Abstract
Background
Citron kinase (CitK), a protein essential to neurogenic cell division in the central nervous system, is polarized in mitotic neural progenitors. The mechanisms that polarize CitK to cellular domains that line the ventricular surface of neuroepithelium are currently not known.
Results
A CitK-Dlg5 interaction was first revealed in a protein array screen of proteins containing PDZ domains, and the interaction was subsequently confirmed by co-immunoprecipitation. In neural precursors CitK polarizes to the VZ surface in metaphase and then concentrates in the cytokinesis furrow and midbody during cytokinesis. In Dlg5−/− mice, however, CitK fails to polarize in metaphase. In addition, the the ratio of ventricular to abventricular mitoses are significantly decreased in Dlg5−/− mutants.
Conclusions
Dlg5 interacts with CitK and the polarization of CitK in mitotic neural progenitors requires Dlg5. Dlg5 is also required for maintaining the normal distribution of apical and basal mitoses within neocortical neuroepithelium. These observations indicate the first biochemical and functional link between a MAGUK protein previously linked to cell polarity and a protein essential to neurogenic cell division.
Background
Neurogenic cell divisions in the developing neocortex are located either along the surface of the ventricular zone, ventricular divisions, or away from the ventricular surface, abventricular divisions. During the period of neuronal production ventricular cell divisions are primarily stem-like divisions that give rise to a fated neuronal precursor that migrates away from the ventricular surface and a radial progenitor that remains attached to the ventricular surface [1,2]. The abventricular cell divisions, in contrast, are thought to primarily give rise to two postmitotic neuronal precursors [1–3]. The final number of neurons generated in the neocortex is largely determined by changes in the numbers of these two types of cell divisions over time.
Mutations in citron kinase (CitK) in rodents revealed that citron kinase is essential to neurogenic cell divisions in the neocortex [4,5]. Animals with mutations in CitK have approximately 50% the number of neurons and this phenotype is not apparent prior to the period of neuronal production. Citron kinase protein is highly polarized in dividing cells at the ventricular surface and the protein is localized to end feet, cytokinesis furrows and cytokinetic midbodies that line the ventricular surface of the lateral ventricles [4]. The mechanisms that polarize CitK to the ventricular surface are not known, however the presence of a consensus QSSV PDZ binding domain at the C-terminus of CitK raised the possibility that it may interact with one of the polarity genes that contain PDZ domains.
Mammalian Discs large 5 (Dlg5) is a membrane-associated guanylate kinase (MAGUK) protein containing four PDZ domains [6]. MAGUK proteins are known to regulate cellular adhesion and plasticity at the developing junctional complex, and Dlg1 is part of the polarity protein complex at the baso-lateral membrane of mammalian cells [7]. A recent study has shown that Dlg5 binds to β-catenin at sites of cell-cell contact and may act as a scaffolding protein [8]. Importantly, Dlg5 is highly expressed in developing brain, and is required for the maintenance of adherens junctions and the maintenance of cell polarity in the developing mammalian brain and kidney [9].
In this study, we report that Dlg5 interacts with CitK and that in Dlg5−/− mutants CitK fails to polarize in mitotic progenitors at the ventricular zone (VZ) surface. In addition, Dlg5−/− neuroepithelium shows a reduction in the number of ventricular cell divisions, and an increase in abventricular cell divisions relative to wildtype. Taken together, we hypothesize that Dlg5 interacts with CitK during neurogenesis and that this interaction is necessary to maintain the proper relative number of ventricular and abventricular cell divisions.
Results
Citron kinase interacts with Discs large 5
We used the Panomics™ PDZ domain array to screen for potential interactors of CitK that contain a PDZ-binding domain. The HIS-tagged C-terminal region of CitK (324 a.a.) containing a PDZ-binding domain was affinity purified and used as a probe on the array. The PDZ domain array IV includes 23 spotted proteins, 33 GST-tagged PDZ domains, and a GST only spot that serves as a negative/background control. Two spots on the array yielded signals above the background control, and these two corresponded to two PDZ domains (PDZ 3 and PDZ 4) of Dlg5 (NP_004738).
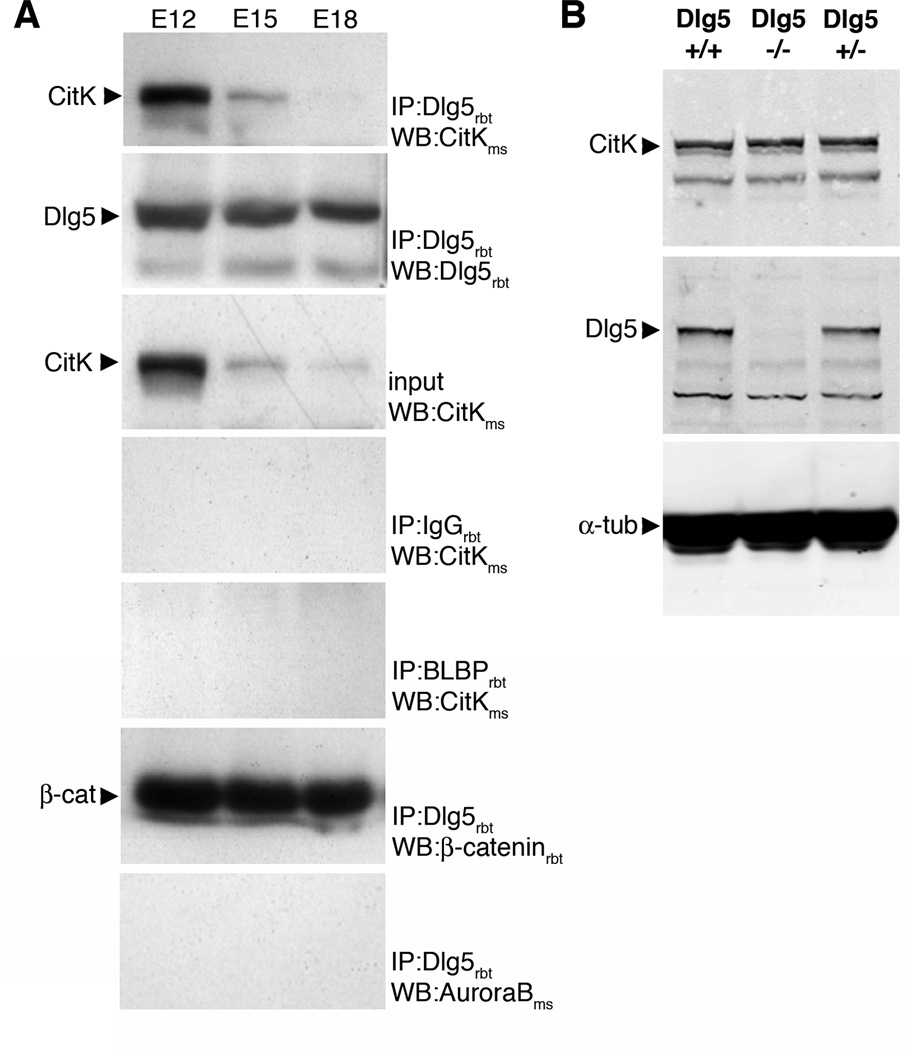
To test for interaction between endogenous Dlg5 and CitK in developing forebrain, we performed co-immunoprecipitation experiments of Dlg5 and CitK from E12, E15, and E18 embryonic forebrain (Fig. 1A). In lysates from E12 brain, an anti-Dlg5 antibody [9] immunoprecipitated Dlg5 (second panel in Fig. 1A) and co-immunoprecipitated CitK (first panel in Fig. 1A). The amount of CitK that co-immunoprecipitated with the anti-Dlg5 antibody in E15 and E18 brain homogenates was decreased relative to that in E12. This decrease in CitK co-immunoprecipitate was correlated with a general decrease in the amount of CitK in the input lysate, and so it is not possible to interpret the decrease as a decrease in interaction between Dlg5 and CitK over time but rather as a decrease in CitK expression (third panel in Fig. 1A). As an indication of specificity we found that CitK did not co-immunoprecipitate with either a rabbit IgG (fourth panel in Fig. 1A) or a rabbit anti-BLBP antibody (fifth panel in Fig. 1A). In addition, as a positive control for Dlg5 co-immunoprecipitation we found that the anti-Dlg5 antibody co-immunoprecipitated β-catenin in developing brain (sixth panel in Fig. 1A) as previously shown in LLC-PK1 cells and brain [8, 9]. To further confirm specificity in these experiments we found that another protein that has been associated with cytokinesis in neural progenitors and localizes to cytokinesis furrows and midbodies, AuroraB, did not co-immunoprecipitate with Dlg5 (last panel in Fig. 1A). These results demonstrate that endogenous Dlg5 interacts with CitK in developing forebrain.
Figure 1. CitK interacts with Dlg5 and its expression level is unaffected in Dlg5−/− mutants.
A, Interaction between endogenous CitK and Dlg5 in rat developing brain. A rabbit polyclonal anti-Dlg5 antibody was used for immunoprecipitation and a mouse anti-CitK antibody (CRIK) was used for western blotting to detect the interactions. In rat embryonic brain lysates (E12, E15, E18), an anti-Dlg5 antibody immunoprecipitated Dlg5 (second panel) and CitK co-immunoprecipitated with Dlg5 (first panel). Note that co-immunoprecipitated CitK was gradually decreased in E15 and E18 brain due to decreased amount of endogenous CitK (third panel). CitK did not co-immunoprecipitate with a rabbit IgG (fourth panel) and a rabbit polyclonal BLBP antibody (fifth panel). β-catenin co-immunoprecipitated with Dlg5 (sixth panel), while AuroraB did not (seventh panel). B, CitK was expressed in E15.5 Dlg5−/− mice brain as well as in Dlg5+/+ and Dlg5+/− brains (first panel). An anti-Dlg5 antibody confirmed that Dlg5 is absent in E15.5 Dlg5−/− mice brain (second panel). α-tubulin was used as internal control (third panel).
Dlg5 is required for polarization of CitK to the VZ surface
Dlg5 knockout mice (Dlg5−/−) were previously generated and characterized [9]. We used these mutants here to determine whether CitK expression is altered by Dlg5 loss of function. Immunoblots of E15.5 wildtype littermate (Dlg5+/+), Dlg5 mutant (Dlg5−/−), and heterozygous (Dlg5+/−) brain lysates probed with an anti-Dlg5 antibody [9] confirmed that there was no Dlg5 protein expressed in Dlg5−/− forebrain (second panel in Fig. 1B). In contrast, the levels of CitK protein were not altered in Dlg5−/− forebrain at E15.5 relative to heterozygous or wildtype littermates (first panel in Fig. 1B).
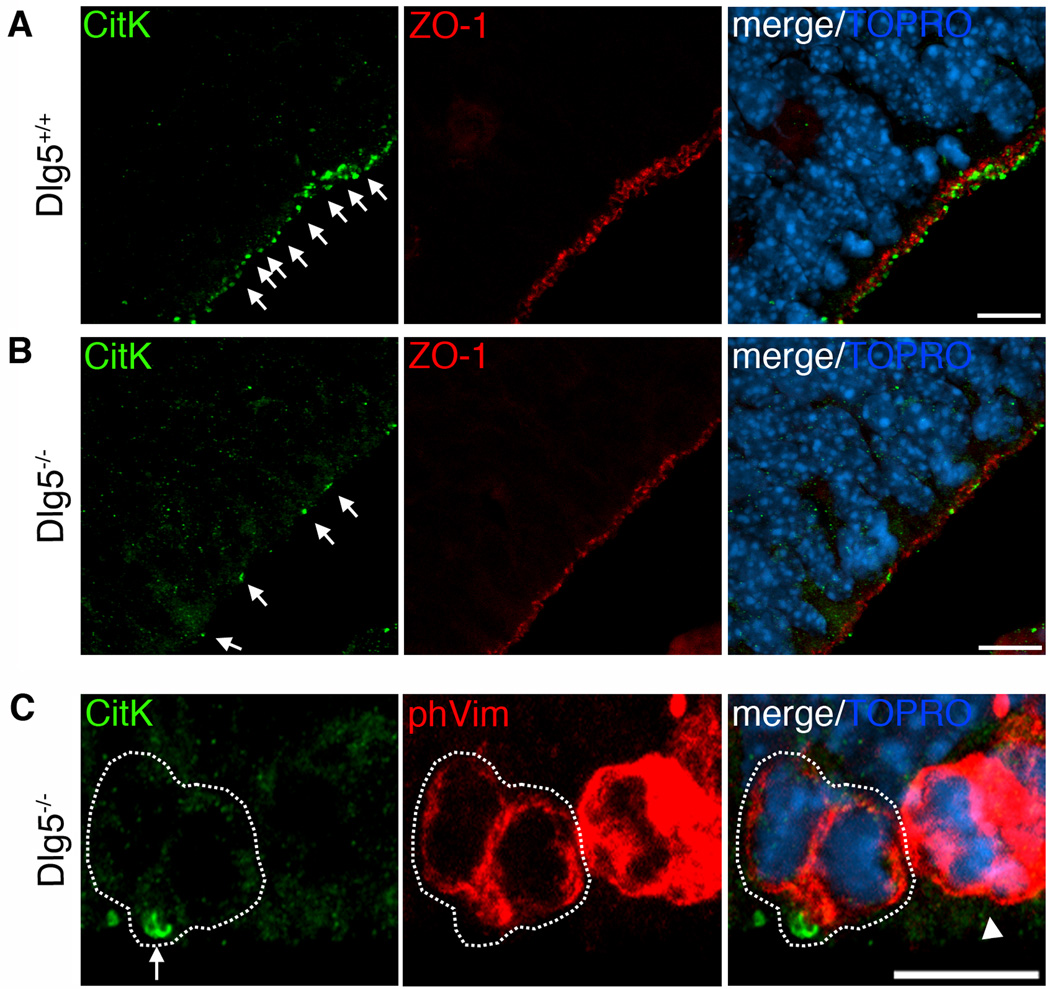
Next, we analyzed E15.5 Dlg5−/− brains by immunohistochemistry and confocal analysis for alterations in CitK distribution in neocortical neuroepithelium. CitK immunostaining showed more diffuse localization at the VZ surface in the Dlg5−/− neocortex (green, Fig. 2B) compared to wildtype neocortex (green, Fig. 2A). Moreover, the number of CitK puncta (arrows) that typically decorate the VZ surface was greatly reduced in Dlg5−/− neocortex (green, Fig. 2B). Although we found that the VZ surface localization of CitK was nearly completely disrupted in the Dlg5 mutant neocortex, we observed that another marker normally polarized to the VZ surface, ZO-1, remained highly polarized along the VZ surface in Dlg5−/− brains. Thus, while CitK polarization is disrupted in the mutant, not all aspects of VZ polarization are lost in Dlg5−/− neocortex.
Figure 2. Dlg5 deficient neocortex displays less CitK polarized to the VZ surface.
A, The E15 wildtype littermate (Dlg5+/+) neocortex shows highly polarized CitK (green, arrows) at the VZ. B, CitK (green, arrows) is diffuse throughout the cell and is less aggregated at the VZ surface of the E15 Dlg5−/− mice neuroepithelium. ZO-1 (red) labels the ventricular zone surface. C, In the Dlg5−/− mice neuroepithelium, CitK (green, arrow) localizes at the midbody in cytokinesis of the VZ surface. Scale bars, 10 µm.
Dlg5 is required for CitK polarization during metaphase
In previous studies, polarized CitK puncta were found to be within cytokinetic midbodies, cytokinesis furrows, and endfeet of interphase progenitors at the ventricular surface [4,10]. In Dlg5−/− mice, although far fewer in number than in wiltype littermates, some CitK immunopositve puncta were distributed along the VZ surface. These puncta tended to be larger in size than the majority of puncta in wildtype brain, and we hypothesized, based on their size and "ringlike" appearance, that these may represent CitK localization at cytokinetic midbodies. In order to assess this idea, we double-labeled with phospho-vimentin55 and CitK antibodies. We found that phospho-vimentin (+) cell pairs that correspond to cells in late telophase reliably had a CitK ring in the position of the midbody (arrow in Fig. 2C).
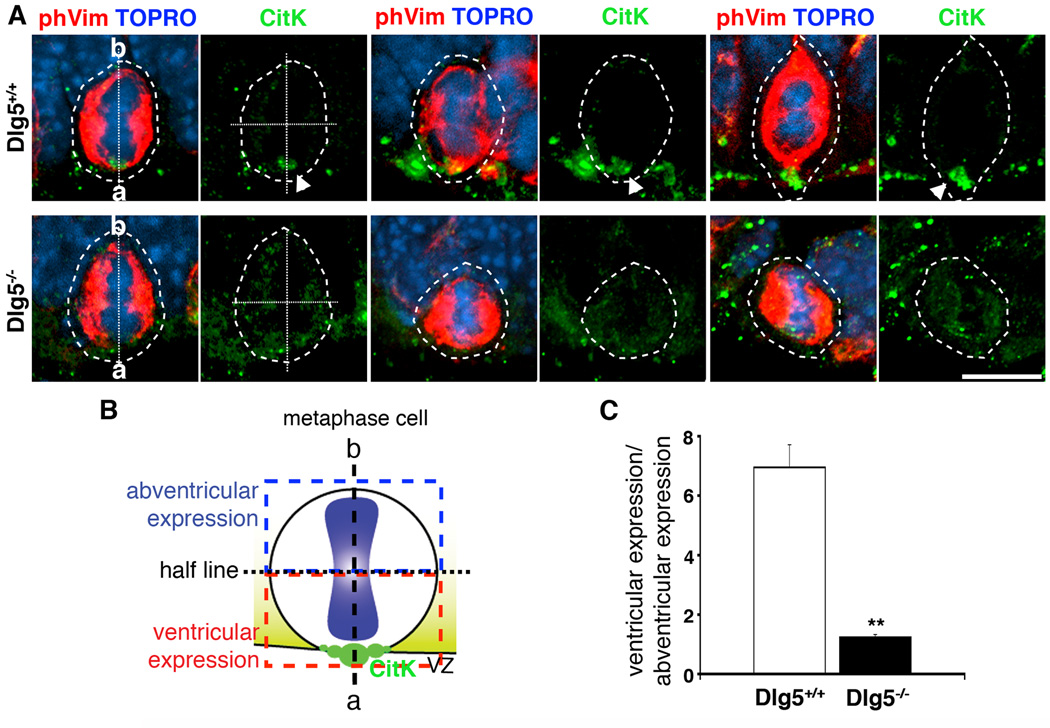
Considering the maintained localization of CitK to cytokinetic midbodies, and yet overall decrease in polarized CitK, we next assessed whether CitK polarization was maintained just prior to cytokinesis. Metaphase cells were identified by a combination of phospho-vimentin55 (phVim, red) immunostaining which labels M-phase cells and TO-PRO®-3 iodide staining that brightly labels the condensed DNA of the metaphase plate. Triple-staining of CitK/phVim/TO-PRO revealed that CitK (green) was polarized to the VZ surface in metaphase cells of wildtype, however, there was a complete lack of VZ polarized CitK in metaphase cells in Dlg5−/− mutants (arrowheads) (Fig. 3A). We quantified the polarity of CitK immunopositivity as described in the methods and Fig. 3B. The ratio of CitK expression at the VZ surface in the Dlg5−/− metaphase cells was 7-fold decreased compared to that in wildtype cells (n=3 brains, 15 cells from each condition, Fig. 3C). Together, these results indicate that while Dlg5 function is not critical to the aggregation of CitK to the cytokinetic midbody, it is essential to CitK polarization at the VZ surface during all other phases of the cell cycle.
Figure 3. Failure of CitK polarization in Dlg5−/− metaphase cells.
A, CitK (green) highly polarizes to the ventricular pole during metaphase (arrowheads in top panels) in wildtype littermates (Dlg5+/+), while CitK localization is diffuse in Dlg5−/− mitoses. A mouse monoclonal phosphovimentin55 antibody (phVim, red) labels M-phase cells, and TO-PRO®-3 iodide staining confirms metaphase cells. Scale bar, 10 µm. The metaphase cells are outlined, and ‘a’ indicates ventricular pole and ‘b’ indicates the abventricular pole of mitotic cells. The dotted lines represent the ventricular-abventricular axis and perpendicular half lines, as depicted in B. The graph in C shows the ratio of CitK immunopositivity in the ventricular half relative to the abventricular half of each cell (B). Dlg5−/− metaphase cells display nearly equal amount of CitK expression in ventricular and abventricular halves of the cells, while Dlg5+/+ metaphase cells maintain higher CitK expression in ventricular side (n=3 brains for either wildtype or Dlg5−/−, 5 cells from each brain and total 15 cells were quantified for each condition).
Absence of Dlg5 causes fewer M-phase cells at the VZ surface
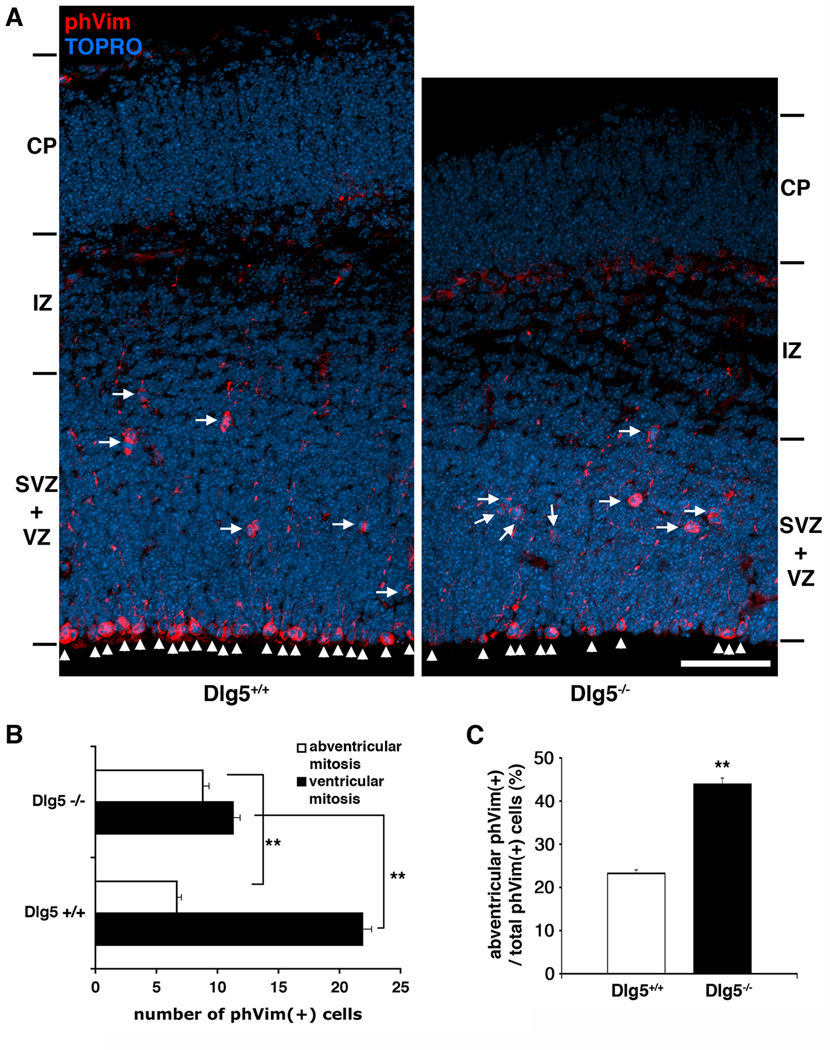
In the developing neocortex there are both ventricular and abventricular mitoses that differentially produce neurons and progenitors or neurons, respectively. We assessed the number of ventricular and abventricular M-phase cells by quantifying the number of phospho-vimentin (+) cells in both wildtype and Dlg5−/− neocortex. The neuroepithelia of Dlg5−/− mice displayed a decreased number of ventricular mitoses (arrowheads), while the number of abventricular mitoses (arrows) was increased, as compared to wildtype neocortices (n=3 brains, 5 sections from each brain, Fig. 4A–B). The proportion of abventricular divisions to ventricular divisions was significantly increased in Dlg5−/− brain (n=3 brains, 5 sections from each brain, Fig. 4C). These results indicate that the absence of Dlg5 decreases the number of M-phase cells at the VZ surface.
Figure 4. Absence of Dlg5 decreases ventricular surface cell divisions.
A, Comparison of the E15.5 neocortex of Dlg5+/+ and Dlg5−/− mice. Fewer M-phase cells (arrowheads) are found at the ventricular surface in E15 Dlg5−/− neuroepithelium (right) compared to wildtype littermates (Dlg5+/+, left). In addition, more non-surface M-phase cells (arrows) are present in the abventricular zone in Dlg5−/− neuroepithelium than in Dlg5+/+. Scale bar, 50 µm. The quantification in B shows comparison between the number of M-phase cells at the ventricular surface and abventricular region in Dlg5−/− and Dlg5+/+ neocortex. The phVim (+) cells were counted in 200 µm wide radial traverse through the dorsal cortex (n=3 brains, 5 sections from each brain). The graph in C shows that the percentage of non-surface M-phase cells significantly increases in Dlg5−/− neocortex (n=3 brains, 5 sections from each brain). **, p<0.01; *, p<0.1, determined by Student’s t-test. Error bars represent ± s.e.m.
Discussion
In this study, we show that Dlg5 interacts with CitK. Analysis of Dlg5 knockout mice further revealed that polarization of CitK at the VZ surface is dependent upon Dlg5. Furthermore, the number of surface M-phase cells was decreased and non-surface mitoses were increased in Dlg5−/− mutants. Previous studies have shown that MAGUKs are involved in the maintenance of epithelial polarity in Drosophila [11] and in mammalian cells [12]. The present data show that Dlg5 contributes to CitK polarity during the cell cycle of endogenous neural progenitors in mammalian neocortex (Fig. 3), while CitK localization to the midbody is independent of Dlg5 (Fig. 2C). We suggest that CitK polarization to the ventricular surface may depend upon general epithelial polarization mechanisms, while concentration of CitK later in the cell cycle, to the midbody, is somewhat independent of epithelial polarity.
Nechiporuk et al. [9] showed that loss of Dlg5 caused severe disruption in the polarization of three junctional proteins, ZO-1, β-catenin, and N-cadherin, in cells lining the third ventricle of developing brain. The loss of cellular polarization observed in this previous study resulted in a fusion of the central aqueduct which caused subsequent hydrocephalus. We found in this study that loss of Dlg5 in the ventricular zone surrounding the lateral ventricles of developing neocortex did not disrupt polarization of ZO-1 (Fig. 2B), but caused a more subtle loss in polarization of CitK. This apparent difference between third and lateral ventricular cells may indicate that Dlg5 has different levels of function in regulating polarity in different neural progenitor types.
We found a significant change in the ratio of ventricular to abventricular cell divisions in the developing neocortex in Dlg5−/− mice. This suggests that as a result of non-polarized CitK there may be a decrease in the number of ventricular stem-like divisions and a corresponding increase in abventricular, neuron-neuron cell divisions. Such a change could result in a premature depletion of progenitors and a corresponding decrease in the total number of neurons produced. Alternatively, the change in ventricular and abventricular cell divisions in the Dlg5−/− mutant could be a result of a parallel non-CitK pathway. Nevertheless, the present results reveal that polarity of a protein critical to neurogenic cell division, CitK, is dependent upon the presence of Dlg5. These results are also the first to our knowledge to show a direct interaction between a polarity control protein, Dlg5, and a cytokinesis control protein, CitK. Such a connection would be predicted to exist in neuroepithelia, and other epithelia, in which mitoses are frequently localized to the surface of epithelia. Dlg5 and CitK interaction may indicate presence of a larger signaling complex that regulates the positioning of mitoses at luminal surfaces of epithelia during development.
Conclusions
Dlg5 and CitK interact in developing rodent neuroepithelia and Dlg5 plays an essential role in polarizing CitK and in positioning neurogenic mitoses within the ventricular zone. Genetic deletion of Dlg5 results in disruption of CitK polarization in metaphase, but does not disrupt localization and concentration of CitK to midbodies suggesting different mechanisms for CitK localization. In addition, Dlg5 functions to maintain a higher ratio of ventricular to abventricular mitoses during neocortical neurogenesis. These observations provide the first evidence of a biochemical and functional connection between a neurogenic cytokinesis protein, CitK, and a MAGUK cell polarity protein, Dlg5.
Materials and methods
Protein domain array system
The PDZ domain array IV from Panomics™ was used to screen proteins interacting with the C-terminus of CitK which includes a terminal QSSV consensus PDZ binding domain. The array membranes were blocked by incubation in 5% nonfat dry milk in TBST for 2 hrs. Sequence coding for the C-terminal 324 residues of CitK (amino acids 1732–2056) were cloned into the vector pET32a (Novagen, Germany). The resulting HIS tagged protein was expressed in Escherichia coli DH5α and affinity purified on Ni-NTA His•Bind Resin. Purified HIS-tagged CitK (pET32a-HIS-CitK-PDZ/SH3) was diluted in TBST to a concentration of 10 µg/ml, and incubated with the array membrane overnight at 4°C. The membrane was then washed three times in TBST and subsequently incubated with a rabbit polyclonal anti-HIS antibody for 3 hrs. After washing three times with TBST, the membrane was incubated with a goat anti-rabbit antibody, and washed three times with TBST and three times with TBS. Bound antibodies were detected with ECL™ (GE Healthcare, Piscataway, NJ).
Co-immunoprecipitation of Dlg5 and CitK
Lysates from rat forebrain at embryonic day 12 (E12), embryonic day 15 (E15), and embryonic day 18 (E18) were subjected to IP with a rabbit polyclonal anti-Dlg5 antibody [9] followed by immunoblotting with a mouse monoclonal anti-CitK antibody (1:1000, BD Pharmingen), a rabbit polyclonal β-catenin antibody (1:10000, Sigma), and a mouse anti-AuroraB antibody (1:250, BD Pharmingen) for detecting interactions and a rabbit polyclonal anti-Dlg5 and a mouse monoclonal anti-CitK antibody for detecting inputs. Rabbit IgG (Sigma) and a rabbit polyclonal BLBP antibody (Abcam) were used for control immunoprecipitation.
Animals
Dlg5 knockout (Dlg5−/−) mice were generated as previously described [9]. Heterozygous (Dlg5+/−) pairs of the Dlg5 knockout mice were time-mated and E15.5 mouse brains were harvested and prepared for cryosection. The genotyping was done as described in Nechiporuk et al. [9].
Immunohistochemistry
Embryonic day 15.5 mice brains were harvested in cold PBS and fixed with 0.5% paraformaldehyde in PBS, cryoprotected, and sectioned on a cryostat at 10–20 µm in the coronal plane. Sections were permeablized/blocked in 1X PBS with 0.5% TritonX-100 and 5% normal goat serum for 1 hr at room temperature, and immunostained with primary antibody solution (0.2% TritonX-100, 2.5% NGS in 1X PBS) overnight at 4°C. The following primary antibodies were used: a rabbit polyclonal anti-Dlg5 antibody [9] (1:100), a mouse monoclonal anti-CitK antibody (1:200, BD Pharmingen), a rabbit polyclonal anti-citron antibody (CT295 [13], 1:200), a mouse monoclonal anti-AuroraB antibody (AIM-1, 1:200, BD Pharmingen), a rat monoclonal anti-ZO-1 antibody (1:300, Development studies Hybridoma bank at the University of Iowa), a rabbit polyclonal anti-phosphorylated histone H3 (1:500, Millipore), and a mouse monoclonal anti-phosphovimentin 55 (1:500, MBL). After three washes (10 min each) with the rinse solution (0.2% TritonX-100, 2.5% NGS in 1X PBS), the sections were incubated with secondary antibodies (0.2% TritonX-100, 2.5% NGS in 1X PBS) for 2 hrs at room temperature. The following secondary antibodies were used: a goat anti-rabbit IgG conjugated Alexa 488 (1:200, Invitrogen), a goat anti-mouse IgG conjugated Alexa 568 (1:200, Invitrogen), a goat anti-rat IgG conjugated Alexa 568 (1:200, Invitrogen), a goat anti-mouse IgG conjugated Alexa 488 (1:200, Invitrogen), and a goat anti-rabbit IgG conjugated Alexa 568 (1:200, Invitrogen). Then the sections were washed with the rinse solution for total 5 times, and DAPI (1:3000, Sigma) and TO-PRO®-3 iodide (1:3000, Molecular probes) were added in the third wash for counter staining. The sections were mounted with Prolong antifade (Invitrogen).
Imaging and statistical analysis
All double- or triple-labeling was viewed with a laser-scanning confocal microscope (Leica TCS SP2, laser lines at 488 nm, 543 nm, 633 nm) using 20X PL APO N.A. 0.7, 40X PL APO N.A. 1.25 and 100X PL APO N.A. 1.40 oil immersion objectives. We used Adobe Photoshop CS3 to assemble all images. The CitK immunopositivity (Fig. 3) was quantified in ImageJ. First, the metaphase cells were outlined, ventricular and abventricular poles were assigned based on metaphase plate indicated by TO-PRO®-3 iodide staining. The half line was operationally defined as the middle of the ventricular-abventricular axis. Then, CitK staining was measured in ventricular or abventricular side with the histogram tool and the ratio was calculated as ventricular expression/abventricular expression. We used two-sample Student’s t-test to compare means of two independent groups and ANOVA. We considered values as significant when p<0.05. All data are presented as means. Error bars represent ± s.e.m.
Acknowledgments
We thank Dr. Mary Kennedy and Leslie Schenker for the anti-citron antibody (CT295). This work has been supported by NIH grant MH056524 to JJL and CA131047 to VV.
List of abbreviations used
- CitK
citron kinase
- Dlg5
discs large 5
- VZ
ventricular zone
- PDZ
PSD95-Discs large-ZO-1
- MAGUK
membrane-associated guanylate kinase
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YC, JJL, RSW participated in the design of the study; YC carried out the experiments and analysis; OK and VV generated, maintained, and provided the Dlg5−/− mice and anti-Dlg5 antibody; YC and JJL are involved in drafting the manuscript and JJL revised the manuscript; VV and RSW contributed to revise the manuscript. All the authors read and approved the final manuscript.
Contributor Information
YoonJeung Chang, Email: yoonjeung.chang@uconn.edu.
Olga Klezovitch, Email: oklezovi@fhcrc.org.
Randall S. Walikonis, Email: randall.walikonis@uconn.edu.
Valera Vasioukhin, Email: vvasiouk@fhcrc.org.
Joseph J. LoTurco, Email: joseph.loturco@uconn.edu.
References
- 1.Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30(1–3):24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- 2.Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Contribution of intermediate progenitor cells to cortical histogenesis. Arch Neurol. 2007;64(5):639–642. doi: 10.1001/archneur.64.5.639. [DOI] [PubMed] [Google Scholar]
- 3.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6(10):777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 4.Sarkisian MR, Li W, Di Cunto F, D'Mello SR, LoTurco JJ. Citron-kinase, a protein essential to cytokinesis in neuronal progenitors, is deleted in the flathead mutant rat. J Neurosci. 2002;22(8):RC217. doi: 10.1523/JNEUROSCI.22-08-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Cunto F, Imarisio S, Hirsch E, Broccoli V, Bulfone A, Migheli A, Atzori C, Turco E, Triolo R, Dotto GP, et al. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron. 2000;28(1):115–127. doi: 10.1016/s0896-6273(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JM. Cell signalling: MAGUK magic. Curr Biol. 1996;6(4):382–384. doi: 10.1016/s0960-9822(02)00501-8. [DOI] [PubMed] [Google Scholar]
- 7.Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi M, Ito T, Mitsushima M, Aizawa S, Ueda K, Amachi T, Kioka N. Interaction of lp-dlg/KIAA0583, a membrane-associated guanylate kinase family protein, with vinexin and beta-catenin at sites of cell-cell contact. J Biol Chem. 2003;278(24):21709–21714. doi: 10.1074/jbc.M211004200. [DOI] [PubMed] [Google Scholar]
- 9.Nechiporuk T, Fernandez TE, Vasioukhin V. Failure of epithelial tube maintenance causes hydrocephalus and renal cysts in Dlg5−/− mice. Dev Cell. 2007;13(3):338–350. doi: 10.1016/j.devcel.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paramasivam M, Chang YJ, LoTurco JJ. ASPM and citron kinase co-localize to the midbody ring during cytokinesis. Cell Cycle. 2007;6(13):1605–1612. doi: 10.4161/cc.6.13.4356. [DOI] [PubMed] [Google Scholar]
- 11.Siegrist SE, Doe CQ. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123(7):1323–1335. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 12.Margolis B, Borg JP. Apicobasal polarity complexes. J Cell Sci. 2005;118(Pt 22):5157–5159. doi: 10.1242/jcs.02597. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Vazquez L, Apperson M, Kennedy MB. Citron binds to PSD-95 at glutamatergic synapses on inhibitory neurons in the hippocampus. J Neurosci. 1999;19(1):96–108. doi: 10.1523/JNEUROSCI.19-01-00096.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]