Abstract
A multiplexed assay was developed by MS to analyze, in a single run, six major human Apos involved in lipoprotein metabolism: ApoA-I, ApoA-II, ApoB100, ApoC-II, ApoC-III, and ApoE. This method was validated in vivo in six subjects who received a 14 h constant infusion of [5,5,5-2H3]L-leucine at 10 μM/kg/h. Plasma lipoprotein fractions were isolated from collected blood samples and were digested with trypsin. Proteotypic peptides were subsequently analyzed by LC/MS/MS. Enrichment measurement data were compared with those obtained by the standard method using GC/MS. The required time to obtain the LC/MS/MS data was less than that needed for GC/MS. The enrichments from both methods were correlated for ApoA-I (r = 0.994; P < 0.0001) and ApoB100 (r = 0.999; P < 0.0001), and the Bland-Altman plot confirmed the similarity of the two methods. Intra- and inter-assay variability calculated for the six Apos of interest did not exceed 10.7 and 12.5%, respectively, and kinetic parameters were similar and/or in agreement with previously reported data. Therefore, LC/MS/MS can be considered as a useful tool for human Apo kinetic studies using stable isotopes.
Keywords: lipoproteins/metabolism, lipoproteins/kinetics, nutrition protein, multiplexed assay, stable isotope, liquid chromatography-tandem mass spectrometry
Lipoprotein kinetic studies using radioactive or stable isotope tracers have been performed for years in humans to gain a better understanding of the mechanisms involved in lipid metabolism disturbances and related diseases (1, 2). Endogenous labeling with an amino acid tracer of Apo, a main component of lipoproteins, is commonly used, assuming the kinetics of Apos represent a good estimate of those of the entire lipoprotein metabolism (1, 3). The incorporation of a labeled amino acid into the protein during its synthesis is subsequently measured over time and analyzed with a compartmental model to obtain the kinetic data (4, 5). The reference method to measure tracer enrichments involves isolation of the Apos by gel electrophoresis followed by acid hydrolysis to obtain unlabeled and labeled amino acids. Then, the amino acids are derivatized for GC/MS analysis (3). These approaches are limited to one or a small number of relatively abundant Apos and remain a time-consuming process. Kinetic studies are therefore mainly focused on the most abundant structural Apos (ApoA-I and ApoB100) and less on others, although they have a central role in lipid metabolism (ApoC-II, ApoC-III and ApoE) (6).
The combination of proteomic tools, such as enzymatic proteolysis and liquid LC/MS/MS, has appeared recently to be a powerful tool to study plasma proteins (7–11). Although promising, one analytical challenge is to use this method in a single run analysis for the determination of concentrations and tracer enrichments of a significant set of plasma proteins with large differences in molecular mass or abundances (6, 11).
We recently published an LC/MS/MS method to simultaneously measure the concentration, tracer enrichment, and average size of Apo(a) (9, 12). In the present study, we aimed to describe the development and the validation of a multiplexed LC/MS/MS method, performing in a single run the enrichment measurements and the quantification of six major human Apos (ApoA-I, ApoA-II, ApoB100, ApoC-II, ApoC-III, and ApoE) in plasma samples obtained from a stable isotope kinetic study in humans.
MATERIALS AND METHODS
Reagents
UPLC/MS-grade acetonitrile, water, and 99% formic acid were purchased from Biosolve (Valkenswaard, The Netherlands). Ammonium bicarbonate (AB), [5,5,5-2H3]L-leucine, DTT, iodoacetamide, sodium deoxycholate (SDC), trypsin, ammonium hydroxide (NaOH), and 37% hydrochloric acid (HCl) were obtained from Sigma-Aldrich (Saint-Quentin Fallavier, France). Synthetic labeled and unlabeled peptides were purchased from Thermo Scientific Biopolymers (Einsteinstrasse, Germany).
Subjects and sample collection
Six overweight male subjects (age: 46 ± 16 years; body mass index: 31.8 ± 1.5 kg/m2) with hypertriglyceridemia (plasma triglycerides: 208 ± 57 mg/dl) were enrolled. After an overnight fast, each subject received a bolus of 10 μM/kg 2H3-leucine, which allowed a faster plasma enrichment plateau, immediately followed by a constant infusion (10 μM/kg/h) of 2H3-leucine for 14 h. Blood samples were collected at 0, 0.75, 1.5, 2.5, 4, 6, 8, 10, 12, and 14 h in EDTA tubes (Venoject, Paris, France), and the plasma was separated by centrifugation at 4°C for 30 min. Plasma lipoprotein fractions, including VLDL, LDL, IDL, and HDL, were separated by sequential ultracentrifugation methods (13, 14) and stored at −80°C until analysis. The Ethics Committee of Nantes University Hospital approved the clinical protocol, and written informed consent was obtained from each subject (reference trial number: NCT01216956).
Selection of peptide markers
Apo sequences were BLAST searched using the UNIPROT tool (www.uniprot.org), and theoretical proteotypic peptides were searched using the free software peptide mass calculator (http://web.expasy.org/peptide_mass). The peptide candidates were selected to maximize sensitivity, specificity, and stability. Therefore, peptides carrying methionine and cysteine residues were not considered due to potential oxidation and peptides having less than seven amino acids were excluded. Furthermore, peptide candidates had to contain at least one leucine residue for 2H3-leucine enrichment measurement. Each putative candidate was then experimentally sought in the appropriate concentrated lipoprotein fraction and then characterized by LC-high resolution MS (LC/HRMS).
Sample preparation for LC/MS/MS analysis
The plasma lipoprotein fractions (100 μl) were desalted and concentrated with 50 mM AB buffer (pH 8; 3 ml) and a 5,000 Da molecular mass cut-off filter. The concentrated samples (100 μl) were mixed with 50 mM AB buffer (pH 8; 88 μl), 10% SDC (10 μl), and 500 mM DTT (2 μl). The samples were reduced for 30 min at 60°C, then alkylated with 2 μl of fresh iodoacetamide solution (1 M in 1 M NaOH) for 60 min at room temperature, and protected from light. The samples were digested overnight with 10 μl of trypsin solution (0.1 mg/ml in 1 mM HCl), and 10 μl of 20% formic acid was added to stop the reaction and to precipitate the SDC. Finally, the samples were centrifuged at 15,000 g at 4°C for 15 min, and the supernatants (150 μl) were transferred to vials for LC/MS/MS analyses. Apos were quantified in plasma and plasma lipoprotein fractions, as previously described (12), using synthetic proteotypic peptides for standard solutions and labeled [13C6, 15N2]K or [13C6, 15N4]R synthetic peptides as internal standards (Table 1). Apo quantification was achieved in three replicates and at three kinetic time points (baseline, 6 h, and 14 h).
TABLE 1.
Summary of the analytical parameters selected for the detection of Apos
| Apo | Peptide Sequence | Fragment | Cone/Collision (V) | MRM Transition (m/z) | Retention Time (min) |
| ApoA-I | |||||
| M0 | ATEHLSTLSEK | y6+ | 30/25 V | 608.3 → 664.3 | 1.6 ± 0.1 |
| M3 | ATEH L ST L SEK | 609.8 → 664.3 + 667.3 | |||
| IS | ATEHLSTLSEK | 612.3 → 672.3 | |||
| ApoA-II | |||||
| M0 | SPELQAEAK | y6+ | 30/30 V | 486.8 → 659.3 | 1.4 ± 0.1 |
| M3 | SPE L QAEAK | 488.3 → 662.3 | |||
| IS | SPELQAEAK | 490.3 → 667.3 | |||
| ApoB100 | |||||
| M0 | NLQNNAEWVYQGAIR | y6+ | 50/30 V | 888.5 → 707.4 | 2.7 ± 0.1 |
| M3 | N L QNNAEWVYQGAIR | 890.0 → 707.4 | |||
| IS | NLQNNAEWVYQGAIR | 893.5 → 717.4 | |||
| ApoC-II | |||||
| M0 | TAAQNLYEK | y4+ | 80/30 V | 1,037.9 → 552.3 | 1.4 ± 0.1 |
| M3 | TAAQN L YEK | 1,040.9 → 555.3 | |||
| IS | TAAQNLYEK | 1,045.9 → 560.3 | |||
| ApoC-III | |||||
| M0 | DALSSVQESQVAQQAR | y8+ | 40/35 V | 858.9 → 887.5 | 2.0 ± 0.1 |
| M3 | DA L SSVQESQVAQQAR | 860.4 → 887.5 | |||
| IS | DALSSVQESQVAQQAR | 863.4 → 897.5 | |||
| ApoE | |||||
| M0 | LGPLVEQGR | y5+ | 25/30 V | 484.8 → 588.3 | 2.1 ± 0.1 |
| M3 | L GP L VEQGR | 486.3 → 588.3 | |||
| IS | LGPLVEQGR | 489.3 → 598.3 |
Underlined L indicates the putative incorporation site(s) of 2H3-leucine. Bold indicates the labeled amino acid [13C6, 15N2]K or [13C6, 15N4]R. IS, internal standard.
LC/MS/MS parameters
Peptide candidates were identified and characterized using a LC/HRMS system composed of a Synapt G2 HDMS® quadrupole-TOF mass spectrometer (Waters Corporation, Milford, MA) with an ESI interface and an Acquity H-Class® UPLCTM device (Waters Corporation). High throughput analyses were then performed on a Xevo® triple-quadrupole mass spectrometer with an ESI interface equipped with an Acquity H-Class® UPLCTM device. Data acquisition and analyses were performed with MassLynx® and TargetLynx® software, respectively (version 4.1; Waters Corporation). Labeled and unlabeled peptides were separated on an Acquity® BEH C18 column (2.1 × 100 mm, 1.7 μm, Waters) at 60°C with a linear gradient of mobile phase B (acetonitrile containing 0.1% formic acid) in mobile phase A (5% acetonitrile in water containing 0.1% formic acid) at a flow rate of 600 μl/min. Mobile phase B was linearly increased from 1 to 50% for 5 min, kept constant for 1 min, returned to the initial condition over 1 min, and kept constant for 1 min before the next injection. Ten microliters of each sample was injected into the LC column. Labeled and unlabeled peptides were then detected by the mass spectrometer with the ESI interface operating in the positive ion mode (capillary voltage, 4 kV; desolvation gas (N2) flow and temperature, 1,000 l/h and 400°C; source temperature, 120°C). The multiple reaction monitoring (MRM) mode was applied for MS/MS detection, and the parameters were optimized for each peptide from synthetic peptide solutions. Selected MRM transitions, cone voltages, and collision energies are described in Table 1.
Conventional GC/MS method for ApoA-I and ApoB100 kinetic measurements
Isolation and measurement of leucine enrichment in ApoB100 and ApoA-I were described previously (14, 15). Briefly, ApoB100- and ApoA-I-containing lipoprotein fractions were isolated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then hydrolyzed with HCl. Amino acids were purified by cation exchange chromatography, derivatized (N-propanol-acetyl chloride and heptafluorobutyric acid), and analyzed by GC/MS to determine [5,5,5-2H3]leucine enrichment. ApoA-I concentrations were measured by immunonephelometry (Behring, Rueil Malmaison, France), and ApoB100 concentrations were obtained by selective precipitation and GC/MS with norleucine as internal standard (16).
Data management
The M3/M0 ratios were calculated using the chromatographic peak areas, where M3 corresponds to the 2H3-leucine-labeled peptide, and M0 corresponds to the unlabeled peptide. For peptides containing two leucines (ApoA-I and ApoE), two M3 isotopomers could form and be detected simultaneously by the selected MRM transitions. Their proportions were assumed to be identical, as described previously (9, 17), and the analytical signal obtained during labeled ApoA-I and ApoE detection was experimentally enhanced 2-fold by the two coeluted isotopomers. In addition, the peptide isotopologues of ApoA-I and ApoE, containing two labeled leucine residues, were not detected in our analytical conditions and were considered as negligible. Therefore, the M3/M0 ratios measured in the biological samples were corrected by dividing the primary result by two, as described previously (17). The M3/M0 ratios measured at baseline were subtracted from the following time point ratios. Chromatographic peaks having signal-to-noise ratios below the limit of quantification of 10:1 were excluded and samples were reanalyzed using higher sample volumes before the concentration step (400 μl). Apo concentrations were calculated using calibration curves plotted from standard solutions, as described previously (12). The primary results were expressed in nanomoles and were converted to milligrams per deciliter assuming 1 mol of peptide equal to 1 mol of protein (10).
Kinetic analysis
Kinetic analysis was achieved using the SAAM II modeling program (Epsilon Group, Charlottesville, VA). HDL-ApoA-I, HDL-ApoE, and VLDL-ApoE fractional catabolic rates (FCRs) were estimated from the 14 h samples with a mono-compartmental model, as described previously (18, 19). We applied the same model for HDL-ApoA-II, HDL-ApoC-III, and VLDL-ApoC-III according to Batal et al. (20) and Chan et al. (21), but also for HDL-ApoC-II and VLDL-ApoC-II. Kinetic data of VLDL-, IDL-, and LDL-ApoB100 were calculated using a three compartmental model, as previously described (22). Plasma leucine was used as precursor pool and pool sizes were considered to be constant, as no significant variation was observed on Apo concentrations at different sampling times (not shown). According to this steady state model, the FCR was equal to the fractional synthetic rate. Production rates (PRs) were calculated by the product of the FCR and the pool sizes of Apos in plasma lipoprotein fractions, assuming a plasma volume of 4.5% of body weight. For ApoE, ApoC-II, and ApoC-III, the concentrations that were not recovered (in the bottom fractions) were considered to be predominantly HDL Apos and these amounts were mathematically added to HDL for calculation of HDL pool sizes (20).
Validation of the multiplexed LC/MS/MS method and statistical analysis
To assess the intra- and inter-assay variability of the LC/MS/MS method, pooled plasma lipoprotein fractions were prepared by mixing 400 μl of plasma lipoprotein fractions from the six subjects at the following kinetic time points: baseline, 45 min, 6 h, and 14 h. These points were chosen to reach baseline, low, intermediate, and high tracer enrichment levels, respectively. The VLDL fractions were used for ApoB100 assay validation, and the HDL fractions were used for the other Apos (i.e., ApoA-I, ApoA-II, ApoC-II, ApoC-III, and ApoE). Each pool was then divided into 18 equal fractions of 100 μl and treated as described above. Six fractions per time point were analyzed, and the analyses were repeated on three consecutive days. The intra- and inter-assay variability of the LC/MS/MS method was calculated [coefficient of variation (CV), percent] with a maximum tolerance level of 15% (23). To assess the accuracy of the LC/MS/MS method, enrichment of ApoA-I in the HDL sample and ApoB100 in the VLDL, IDL, and LDL samples were measured by conventional methods using GC/MS (six subjects, 10 kinetic time points per plasma lipoprotein fraction). The paired results, baseline excluded, obtained for ApoA-I (HDL, n = 54) and ApoB100 (VLDL, IDL, and LDL, n = 162) with both analytical methods were analyzed using a Pearson correlation test. A Bland-Altman plot was also generated to test the similarity of both methods accurately (24). Graphics and statistical analyses were achieved with GraphPad Prism software (version 6.0; GraphPad Software Inc., La Jolla, CA).
RESULTS
Selection of peptide markers
The selection of specific peptide markers was performed successfully for each target Apo by LC/HRMS. The in silico selection of the proteotypic peptides led to 2 (ApoA-II and ApoC-III), 3 (ApoC-II), 16 (ApoE), 19 (ApoA-I), and 73 (ApoB100) peptide candidates. The most specific and detectable of them were selected to optimize the assay sensitivity and specificity. As shown in Table 1, each candidate was detected as a doubly charged precursor ion, except for ApoC-II. After MS/MS fragmentations, each precursor ion yielded between 10 (ApoE) and 29 (ApoC-III) specific and singly charged product ions (supplementary Fig. 1A, B). As an example, for ApoA-I and ApoB100, the complete characterization of the fragmentation patterns identified the peptide sequences underlying their specificity. The most intense product ion was then selected for MRM transitions leading to the specific detection of the target peptides (supplementary Fig. 1C). As illustrated for ApoE (supplementary Fig. 2), the MRM mode allowed the specific detection of both labeled and unlabeled target peptides. As expected, the chromatographic peak intensities corresponding to the labeled ApoE peptide (M3) increased during the course of the labeled tracer perfusion.
Comparison of LC/MS/MS with conventional GC/MS methods
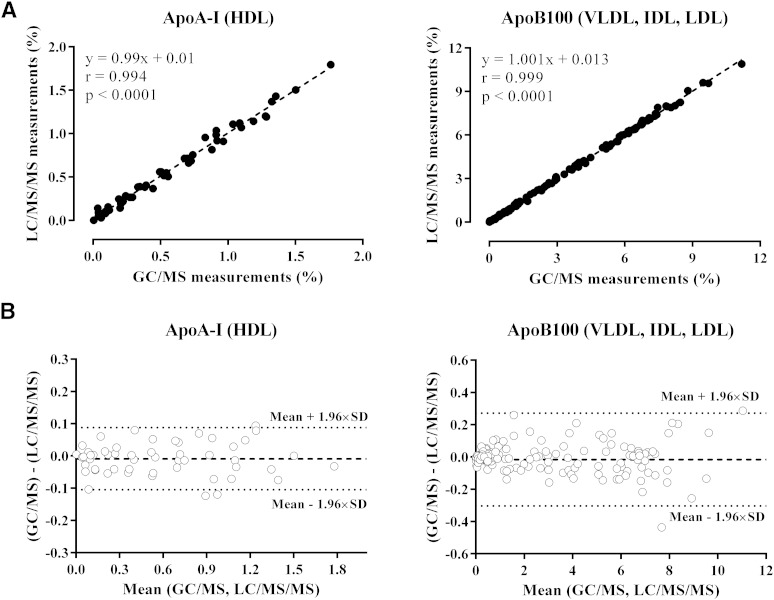
As illustrated in Fig. 1A, the data obtained with the two methods were not statistically different and were significantly correlated with a slope close to one for ApoA-I in HDL (r = 0.994, P < 0.0001, y = 0.99x + 0.01) and ApoB100 in VLDL/IDL/LDL (r = 0.999, P < 0.0001, y = 1.001x + 0.013). For the Bland-Altman plot, the mean difference and the limits of agreement, corresponding to the 95% confidence level (i.e., mean ± 1.96 × SD), were drawn (Fig. 1B), and 94 and 98% of the points were between the limits of similarity for ApoA-I and ApoB100, respectively.
Fig. 1.
Comparison of the conventional GC/MS method and the LC/MS/MS method for 2H3-leucine incorporation measurements in ApoA-I (HDL, n = 54) and ApoB100 (VLDL, IDL, and LDL, n = 162). Linear correlation obtained between the two methods (A) and comparison of the two methods using the Bland-Altman plot (B). For the Bland-Altman plot, the average enrichment levels (percent) obtained by both methods were calculated and then plotted against the difference of the two measurements.
Validation of the LC/MS/MS method
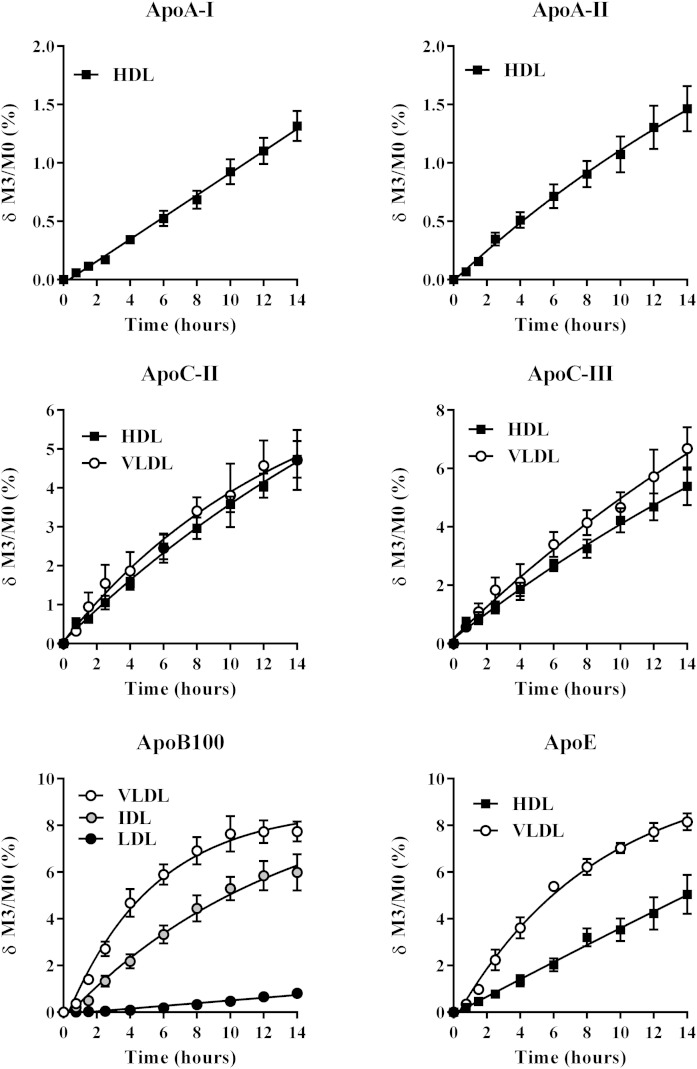
The accuracy of the LC/MS/MS method was established by comparing the ApoA-I and ApoB100 enrichment measurements with those obtained by the reference methods used for many years, as described above. The precision of the LC/MS/MS measurements was determined by CVs calculated from six replicates per enrichment level and over three distinct experiments. The intra- and inter-assay variability did not exceed 10.7 and 12.5%, respectively, for any of the Apos or for various enrichments ranging from 0.04 to 7.72% (supplementary Table 1). Finally, the LC/MS/MS efficiency is illustrated in Fig. 2, which shows the kinetic enrichment curves of the six Apos. The kinetic measurements were assessed simultaneously in 240 samples within 1 week (6 subjects, 10 kinetic time points, and 4 plasma lipoprotein fractions).
Fig. 2.
Mean changes in 2H3-leucine incorporation over the course of the tracer infusion in six hypertriglyceridemic patients. Basal enrichment was subtracted (δ M3/M0). Results were obtained for ApoA-I (HDL), ApoA-II (HDL), ApoB100 (VLDL, IDL, and LDL), ApoC-II (VLDL, HDL), ApoC-III (VLDL, HDL), and ApoE (VLDL, HDL). Values are presented as the mean ± SEM.
Kinetic parameters of Apos
Apos were successfully quantified in plasma and lipoprotein fractions by LC/MS/MS. Total recoveries (mean ± SEM) of ApoE, ApoC-II, and ApoC-III in plasma lipoprotein fractions separated by ultracentrifugation were 69.7 ± 2.5%, 79.1 ± 6.9%, and 67.5 ± 2.7%, respectively, and ranged from 96.2 to 104.7% for ApoA-I, ApoA-II, and ApoB100. As CVs did not exceed 12.4% and Apo concentrations were stable throughout the kinetics, the mean values were kept as final concentrations (Table 2). ApoA-I/ApoA-II concentration ratio was of 4.0 ± 0.7 in HDL and ApoC-III/ApoC-II concentration ratios were of 3.3 ± 0.3 and 3.3 ± 0.9 in HDL and VLDL, respectively. As shown in Table 2, ApoA-I and ApoB100 concentrations measured by LC/MS/MS and conventional methods were not different. Kinetic parameters of Apos were also similar with both methods for ApoB100 and ApoA-I. Compared with previous reported data, the results obtained for ApoA-II, ApoC-II, ApoC-III, and ApoE were in similar ranges (Table 2, supplementary Table 2).
TABLE 2.
Kinetic parameters of Apos
| Apos | Concentration (mg/dl) | FCR (pool/day) | PR (mg/kg/day) |
| HDL-ApoA-I, LC/MS/MS | 139.0 ± 10.5 | 0.28 ± 0.04 | 17.7 ± 3.0 |
| HDL-ApoA-I, GC/MS | 136.3 ± 7.6 | 0.28 ± 0.04 | 17.2 ± 2.6 |
| HDL-ApoA-II, LC/MS/MS | 38.8 ± 6.4 | 0.36 ± 0.06 | 6.0 ± 1.0 |
| VLDL-ApoB100, LC/MS/MS | 8.8 ± 1.1 | 5.44 ± 1.1 | 20.7 ± 2.8 |
| VLDL-ApoB100, GC/MS | 8.7 ± 1.2 | 5.65 ± 0.9 | 21.3 ± 2.8 |
| IDL-ApoB100, LC/MS/MS | 3.9 ± 0.5 | 5.66 ± 1.15 | 10.0 ± 2.5 |
| IDL-ApoB100, GC/MS | 3.7 ± 0.4 | 5.60 ± 1.12 | 9.9 ± 2.6 |
| LDL-ApoB100, LC/MS | 52.5 ± 8.3 | 0.30 ± 0.04 | 7.0 ± 1.3 |
| LDL-ApoB100, GC/MS | 49.5 ± 6.5 | 0.31 ± 0.05 | 6.8 ± 1.3 |
| HDL-ApoC-II, LC/MS/MS | 1.5 ± 0.3 | 1.39 ± 0.30 | 0.9 ± 0.2 |
| VLDL-ApoC-II, LC/MS/MS | 5.4 ± 1.5 | 1.44 ± 0.45 | 3.3 ± 1.1 |
| HDL-ApoC-III, LC/MS/MS | 4.5 ± 0.9 | 1.11 ± 0.12 | 3.2 ± 0.8 |
| VLDL-ApoC-III, LC/MS/MS | 13.2 ± 1.7 | 1.17 ± 0.18 | 13.0 ± 2.0 |
| HDL-ApoE, LC/MS/MS | 2.5 ± 0.5 | 0.58 ± 0.09 | 0.7 ± 0.3 |
| VLDL-ApoE, LC/MS/MS | 1.6 ± 0.3 | 2.70 ± 0.46 | 2.0 ± 0.6 |
Values are mean ± SEM.
DISCUSSION
The current approaches with the conventional GC/MS method to measure the Apo kinetics of lipoproteins using stable isotope-labeled tracers are often limited to a small number of relatively abundant proteins, such as ApoB100 and ApoA-I, and require a complex and time-consuming preparation. This study demonstrated that enzymatic proteolysis and subsequent LC/MS/MS analysis can overcome this pitfall.
The choice of the peptide candidates is a critical point to conduct Apo measurements by LC/MS/MS. They must be selected to not interfere with other nontargeted proteins. For example, the amino acid sequence of ApoB48 is 48%, identical to that of the N-terminal ApoB100 sequence (25). The trypsin digestion of ApoB100 yields a set of peptides indistinguishable from those generated for ApoB48; therefore, they must not be considered (not shown). All the candidates shown in Table 1 meet this criteria and some of them (ApoA-I, ApoA-II, and ApoE) were identical to those selected by Ceglarek et al. (11). The high level of reproducibility (CVs <15%) reinforced their relevance for the kinetic enrichment measurements of the targeted Apos.
We confirmed that LC/MS/MS is able to accurately quantify plasma proteins, as previously published (6, 8, 10, 11). We measured similar ApoB100 and ApoA-I concentrations whatever the analytical method previously employed. For the other Apos, the concentrations were in the same range compared with other studies involving either LC/MS/MS (6, 8, 11) or conventional methods (5, 14, 15, 18–22, 26–28). In addition, ApoA-I/ApoA-II and ApoC-III/ApoC-II concentration ratios were also in the reported ranges (6, 11, 19, 21, 26). However, some drawbacks may be encountered when using LC/MS/MS. The use of proteotypic peptides involves an optimal proteolysis to obtain a full recovery; otherwise the concentrations could be underestimated. Using our protocol and an overnight trypsin digestion, the hydrolysis was complete for each target protein (6, 9–11). LC/MS/MS detector responses could also be altered by matrix effects. It was unfortunately impossible to accurately quantify Apos directly in desalted samples of lipoproteins (5,000 Da molecular mass cut-off filter) because we did not find solution to assess total Apo recoveries during the desalting process. For quantification, samples were therefore assayed separately and the sample dilution (1/25) minimized matrix effects (12) without significantly affecting the limit of quantification of the Apos analyzed in this study (signal-to-noise ratio >10 in each sample). LC/MS/MS is based on proteotypic peptide analysis and results are primarily expressed as moles per liter. To convert concentrations in milligrams per deciliter, protein molecular masses are used, assuming a single polymorphic isoform for each Apo. This is not the case for ApoE and ApoC-III (20). The accurate detection of isoforms could be assessed by LC/MS/MS (10, 12), but this is a complex analysis. We have assumed that these polymorphic variations did not significantly alter the molecular masses of the targeted Apos. This is another limitation of LC/MS/MS, but the comparison with the other methods used for concentration measurements is reassuring and suggests that this pitfall is probably not critical. The protocol used (primed and constant infusion of the tracer) is another limitation, as tracer boluses are often preferred for proteins with slow turnover rates (29). With this latter study design, the peak enrichments are different compared with the range we have analyzed. Additional studies are warranted to validate this LC/MS/MS method with the bolus tracer study design.
To compare and validate our LC/MS/MS method with the conventional GC/MS method for enrichment measurements, we used 240 biological samples (6 subjects, 10 kinetic time points, 4 plasma lipoprotein fractions). From isolation by gel electrophoresis (VLDL-, IDL,-, LDL-ApoB100, and HDL-ApoA-I) to GC/MS processing, it took 3 weeks to obtain results, while the LC/MS/MS results were obtained within 5 days. We acknowledge that this observation relied on the laboratory technical resources, but the LC/MS/MS method was also able to provide measurements for additional Apos (Fig. 2), which would have required some additional time with the conventional method. As suggested above, one limitation of the LC/MS/MS method measurements was the loss of accuracy for chromatographic peaks having signal-to-noise ratios below 10. For the six Apos studied, only seven samples (five VLDL and two IDL samples, ApoB100 only) needed to be reanalyzed. The problem was overcome by using higher sample volumes (400 μl) during the concentration step. As shown by the Bland-Altman analysis, the enrichments measured by LC/MS/MS in this study were identical to those measured with the conventional GC/MS method and were in the same range as those reported previously by others using similar stable isotope protocols and the GC/MS method for ApoA-I (15, 26) and ApoB100 (20, 27). Similar results have been also reported for ApoA-II (21, 26), ApoE (19, 20), and ApoC-III (20, 28). To the best of our knowledge, no data have been published for ApoC-II with GC/MS measurements.
The kinetics of Apos were in good agreement with those previously published in several studies (see supplementary Table 2). For HDL-ApoA-I- and HDL-ApoB100-containing lipoproteins, FCRs and PRs were similar whatever the analytical method employed and in the same range as those already published (5, 14, 18–22, 26, 27). In addition, HDL-ApoA-II FCRs were close to those obtained for HDL-ApoA-I and ensuing PRs were also consistent with previous estimates (21, 26). Similar findings were observed for ApoE and ApoC-III (19, 20, 28), although the patients studied and the study design were not perfectly comparable. As previously explained (28) and unlike ApoE (19, 20), ApoC-III FCRs calculated in VLDL and HDL were similar, supporting rapid exchanges of ApoC-III between both particles. We found similar data for ApoC-II but, to our knowledge, no previous work can support this finding. Despite the lack of direct comparisons in our study with the conventional GC/MS method for ApoA-II, ApoC-II, ApoC-III, and ApoE, the comparison of our kinetic data with already published works supported the efficiency of the LC/MS/MS method.
Finally, we could easily add the kinetic analysis for Apo(a) that we have reported previously (12) to this multiplexed LC/MS/MS analysis. Along with others, we have recently shown that LC/MS/MS can also be used to quantify plasma proteins and assess protein sequence modifications, such as polymorphic size (6, 10–12). In this study, we have focused on the concentrations and the tracer enrichments of six major human Apos, measured using a simple and fast protocol. These analytical methods could be merged and adapted continually to obtain, in a single run, the concentrations, polymorphic modifications, and stable isotope enrichments of most of the human Apos, making LC/MS/MS a useful tool for human lipoprotein kinetic studies.
Supplementary Material
Acknowledgments
The authors thank the staff of the Clinical Investigation Center of the University Hospital in Nantes, especially Eliane Hiverneau for her invaluable help with patients and blood collection. They also thank Stéphanie Billon-Crossouard and Audrey Aguesse for their technical assistance for developing this method.
Footnotes
Abbreviations:
- AB
- ammonium bicarbonate
- CV
- coefficient of variation
- FCR
- fractional catabolic rate
- HCl
- hydrochloric acid
- LC/HRMS
- LC-high resolution MS
- M0
- unlabeled peptide
- M3
- 2H3-leucine-labeled peptide
- MRM
- multiple reaction monitoring
- NaOH
- ammonium hydroxide
- PR
- production rate
- SDC
- sodium deoxycholate
This work was supported by the Biogenouest CORSAIRE core facility.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Foster D. M., Barrett P. H., Toffolo G., Beltz W. F., and Cobelli C.. 1993. Estimating the fractional synthetic rate of plasma apolipoproteins and lipids from stable isotope data. J. Lipid Res. 34: 2193–2205. [PubMed] [Google Scholar]
- 2.Lam S. M., and Shui G.. 2013. Lipidomics as a principal tool for advancing biomedical research. J. Genet. Genomics. 40: 375–390. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein A. H., Cohn J. S., Hachey D. L., Millar J. S., Ordovas J. M., and Schaefer E. J.. 1990. Comparison of deuterated leucine, valine, and lysine in the measurement of human apolipoprotein A-I and B-100 kinetics. J. Lipid Res. 31: 1693–1701. [PubMed] [Google Scholar]
- 4.Barrett P. H., and Watts G. F.. 2003. Kinetic studies of lipoprotein metabolism in the metabolic syndrome including effects of nutritional interventions. Curr. Opin. Lipidol. 14: 61–68. [DOI] [PubMed] [Google Scholar]
- 5.Matthan N. R., Jalbert S. M., Lamon-Fava S., Dolnikowski G. G., Welty F. K., Barrett H. R., Schaefer E. J., and Lichtenstein A. H.. 2005. TRL, IDL, and LDL apolipoprotein B-100 and HDL apolipoprotein A-I kinetics as a function of age and menopausal status. Arterioscler. Thromb. Vasc. Biol. 25: 1691–1696. [DOI] [PubMed] [Google Scholar]
- 6.Pan Y., Zhou H., Mahsut A., Rohm R. J., Berejnaia O., Price O., Chen Y., Castro-Perez J., Lassman M. E., McLaren D., et al. 2014. Static and turnover kinetic measurement of protein biomarkers involved in triglyceride metabolism including apoB48 and apoA5 by LC/MS/MS. J. Lipid Res. 55: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee A. Y., Yates N. A., Ichetovkin M., Deyanova E., Southwick K., Fisher T. S., Wang W., Loderstedt J., Walker N., Zhou H., et al. 2012. Measurement of fractional synthetic rates of multiple protein analytes by triple quadrupole mass spectrometry. Clin. Chem. 58: 619–627. [DOI] [PubMed] [Google Scholar]
- 8.Lassman M. E., McLaughlin T. M., Somers E. P., Stefanni A. C., Chen Z., Murphy B. A., Bierilo K. K., Flattery A. M., Wong K. K., Castro-Perez J. M., et al. 2012. A rapid method for cross-species quantitation of apolipoproteins A1, B48 and B100 in plasma by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 26: 101–108. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H., Castro-Perez J., Lassman M. E., Thomas T., Li W., McLaughlin T., Dan X., Jumes P., Wagner J. A., Gutstein D. E., et al. 2013. Measurement of apo(a) kinetics in human subjects using a microfluidic device with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 27: 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassman M. E., McLaughlin T. M., Zhou H., Pan Y., Marcovina S. M., Laterza O., and Roddy T. P.. 2014. Simultaneous quantitation and size characterization of apolipoprotein(a) by ultra-performance liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 28: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 11.Ceglarek U., Dittrich J., Becker S., Baumann F., Kortz L., and Thiery J.. 2013. Quantification of seven apolipoproteins in human plasma by proteotypic peptides using fast LC-MS/MS. Proteomics Clin. Appl. 7: 794–801. [DOI] [PubMed] [Google Scholar]
- 12.Croyal M., Ouguerram K., Passard M., Ferchaud-Roucher V., Chétiveaux M., Billon-Crossouard S., de Gouville A. C., Lambert G., Krempf M., and Nobécourt E.. 2015. Effects of extended-release nicotinic acid on apolipoprotein (a) kinetics in hypertriglyceridemic patients. Arterioscler. Thromb. Vasc. Biol. 35: 2042–2047. [DOI] [PubMed] [Google Scholar]
- 13.Havel R. J., Eder H. A., and Bragdon J. H.. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouguerram K., Chetiveaux M., Zair Y., Costet P., Abifadel M., Varret M., Boileau C., Magot T., and Krempf M.. 2004. Apolipoprotein B100 metabolism in autosomal-dominant hypercholesterolemia related to mutations in PCSK9. Arterioscler. Thromb. Vasc. Biol. 24: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 15.Chétiveaux M., Ouguerram K., Zair Y., Maugère P., Falconi I., Nazih H., and Krempf M.. 2004. New model for kinetic studies of HDL metabolism in humans. Eur. J. Clin. Invest. 34: 262–267. [DOI] [PubMed] [Google Scholar]
- 16.Beghin L., Duhal N., Poulain P., Hauw P., Lacroix B., Lecerf J. M., Bonte J. P., Fruchart J. C., and Luc G.. 2000. Measurement of apolipoprotein B concentration in plasma lipoproteins by combining selective precipitation and mass spectrometry. J. Lipid Res. 41: 1172–1176. [PubMed] [Google Scholar]
- 17.Brunengraber H., Kelleher J. K., and Des Rosiers C.. 1997. Applications of mass isotopomer analysis to nutrition research. Annu. Rev. Nutr. 17: 559–596. [DOI] [PubMed] [Google Scholar]
- 18.Frénais R., Ouguerram K., Maugeais C., Mahot P., Maugère P., Krempf M., and Magot T.. 1997. High density lipoprotein apolipoprotein AI kinetics in NIDDM: a stable isotope study. Diabetologia. 40: 578–583. [DOI] [PubMed] [Google Scholar]
- 19.Bach-Ngohou K., Ouguerram K., Frénais R., Maugère P., Ripolles-Piquer B., Zaïr Y., Krempf M., and Bard J. M.. 2005. Influence of atorvastatin on apolipoprotein E and AI kinetics in patients with type 2 diabetes. J. Pharmacol. Exp. Ther. 315: 363–369. [DOI] [PubMed] [Google Scholar]
- 20.Batal R., Tremblay M., Barrett P. H., Jacques H., Fredenrich A., Mamer O., Davignon J., and Cohn J. S.. 2000. Plasma kinetics of apoC-III and apoE in normolipidemic and hypertriglyceridemic subjects. J. Lipid Res. 41: 706–718. [PubMed] [Google Scholar]
- 21.Chan D. C., Watts G. F., Nguyen M. N., and Barrett P. H.. 2006. Factorial study of the effect of n–3 fatty acid supplementation and atorvastatin on the kinetics of HDL apolipoproteins A-I and A-II in men with abdominal obesity. Am. J. Clin. Nutr. 84: 37–43. [DOI] [PubMed] [Google Scholar]
- 22.Maugeais C., Ouguerram K., Krempf M., Maugeais P., Gardette J., Bigot E., and Magot T.. 1996. A minimal model using stable isotopes to study the metabolism of apolipoprotein B-containing lipoproteins in humans. Diabetes Metab. 22: 57–63. [PubMed] [Google Scholar]
- 23.Viswanathan C. T., Bansal S., Booth B., DeStefano A. J., Rose M. J., Sailstad J., Shah V. P., Skelly J. P., Swann P. G., and Weiner R.. 2007. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm. Res. 24: 1962–1973. [DOI] [PubMed] [Google Scholar]
- 24.Bland J. M., and Altman D. G.. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1: 307–310. [PubMed] [Google Scholar]
- 25.Jackson K. G., and Williams C. M.. 2004. Apolipoprotein B-48: comparison of fasting concentrations measured in normolipidaemic individuals using SDS-PAGE, immunoblotting and ELISA. Atherosclerosis. 176: 207–217. [DOI] [PubMed] [Google Scholar]
- 26.Okubo K., Ikewaki K., Sakai S., Tada N., Kawaguchi Y., and Mochizuki S.. 2004. Abnormal HDL apolipoprotein A-I and A-II kinetics in hemodialysis patients: a stable isotope study. J. Am. Soc. Nephrol. 15: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 27.Frischmann M. E., Ikewaki K., Trenkwalder E., Lamina C., Dieplinger B., Soufi M., Schweer H., Schaefer J. R., König P., Kronenberg F., et al. 2012. In vivo stable-isotope kinetic study suggests intracellular assembly of lipoprotein(a). Atherosclerosis. 225: 322–327. [DOI] [PubMed] [Google Scholar]
- 28.Ooi E. M., Chan D. T., Watts G. F., Chan D. C., Ng T. W., Dogra G. K., Irish A. B., and Barrett P. H.. 2011. Plasma apolipoprotein C–III metabolism in patients with chronic kidney disease. J. Lipid Res. 52: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett P. H. R., Chan D. C., and Watts G. F.. 2006. Design and analysis of lipoprotein tracer kinetics studies in humans. J. Lipid Res. 47: 1607–1619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.