Abstract
Estrogens are potent mitogens for some target organs, such as the uterus, and cancers that develop in this organ might be linked to the proliferative action of these hormones. However, the mechanism by which estrogens influence the cell cycle machinery is not known. We found that a null mutation for the insulin receptor substrate (IRS)-1, a docking protein that is important for IGF-1 signaling, compromised hormone-induced mitosis in the uterine epithelium; BrdU incorporation was not affected. This selective effect on mitosis was associated with a reduction in uterine cyclin B-associated kinase activity; cyclin A-associated kinase activity was not changed. The null mutation also reduced the extent of hormone-induced phosphorylation of endogenous uterine histone H1, as determined with phosphospecific antiserum. Uterine epithelial cdk1 was induced in response to hormone, but the level of the kinase protein, as determined by immunoblotting, was noticeably less in the irs1 null mutant when compared to that of the wild-type mouse, especially around the time of peak mitosis (24 hours). Since IRS-1 binds/activates PI 3-kinase, the absence of this docking protein could impair signaling of a known pathway downstream of Akt that stimulates translation of cell cycle components. Indeed, we found that phosphorylation of uterine Akt (Ser473) in irs-1 null mutants was less than that in wild-types following treatment. Based on earlier studies, it is also possible that an IGF-1/IRS-1/PI 3-kinase/Akt pathway regulates posttranslational changes in cdk1. This model may provide insights as to how a growth factor pathway can mediate hormone action on cell proliferation.
Keywords: estradiol, insulin-like growth factor-1, insulin receptor substrate-1, cdk-1(p34), cyclin B, Akt, uterus
Introduction
It is well known from laboratory studies of rodents that estradiol (E2) is a potent mitogen for uterine luminal and glandular epithelial cells (Tong & Pollard 2002). This mitotic action extends to other congeners of E2 and may contribute to the increased risk of endometrial carcinoma observed in postmenopausal females given estrogen replacement therapy (Gambrell et al. 1983). The proliferative response to E2 occurs naturally during the ovarian cycle and during early pregnancy. Hormone interaction with the uterine estrogen receptor-α (ERα) is apparently essential for this action since ovariectomized ERα knockout (αERKO) mice exhibit negligible uterine cell DNA synthesis or proliferation in response to E2 (Couse et al. 1995). Injection of E2 into the ovariectomized rodent increases both the expression of cell cyclins and the activation of cyclin-dependent kinases. Transcripts for uterine cyclin D (D1–D3), E, A, and B increase with each cyclin having a specific temporal pattern following estrogen exposure (Altucci et al. 1997; Tong & Pollard 1999). In mammalian cells, cyclin A is reported to function during S phase, as well as the G2/M transition, whereas cyclin B controls entry into mitosis (Minshull et al 1990; Nurse 1990). There is limited knowledge of the local pathways that link activation of the estrogen receptor with the uterine epithelial cell cycle machinery. For over two decades investigators have considered the notion that hormone-induced growth factors can serve as mediators of cell proliferation. In this regard, experimental evidence is compiling that supports a functional role for the insulin-like growth factor (IGF-1) in estrogen-induced proliferation of uterine epithelial cells. E2 is known to increase the level of IGF-1 transcripts in the uterus of some mammals. For instance, IGF-1 mRNA increases by 14-fold in the uterus of the ovariectomized rat as early as 6 hours after treatment with E2 (Murphy et al. 1987). This elevation of the uterine IGF-1 ligand leads to activation of the IGF-1 receptor and downstream signaling pathways in the uterus (Richards et al. 1996; Richards et al. 1998; Klotz et al. 2000). Other studies show that the insulin receptor substrate-1 (IRS-1), which functions as a primary substrate for the insulin and IGF-1 receptors, is critical for the mitogenic response of these factors (Waters et al. 1993; Rose et al. 1994; Bruning et al. 1997). The omission of IRS-1 in embryonic fibroblasts by null mutation results in a marked reduction in IGF-1–stimulated cell proliferation and phosphatidylinositol (PI) 3-kinase activity (Bruning et al. 1997). In situ hybridization studies suggest that the interaction of IGF-1 with the uterine epithelial IGF-1 receptor occurs through a paracrine-type mechanism, with IGF-1 originating in proximal stromal cells (Baker et al. 1996; Cooke et al. 1997). A complex containing the IGF-1R, IRS-1, and PI 3-kinase occurs in uterine extracts of mice treated with E2 or IGF-1 (Richards et al. 1998). Formation of this hormone-induced complex is severely compromised in αERKO mice (Klotz et al. 2000) and also in IGF-1m/m mice (Richards et al. 1998), which have a deficiency in IGF-1 (Lembo et al. 1996).
It is well known that IGF-1 can stimulate proliferation of a variety of cells in vitro (Lowe 1991). This growth factor was originally referred to as a “G1 progression factor” as an outcome of these earlier studies (Leof et al. 1982; Campisi & Pardee 1984). However, a more recent investigation of cell cycle kinetics in fibroblasts derived from igf1−/− null mice reveals a large increase in the duration of the G2/M phase when compared to that of wild-type cells (Sell et al. 1994). Moreover, when intact igf1−/− null female mice are treated with E2, the proportion of uterine epithelial cells in G1- and S-phases by 20 hours is not different between wild-type and mutant mice; however, cell number and mitotic figures are markedly reduced in the igf1−/−null mice (Adesanya et al. 1999). This is direct evidence that IGF-1 provides a mitogenic stimulus in the uterus in response to E2 and does so by influencing transit time through G2/M, rather than G1.
The major goal of the present study is to identify components of the cell cycle in the uterus that are regulated by the hormone-induced IGF-1 signaling pathway. To achieve this, we use the uterine epithelium of hormone-treated ovariectomized mice as an experimental model (Fagg et al. 1979). This model permits analysis of signaling and cell cycle proteins in the uterine epithelium, while providing the permissive microenvironment for E2 to stimulate cell proliferation (Cooke et al. 2002). Since adult igf1−/− null mutant mice are extremely small and and can exhibit a higher frequency of neonatal lethality (Liu et al. 1993), irs1−/− null mutants (Araki et al. 1994) were selected for most of the experiments. As described above, the uterus of the irs1−/− mouse should have a compromised IGF-1 signaling pathway. Our initial experiments used igf1−/− mice to establish that this ligand is critical for E2-stimulated association of PI 3-kinase (p85) with IRS-1. Following this, we sought to determine whether irs1−/− null mutants exhibit compromised mitosis in the uterine epithelium in response to E2, as was previously observed with igf1−/− null mutants (Adesanya et al. 1999). Finally, we attempted to identify what component(s) of the cell cycle might be regulated by the IGF-1/IRS-1 pathway. Our findings indicate that the IGF-1/IRS-1 pathway contributes to the mitogenic action of E2 by stimulation of cyclin B-associated cdk1 kinase. This finding may have broad implications with regard to the mechanism by which IGF-1 provides a stimulus for cell proliferation in various tissues or neoplasias.
Materials and Methods
Mice, treatments, and tissue collection
IRS-1 wild-type (IRS-1+/+), and IRS-1 null mutant (IRS-1−/−) mice on a C57Bl/6 × 129Sv background were generated in our laboratory from heterozygous IRS-1 breeding pairs obtained from Taconic (Germantown, NY) with the permission of Dr. Ronald Kahn (Joslin Diabetes Center, Boston, MA). Null mutants were also generated by breeding irs1−/− males with irs1+/− females. The weight of female irs1−/− mice was approximately 70% that of corresponding wild-types. IGF-1 wild-type (igf1+/+) and IGF-1 null mutant (igf1−/−) mice were generated from heterozygous IGF-1 breeding pairs (MF1 X 129/Sv hybrids) provided by Dr. Argiris Efstradiatis (Columbia University, New York, NY). Tail genomic DNA was screened by PCR to determine the genotype. All of the wild-type, irs1−/−, and igf1−/− mice were ovariectomized at 8–13 weeks of age. Procedures for surgery, treatments, and euthanasia were conducted in compliance with the guidelines of the NIEHS, National Institutes of Health Animal Care and Use Committee.
To assess BrdU incorporation, ovariectomized irs1−/− mice were treated with estradiol (0.08 µg/g) or vehicle (4% v/v ethanol phosphate-buffered saline) subcutaneously and euthanized after 18h; BrdU (Sigma, 0.1 mg/g intraperitoneally) was injected two hours before euthanasia. For mitosis experiments, mice were euthanized 22 h after hormone treatment; the antimitotic compound demecolcine (Colcemid, Sigma, 5 µg/g intraperitoneally) was injected two hours before euthanasia. Uteri were excised and a 5 mm-long transverse section in the middle of each horn was taken and immersed in 10% neutral-buffered formalin. Paraffin sections were prepared by standard procedures. BrdU was detected in sections with a rat anti-BrdU monoclonal antibody that was part of a Vector kit (no. PK4004). All mice were treated with hormone no earlier than 14 days after ovariectomy.
Apoptotic cells were identified in uterine sections with a TACS™ 2 TdT (TBL) In Situ Apoptosis Detection Kit (Trevigen, Gaithersburg, MD) according to instructions; negative and positive control slides were included in the kit.
Epithelial cell extracts
Mice were treated with hormone as described above and euthanized at various times after treatment. The specific intervals (up to 48h) following treatment for the different experiments are shown in the figures or figure legends. For the 0 time point, mice were treated with 4% ethanol phosphate-buffered saline and immediately euthanized. Uterine epithelial cells were removed as previously described (Fagg et al. 1979; Tong & Pollard 1999). Briefly, uteri were slit lengthwise and placed in 15 ml-tissue culture tubes with chilled extraction buffer [1% Triton X-100, 150 mM NaCl, 1 mM Na3VO4, 1 mM NaF, 50 mM Na2MO4, 20 µg/ml aprotinin, 20 µg/ml leupeptin, and 4 µg/ml 4-amidophenylmethylsulfonyl fluoride (APMSF) in 50 mM Tris-HCl, pH 7.4]. A vol. of 580 and 400 µls buffer was used for each wild-type and irs1−/− uterus, respectively. The uteri were agitated in the presence of seven glass beads (3.0 mm diameter) on a Vortex-Genie mixer (30 sec on/30 sec off) for a total of 5 min. The resulting cell suspension was drawn off and then disrupted ultrasonically (3 × 5 sec bursts) followed by brief centrifugation. The supernatant was transferred to a microfuge tube and stored at −80°C. Protein concentration was determined with a Pierce BCA assay kit (Pierce Chemical, Rockford, IL) with bovine albumin (2mg/ml) as a standard.
Western immunoblotting and immunoprecipitation
To remove endogenous IgGs prior to immunoprecipitation, aliquots of supernatants were cleared by incubating with 100 µl of washed protein A-Sepharose (CL-4B, Amersham Pharmacia Biotech, Piscataway, NJ) for 30 min at 4°C. For immunoprecipitation, the cleared aliquots (300–450 µg protein) were incubated with 5 µg of antibody for 2 h at 4°C. After incubation with the antibody, supernatants were incubated with the protein A-Sepharose for an additional 2 h at 4°C. Antibody/protein A-Sepharose pellets were washed three times with the extraction buffer and subjected to kinase assays or boiled for 5 min in Laemmli sample buffer. After boiling, precipitates were stored at −20°C. Aliquots of mouse uterine epithelial cell extracts or immunoprecipitates were boiled for an additional 2 min, subjected to SDS-PAGE, and then transferred to polyvinylidene fluoride (Immobilon-P) membrane (Millipore, Bedford, MA). The membrane was blocked with either 5% bovine albumin/Tris-buffered saline with 0.1% Tween 20 or 5% nonfat dry milk/PBS and incubated with a dilution of specific antibody. For cdk1, where cytokeratin 18 was used as a loading control, the membrane was cut just above the 37 kDa protein marker. The lower portion (cdk1) was blocked with 1% BSA/TBS-Tween and the upper portion (cytokeratin 18) blocked with 5% nonfat dry milk/PBS.
Antibodies [catalog number] to IRS-1 [06-248], p85 [06-195], cdk1 [06-141], and cdk2 [05-596], were purchased from Upstate (Temecula, CA); antibodies to cyclin A [sc-596], cyclin B1 [sc-595] and cytokeratin 18 [sc-28264] were from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies to Akt [9272] and phospho-Akt (Ser473) [9271] were from Cell Signaling Technology (Beverly, MA).
Each membrane was then incubated with a horseradish peroxidase-conjugated donkey anti-rabbit [NA9340] (Amersham Biosciences, Piscataway, NJ) or goat anti-mouse IgG secondary antibody [A3682] from Sigma (St. Louis, MO). Immunoreactive proteins were detected using enhanced chemiluminescence (Amersham Pharmacia Biotech).
Densitometric scans of bands corresponding to cdk-1 and cytokeratin 18 on immunoblot films were made with an Alpha Innotech FluorChem® FC2 Imaging System.
Microarray analysis
Fold changes in mouse uterine cdk1 and cdk2 transcripts following treatment with estradiol were retrieved from the microarray data offered by Sylvia C. Hewitt and Dr. Bonnie Deroo (NIEHS, NIH). Details of the experimental design have been published (Hewitt et al. 2003) and the repository for the microarray data is accessible at NCBI’s Gene Expression Omnibus(GEO, http://www.ncbi.nlm.nih.gov/geol/); accession numbers GSE4664 and GSE4615.
Quantitative real-time PCR
Each uterus removed was immediately placed on ice in a tube containing 1.5 ml RNAlater Stabilization Reagent (Ambion, Austin, TX). RNA was extracted with the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Uteri were transferred to the RLT buffer (600 ml per uterus containing β-mercaptoethanol (15 µl per ml), and homogenized with an Omni Model TH-115 hand-held homogenizer (7 mm sawtooth probe with three bursts at full power, each ten seconds). RNA isolation was followed by a DNA removal step with DNase I. One µg of total RNA was then reverse transcribed (RT) using a Gene Amp PCR System 9700 (Applied Biosystems, Foster City, CA) and the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA).
Quantitative real-time PCR was performed with the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) on a Bio-Rad iCycler (Bio-Rad, Hercules, CA). The primers for mouse cdk1 were 5’-GGA-CCT-CAA-GAA-GTA-CCT-GGA-C-3’ (forward) and 5’-CCC-TGG-AGG-ATT-TGG-TGT-AAG-3’ (reverse). The primers for mouse actin were 5’-TGA-CAG-GAT-GCA-GAA-GGA-GA-3’ (forward) and 5’-CGC-TCA-GGA-GGA-GCA-ATG-3’ (reverse). The cDNAs were amplified for 40 cycles (95°C/10 min → [95°C/15 sec → 60°C/30 sec]). Controls included the omission of template or primers. Melt curves of both amplicons indicated the presence of a single product and the absence of primer-dimer formation. Relative quantitation of cdk1 expression was determined by the comparative CT method (Pfaffl 2001) with actin as the endogenous reference gene. Real-time PCR efficiencies for the cdk1 (E=1.97) and actin (E=1.98) were determined from the Q5 Optical System Software (Bio-Rad).
Cyclin-associated kinase assay
Uterine epithelial extracts were prepared as described above and stored at −80°C. Equal amounts of WT and KO extracts (~400 µg protein) were diluted to 0.5 ml with the extraction buffer after preclearing the sample with protein A-Sepharose, 5 µg of anti-cyclin B (Upstate) or anti-cyclin A (Santa Cruz) were reacted with the sample for 2.5 h. Protein A-Sepharose was added and immunoprecipitates collected were washed three times with the extration buffer and then three times with the Cdk1/cdc2 kinase assay buffer (20 mM MOPS, pH 7.2, 25 mM glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate, and 1 mM dithiothreitol). The kinase reaction was carried out according to the instructions of the cdk1/cdk2 kinase assay kit (Upstate). Kinase activity of the immunoprecipitate was carried out in the assay buffer containing 10 µCi [γ-32P]ATP (3000 Ci/mmol), 90 µM ATP, and 14 mM MgCl2. Histone H1 (0.4 µg/µl) was the exogenous substrate. The mixture was incubated for 10 min at 30°C. The assay was stopped by placing the reaction tubes on ice and adding gel sample loading buffer. Sample proteins were separated on 4–20% Tris Ready Gels (Bio Rad) and transblotted to Immobilion-P membrane. Detection was made by autoradiography and phosphoimaging (Storm 860 Molecular Imager).
In situ Histone (H1) phosphorylation
Nuclei were isolated from whole uteri following homogenization in a detergent-containing buffer, as previously described (Archer et al. 1991). Nuclei were resuspended in 0.4N H2SO4 and proteins in the soluble fraction precipitated with 5 volumes of ethanol. Ten µg of isolated histones were separated by SDS-PAGE and transferred to polyvinylidene fluoride membrane. Immunoblotting procedures were similar to those described above. The membrane was probed with antibodies (1–2 µg/ml) developed in rabbits against mouse H1.3 amino acids 15–27 containing a phosphate at Thr18 (Deterding et al. 2008). A test of the antibody against chromatographically purified H1 isoforms revealed recognition of only H1.3 and H1.4 isoforms in mouse cells; the antibody did not detect an H1.3 peptide phosphorylated at Ser173.
Results
IGF-1 is required for E2-induced IRS-1 activation
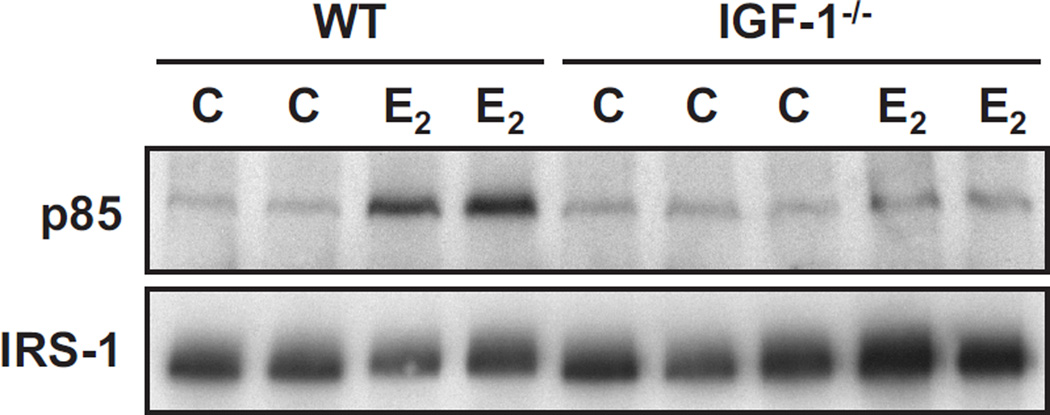
Since we intended to use the irs1−/− mice to compromise the IGF-1R signaling pathway, it was important to ascertain at the outset that the IRS-1 docking protein requires IGF-1 for activation of PI 3-kinase in response to E2. To demonstrate this, ovariectomized female wild-type and igf1−/− mice were treated with E2 (6 h) and immunoprecipitates of IRS-1 from uterine epithelial extracts were evaluated for the PI 3-kinase regulatory subunit (p85). As shown in Figure 1, immunoprecipitates of uterine IRS-1 from wild-type mice (lanes 1–4) revealed the expected increased association of p85 as a result of hormone treatment (Richards et al. 1998). In contrast, uterine IRS-1 from hormone-treated igf1−/− mice (lanes 5–9) was not associated with p85. These findings indicate that the IGF-1 ligand is a required intermediate for estrogens to activate IRS-1 and stimulate binding of p85 to IRS-1 in the uterus. In addition, these data rule out the participation of another IGF-1R ligand, e.g. IGF-2, that might function in the absence of IGF-1 to stimulate activation of IGF-1R and IRS-1 (Osborne et al. 1989).
Fig. 1. Binding of the PI 3-kinase regulatory subunit (p85) to uterine IRS-1 in wild-type (WT) and igf1−/− mice treated with estradiol.
Ovariectomized mice were treated with estradiol (E2) or vehicle (C, control) and uterine epithelial extracts obtained six hours later. Aliquots of extracts were incubated with anti-IRS-1 antiserum. Immunprecipitates were separated by SDS-7.5% PAGE and then immunoblotted with anti-p85 antiserum (upper panel) or anti-IRS-1 antiserum (lower panel). Other details of the treatment, extraction, and immunoblotting procedures were previously published (Richards et al. 1996). Each lane represents analyses of a different animal.
E2-Stimulated uterine epithelial proliferation is compromised in irs1−/− mice
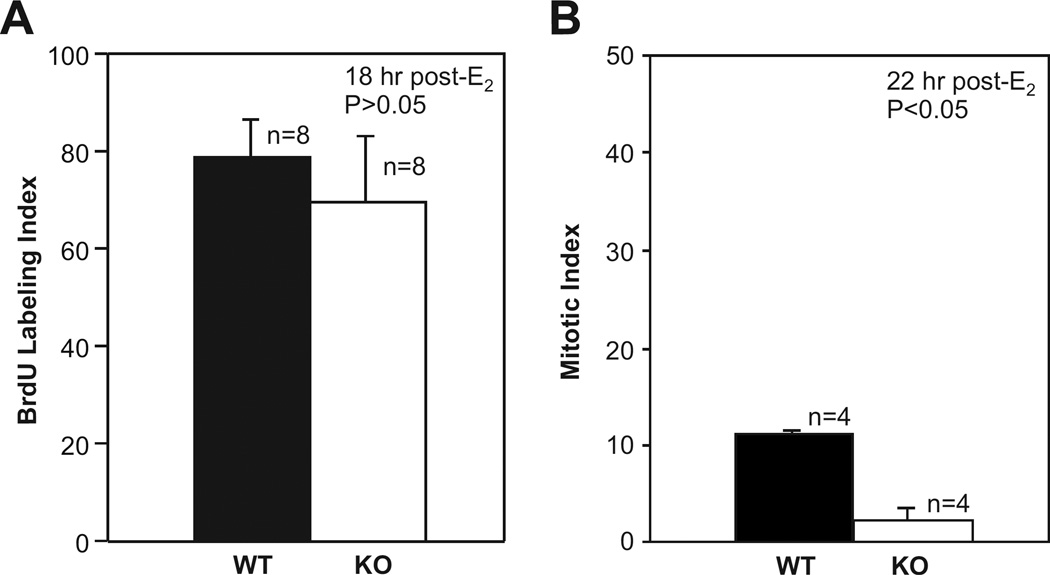
Earlier reports showed convincingly that IRS-1 is essential for the mitogenic action of both IGF-1 and insulin for cells in culture (Waters et al. 1993; Rose et al. 1994; Bruning et al. 1997). Since IGF-1 was critical for E2-induced activation of uterine IRS-1, mice with an IRS-1 null mutation could provide insights as to the importance of an IGF-1/IGF-1R/IRS-1 pathway in the proliferative response of uterine epithelial cells to E2. In order to determine this, ovariectomized adult irs1−/− and wild-type (irs1+/+) female mice were treated with E2 and the uterine epithelium examined for the proportion of cells in S phase and in mitosis. The proliferative response of the uterine epithelial cells to E2 in the adult mouse is considered to begin with a drastic shortening of the G1-S transition rate (Tong & Pollard 2002). The epithelial cells exhibit a near-synchronous wave of DNA synthesis beginning about 10 h after hormone treatment; this is followed by mitosis that peaks around 20–24 h. To identify cells in S phase, BrdU was injected into animals that were previously treated with E2 16 hours earlier. Negligible incorporation of BrdU into uterine epithelial cells occurred when wild-type or mutant mice were injected with vehicle. Incorporation of BrdU was apparent in both groups of mice and the proportion of cells that incorporated BrdU in irs1−/− mice was not significantly different from that of wild-types (Fig. 2A). The fraction of epithelial cells undergoing mitosis was determined at 22 h after hormone treatment. At this time, epithelial cells of the irs1−/− mice had approximately one-sixth the frequency of mitotic figures as that of wild-types (Fig. 2B). These data suggest that the IRS-1 null mutation affects the rate of transit of uterine epithelial cells through G2/M without impairing recruitment of cells into G1/S in response to E2. The effects obtained with the irs1−/− mice with regard to hormone stimulation of the uterine epithelial cell cycle are similar to that previously reported for igf1−/− mice (Adesanya et al. 1999). In both cases, the null mutation affected the number of cells in mitosis but not cells in S phase. This functional overlap indicates that IGF-1 and IRS-1 are part of the same signaling pathway that mediates hormonal stimulation of mitosis of cells in the uterine epithelium. The decrease in the number of mitotic figures observed at 22 h after E2 in irs1−/− mice could not be explained by a difference in apoptosis. Evaluation of uterine sections at this time point for detection of fragmented DNA in situ end labeling showed no detectable apoptosis in the epithelium of wild-type or irs1−/− mutant mice (data not shown).
Fig. 2. BrdU uptake and mitosis in uterine epithelial cells of estradiol-treated wild-type and irs1−/− mice.
(A) Mice were treated with estradiol and euthanized 18 h later. BrdU was injected two hours prior to euthanasia. Standard methods were used to prepare uterine sections for examination by light microscopy. BrdU was detected in epithelial cells with a rat anti-BrdU monoclonal antibody.
(B) Mitosis in uterine epithelial cells was determined by injecting demecolcine into mice 20 h after hormone treatment, followed by euthanasia at 22 h. Further details of the procedures used are described in Materials and Methods. In A and B, each bar represents the mean ± standard deviation; n is the number of mice used in each group. For both BrdU uptake (A) and mitosis (B), approximately 500 epithelial cells were counted in each uterine section. The mitotic index of IRS-1-knockout (KO), or irs1−/− mice, was significantly different from that of wild-type at p < 0.05.
The IRS-1 null mutation reduces the E2-induced increase in cdk1 (p34) protein but not mRNA
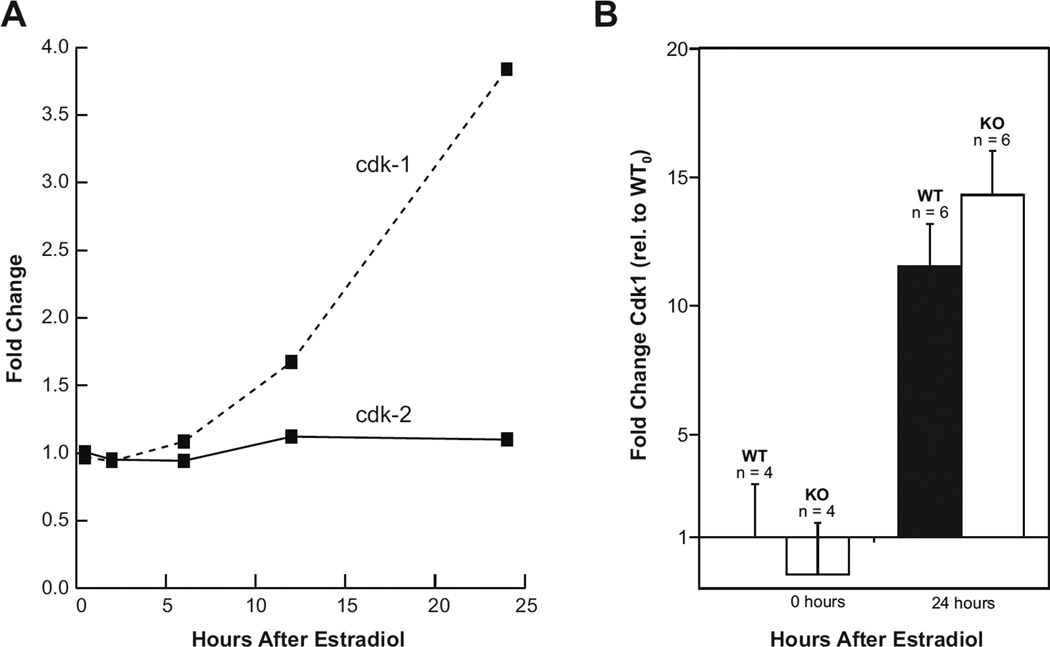
The significant decrease in hormone-induced mitosis in the uterine epithelium of irs1−/− mice prompted us to consider an effect of the mutation on the major mitotic kinase cdk1. In the mammal, cdk1 kinase is poorly expressed in normal quiescent cells but accumulates during S-phase and peaks during G2 and M phases of the cell cycle (Kim et al. 1992; Loyer et al. 1994). As shown in Figure 3A, microarray analysis of wild-type mouse uterine RNA reveals that the level of cdk1 transcripts increases markedly by 24 h after treatment with E2, whereas the level of cdk2 transcripts was essentially invariate during this period. The influence of E2 on uterine cdk1 mRNA in wild-type mice was compared with that of irs1−/− by quantitative PCR analyses using the comparative (CT) method with actin as the reference gene. As shown in Figure 3B, cdk1 transcripts increased in both groups by 24 h after estrogen treatment. Interestingly, the level of uterine cdk1 mRNA in the IRS-1 null mutants was significantly greater (p<0.05) than that of the wild-type mice at this time point.
Fig. 3. Changes in uterine cdk1 transcripts in wild-type and irs1−/− mice after treatment with estradiol.
(A) Total RNA was isolated from whole uteri of C57BL/6 (ovariectomized) mice at the indicated times after treatment with estradiol. The plots compare the estradiol-induced fold-changes in uterine gene expression of cdk1 against that of cdk2, as determined by microarray analyses. Details of the microarray are given in Materials and Methods and in ref. (Hewitt et al. 2003). The plots were constructed from values given at the website in this reference.
(B) Total RNA was isolated from whole uteri of ovariectomized wild-type and irs1−/− mice before and after treatment with estradiol. cDNA was prepared from 1µg RNA and used for quantitative real-time PCR. Quantitation was made by the comparative CT method with actin as the endogenous reference gene. After calculating the ΔCT (cdk1 minus actin), the values (ΔΔCT) were determined relative to that for untreated wild-types. Each bar represents the mean ± SD; n is the number of mice used in each group. Note that the mean value for the untreated IRS-1 (KO) was less than 1.0. Cdk1 transcript level was increased in wild-type (WT) and KO animals 24 h after hormone treatment; the value for KO (24 h) was significantly greater than that of WT (24 h) at p < 0.05. Further details of the procedures and primer sequences are described in Materials and Methods.
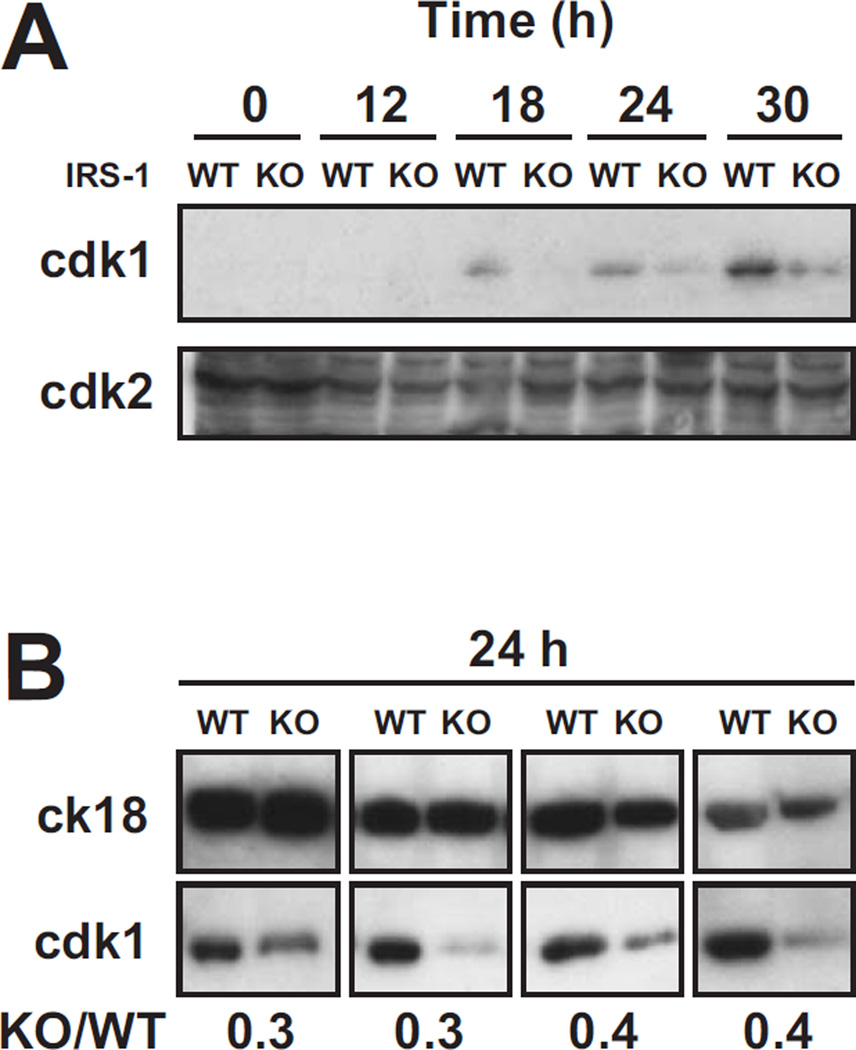
To compare the levels of cdk1 protein, extracts of wild-type and mutant uterine epithelium were prepared at various times after hormone treatment and evaluated by immunoblotting. In accord with the microarray and quantitative PCR data, cdk1 (p34) protein in the quiescent uterine epithelium of ovariectomized mice (time 0), wild-type or irs1−/−, was below the level of detection (Fig. 4A). When mice were treated with E2, uterine cdk1 increased and could be readily detected by 18 h in wild-type mice. In contrast, the uterine cdk1 abundance in irs1−/− mice was generally much less than that observed in wild-types at the corresponding intervals after hormone treatment (Fig. 4A). In repeated trials that analyzed extracts from uteri obtained 24 h after estrogen, the levels of cdk1 protein in wild-types were consistently greater than that of mutants (Fig. 4B). For both wild types and mutants, the level of hormone-induced cdk-1 was highest at the 30 hour time point, which is after peak mitosis in this model. It is not known whether treatment with estradiol alone (“unopposed”) favors this pattern, which might differ considerably from that of the intact female exposed to sequential cycles of estrogens and progestins. Levels of cdk2 (p33) kinase, which forms a heterodimer with cyclin A or cyclin E, were also examined in uterine extracts of wild-type and irs1−/− mice treated with hormone. As shown in Figure 4 (upper panel), cdk2, in contrast to cdk1, was detected in extracts before and after hormone treatment. Hormone treatment did not elevate uterine cdk2 protein levels, which is in accord with the microarray findings (Fig. 3A). The discrepancy observed for cdk1 in wild-type and irs1−/− mice was not apparent for cdk2.
Fig. 4. Time-course analyses of cdk1 and cdk2 (immunoreactivity) in uterine epithelial extracts of wild-type and irs1−/− mice.
Ovariectomized wild-type (WT) and irs1−/− (KO) mutant mice were treated with estradiol and uterine epithelial extracts obtained at the times indicated. Mice at the 0 time point did not receive hormone. Proteins (~30 µg) in aliquots from the extracts were separated by SDS-PAGE and immunoblotted for cdk-1 or cdk-2. Further details of the extraction and immunoblotting are given in Materials and Methods. In A, immunoblots of uterine cdk-1 and cdk-2 are compared for up to 30 h after estradiol. The results are representative of six different trials. Extracts for each trial (time course) were prepared on separate days; each lane represents an extract from one mouse uterus. In B, immunoblots of cdk-1 from wild-type (WT) and irs1−/− (KO) mouse uterine extracts are compared. All the extracts were obtained from uteri 24 h after hormone treatment. Extracts from each of the four pairs of blots (WT vs. KO) were prepared on different days; each lane represents an extract from one mouse uterus. Cytokeratin 18 (ck18), an epithelial marker, was a loading control.
The number below each pair of blots represents the relative amount of cdk-1 (KO/WT) in the samples, as determined by densitometry. The level of cdk-1 was corrected for the amount of the cytokeratin 18 (loading control).
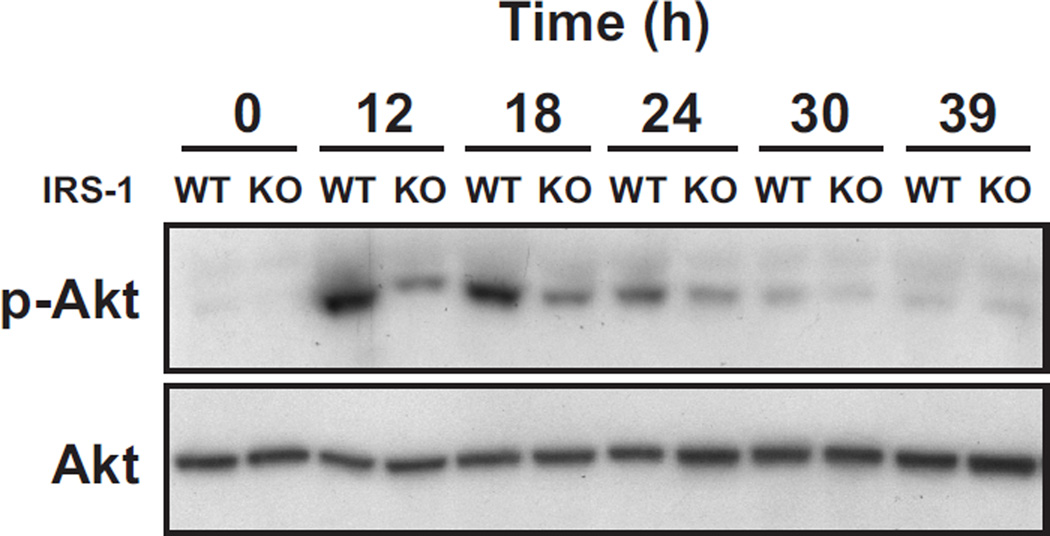
The stimulation of Akt phosphorylation and cyclin B-associated kinase by E2 is reduced in the irs1−/− mutant
Since hormone induced mitosis was inhibited by the irs1−/− null mutation, we wanted to resolve whether the mutation compromised mitotic kinase activity. Cdk1 acquires the potential to become a mitotic kinase in mammalian cells when bound to cyclin B (Meijer et al. 1989) It is possible that the observed reduction of cdk1 (protein) in mutants prevented accumulation of enough mitotic kinase for mitosis. Alternatively, the mutation might affect posttranslational changes that regulate cdk1. Activation and inhibition of cdk1 kinase are known to occur by phosphorylation and dephosphorylation of specific sites and it is likely that some of these modifications are integrated with growth factor pathways. The activation of Akt (Ser473 phosphorylation) can be demonstrated in our experimental model, as shown by the time-course study in Figure 5. Akt phosphorylation is clearly evident in extracts from wild-type uterine epithelium obtained at 12 h and 18 h following estradiol treatment. The corresponding bands from IRS-1 KO animals were much weaker. After 24 h, the level of Akt phosphorylation was very weak in both groups (Fig. 5). These findings support that an IRS-1-dependent pathway is utilized for the hormonal activation of uterine epithelial Akt. Other (non IRS-1) signaling pathways probably account for the phosphorylation of Akt observed in mutant animals.
Fig. 5. Akt phosphorylation in the uterine epithelium of estradiol-treated wild-type mice and irs1−/− (KO) mice.
Ovariectomized wild-type (WT) and irs1−/− (KO) mice were treated with estradiol and uterine epithelial extracts obtained at the times indicated. Mice at the 0 time point did not receive hormone. Each lane represents analyses of one uterus. Equal amounts of extracted protein (300–400 µg) were used for WT and KO mice in each experiment. Akt was immunoprecipitated from extracts with 5 µg anti-Akt antibody and immunoblots made with antiphospho-Akt (Ser 473) and anti-Akt antibodies. Further details are given in Materials and Methods. The results shown are representative of four separate trials with different animals. The known increase in mouse uterine Akt transcripts in response to estradiol (Hewitt et al. 2003) probably accounts for the increase in Akt immunoreactivity observed at 24, 30, and 39 h.
The relative amounts (p-Akt/Akt) for KO/WT as determined by densitometry for the 12, 18, and 24 h time points of this experiment were 0.4, 0.5, and 0.4, respectively.
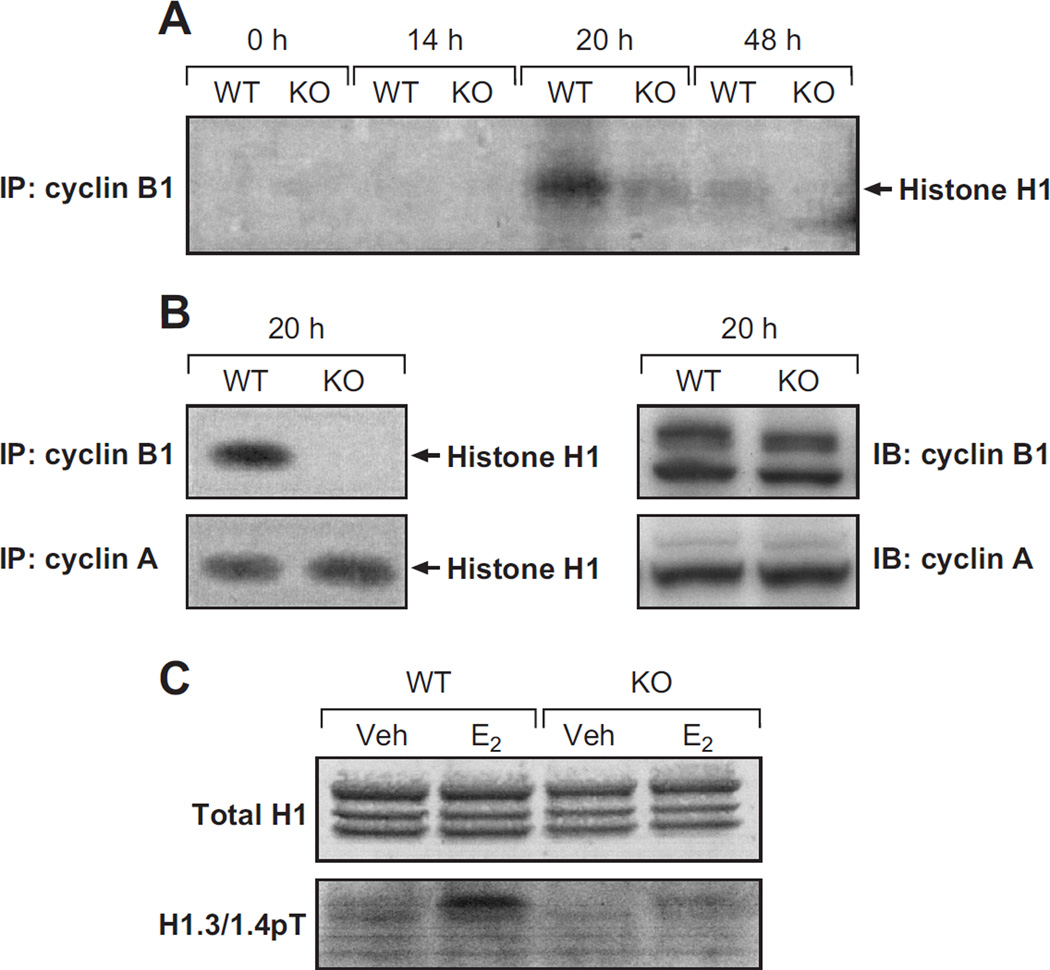
To determine whether the irs-1 null mutation can affect mitotic kinase activity uterine cyclin B-associated kinase activity was examined for up to 48h following treatment of the mice with E2. The phosphorylation of the histone H1 (exogenous) substrate was clearly evident at 20 h after hormone treatment in wild-type samples; however, much less kinase activity was observed at 48 h (Fig. 6A). This temporal pattern is in keeping with the importance of cdk1/cyclin B kinase activity for entry into the mitotic phase of the cell cycle (Lew & Kornbluth 1996). The corresponding time-course study with samples from irs1−/− uteri revealed much less phosphorylation of histone H1 than that obtained with wild-type samples. This effect of the IRS-1 null mutation on hormonal activation of cyclin B-associated kinase activity was not observed with cyclin A-associated kinase activity. As shown in Figure 6B, the32P-labeling of the histone H1 substrate by kinase associated with cyclin A precipitates was comparable in extracts from wild-type and irs1−/− mice. Immunoblots were made of cyclin B and A in extracts obtained after 20 hours. The amount of each cyclin appeared comparable in wild-type and irs1−/− samples (Fig. 6B). Therefore, it is unlikely that the disparity between hormone-induced cyclin B-associated kinase activity in irs1−/− and wild-type uteri could be explained by lower cyclin B levels.
Fig. 6. Cyclin A- and B- associated kinase activities in the wild-type and irs1−/− uterine epithelium.
(A) Uterine epithelial extracts were obtained from ovariectomized wild-type (WT) and irs1−/− (KO) mice after the indicated interval following treatment with estradiol. Mice at the 0 time point did not receive estradiol. Cyclin B was immunoprecipitated from equal amounts (~400 µg protein) of extracts from WT and KO uteri and analyzed for histone H1 kinase activity. More details of the immunoprecipitation and the kinase assay are given in Materials and Methods. The kinase samples were run on 4–20% Tris Ready Gels, and then transblotted to Immobilon-P membrane.
Detection of the32P-labeled histone H1, shown here prominently 20 h (WT) after estradiol treatment, was obtained by autoradiography. Specific quantitation of the32P-labeled histone in the 20 h WT and KO samples was made by phosphoimaging. The KO/WT ratio determined at equivalent cyclin B loading was 0.38 ± 0.14 (mean ± SD) from five independent experiments.
(B, left) Cyclin A- and cyclin B1-associated kinase activities were measured in uterine epithelial extracts obtained 20 h after hormone treatment.
(B, right) Immunoblots were made of cyclin A and cyclin B1 in uterine epithelial extracts obtained from wild-type and mutant mice 20 h after hormone treatment.
(C) Histones were enriched from whole uterine nuclear fractions, separated by 16% SDS-PAGE, and transblotted. The upper panel represents staining with Coomassie Blue; in the lower panel the blot was probed with antiserum to mouse phospho (Thr18) histone H1.3/1.4 (Deterding et al. 2008). Veh, represents extracts from vehicle-treated, or control, mice.
The data in A and B are representative of five separate trials; the data in C is representative of three separate trials. Each trial used extracts from different mice and each lane represents an extract from a single mouse uterus.
Phosphorylation of H1 histone during mitosis entry is considered a downstream event of cdk1/cyclin B (Langan et al. 1989). Therefore, we examined the effect of the IRS-1 null mutation on hormone-induced phosphorylation of endogenous H1 histone with a phosphospecific (pThr18) antiserum to a cognate sequence of phospho (Thr18) mouse histone H1.3. As shown in Figure 6C, three major bands corresponding to H1 isoforms were detected in acid extracts of isolated uterine nuclei from wild-type and irs1−/− mice. Twenty hours following hormone treatment, an immunoreactive band was observed in wild-type extracts that comigrated with the H1.3/H1.4 isoforms. In contrast, hormone induced phosphorylation of these isoforms isolated from irs1−/− mice was much less than that observed with wild-type mice. This finding suggests that the activation of the IGF-1/IRS-1 pathway by E2 can result in phosphorylation of endogenous histone.
Discussion
The use of the whole animal as an experimental model is important in accurately resolving the qualitative and quantitative responses of hormones. Cell cultures are generally inappropriate for studying estrogen-stimulated proliferation because of the requirement for normal epithelial/stromal interactions (Cooke et al. 1997; Cooke et al. 2002). Cell cultures may not only be refractory to hormone action but also impose artifacts as a consequence of immortalization, high rate of cell turnover, or inappropriate activation of signaling pathways. This later aspect is particularly important in the present investigation where we are trying to link an E2-induced growth factor pathway with components of the cell cycle.
Since E2 treatment of ovariectomized igf1−/− female mice failed to stimulate binding of p85 (PI 3-kinase) to IRS-1 in uterine epithelial cells, mice with the IRS-1 null mutation should reflect the importance of this adaptor protein as well as IGF-1 receptor signaling in response to E2. Earlier studies in this laboratory showed that PI 3-kinase was the only SH2 protein that bound to uterine IRS-1 following E2 treatment (Richards et al. 1998). Thus, the absence of IRS-1 should-barring any adaptive changes in the null mutant-selectively deprive the uterine epithelial cell of that pool of PI 3-kinase that becomes catalytically active by binding to IRS-1 as a consequence of E2-stimulation of the IGF-1 receptor pathway. It was previously reported that stimulation of PI 3-kinase activity by IGF-1 in irs1−/− mouse embryonic fibroblasts was reduced by 70% when compared to that of irs1+/+ cells (Bruning et al. 1997). Mouse embryonic cells, along with uterine cells, contain IRS-2, which can also bind and activate PI 3-kinase (Sun et al. 1995). This IRS isoform does not functionally supplant IRS-1 in the embryonic fibroblasts. In addition, after E2-treatment, uterine IRS-2 is degraded through an IGF-stimulated, proteasome-dependent pathway while levels of IRS-1 remain essentially unchanged (Richards et al. 2001).
The IRS-1 null mutation did not significantly alter the fraction of uterine epithelial cells undergoing DNA synthesis in response to E2; however, this mutation markedly reduced the fraction of cells undergoing mitosis. These data are very similar to the cytokinetic response to E2 in uterine cells of igf1−/− mice (Adesanya et al. 1999). In a broad sense, these findings indicate that a growth factor pathway can regulate a specific stage, e.g., mitosis, of the cell cycle. Thus, we can propose that an IGF-1R/IRS-1/PI 3-kinase pathway stimulates the rate of mitosis in the uterine epithelium in response to estrogens. There are several reports where cell lines were used to demonstrate that a signaling pathway downstream from PI 3-kinase/Akt functions in the G2 → M transition of the cell cycle. In synchronized HeLa or MDCK cells, Akt activity was maximal during G2/early M phase and then dropped precipitously as cells completed mitosis (Shtivelman et al. 2002). Inhibition of PI 3-kinase in these cells prevents activation of cyclin B-associated kinase and elicits G2 arrest that can be abolished by activated Akt. In HEK293 cells, inhibition of PI 3-kinase also induces a G2 cell cycle arrest that could be alleviated by activated Akt. Mouse embryonic fibroblasts with an Akt1 null mutation revealed a delayed transition from G2/M to G1 (Kandel et al. 2002). In the present study, the reduction of PI 3-kinase/Akt activation caused by the IRS-1 null mutation apparently led to a loss of specific stimulation of the G2 → M transition. The component(s) of the cell cycle regulated by the hormonally-stimulated IRS-1/PI 3-kinase/Akt that would specifically influence the mitotic phase is unknown.
In accord with the reduced rate of E2-induced mitosis in uterine cells of irs1−/− mice, there was a marked reduction of cyclin B-associated kinase activity. Other cdks cannot supplant the function of cdk1 as a mitotic kinase in mammalian cells (Nurse 1990; Nigg 1993). On the other hand, cdk1 can associate with A- and B- type cyclins in mammalian cells to phosphorylate histone H1 (Loyer et al. 1994; Swank et al. 1997). Since levels of cyclin A, cdk2, and cyclin A-associated H1 kinase activity were not affected by the IRS-1 null mutation, it is likely that cyclin A predominantly functions in uterine cells as a complex with cdk2. This would favor the notion that IGF-1R signaling in these cells targets cdk1, and not other cdks, such as cdk2. The level of cdk1 in the IRS-1 null mutants prior to mitosis was less than that in wild-types, which could delay formation of the threshold concentration of the mitotic kinase (heterodimer). Since the lower levels of cdk1 protein could not be explained by a corresponding decrease in transcript, altered signaling in the mutant might have reduced translation or half-life of cdk1. Earlier studies showed that IRS-1 and PI 3-kinase were required for insulin to stimulate eIF-4E/4E-BP-1 phosphorylation and translation in cultured cells (Mendez et al. 1996). Using phosphospecific antisera, we demonstrated that the translation effectors mTOR, tuberin, 4EBP1, eIF-4E, p70S6 kinase, and GSK-3 are activated in response to estradiol; however, we could not demonstrate an effect of the IRS-1 null mutation on specific phosphorylation of these translational components (data not shown). To our knowledge, this specific effect of the IGF-1R signaling on cdk1 protein levels has not been previously reported. One study did suggest that translational control of cdk1 (p34cdc2) was associated with meiotic competence of mouse oocytes; incompetent oocytes have abundant cdk1 mRNA but low levels of cdk1 protein (deVantery et al. 1997). Our experimental model may have allowed us to identify changes in cell cycle components that do not occur or are obscured by most cells in culture. With many immortalized cell lines, for instance, the cdk1 protein is expressed at all stages of the cell cycle (McGowan et al. 1990; Welch & Wang 1992; Draetta & Beach 1998). However, there are aspects of cdk1 transcripts in mammalian cell cultures that overlap with those observed in animal studies. For instance, cdk1 transcription in HeLa cells is elevated in S and G2 phases and low in G1 phase of the cell cycle, based on nulear run-on assays (Dalton 1992). Likewise, other studies with HeLa cells revealed that the abundance of cdk1 mRNA and rate of synthesis of cdk1 protein (p34) increases dramatically as the cells pass through S/G2 (Welch & Wang 1992).
It was reasonable to expect in our model that the IRS-1 null mutation would cause a pronounced reduction in Akt activation. Previous reports suggest that enzymes that regulate phosphorylation and activity of the mitotic kinase are proximal targets of Akt. For instance, phosphorylation at Thr14 and Tyr15 of cdk1 by Myt1, a member of the Wee family of kinases, inhibits the kinase activity of cdk1 (Mueller et al. 1995). In a study with starfish oocytes, it was shown that Akt, acting downstream from PI 3-kinase, phosphorylates Myt1 and downregulates its activity, which favors the meiotic G2/M-phase transition (Okumura et al. 2002). A related study showed that Akt can phosphorylate Wee 1 in 293T or Hela cells, which promotes cytoplasmic localization at the kinase (by binding to 14-3-3q), and G2/M cell progression (Katayama et al. 2005). Thus, it is possible that a deficient PI 3-kinase/Akt signaling pathway in the IRS-1 null mutants increases phosphorylation at Thr14/Tyr15 of cdk1 and thereby compromises its kinase activity. Alternatively, Akt might also be activating Cdc25C, which dephosphorylates Thr14/Tyr15 of cdk1. Inhibition of PI 3-kinase by LY294002 in HeLa cells delays hyperphosphorylation of Cdc25C and cells entering mitosis (Dangi et al. 2003). However, it is still not known whether a PI 3-kinase/Akt pathway is activating for Cdc25C, directly or indirectly. In another study, wortmannin, a potent inhibitor of PI 3-kinase, partially inhibited cyclin B translation and inhibited dephosphorylation of the tyrosine (inhibitory) site of cdk1 of sea urchin oocytes (Salaün et al. 2002). In the later study, the effects of wortmannin on cyclin B synthesis (triggered by fertilization) were also correlated with the effects of this compound on cap-dependent translation machinery, which included 4E-BP/eIF-4E dissociation. Akt facilitates the G2/M phase transition of mouse PC12 neuronal cells (Lee et al. 2005). Stable expression of a dominant negative form of Akt suppressed expression of both cdk1 and cyclin B1 at the mRNA and protein levels without affecting the cyclin A or cdk2 protein levels. A constitutively active form of Akt (myristoylated-Akt) elevated cyclin B and cdk1 mRNA and protein levels and could override cell-growth arrest at G2/M (induced by etopside).
The present finding that the hormone-induced phosphorylation of an endogenous histone H1 isoform in wild-types clearly exceeded that of irs1−/− mice is in accord with the observed decrease of cyclin B-associated kinase activity in the mutant animals. The purified cdk1/cyclin B heterodimer phosphorylates specific sites on histone H1 in vitro (Swank et al. 1997). Earlier studies had also provided evidence to show that the cdks are the major in vivo kinases for H1 at cell cycle transitions with cdk1 implicated as the G2 kinase for H1 (Langan et al. 1989). The phosphorylation of histone H1 in vivo by a cdk is considered to destabilize H1-chromatin interactions and, thereby, facilitate a more open chromatin structure (Contrera et al. 2003). Thus, by regulating cyclin B-associated kinase activity an IGF-1R/IRS-1/PI 3 kinase-stimulated signaling cascade mediates hormonal effects on the state of chromatin condensation. A query to consider for postmenopausal females receiving prolonged exposure to estrogens is whether the perturbation of chromatin structure by this signaling pathway facilitates genomic instability and increased risk of endometrial cancer (Enders & Maude 2006).
Our cumulative findings support that the stimulation of uterine epithelial cell proliferation by estradiol is mediated by an IGF-1/IRS-1/PI 3-kinase/Akt pathway and that this pathway targets the mitotic kinase cdk1/cyclin B. A specific goal of future work should be to determine whether impairment of Akt activity by the IRS-1 null mutation affects the activity of enzymes or other proteins that regulate post-translational changes or catalytic activity of cdk1. Other cell populations might have mechanisms to regulate mitotic kinase activity that are similar to or overlap with those observed in the present study. In a broad sense, our experimental findings are probably part of a more general phenomenon, where individual growth factors exert stage-specific regulation of the cell cycle by activating or suppressing specific cyclin-associated kinases.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, NIH; project number Z01 ES071006-09.
We thank Drs. C. Ronald Kahn and Argiris Efstratiadis for providing mice with an IRS-1 null allele and IGF-1 null allele, respectively. We thank Jesse DeGraff for supervising the breeding of the mutant mice. The assistance given by Janice Hicks, Maureen Trogdon, Ervin Smallwood, Coleen Anna, and Wes Gladwell is also greatly appreciated. We thank James Clark and Page Meyers for reliably preparing ovariectomized mice throughout the study. Sylvia C. Hewitt and Dr. Bonnie Deroo are gratefully acknowledged for providing the mouse uterine microarray data. The critique of the manuscript by Tom Gray and Sylvia C. Hewitt is also greatly appreciated.
Footnotes
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Adesanya OO, Zhou J, Samathanam C, Powell-Braxton L, Bondy CA. Insulin-like growth factor 1 is required for G2 progression in the estradiol-induced mitotic cycle. PNAS. 1999;96:3287–3291. doi: 10.1073/pnas.96.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altucci L, Addeo L, Cicatiello L, Germano D, Pacilio C, Battista T, Cancemi M, Petrizzi VB, Bresciani F, Weisz A. Estrogen induces early and timed activation of cyclin-dependent kinases 4, 5, and 6 and increases cyclin messenger ribonucleic acid expression in rat uterus. Endocrinology. 1997;138:978–984. doi: 10.1210/endo.138.3.5002. [DOI] [PubMed] [Google Scholar]
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag BL, III, Johnson RS, Kahn CR. Alternative pathway of insulin signaling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- Archer TK, Cordinley MG, Wolford RG, Hager GL. Transcription factor access is mediated by accurately positioned nucleosomes on the mouse mammary tumor virus promoter. Molecular and Cellular Biology. 1991;11:688–698. doi: 10.1128/mcb.11.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellvre AR, Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Molecular Endocrinology. 1996;10:903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Winnay J, Cheatham B, Kahn CR. Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Molecular and Cellular Biology. 1997;17:1513–1521. doi: 10.1128/mcb.17.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Pardee AB. Post-translational control of the onset of DNA synthesis by an insulin-like growth factor. Molecular and Cellular Biology. 1984;4:1807–1814. doi: 10.1128/mcb.4.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrera AA, Hale TK, Stenoien DL, Rosen JM, Mancini MA, Herrera RE. The dynamic mobility of histone H1 is regulated by cyclin/cdk phosphorylation. Molecular and Cellular Biology. 2003;23:8626–8636. doi: 10.1128/MCB.23.23.8626-8636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. PNAS. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Kurita T, Lubahn DB, Cunha GR. Role of stromal-epithelial interactions in hormonal responses in the uterus. In: Glasser SR, Alpin JD, Giudice LC, Tabibzadeh S, editors. The Endometrium. London: Taylor & Francis; 2002. pp. 151–166. [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Molecular Endocrinology. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- Dalton S. Cell cycle regulation of the human cdc2 gene. EMBO Journal. 1992;11:1797–1804. doi: 10.1002/j.1460-2075.1992.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi S, Cha H, Shapiro P. Requirement for phosphatidylinositol-3 kinase activity during progression through S-phase and entry into mitosis. Cellular Signaling. 2003;15:667–675. doi: 10.1016/s0898-6568(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Deterding LJ, Bunger MK, Banks GC, Tomer KB, Archer TK. Global changes in and characterization of specific sites of phosphorylation in mouse and human histone H1 isoforms upon CDK inhibitor treatment using mass spectrometry. Journal of Proteome Research. 2008;7:2368–2379. doi: 10.1021/pr700790a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deVantery C, Stutz A, Vassalli JD, Schorderet-Slatkine S. Acquisition of meiotic competence in growing mouse oocytes is controlled at both translational and posttranslational levels. Developmental Biology. 1997;187:43–54. doi: 10.1006/dbio.1997.8599. [DOI] [PubMed] [Google Scholar]
- Draetta G, Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1998;54:17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Enders GH, Maude SL. Traffic safety for the cell: Influence of cyclin-dependent kinase activity on genomic stability. Gene. 2006;371:1–6. doi: 10.1016/j.gene.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Fagg B, Martin L, Rogers LA, Clark BF, Quarmby VE. A simple method for preparing pure samples of uterine cells. Journal of Reproduction and Fertility. 1979;57:335–345. doi: 10.1530/jrf.0.0570335. [DOI] [PubMed] [Google Scholar]
- Gambrell RD, Bagnell CA, Greenblatt RB. Role of estrogens and progesterone in the etiology and prevention of endometrial cancer: review. American Journal of Obstetrics and Gynecology. 1983;146:696–707. doi: 10.1016/0002-9378(83)91014-1. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Molecular Endocrinology. 2003;17:2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- Kandel ES, Keen JS, Majewski N, DiCristofano A, Pandolfi PP, Feliciano CS, Gartel A, Hay N. Activation of Akt/protein kinase B overcomes a G2/M cell cycle checkpoint induced by DNA damage. Molecular and Cellular Biology. 2002;22:7831–7841. doi: 10.1128/MCB.22.22.7831-7841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Fujita N, Tsuroro T. Akt/protein kinase B-dependent phosphorylation and inactivation of WEE1Hu promote cell cycle progression at G2/M transition. Molecular and Cellular Biology. 2005;25:5725–5737. doi: 10.1128/MCB.25.13.5725-5737.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Proust JJ, Buchholz MJ, Chrest FJ, Nordin AA. Expression of the murine homologue of the cell cycle control protein p34cdc2 in T lymphocytes. Journal of Immunology. 1992;149:17–23. [PubMed] [Google Scholar]
- Klotz DM, Curtis Hewitt S, Korach KS, DiAugustine RP. Activation of a uterine insulin-like growth factor I signaling pathway by clinical and environmental estrogens: Requirement of estrogen receptor-α. Endocrinology. 2000;141:3430–3439. doi: 10.1210/endo.141.9.7649. [DOI] [PubMed] [Google Scholar]
- Langan TA, Gautier J, Lohka M, Hollingsworth R, Moreno S, Nurse P, Maller J, Sclafani RA. Mammalian growth-associated H1 histone kinase: a homolog of cdc2 +/cdc28 protein kinases controlling mitotic entry in yeast and frog cells. Molecular and Cellular Biology. 1989;9:3860–3868. doi: 10.1128/mcb.9.9.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-R, Park JH, Park EK, Chung CHS, Kang S-S, Bang OS. Akt-induced promotion of cell-cycle progression at G2/M phase involves upregulation of NF-Y binding activity in PC12 cells. Journal of Cellular Physiology. 2005;205:270–277. doi: 10.1002/jcp.20395. [DOI] [PubMed] [Google Scholar]
- Lembo G, Rockman HA, Hunter JJ, Steinmetz H, Koch WJ, Ma L, Prinz MP, Ross K, Jr, Chien KR, Powell-Braxton L. Elevated blood pressure and enhanced myocardial contractility in mice with severe IGF-1 deficiency. Journal of Clinical Investigation. 1996;98:2648–2655. doi: 10.1172/JCI119086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof EB, Wharton W, van Wyk JJ, Pledger WJ. Epidermal growth factor (EGF) and somatodmedin C regulate G1 progression in competent BALB/c-3T3 cells. Experimental Cell Research. 1982;141:107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Lew DJ, Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Current Opinion in Cell Biology. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor 1 (IGF-1) and type 1 IGF receptor (IGF1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Lowe WL. Biological actions of the insulin-like growth factor. In: LeRoith D, editor. Insulin-like growth factors: molecular and cellular aspects. Boca Raton: CRC Press; 1991. pp. 49–85. [Google Scholar]
- Loyer P, Glaise D, Cariou S, Baffet G, Meijer L, Guguen-Guillouzo C. Expression and activation of cdks (1 and 2) and cyclins in the cell cycle progression during liver regeneration. Journal of Biological Chemistry. 1994;269:2491–2500. [PubMed] [Google Scholar]
- McGowan CH, Russell P, Reed SI. Periodic biosynthesis of the human M-phase promoting factor catalytic subunit p34 during the cell cycle. Molecular and Cellular Biology. 1990;10:3847–3851. doi: 10.1128/mcb.10.7.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Arion D, Golsteyn R, Pines J, Brizuela L, Hunt T, Beach D. Cyclin is a component of the sea urchin egg M-phase specific histone H1 kinase. EMBO Journal. 1989;8:2275–2282. doi: 10.1002/j.1460-2075.1989.tb08353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R, Myers MG, Jr, White MF, Rhoads RE. Stimulation of protein synthesis, eukaryotic translation initiation factor 4E phosphorylation, and PHAS-I phosphorylation by insulin requires insulin receptor substrate 1 and phosphatidylinositol 3-kinase. Molecular and Cellular Biology. 1996;16:2857–2864. doi: 10.1128/mcb.16.6.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J, Goldsteyn R, Hill CS, Hunt T. The A- and B- type cyclin associated cdc2 kinases in xenopus turn on and off at different times in the cell cycle. EMBO Journal. 1990;9:2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Murphy LJ, Murphy LC, Friesen HG. Estrogen induces insulin-like growth factor-1 expression in the rat uterus. Molecular Endocrinology. 1987;1:445–450. doi: 10.1210/mend-1-7-445. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Cellular substrates of p34cdc2 and its companion cyclin-dependent kinases. Trends in Cell Biology. 1993;3:296–301. doi: 10.1016/0962-8924(93)90011-o. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature (Lond.) 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Okumura E, Fukuhara T, Yoshida H, Hanada S, Kozutsumi R, Mori M, Tachibana K, Kishimoto T. Akt inhibits Myt1 in the signaling pathway that leads to meiotic G2/M-phase transition. Nature Cell Biology. 2002;4:111–116. doi: 10.1038/ncb741. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Coronado EB, Kitten LJ, Arteaga CI, Fuqua SA, Ramasharma K, Marshall M, Li CH. Insulin-like growth factor-II (IGF-II): a potential autocrine/paracrine growth factor for human breast cancer acting via the IGF-1 receptor. Molecular Endocrinology. 1989;3:1701–1709. doi: 10.1210/mend-3-11-1701. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. (online). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RG, DiAugustine RP, Petrusz P, Clark GC, Sebastian J. Estradiol stimulates tyrosine phosphorylation of the insulin-like growth factor-1 receptor and insulin receptor substrate-1 in the uterus. PNAS. 1996;93:12002–12007. doi: 10.1073/pnas.93.21.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RG, Klotz DM, Bush MR, Walmer DK, DiAugustine RP. E2-induced degradation of uterine insulin receptor substrate-2: requirement for an IGF-1-stimulated, proteasome-dependent pathway. Endocrinology. 2001;142:3842–3849. doi: 10.1210/endo.142.9.8370. [DOI] [PubMed] [Google Scholar]
- Richards RG, Walker MP, Sebastian J, DiAugustine RP. Insulin-like growth factor-1 (IGF-1) receptor-insulin receptor substrate complexes in the uterus. Altered signaling response to estradiol in the IGF-1m/m mouse. Journal of Biological Chemistry. 1998;273:11962–11969. doi: 10.1074/jbc.273.19.11962. [DOI] [PubMed] [Google Scholar]
- Rose DW, Saltile AR, Majumdar M, Decker SJ, Olefsky JM. Insulin receptor substrate 1 is required for insulin-mediated mitogenic signal transduction. PNAS. 1994;91:797–801. doi: 10.1073/pnas.91.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaün P, LeBreton M, Morales J, Belle R, Boulben S, Mulner-Lorillon O, Cormier P. Signal transduction pathways that contribute to CDK1/cyclin B activation during the first mitotic division in sea urchin embryos. Experimental Cell Research. 2002;296:347–357. doi: 10.1016/j.yexcr.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the type 1 IGF receptor gene on growth and transformation of mouse embryo fibroblasts. Molecular and Cellular Biology. 1994;14:3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E, Sussman JS, Stokoe D. A role for PI 3-kinase and PKB activity in the G2/M phase of the cell cycle. Current Biology. 2002;12:919–924. doi: 10.1016/s0960-9822(02)00843-6. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Wang LM, Zhang Y, Yenush L, Myers MG, Jr, Glasheen EM, Lane WS, Pierce JH, White MF. Role of IRS-2 in insulin and cytokine signaling. Nature. 1995;377:173–177. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- Swank RA, Th’ng JPH, Guo X-W, Valdez J, Bradbury EM, Gurley LR. Four distinct cyclin-dependent kinases phosphorylate histone H1 at all of its growth-related phosphorylation sites. Biochemistry. 1997;36:13761–13768. doi: 10.1021/bi9714363. [DOI] [PubMed] [Google Scholar]
- Tong W, Pollard JW. Female sex steroid hormone regulation of cell proliferation in the endometrium. In: Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. The Endometrium. London: Taylor & Francis; 2002. pp. 94–109. [Google Scholar]
- Tong W, Pollard JW. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E-and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Molecular and Cellular Biology. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SB, Yamauchi K, Pessin JE. Functional expression of insulin receptor substrate-1 is required for insulin-stimulated mitogenic signaling. Journal of Biological Chemistry. 1993;268:22231–22234. [PubMed] [Google Scholar]
- Welch PJ, Wang JYJ. Coordinated synthesis and degradation of cdc2 in the mammalian cell cycle. PNAS. 1992;89:3093–3097. doi: 10.1073/pnas.89.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]