2/STAT‐1 signals are involved in preferential inhibition of iNOS over COX‐2 gene expression by newly synthesized tetrahydroisoquinoline alkaloid, CKD712, in cells activated with lipopolysacchride. Cell Signal 2008;20:1839–1847. [DOI] [PubMed] [Google Scholar]
- 21. Li Q, Guo Y, Ou Q et al. Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase‐1 via a nuclear factor‐{kappa}B‐dependent pathway. Circulation 2009;120:1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 2008;60:79–127. [DOI] [PubMed] [Google Scholar]
- 23. Ryter SW, Morse D, Choi AM. Carbon monoxide and bilirubin: Potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol 2007;36:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woo J, Iyer S, Mori N et al. Alleviation of graft‐versus‐host disease after conditioning with cobalt‐protoporphyrin, an inducer of heme oxygenase‐1. Transplantation 2000;69:623–633. [DOI] [PubMed] [Google Scholar]
- 25. Kawamoto S, Flynn JP, Shi Q et al. Heme oxygenase‐1 induction enhances cell survival and restores contractility to unvascularized three‐dimensional adult cardiomyocyte grafts implanted in vivo. Tissue Eng Part A 2011;17:1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. L'Abbate A, Neglia D, Vecoli C et al. Beneficial effect of heme oxygenase‐1 expression on myocardial ischemia‐reperfusion involves an increase in adiponectin in mildly diabetic rats. Am J Physiol Heart Circ Physiol 2007;293:H3532–3541. [DOI] [PubMed] [Google Scholar]
- 27. Cai C, Teng L, Vu D et al. The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal‐regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. J Biol Chem 2012;287:33720–33732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng K, Gupta S. Quantitative tools for assessing the fate of xenotransplanted human stem/progenitor cells in chimeric mice. Xenotransplantation 2009;16:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He JQ, Vu DM, Hunt G et al. Human cardiac stem cells isolated from atrial appendages stably express c‐kit. PLoS One 2011;6:e27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Becker KF, Schott C, Hipp S et al. Quantitative protein analysis from formalin‐fixed tissues: Implications for translational clinical research and nanoscale molecular diagnosis. J Pathol 2007;211:370–378. [DOI] [PubMed] [Google Scholar]
- 31. Rosova I, Dao M, Capoccia B et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008;26:2173–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pasha Z, Wang Y, Sheikh R et al. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res 2008;77:134–142. [DOI] [PubMed] [Google Scholar]
- 33. Tang YL, Tang Y, Zhang YC et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia‐regulated heme oxygenase‐1 vector. J Am College Cardiol 2005;46:1339–1350. [DOI] [PubMed] [Google Scholar]
- 34. Reinecke H, Zhang M, Bartosek T et al. Survival, integration, and differentiation of cardiomyocyte grafts: A study in normal and injured rat hearts. Circulation 1999;100:193–202. [DOI] [PubMed] [Google Scholar]
- 35. Toma C, Pittenger MF, Cahill KS et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002;105:93–98. [DOI] [PubMed] [Google Scholar]
- 36. Duran JM, Makarewich CA, Sharp TE et al. Bone‐derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res 2013;113:539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams AR, Hatzistergos KE, Addicott B et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 2013;127:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mirotsou M, Jayawardena TM, Schmeckpeper J et al. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol 2011;50:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou P, Wirthlin L, McGee J et al. Contribution of human hematopoietic stem cells to liver repair. Semin Immunopathol 2009;31:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen L, Tredget EE, Wu PY et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gnecchi M, Zhang Z, Ni A et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 2008;103:1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair 2008;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shintani S, Kusano K, Ii M et al. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med 2006;3(suppl 1):S123–128. [DOI] [PubMed] [Google Scholar]
- 44. Stastna M, Abraham MR, Van Eyk JE. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett 2009;583:1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stastna M, Chimenti I, Marban E et al. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics 2010;10:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Czibik G, Derumeaux G, Sawaki D et al. Heme oxygenase‐1: An emerging therapeutic target to curb cardiac pathology. Basic Res Cardiol 2014;109:450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


