Abstract
Purpose of review
In 1954 Harman proposed the free radical theory of aging, and in 1972 he suggested that mitochondria are both the source and the victim of toxic free radicals. Interestingly, hypertension is age-associated disease and clinical data show that by age 70, 70% of the population has hypertension and this is accompanied by oxidative stress. Antioxidant therapy however is not currently available and common antioxidants like ascorbate and vitamin E are ineffective in preventing hypertension. The present review focuses on molecular mechanisms of mitochondrial oxidative stress and therapeutic potential of targeting mitochondria in hypertension.
Recent findings
In the past several years, we have shown that the mitochondria become dysfunctional in hypertension and have defined novel role of mitochondrial superoxide radicals in this disease. We have shown that genetic manipulation of mitochondrial antioxidant enzyme superoxide dismutase (SOD2) affects blood pressure and have developed mitochondria-targeted therapies such as SOD2 mimetics that effectively lower blood pressure. The specific mechanism of mitochondrial oxidative stress in hypertension, however, remains unclear. Recent animal and clinical studies have demonstrated several hormonal, metabolic, inflammatory, and environmental pathways contributing to mitochondrial dysfunction and oxidative stress.
Summary
Nutritional supplements, calorie restriction, and life style change are the most effective preventive strategies to improve mitochondrial function and reduce mitochondrial oxidative stress. Aging associated mitochondrial dysfunction, however, reduces efficacy of these strategies. Therefore, we propose that new classes of mitochondria-targeted antioxidants can provide high therapeutic potential to improve endothelial function and reduce hypertension.
Keywords: Mitochondria, Hypertension, oxidative stress, reactive oxygen species, antioxidants
INTRODUCTION
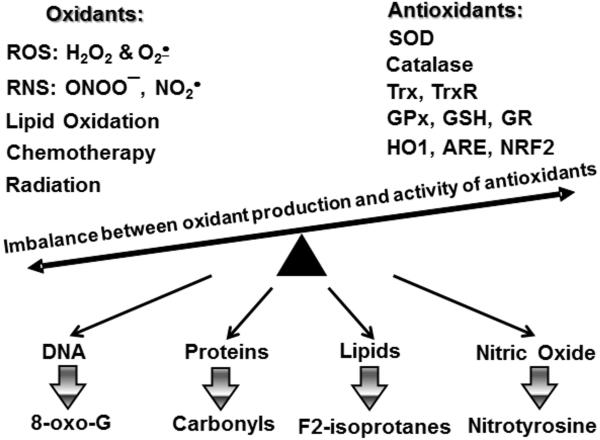
Hypertension is a multifactorial disorder involving perturbations of the vasculature, the kidney and the central nervous system (1). Despite treatment with multiple drugs, 37% of hypertensive patients remains hypertensive (2), likely due to the mechanisms contributing to blood pressure elevation that are not affected by current treatments. Human hypertension is associated with reduced activity of antioxidant enzymes and increased production of reactive oxygen species (ROS: O2• and H2O2) leading to oxidative stress as measured by lipid and DNA oxidation (Figure 1) (3, 4). Indeed, in almost all experimental models of hypertension ROS are increased in multiple organs, including critical centers of the brain, the vasculature, and the kidney. In the brain, ROS promote neuronal firing, ultimately increasing sympathetic outflow (5, 6). In the kidney, ROS act in multiple sites to promote sodium resorption and volume retention (7). In the vasculature ROS promote vasoconstriction and remodeling, increasing systemic vascular resistance (8). Our group has revealed several sources of ROS contributing to hypertension, including the NADPH oxidase, uncoupled nitric oxide synthase, and the mitochondria (9**) and defined their interaction (10, 11). Meanwhile, antioxidant therapy is not currently available, and common antioxidants like ascorbate and vitamin E are ineffective in preventing cardiovascular diseases and hypertension (12), since these agents likely do not reach important sites of ROS production such as mitochondria.
Figure 1.
Oxidative stress is an imbalance between oxidant production and activity of antioxidant system leading to oxidation of multiple biological targets such as DNA, proteins, lipids and nitric oxide which can be followed by accumulation of markers of oxidative stress.
REGULATION OF MITOCHONDRIAL ROS
The major biological function of mitochondria is ATP synthesis (13). This process is based on transfer of electrons through the mitochondrial respiratory chain coupled with transporting protons (H+) from the matrix to the intermembrane space to generate the proton motive force which is used for conversion of ADP to ATP. Several sites of the electron transport chain “leak” electrons to O2 creating O2• (14, 15). This is not a “spontaneous” process, and the production of mitochondrial ROS is highly regulated (9**, 10). It strongly depends on the pH gradient across the inner membrane (16), activation of mitochondrial ATP-sensitive potassium channels (mitoK+ATP) (17, 18), and opening of mitochondrial permeability transition pore (mPTP) (19, 20). Mitochondrial O2• is rapidly converted to H2O2 by SOD2 (21, 22), and H2O2 is a neutral molecule which easily leaves mitochondria. We have previously shown that activation of NADPH oxidases increases the production of mitochondrial ROS and vice versa: increase in mitochondrial ROS activates NADPH oxidases (19, 23). Production of mitochondrial ROS, therefore, is redox-dependent and represents an ongoing feed-forward cycle (10). It is recognized that mitochondrial ROS play an important physiological function (24); however, excessive stimulation of mitochondria leads to ROS overproduction and depletion of antioxidants resulting in imbalance between oxidant production and antioxidant defense system which constitutes an oxidative stress (Figure 2).
Figure 2.
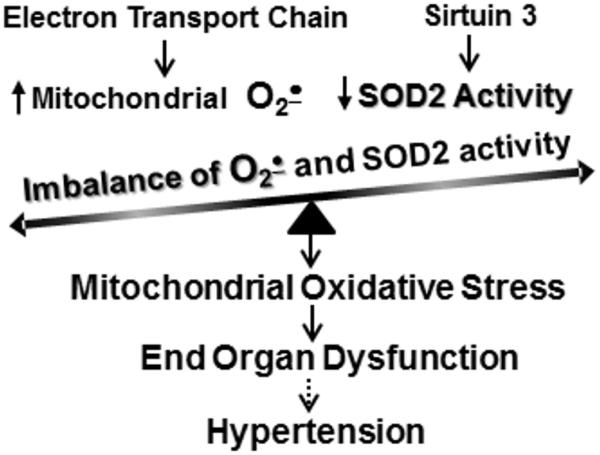
Overproduction of mitochondrial superoxide and reduced SOD2 activity leads to mitochondrial oxidative stress which contributes to end organ dysfunction and hypertension.
SIRTUIN 3 AND MITOCHONDRIAL SUPEROXIDE DISMUTASE (SOD2)
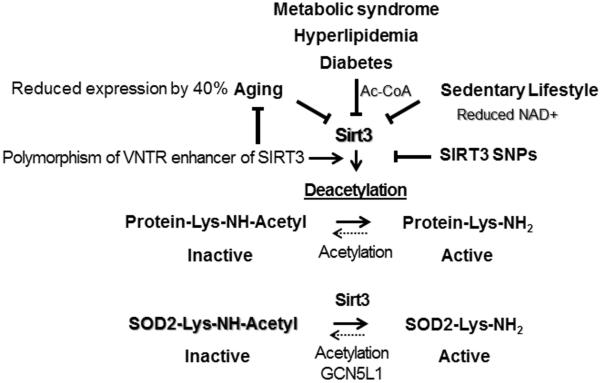
Mitochondrial SOD2 is a key antioxidant enzyme and acetylation represents a major post-translational regulation of SOD2 activity. SOD2 in vivo is activated by Sirtuin 3 mediated deacetylation of specific lysine residues (25). NAD+-dependent mitochondrial Sirtuin 3 (Sirt3) acts as a metabolic sensor, using intracellular metabolites such as NAD+ and acetyl-CoA to modulate mitochondrial function to match nutrient supply (Figure 3) (26). Access of Acetyl-CoA leads to SOD2 hyperacetylation resulting in SOD2 inactivation, and deacetylation by Sirt3 restores SOD2 activity (27). The profound increase of hypertension with age is associated with the decline of both mitochondrial energy metabolism (28) and the NAD+ dependent deacetylase activity (29). Sirt3 is a nuclear-encoded protein, however, the majority of Sirt3 translocates to mitochondria (29). Clinical studies have shown that Sirt3 expression declines by 40% by age 65 paralleling the increased incidence of hypertension (28, 30). Angiotensin II and inflammation also mediate the decline in Sirt3 activity (31). Interestingly, SOD2 expression is not changed with age but the activity of Sirt3 and SOD2 are diminished (32) suggesting that SOD2 acetylation contributes to hypertension. Indeed, we have shown that SOD2 overexpression attenuates hypertension (23), while SOD2 depletion enhances oxidative stress and hypertension.
Figure 3.
Critical role of Sirt3 in regulation of mitochondrial metabolism and activation of mitochondrial superoxide dismutase (SOD2). Aging, metabolic conditions and lifestyle alterations reduce Sirt3 activity and increase SOD2 acetylation leading to SOD2 inactivation which contributes to mitochondrial oxidative stress.
There are several genetic and metabolic factors which affect Sirt3 expression and activity. Bellinzi et al. have discovered a variable number tandem repeat (VNTR) polymorphism which has an allele-specific enhancer activity and this activity is virtually absent in men older than 90 years (33). Authors suggested that Sirt3 underexpression can be detrimental for longevity as it occurs in animal models (34). Dransfeld et al. have recently identified two non-synonymous human SIRT3 SNPs and evaluated their impact on SIRT3 activity and stability (35). It is interesting that multiple risk factors for hypertension are associated with the reduced Sirt3 expression and activity. For example, Sirt3 activity is metabolically downregulated by increased Acetyl-CoA and reduced NAD+ in metabolic syndrome, hyperlipidemia, diabetes and sedentary lifestyle while aging and smoking reduces Sirt3 expression (Figure 3) (30, 36*). It has been suggested that dietary supplementation of Sirt3 activating compounds such as resveratrol can be beneficial (37, 38). Resveratrol supplementation reduced renal inflammation and attenuated hypertension in the animal models (39*); however, recent clinical studies showed mixed results of resveratrol supplementation in cardiovascular disease and human hypertension (40, 41).
AGING AND MITOCHONDRIAL IMPAIRMENT
Silencing information regulator 2 (SIR2) extends lifespan in yeast, worms, and flies (42), and sirtuins are SIR2 homologs in mammals (43). SIRT3 is suppressed with aging and SIRT3 upregulation in aged hematopoietic stem cells improves their regenerative capacity (32). Therefore, Sirt3 is one of the potential candidates for age-associated development of cardiovascular diseases. Indeed, Sirt3 expression declines by 40% by age 65 paralleling the increased incidence of hypertension (30). Sirt3 activation of mitochondrial metabolism by deacetylation of key Krebs cycle enzymes, promotes fatty acid β-oxidation, activates complex I, improves antioxidant defense by activation of NADPH producing isocitrate dehydrogenase and increasing SOD2 activity (27, 44). There is compelling evidence for accumulation of oxidative damage to specific mitochondrial proteins which leads to the progressive mitochondrial dysfunction with aging (45). The role age-associated mitochondrial oxidative stress in hypertension was highlighted in the studies of mice deficient in mitochondrial superoxide dismutase (SOD2−/+) which have age-associated hypertension and increased sensitivity to salt (46). Recent studies further support the role of mitochondrial oxidative stress in the aging which depresses respiratory function and contribute to muscle atrophy (47). The early dysfunction appears to be reversible based on improved mitochondrial function and elevated mitochondrial gene expression after exercise training (48).
ANGIOTENSIN II AND MITOCHONDRIAL DYSFUNCTION
Interestingly, angiotensin-converting enzyme inhibitor enalapril and angiotensin II type I receptor (AT1R) blocker losartan attenuate age-associated mitochondrial dysfunction (49). Angiotensin II stimulates ROS production by non-phagocytic NADPH oxidases such as Nox1 and Nox2. We have investigated the potential cross talk between mitochondria and NADPH oxidases (10). It was found that depletion of p22phox and Nox1 subunits of NADPH oxidases in endothelial cells completely prevented angiotensin II induced mitochondrial oxidative stress (9**, 19). Furthermore, scavenging of mitochondrial ROS by acute treatments with mitochondria-targeted antioxidants mitoTEMPO and mitoEbselen attenuated NADPH oxidase activity and reduced O2• production in the cytoplasm (9**, 50). These data demonstrate redox-dependent cross-talk between cytoplsamic Nox2 and mitochondrial ROS in endothelial cells. Other cell types, however, have different Nox isoforms. Vascular smooth muscle cells, for example, express Nox1 which is upregulated by angiotensin II (51, 52) and, therefore, Nox1 can play an important role in cross-talk between mitochondrial ROS and NADPH oxidase in vascular smooth muscle cells. Indeed, both angiotensin II and mitoK+ATP activator diazoxide stimulated mitochondrial ROS and NADPH oxidase in rat vascular smooth muscle cells in vitro and in rat aorta (53).
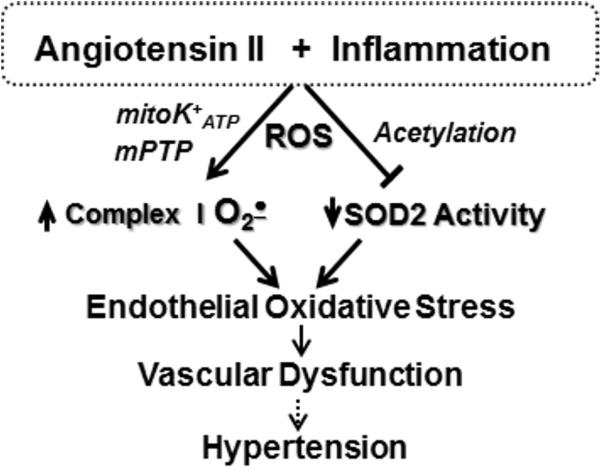
The cross-talk between NADPH oxidases and mitochondrial ROS is bi-directional. Angiotensin II activates NADPH oxidases via AT1R receptor, while expression of AT1R is redox dependent and thereby overproduction of mitochondrial ROS may enhance stimulation of AT1R mediated pathways (11). NADPH oxidases produce O2• and H2O2 which increase intracellular Ca2+ and stimulate redox dependent mitoK+ATP and PKCε (Figure 4) which triggers Ca2+ and redox induced production of mitochondrial ROS (18, 50, 54). Activation of c-Src is redox sensitive and is stimulated by H2O2 (55), which appears to represent a feed-forward mechanism, whereby mitochondrial H2O2 amplifies NADPH oxidase activity (9**). Mitochondria apparently regulate both expression (56) and activity of NADPH oxidases (57). Partial depolarization of mitochondrial membrane potential reduces Ca2+ uptake by mitochondrial uniporter and increases Ca2+ dependent activation of NADPH oxidases (57), while depletion of SOD2 results in increase of basal and stimulated vascular NADPH oxidase activity (23). Therefore, overproduction of mitochondrial ROS may result in overstimulation of cytoplasmic NADPH oxidases and dysregulation of cellular signaling leading to the development of vicious cycle of oxidative stress (23).
Figure 4.
Angiotensin II and inflammation increases ROS production by NADPH oxidases which lead to opening of mitochondrial redox sensitive mitochondrial channels (mPTP and mitoK+ATP) and increased acetylation of mitochondrial enzymes. In endothelial cells this stimulates superoxide production by complex I and reduces SOD2 activity leading to endothelial oxidative stress, impaired vasodilatation and increased hypertension.
CALORIE RESTRICTION AND MITOCHONDRIAL ANTIOXIDANTS
It has been previously shown that calorie restriction attenuates hypertension in rat models (58); however, the exact mechanisms and therapeutic potential of this intervention is not clear. It has been suggested that calorie restriction activates AMPK pathway which improves NO synthase phosphorylation and endothelial function (58). Calorie restriction increases expression of endothelial and neuronal NO synthase and enhances NO-mediated mitochondrial biogenesis (59, 60). Other groups have shown that calorie restriction (25) increases Sirt3 expression and activity which may suggest that increased Sirt3 activity may reduce hypertension. Recent clinical studies showed substantial decrease in systolic blood pressure by 20 mm Hg in subjects following one year of calorie restriction (61) which can be mediated by a number of pathways. One potential mechanism may include upregulation of Sirt3 by calorie restriction and Sirt3 stimulated mitochondrial antioxidant defense system (Figure 5) (62). The mitochondrial NAD+-dependent Sirt3 is upregulated in response to fasting and calorie restriction while high-fat diet downregulates Sirt3 leading to mitochondrial protein hyperacetylation and oxidative stress (63).
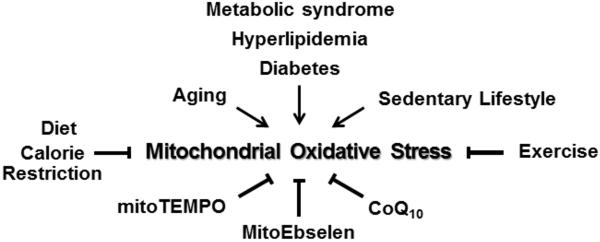
Figure 5.
Multiple risk factors for hypertension such as aging, metabolic conditions and smoking and sedentary lifestyle increase mitochondrial oxidative stress. This can be reduced by diet, calorie restriction, exercise, co-enzyme CoQ10 supplementation or directly with mitochondria-targeted antioxidants such as mitoTEMPO and mitoEbselen.
Calorie restriction activates Sirt1 and Sirt3, therefore, new sirtuin activators has been developed as calorie restriction mimetics which can be beneficial in age-related disorders (64). Indeed, preclinical and clinical studies support the role of sirtuin activator resveratrol in the treatment of cardiovascular diseases (65**). Furthermore, the enzymatic activities of isocitrate dehydrogenase 2, glutathione peroxidase, and SOD2, as well as deacetylation of SOD2 were increased by resveratrol treatment in endothelial cells supporting the effect of resveratrol through Sirt3 signaling pathway (66).
PHYSICAL ACTIVITY AND MITOCHONDRIA
Physical activity affects mitochondrial function through multiple pathways which include shear stress stimulation of NO-mediated mitochondrial biogenesis (60, 67), metabolic regulations (68**), increased antioxidant expression (69), and NAD+ dependent Sirt3 activation (70). Endurance exercise attenuates age-associated reduction in Sirt3 expression and improved mitochondrial function (Figure 5) (28). It is important to note that excessive exercise can induce mitochondrial damage followed by excessive ROS production, NFκB activation, and proinflammatory cytokines formation (71). Moreover, high-intensity exercise in elderly subjects does not reverse age-related mitochondrial damage and dysfunction but can contribute to further alteration of mitochondrial morphology (71). It is clear that regular exercise reduces mitochondrial oxidative stress and improves mitochondrial function, however, high-intensity exercise should be avoided, particularly in elderly subjects.
SMOKING AND MITOCHONDRIAL OXIDATIVE STRESS
Smoking is one of the major risk factors for development of hypertension (72). Smoking increases blood pressure both in normotensive and hypertensive subjects (73, 74); however, smoking cessation success is very limited (7%) (75, 76), and risk for cardiovascular diseases remains elevated long after quitting smoking (77, 78). Smoking increases oxidative stress (79) and causes mitochondrial dysfunction (80, 81). Cigarette smoke increases O2• production (82) and reduces Sirt3 levels (36*) which can further exacerbate mitochondrial oxidative stress. The precise mechanisms of smoking induced mitochondrial oxidative stress remain elusive.
Clinical studies showed that accumulation of lipid oxidation product malondialdehyde in blood plasma of smokers was increased by 2.5-fold while activity of major antioxidant enzymes catalase, superoxide dismutase and glutathione peroxidase were significantly reduced (83). This leads to oxidation of cysteine and glutathione, and the level of reduced glutathione is diminished in kidney by 2-fold in mice exposed to cigarette smoke for four days (84). The resultant alteration in the thiol redox status impairs cellular redox signaling and can contribute to cellular and mitochondrial dysfunction. Indeed, smoking a single cigarette rapidly reduces endothelial nitric oxide production and significantly diminishes blood plasma antioxidants (85). It has been proposed that cigarette smoke induces O2• production in endothelial cells leading to NO inactivation and NO synthase uncoupling (86). Finally, cigarette smoke induces metabolic reprograming in mitochondria which contributes to cancer development (81, 87). These metabolic alterations are associated with NADH accumulation (81) resulting in increased mitochondrial O2• and reduced SOD2 activity (88). Interestingly, hypertensive patients with smoking have significantly reduced responses to common classes of antihypertensive drugs potentially due to metabolic interference between cigarette smoking and drugs (72), therefore, new classes of antihypertensive agents could add to the currently available therapeutic armamentarium to improve treatment of hypertension. It is possible that new mitochondria-targeted therapeutic approaches can be useful in treatment of subjects with history of smoking.
TARGETING MITOCHONDRIAL OXIDATIVE STRESS IN HYPERTENSION
We have developed a mitochondria-targeted SOD2 mimetic, mitoTEMPO, by conjugating the lipophilic triphenylphosphonium cation to an antioxidant moiety SOD mimetic TEMPO (23). Our data indicate that mitoTEMPO accumulates several-hundredfold within mitochondria, likely due to its positive charge and driven by the mitochondrial membrane potential. This markedly enhances scavenging of mitochondrial O2• and provides protection of mitochondrial and cellular function from O2• overproduction (Figure 5). Inhibition of mitochondrial oxidative stress with mitoTEMPO supplementation attenuates endothelial oxidative stress, restores NO• production, improves endothelium-dependent vasodilatation and reduces blood pressure in angiotensin II and DOCA-salt models of hypertension (23).
We have recently tested hypothesis that mitochondrial H2O2 stimulates mitochondrial O2• production and contributes to vicious cycle of oxidative stress which can be interrupted at the mitochondrial site by mitochondria targeted H2O2 scavenger mitoEbselen (9**). Indeed, supplementation of endothelial cells with mitoEbselen abolished angiotensin II-induced mitochondrial O2• production and acute treatment with mitoEbselen after onset of mitochondrial oxidative stress reduced mitochondrial O2• and diminished cytoplasmic O2• production by NADPH oxidases (9**, 50). Furthermore, treatment of hypertensive mice with mitoEbselen after onset of angiotensin II-induced hypertension diminished vascular oxidative stress and significantly reduced blood pressure (9**). Despite the fact that mitochondria-targeted scavengers of O2• and H2O2 have shown significant antihypertensive activity additional translational studies are required in order to move mitochondria-targeted antioxidants from bench to the bedside.
There are two currently available mitochondria directed strategies: supplementation with mitochondrial co-factor ubiquinone (CoQ10) and treatment with cardiolipin-protective compound bendavia. Ubiquinone is reduced in hypertension, and CoQ10 supplementation has an antihypertensive effect (Figure 5) (89). It has been suggested that ubiquinone acts as mitochondrial antioxidant. Our studies, however, do not support significant free radical scavenging by Ubiquinone (90). Ubiquinone primarily functions as electron carrier and it is a critical cofactor in the enzymatic electron transfer in mitochondrial electron transfer chain. Therefore, Ubiquinone can potentially improve oxidative phosphorylation and reduce “electron leakage” in mitochondria. Bendavia (Stealth BioTherapeutics) is a candidate drug that inhibits cytochrome c/cardiolipin peroxidase activity (91). As a result, Bendavia protects the structure of mitochondrial cristae and promotes oxidative phosphorylation (92). In preclinical studies Bendavia reduced renal and cardiac ischemic injury, mitigated microvascular rarefaction and fibrosis, prevented acute kidney injury and improved postinfarction cardiac function (93**-95). Multiple Phase 1 and phase 2 trials studying Bendavia in mitochondrial conditions have found that the drug is well tolerated.
CONCLUSION
In the past decade it has become clear that mitochondrial oxidative stress contributes to hypertension. Several mitochondria-targeted strategies have been developed. Meanwhile, there are still many questions left unanswered: the precise molecular mechanisms leading to mitochondrial oxidative stress in hypertension and end organ dysfunction remain unclear; the response of specific cells and tissue to mitochondria-targeted treatments is not known; the therapeutic potential of targeting mitochondrial regulators of ROS production and antioxidant activity has not been validated. Additional studies are required to address these questions. In the interim, careful attention to preventive strategies and lifestyle modification must be used. The strengths and weaknesses of each approach will help clinicians and researchers to determine which measures are best for each particular situation.
KEY POINTS.
Hypertension is associated with increased production of mitochondrial superoxide and reduced activity of antioxidant defense system which contributes to the pathogenesis of hypertension and end organ dysfunction;
Several risk factors for hypertension such as aging, smoking and metabolic conditions increases mitochondrial oxidative stress and lifestyle modification reduces mitochondrial dysfunction and attenuate hypertension.
Future studies must be directed to determine the precise molecular mechanisms of mitochondrial oxidative stress in hypertension and validate the therapeutic potential different of mitochondria-targeted strategies.
Acknowledgments
We would like to thank Dr. David G. Harrison for his support of our studies.
Financial support and sponsorship
This work is supported by funding from National Institute of Health (R01HL124116), Vanderbilt University (VR7040) and American Heart Association (15IRG22730049).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol. 2012;3:128. doi: 10.3389/fphys.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd JB, Zeng C, Tavel HM, Magid DJ, O'Connor PJ, Margolis KL, et al. Combination therapy as initial treatment for newly diagnosed hypertension. Am Heart J. 2011;162(2):340–6. doi: 10.1016/j.ahj.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redon J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41(5):1096–101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- 4.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44(3):248–52. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, et al. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91(11):1038–45. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 6.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension. 2013;61(2):382–7. doi: 10.1161/HYPERTENSIONAHA.111.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, et al. Oligoclonal CD8+ T Cells Play a Critical Role in the Development of Hypertension. Hypertension. 2014;64(5):1108–15. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10(6):1045–59. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling K, et al. Nox2-induced production of mitochondrial superoxide in angiotensin II - mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal. 2014;20(2):281–94. doi: 10.1089/ars.2012.4918. [This article demonstrates therapeutic potential of targeting of mitochondrial ROS in hypertension and aaplication of mitochondria-targeted antioxidants as a research tool.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51(7):1289–301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal. 2013;19(10):1085–94. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griendling KK, Harrison DG. Out, damned dot: studies of the NADPH oxidase in atherosclerosis. J Clin Invest. 2001;108(10):1423–4. doi: 10.1172/JCI14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955;217(1):409–27. [PubMed] [Google Scholar]
- 14.Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 1984;105:429–35. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- 15.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278(8):5557–63. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 16.Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004;382(Pt 2):511–7. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doughan AK, Harrison DG, Dikalov SI. Molecular Mechanisms of Angiotensin II–Mediated Mitochondrial Dysfunction. Linking Mitochondrial Oxidative Damage and Vascular Endothelial Dysfunction. Circ Res. 2008;102(4):488–96. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 18.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291(5):H2067–74. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 19.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II mediated mitochondrial dysfunction. Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102(4):488–96. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 20.Mantel C, Messina-Graham SV, Broxmeyer HE. Superoxide flashes, reactive oxygen species, and the mitochondrial permeability transition pore: potential implications for hematopoietic stem cell function. Curr Opin Hematol. 2011;18(4):208–13. doi: 10.1097/MOH.0b013e3283475ffe. [DOI] [PubMed] [Google Scholar]
- 21.Cadenas E, Boveris A, Ragan CI, Stoppani AO. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977;180(2):248–57. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- 22.Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353(Pt 2):411–6. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107(1):106–16. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35(9):505–13. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–7. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Hirschey MD, Shimazu T, Huang JY, Verdin E. Acetylation of mitochondrial proteins. Methods Enzymol. 2009;457:137–47. doi: 10.1016/S0076-6879(09)05008-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, Park SH, Ozden O, Kim HS, Jiang H, Vassilopoulos A, et al. Exploring the electrostatic repulsion model in the role of Sirt3 in directing MnSOD acetylation status and enzymatic activity. Free Radic Biol Med. 2012;53(4):828–33. doi: 10.1016/j.freeradbiomed.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57(11):2933–42. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houtkooper RH, Auwerx J. Exploring the therapeutic space around NAD+. J Cell Biol. 2012;199(2):205–9. doi: 10.1083/jcb.201207019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhry KN, Chavez P, Gasowski J, Grodzicki T, Messerli FH. Hypertension in the elderly: some practical considerations. Cleve Clin J Med. 2012;79(10):694–704. doi: 10.3949/ccjm.79a.12017. [DOI] [PubMed] [Google Scholar]
- 31.Capettini LS, Montecucco F, Mach F, Stergiopulos N, Santos RA, da Silva RF. Role of renin-angiotensin system in inflammation, immunity and aging. Curr Pharm Des. 2012;18(7):963–70. doi: 10.2174/138161212799436593. [DOI] [PubMed] [Google Scholar]
- 32.Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3(2):319–27. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85(2):258–63. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Giblin W, Skinner ME, Lombard DB. Sirtuins: guardians of mammalian healthspan. Trends Genet. 2014;30(7):271–86. doi: 10.1016/j.tig.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dransfeld CL, Alborzinia H, Wolfl S, Mahlknecht U. SIRT3 SNPs validation in 640 individuals, functional analyses and new insights into SIRT3 stability. Int J Oncol. 2010;36(4):955–60. doi: 10.3892/ijo_00000574. [DOI] [PubMed] [Google Scholar]
- 36*.Li Y, Yu C, Shen G, Li G, Shen J, Xu Y, et al. Sirt3-MnSOD axis represses nicotine-induced mitochondrial oxidative stress and mtDNA damage in osteoblasts. Acta Biochim Biophys Sin (Shanghai) 2015;47(4):306–12. doi: 10.1093/abbs/gmv013. [This article highlights the potential utility of Sirt3-SOD2 pathway in metigation of smoking induced dysfunction.] [DOI] [PubMed] [Google Scholar]
- 37.Schirmer H, Pereira TC, Rico EP, Rosemberg DB, Bonan CD, Bogo MR, et al. Modulatory effect of resveratrol on SIRT1, SIRT3, SIRT4, PGC1alpha and NAMPT gene expression profiles in wild-type adult zebrafish liver. Mol Biol Rep. 2012;39(3):3281–9. doi: 10.1007/s11033-011-1096-4. [DOI] [PubMed] [Google Scholar]
- 38.Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci. 2013;5:48. doi: 10.3389/fnagi.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Javkhedkar AA, Quiroz Y, Rodriguez-Iturbe B, Vaziri ND, Lokhandwala MF, Banday AA. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2015;308(10):R840–6. doi: 10.1152/ajpregu.00308.2014. [This article shows potential utility of Sertuin activator resveratrol to reduce oxidative stress and hypertension.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Ma W, Zhang P, He S, Huang D. Effect of resveratrol on blood pressure: a meta-analysis of randomized controlled trials. Clin Nutr. 2015;34(1):27–34. doi: 10.1016/j.clnu.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM, et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors--Results from a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2015;189:47–55. doi: 10.1016/j.ijcard.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–8. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- 43.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–91. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–5. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell. 2004;3(1):13–6. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol. 2007;102(1):255–60. doi: 10.1152/japplphysiol.00513.2006. [DOI] [PubMed] [Google Scholar]
- 47.Hepple RT. Mitochondrial involvement and impact in aging skeletal muscle. Front Aging Neurosci. 2014;6:211. doi: 10.3389/fnagi.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conley KE, Marcinek DJ, Villarin J. Mitochondrial dysfunction and age. Curr Opin Clin Nutr Metab Care. 2007;10(6):688–92. doi: 10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]
- 49.de Cavanagh EM, Piotrkowski B, Fraga CG. Concerted action of the renin-angiotensin system, mitochondria, and antioxidant defenses in aging. Mol Aspects Med. 2004;25(1-2):27–36. doi: 10.1016/j.mam.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Nazarewicz RR, Dikalova AE, Bikineyeva A, Dikalov SI. Nox2 as a potential target of mitochondrial superoxide and its role in endothelial oxidative stress. Am J Physiol Heart Circ Physiol. 2013;305(8):H1131–40. doi: 10.1152/ajpheart.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112(17):2668–76. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 52.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2010;299(3):H673–9. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45(3):438–44. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 54.Costa AD, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT. Am J Physiol Heart Circ Physiol. 2008;295(2):H874–82. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21(4):489–95. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- 56.Wosniak J, Jr., Santos CX, Kowaltowski AJ, Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11(6):1265–78. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- 57.Dikalov SI, Li W, Doughan AK, Blanco RR, Zafari AM. Mitochondrial reactive oxygen species and calcium uptake regulate activation of phagocytic NADPH oxidase. Am J Physiol Regul Integr Comp Physiol. 2012;302(10):R1134–42. doi: 10.1152/ajpregu.00842.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, et al. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56(3):412–21. doi: 10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- 59.Cerqueira FM, Cunha FM, Laurindo FR, Kowaltowski AJ. Calorie restriction increases cerebral mitochondrial respiratory capacity in a NO*-mediated mechanism: impact on neuronal survival. Free Radic Biol Med. 2012;52(7):1236–41. doi: 10.1016/j.freeradbiomed.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310(5746):314–7. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 61.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101(17):6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han C, Someya S. Maintaining good hearing: calorie restriction, Sirt3, and glutathione. Exp Gerontol. 2013;48(10):1091–5. doi: 10.1016/j.exger.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green MF, Hirschey MD. SIRT3 weighs heavily in the metabolic balance: a new role for SIRT3 in metabolic syndrome. J Gerontol A Biol Sci Med Sci. 2013;68(2):105–7. doi: 10.1093/gerona/gls132. [DOI] [PubMed] [Google Scholar]
- 64.Alcain FJ, Villalba JM. Sirtuin activators. Expert Opin Ther Pat. 2009;19(4):403–14. doi: 10.1517/13543770902762893. [DOI] [PubMed] [Google Scholar]
- 65**.Zordoky BN, Robertson IM, Dyck JR. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta. 2015;1852(6):1155–77. doi: 10.1016/j.bbadis.2014.10.016. [This paper review challenges in translating pre-clinical resveratrol findings to improved patient outcomes.] [DOI] [PubMed] [Google Scholar]
- 66.Zhou X, Chen M, Zeng X, Yang J, Deng H, Yi L, et al. Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis. 2014;5:e1576. doi: 10.1038/cddis.2014.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis ME, Cai H, Drummond GR, Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res. 2001;89(11):1073–80. doi: 10.1161/hh2301.100806. [DOI] [PubMed] [Google Scholar]
- 68**.Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv290. [This paper discusses therapuetic potential of Sirtuin activation strategies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morrison D, Hughes J, Della Gatta PA, Mason S, Lamon S, Russell AP, et al. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med. 2015 doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 70.White AT, Schenk S. NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab. 2012;303(3):E308–21. doi: 10.1152/ajpendo.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee S, Kim M, Lim W, Kim T, Kang C. Strenuous exercise induces mitochondrial damage in skeletal muscle of old mice. Biochem Biophys Res Commun. 2015;461(2):354–60. doi: 10.1016/j.bbrc.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 72.Leone A. Does Smoking Act as a Friend or Enemy of Blood Pressure? Let Release Pandora's Box. Cardiol Res Pract. 2011;2011:264894. doi: 10.4061/2011/264894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Groppelli A, Giorgi DM, Omboni S, Parati G, Mancia G. Persistent blood pressure increase induced by heavy smoking. J Hypertens. 1992;10(5):495–9. doi: 10.1097/00004872-199205000-00014. [DOI] [PubMed] [Google Scholar]
- 74.Minami J, Ishimitsu T, Matsuoka H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension. 1999;33(1 Pt 2):586–90. doi: 10.1161/01.hyp.33.1.586. [DOI] [PubMed] [Google Scholar]
- 75.Quitting Smoking Among Adults - United States -. Weekly. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- 76.Deppen SA, Grogan EL, Aldrich MC, Massion PP. Lung cancer screening and smoking cessation: a teachable moment? J Natl Cancer Inst. 2014;106(6):dju122. doi: 10.1093/jnci/dju122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, et al. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. Archives of internal medicine. 1994;154(2):169–75. [PubMed] [Google Scholar]
- 78.World Health Organization. International Agency for Research on Cancer. Reversal of risk after quitting smoking. Risk of cardiovascular diseases after smoking cessation. IARC Handbook of Cancer Prevention. 2007:227–68. [Google Scholar]
- 79.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332(18):1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 80.Agarwal AR, Zhao L, Sancheti H, Sundar IK, Rahman I, Cadenas E. Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox status independent of inflammatory responses in mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2012;303(10):L889–98. doi: 10.1152/ajplung.00219.2012. [DOI] [PubMed] [Google Scholar]
- 81.Rahman SMJ, Ji X, Zimmerman JL, Li M, Harris KB, Hoeksema DMD, et al. Metabolic reprogramming in the airway epithelium of high risk individuals. J Clin Invest. 2015 In Press. [Google Scholar]
- 82.Peluffo G, Calcerrada P, Piacenza L, Pizzano N, Radi R. Superoxide-mediated inactivation of nitric oxide and peroxynitrite formation by tobacco smoke in vascular endothelium: studies in cultured cells and smokers. Am J Physiol Heart Circ Physiol. 2009;296(6):H1781–92. doi: 10.1152/ajpheart.00930.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Metta S, Basalingappa DR, Uppala S, Mitta G. Erythrocyte Antioxidant Defenses Against Cigarette Smoking in Ischemic Heart Disease. J Clin Diagn Res. 2015;9(6):BC08–11. doi: 10.7860/JCDR/2015/12237.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raza H, John A, Nemmar A. Short-term effects of nose-only cigarette smoke exposure on glutathione redox homeostasis, cytochrome P450 1A1/2 and respiratory enzyme activities in mice tissues. Cell Physiol Biochem. 2013;31(4-5):683–92. doi: 10.1159/000350087. [DOI] [PubMed] [Google Scholar]
- 85.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation. 2002;105(10):1155–7. doi: 10.1161/hc1002.105935. [DOI] [PubMed] [Google Scholar]
- 86.Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol. 2007;5(4):276–92. doi: 10.2174/157016107782023406. [DOI] [PubMed] [Google Scholar]
- 87.Nagathihalli NS, Massion PP, Gonzalez AL, Lu P, Datta PK. Smoking induces epithelial-to-mesenchymal transition in non-small cell lung cancer through HDAC-mediated downregulation of E-cadherin. Mol Cancer Ther. 2012;11(11):2362–72. doi: 10.1158/1535-7163.MCT-12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Margaret AL, Syahruddin E, Wanandi SI. Low activity of manganese superoxide dismutase (MnSOD) in blood of lung cancer patients with smoking history: relationship to oxidative stress. Asian Pac J Cancer Prev. 2011;12(11):3049–53. [PubMed] [Google Scholar]
- 89.Ho MJ, Bellusci A, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database Syst Rev. 2009;(4):CD007435. doi: 10.1002/14651858.CD007435.pub2. [DOI] [PubMed] [Google Scholar]
- 90.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal. 2007;9(11):1825–36. doi: 10.1089/ars.2007.1693. [DOI] [PubMed] [Google Scholar]
- 91.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol. 2014;171(8):2017–28. doi: 10.1111/bph.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029–50. doi: 10.1111/bph.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93**.Liu S, Soong Y, Seshan SV, Szeto HH. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am J Physiol Renal Physiol. 2014;306(9):F970–80. doi: 10.1152/ajprenal.00697.2013. [This article demonstrate therapuetic potential of targeting cardiolipin oxidation to mitigate renal dysfunction.] [DOI] [PubMed] [Google Scholar]
- 94.Tabara LC, Poveda J, Martin-Cleary C, Selgas R, Ortiz A, Sanchez-Nino MD. Mitochondria-targeted therapies for acute kidney injury. Expert Rev Mol Med. 2014;16:e13. doi: 10.1017/erm.2014.14. [DOI] [PubMed] [Google Scholar]
- 95.Dai W, Shi J, Gupta RC, Sabbah HN, Hale SL, Kloner RA. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J Cardiovasc Pharmacol. 2014;64(6):543–53. doi: 10.1097/FJC.0000000000000155. [DOI] [PubMed] [Google Scholar]