Abstract
Oxysterols are intermediates of cholesterol metabolism and are generated from cholesterol via either enzymatic or nonenzymatic pathways under oxidative stress conditions. Cholestan-3β,5α,6β-triol (C-triol) and 7-ketocholesterol (7-KC) have been proposed as new biomarkers for the diagnosis of Niemann-Pick type C (NP-C) disease, representing an alternative tool to the invasive and time-consuming method of fibroblast filipin test. To test the efficacy of plasma oxysterol determination for the diagnosis of NP-C, we systematically screened oxysterol levels in patients affected by different inherited disorders related with cholesterol metabolism, which included Niemann-Pick type B (NP-B) disease, lysosomal acid lipase (LAL) deficiency, Smith-Lemli-Opitz syndrome (SLOS), congenital familial hypercholesterolemia (FH), and sitosterolemia (SITO). As expected, NP-C patients showed significant increase of both C-triol and 7-KC. Strong increase of both oxysterols was observed in NP-B and less pronounced in LAL deficiency. In SLOS, only 7-KC was markedly increased, whereas in both FH and in SITO, oxysterol concentrations were normal. Interestingly, in NP-C alone, we observed that plasma oxysterols correlate negatively with patient’s age and positively with serum total bilirubin, suggesting the potential relationship between oxysterol levels and hepatic disease status. Our results indicate that oxysterols are reliable and sensitive biomarkers of NP-C.
Keywords: oxysterols, Niemann-Pick type C disease, Niemann-Pick type B disease, acid lipase deficiency, Smith-Lemli-Opitz syndrome, congenital familial hypercholesterolemia
The oxysterols are the resulting 27-carbon intermediates or end products of cholesterol metabolism that are readily able to cross lipophilic membranes. Thereby, oxysterol production is a mechanism by which some cells may eliminate cholesterol excess. Moreover, they are important intermediates in a number of hepatic and extrahepatic catabolic pathways, most of which generate water-soluble bile acids as final products, while a small portion of cholesterol is converted into steroid hormones (1).
A number of experimental studies have consistently shown that cholesterol oxidation products, in particular oxysterols, possess a chemical reactivity one or even two orders of magnitude higher than cholesterol; thus, oxysterol excess in cells and tissues could indeed contribute to the pathogenesis of various disease processes, much more than cholesterol itself (2).
Recent studies show that the level and type of oxysterols generated under oxidative stress conditions are not related only to cholesterol availability or to reactive oxygen and nitrogen species, because the different oxysterols may reflect pathological processes in specific sites or organs (3). Two specific oxysterols, cholestan-3β,5α,6β-triol (C-triol) and 7-ketocholesterol (7-KC), have been found elevated in Niemann-Pick type C (NP-C) disease and proposed as diagnostic biomarkers (4). NP-C is a rare autosomal recessive neurovisceral disorder caused by mutations in either the NPC1 (95% cases) or the NPC2 gene (∼5% cases), which lead to impaired intracellular lipid trafficking, with accumulation of unesterified cholesterol and glycolipids in the lysosomal/late endosomal system (5).
In order to test the potential contribution of plasma oxysterol determination in the differential diagnosis of NP-C with other cholesterol metabolism-related disorders (CMRDs), we systematically tested plasma C-triol and 7-KC levels in a large series of CMRDs, including patients with NP-C and Niemann-Pick type B (NP-B) disease, lysosomal acid lipase (LAL) deficiency, Smith-Lemli-Opitz syndrome (SLOS), congenital familial hypercholesterolemia (FH), and sitosterolemia (SITO).
METHODS
Plasma oxysterol analysis
C-triol and 7-KC were analyzed as dimethylaminobutyrate (DMAB) esters (DMAB derivatives) by LC/MS/MS using electrospray ionization source. Chromatography was performed on an Agilent series 1290 pump equipped with autosampler. The column for chromatographic separation was a Phenomenex Synergy Fusion C18, 50 mm × 2.0 mm internal diameter, 4 μm. Chromatographic separation of metabolites was obtained with gradient elution of two solutions as previously described (6). Total run was 5.1 min. Elution times were 1.58 min for C-triol and 2H7-C-triol and 1.82 min for 7-KC and 2H7-7-KC. 7-Hydroxycholest-4-en-3-one (C4) standard was obtained from Avanti Polar Lipids (AL). For C4, the same 7-KC transition m/z 514.5/132.1 was monitored.
Tandem mass spectrometry experiments were carried out on a 4000-QTrap mass spectrometer (ABSciex, Toronto, Ontario, Canada), equipped with a Turbo Ion Spray Source operating in positive ion mode. The monitored multiple reaction monitoring (MRM) transitions for C-triol-DMAB and 7-KC-DMAB derivatives were m/z 534/132 and 514/132, respectively.
Sample treatment procedure
Plasma was obtained from blood samples collected in EDTA K2 tubes immediately centrifuged for 5 min at 3,000 rpm. Plasma was separated from red cells and immediately stored frozen at −80°C, in 60 μl aliquots, as plasma samples used for the oxysterol analysis are sensitive to thawing and stable for about 8 months at −80°C (for ∼3 months at −20°C). Briefly, 5.0 μl of 500 ng/ml 2H7-C-triol and 2H7-7KC standard solution, prepared in methanol, were mixed with 50 μl of plasma sample in an Eppendorf tube. Liquid-liquid extraction was performed adding 500 μl of ethyl acetate and mixing by vortex for 2 min. After centrifugation for 5 min at 14,000 rpm, the supernatant fluid was transferred to a clean glass vial and dried under nitrogen steam. Derivatization was performed adding 20 μl of derivatizing solution (100 mM dimethyl-aminobutyric acid imidazolide) at 65°C for 15 min. Finally, the sample was dried again and reconstituted with 200 μl solvent, and 5 μl was injected for the detection and quantification of free or unesterified oxysterols, C-triol, and 7-KC, as DMAB monoderivatives (6).
Patients and controls
We collected 51 samples obtained from 16 patients affected by NP-C, 5 samples from 2 NP-B patients, 6 samples from 2 patients affected by LAL deficiency, 7 samples from 4 SLOS patients, 3 samples from 3 patients with FH, and 1 sample from 1 patient with SITO. All patients had biochemical, enzymatic and/or molecular diagnosis. Twelve out of 16 NP-C patients were those reported in the previous study (6), but, in the present study, for those patients, supplemental sample results were added. All patients were followed at the Division of Metabolism, “Bambino Gesù” Children’s Hospital.
Serum total bilirubin (t-bil), total cholesterol (t-chol), LDL, and HDL were analyzed, immediately after sampling, by automated assay (ADVIA 2400 Chemistry System; Siemens Healthcare Diagnostics, Forchheim, Germany).
Blood samples were collected after obtaining informed consent during routine clinical evaluation following an overnight fast. The study was approved by Bambino Gesù Children’s Hospital Ethics Committee (no. 1002_OPBG_2015).
Controls samples were obtained from 135 healthy age-matched subjects. Child controls were subjects with normal neurological and liver function or subjects with traumatic injuries, and adults’ plasma was obtained from healthy blood donors.
Statistical analysis
SPSS version 11.5.1 (SPSS Inc., Chicago, IL) was used as the statistical software. Descriptive statistics were presented as median. The preliminary Kolmogorov-Smirnov test was used to check variables that were under a normal distribution, to further use parametric or nonparametric tests. Statistically significant differences between groups were analyzed using Student’s t-test to normal variables and Mann-Whitney test to nonnormal variables. A value of P < 0.001 was considered extremely statistically significant, a value of P from 0.001 to 0.01 was considered very statistically significant, and a value of P from 0.01 to 0.05 was considered statistically significant.
C-triol and 7-KC values for control values were expressed as median and 2.5th to 97.5th percentiles of the distributions. Plasma C-triol and 7-KC values were expressed as median and minimum/maximum range of the distributions because of the small number of samples for each patient. Pearson coefficients were used to evaluate the relationship between continuous variables and the strength of their relationships. A value of P < 0.05 was considered statistically significant.
RESULTS
Biological variation of C-triol and 7-KC in plasma
We measured C-triol and 7-KC concentrations in plasma samples of 135 healthy subjects from 0.2 to 38 years old, the age range that covers the ages of our patients. C-triol control values were median 4.1 ng/ml and 2.5th to 97.5th percentile 1.1–21.9 ng/ml, respectively; 7-KC control values were median 16.1 ng/ml and 2.5th to 97.5th percentile 3.8–39.8 ng/ml, respectively.
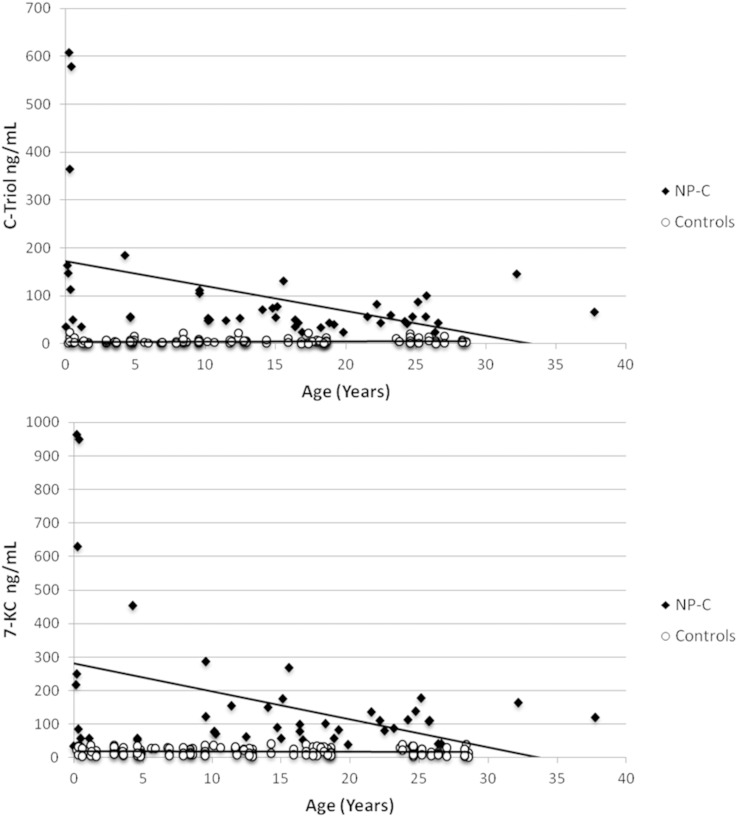
Data showed a significant direct correlation between C-triol and 7-KC (Pearson 0.404, P < 0.001), whereas their values were not correlated with age.
Patients’ results
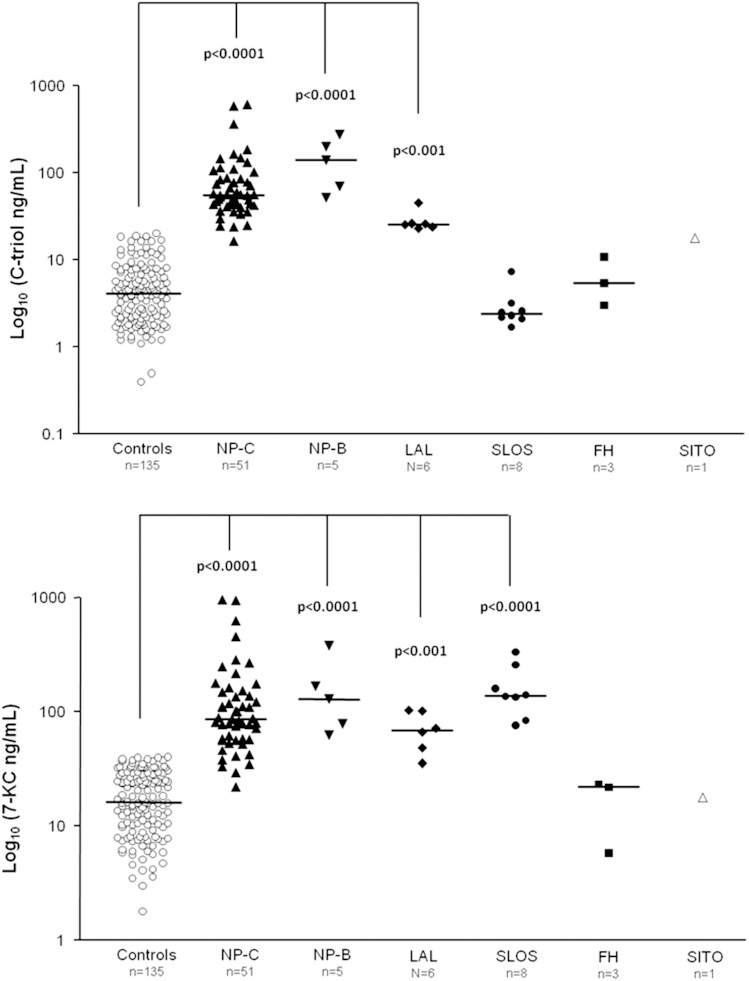
Overall, the 16 NP-C patients showed a significant increase of both oxysterols: C-triol median value was 55.3 ng/ml (range 16.3–608, P < 0.001), and the 7-KC median value was 86.0 ng/ml (range 21.9–963, P < 0.001). Only in 1 out of 51 NP-C samples did we find a C-triol value within the control range, whereas in 4 out of 51 samples, 7-KC plasma concentrations were found to be normal (Table 1).
TABLE 1.
Individual data of plasma oxysterols in CMRD patients expressed as median (minimum–maximum) according to the age at blood sampling (years)
| Patient | Age at Blood Sampling | Disease | C-triol (ng/ml) | 7-KC (ng/ml) |
| 1 | 0.1 | NP-C | 163 | 217 |
| 2 | 0.3 (0.2–0.4) | NP-C | 578 (365–608) | 950 (630–963) |
| 3 | 0.4 (0.2–1.1) | NP-C | 81.1 (49.2–148.1) | 72.3 (56.3–250) |
| 4 | 4.2 | NP-C | 184 | 453 |
| 5 | 4.6 (4.1–5.1) | NP-C | 55.9 (55.1–56.6) | 122 (52.4–56.9) |
| 6 | 9.5 (9.5–10.3) | NP-C | 105 (49.3–111) | 122 (71.6–287) |
| 7 | 10.8 (10.2–12.4) | NP-C | 50.3 (47.8–52.8) | 76.5 (75.7–154) |
| 8 | 15.1 (14 0–16.2) | NP-C | 76.3 (55.3–131) | 88.8 (58.3–269) |
| 9 | 16.5 (16.4–16.9) | NP-C | 39.5 (43–78.2) | 65.6 (52.9–99.5) |
| 10 | 19.2 (18.2–20.3) | NP-C | 33.4 (16.3–43.3) | 56.3 (29.3–102) |
| 11 | 22.5 (21.5–23.7) | NP-C | 55.8 (29.8–82.6) | 87.1 (45.9–136) |
| 12 | 25.2 (24.2–25.7) | NP-C | 87.1 (46.9–87.l) | 112 (112–179) |
| 13 | 25.5 (24.4–26.6) | NP-C | 42.0 | 34.8 |
| 14 | 25.7 (24.7–26.9) | NP-C | 41.1 (24.7–56.9) | 79.2 (33.3–139) |
| 15 | 32.2 | NP-C | 145 | 163 |
| 16 | 37.8 | NP-C | 66.9 | 119 |
| 17 | 4.1 (3.5–4.6) | NP-B | 171 (69.8–271) | 149 (78.3–383) |
| 18 | 13.5 | NP-B | 52.3 | 62.8 |
| 19 | 8.0 (7.8–8.7) | LAL deficiency | 25.7 (23.9–45.1) | 86.9 (66.2–103) |
| 20 | 14.7 (14.4–15.0) | LAL deficiency | 24.2 (22.8–25.6) | 42.0 (35.5–48.5) |
| 21 | 3.6 | SLOS | 2.1 | 337 |
| 22 | 7.4 (7.3–7.5) | SLOS | 2.9 (2.6–3.2) | 110 (84.0–135) |
| 23 | 15.5 (15.3–15.7) | SLOS | 2.3 (2.2–2.3) | 119 (76.4–161) |
| 24 | 17.3 (16.8–17.9) | SLOS | 4.5 (1.7–7.4) | 139 (137–141) |
| 25 | 5.5 | FH | 3.0 | 22.0 |
| 26 | 9.9 | FH | 10.8 | 23.1 |
| 27 | 10.7 | FH | 5.4 | 5.8 |
| 28 | 2.0 | SITO | 15.7 | 17.5 |
| Controls (n = 135) | 2.5th to 97.5th percentile | 1.1–21.9 | 3.8–39.8 |
In the two NP-B patients, we found the highest increase of both oxysterols observed in our CMRDs series: C-triol median value 140 ng/ml (range 52–271, P < 0.0001) and 7-KC median value 129 ng/ml (range 62.8–383, P < 0.0001) (Table 1).
In plasma samples from the 2 LAL deficiency patients, both oxysterols were increased, however, to a lesser extent if compared with other CMRDs. The median value of C-triol was 25.5 ng/ml (range 22.8–45.1, P < 0.001), and of 7-KC 69.0 ng/ml (range 35.5–103, P < 0.001) (Table 1).
In patients with SLOS, we found normal concentrations of C-triol (median 2.4 ng/ml, range 1.7–7.4 ng/ml), whereas 7-KC concentrations were strongly elevated in all determinations (median 139 ng/ml, range 76.4–337 ng/ml; P < 0.001) (Table 1). We excluded that 7-KC coeluted with its isomer 7-hydroxycholest-4-en-3-one (C4) by testing, with the same derivatization procedure, the C4 standard solution. The retention time was 1.60 min (data not shown).
Despite a massive increase of total plasma cholesterol (median 336 mg/dl, control values 120–200), in the three FH patients plasma oxysterols were within the control range (Table 1).
Figure 1 shows the relative concentrations of the two oxysterols in CMRDs in comparison with controls.
Fig. 1.
Levels of C-triol and 7-KC in patients affected by NP-C (P < 0.0001), NP-B (P < 0.0001), and LAL deficiency (P < 0.001). C-triol in SLOS patients was normal (P > 0.05), whereas 7-KC was increased compared with control values (P < 0.0001). Levels of C-triol and 7-KC in patients affected by FH and SITO were normal (P > 0.05).
Although the oxysterols are known to be cholesterol oxidation products, no correlations were found between the plasma concentrations of the two oxysterols in CMRDs with the levels of t-chol, LDL, or HDL when analyzed in the same sample (Pearson <0.3, P > 0.01) (supplementary Table 3). Indeed, oxysterols in CMRDs were not correlated with t-bil or with age. Remarkably, in NP-C patients alone we found significant correlations between the plasma concentrations of oxysterols and both t-bil and age, as shown in Table 2, Fig. 2, and Fig. 3.
TABLE 2.
Correlations between C-triol, 7-KC, t-bil, and age in the NP-C patient group
| C-triol | 7-KC | t-Bil | Age | ||
| C-triol | Pearson correlation | 1 | 0.895a | 0.726a | −0.468a |
| Significance (two-tails) | . | 0.0001 | 0.0001 | 0.002 | |
| N | 45 | 45 | 41 | 45 | |
| 7-KC | Pearson correlation | 0.895a | 1 | 0.601a | −0.434a |
| Significance (two-tails) | 0 | . | 0.001 | 0.004 | |
| N | 45 | 45 | 41 | 45 | |
| t-Bil | Pearson correlation | 0.726a | 0.601a | 1 | −0.547a |
| Significance (two-tails) | 0 | 0.001 | . | 0.003 | |
| N | 41 | 41 | 41 | 41 | |
| Age | Pearson correlation | −0.468a | −0.434a | −0.547a | 1 |
| Significance (two-tails) | 0.002 | 0.004 | 0.003 | . | |
| N | 45 | 45 | 41 | 45 |
“.” indicates that the significance of the correlation between one parameter and itself is maximum (unquantifiable number<0.00001).
P < 0.01.
P < 0.05.
Fig. 2.
Correlations between plasma oxysterols and age (years) in NP-C patients and in aged-matched healthy controls.
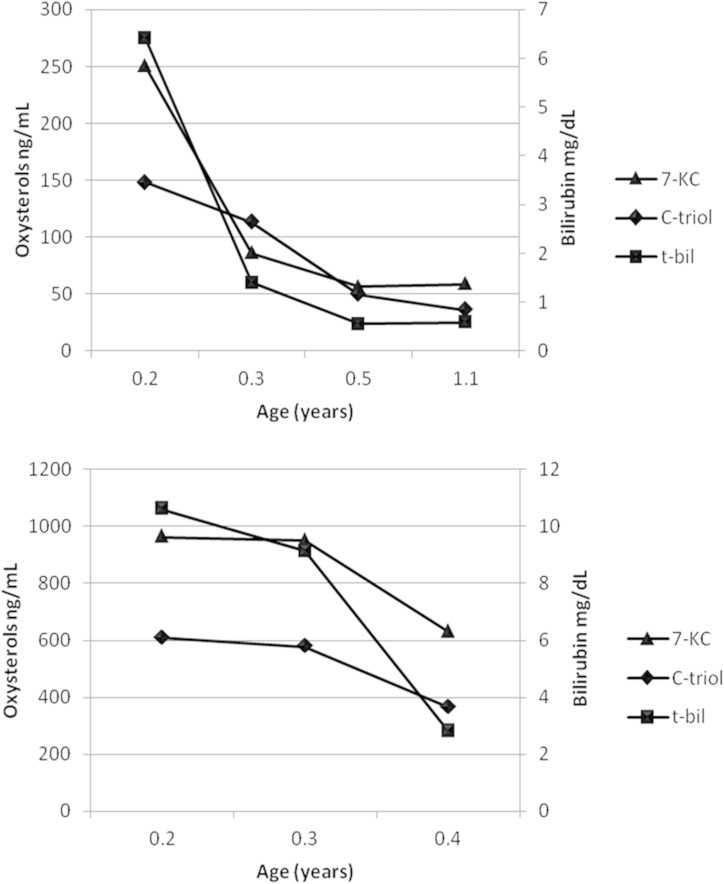
Fig. 3.
Oxysterols correlate with t-bil in NP-C patients: subsequent samples at onset from patient 2 (A) and from patient 3 (B); age at blood sampling is expressed as years.
DISCUSSION
The conversion of cholesterol to oxysterols occurs by enzymatic and nonenzymatic reactions. The nonenzymatic reactions mainly affect the sterol ring, whereas the enzymatic ones react in the side chain of sterol structures by enzymatic cholesterol hydroxylation, catalyzed by different types of hydroxylases. However, some exceptions exist: 25-hydroxycholesterol and 7α-hydroxy-cholesterol can be generated by either mechanism (7). Oxysterol production from nonenzymatic reactions is not controlled by enzymatic mechanisms, so their formation depends on the concentration of reactive oxygen species and the structure of the target lipids to be oxidized (8). The increase of plasma C-triol and 7-KC observed in NP-C disease is the consequence of the nonenzymatic cholesterol oxidation promoted by oxidative stress, as reported in human NP-C mutant fibroblasts (9, 10), in tissues from NPC1 mutant mice (11), and in NP-C patients (12). The defect of NPC1 or NPC2 transporters, occurring in NP-C disease, makes available for the various cell functions a very small amount of free cholesterol from lysosomes, causing an impaired lipid trafficking and cholesterol synthesis upregulation (13). Subsequently, free cholesterol accumulation leads to high oxysterol generation. In particular, C-triol is produced by epimeric 5,6-epoxides, whereas 7-KC is produced by an attack of cholesterol by oxidative radical species resulting in the formation of 7-hydroperoxy-cholesterol, which decomposes to give either 7-KC or 7α- or 7-β-hydroxy-cholesterol, under increased cellular oxidative stress conditions. The current results confirmed that C-triol has a higher sensitivity in detecting NP-C patients when compared with 7-KC (Fig. 1) (6, 14). Remarkably, in NP-C patients we observed two novel significant correlations between the plasma oxysterols and total bilirubin and patients’ age. This seems to indicate that in NP-C the elevation of oxysterols reflects liver involvement, following different stages of the disease (13). It is well known that disease phenotype changes according to the patient’s age, ranging from a picture dominated by visceral (i.e., hepatic) manifestations in the early onset cases, moving to a “pure” (nonhepatic) neurological phenotype in adulthood. Following these correlations, it was possible to track the hepatic disease course in two early onset infants both presenting initially with severe cholestasis and showing with age a parallel decline of oxysterols and total bilirubin.
Besides NP-C disease, two recent studies, based on the determination of 7-KC alone, showed its increase in NP-B disease and in SLOS, two inherited disorders due to deficient activity of the lysosmal enzyme sphingomyelinase and of 7-dehydrocholesterol reductase, respectively (15, 16). A further study, showed that both 7-KC and C-triol were elevated in NP-B patients (14). A very recent study described high levels of C-triol in NP-C, cerebrotendinous xanthomatosis, and LAL deficiency (17).
In NP-B disease, the deficient activity of acid sphingomyelinase impairs the conversion of SM to ceramide, which causes massive intracellular accumulation of SM. Interestingly, a recent study showed that the free cholesterol transporter NPC2 is inhibited by SM excess and upregulated by ceramide, indicating that sphingomyelinase also participates in the complex machinery that regulates the secretion of free cholesterol from the lysosomal compartment (18). The effects of SM excess on cellular cholesterol transport have been demonstrated in NP-B fibroblasts (19). By confocal microscopy and cellular lipid mass measurement, this study showed the lysosomal cosequestration and trapping of SM and free cholesterol in mutant NP-B cell lines. Furthermore, NP-B fibroblasts showed also a significant increase of de novo cholesterol biosynthesis when compared with control fibroblast. These results demonstrate that free cholesterol accumulation in lysosomes, caused by an impairment of its transport out of the organelle, upregulates the endogenous cholesterol synthesis, which further increases the cellular storage. Interestingly, a recent study showed that cholesterol transfer mediated by NPC2 protein is inhibited by SM excess, indicating that sphingomyelinase participates in the complex machinery that regulates the secretion of free cholesterol from the lysosomal compartment (19). Therefore, the high oxysterol levels observed in NP-B patients are likely to reflect the lysosomal free cholesterol accumulation with a mechanism similar to NP-C.
We found that plasma oxysterol concentrations were also elevated in LAL deficiency patients, however, to a lesser extent when compared with NP-C or NP-B. The finding of increased C-triol and 7-KC in LAL defect is difficult to explain because the intracellular free cholesterol concentration would presumably be reduced in LAL deficiency. Following the receptor-mediated cell endocytosis of LDL, the hydrolysis of LDL-derived cholesteryl esters is catalyzed in the lysosome by LAL. Therefore, a deficiency of this enzyme would result in the lysosmal accumulation of esterified cholesterol and in a relative depletion of free cholesterol, which may likely cause the activation of endogenous cholesterol biosynthesis by the upregulation of HMG-CoA reductase, resulting in the characteristic hypercholesterolemia observed in these patients and contributing to the formation of oxysterols (20, 21).
In SLOS patients, the defect of 7-dehydrocholesterol reductase causes a massive accumulation of 7-dehydrocholesterol (7-DHC) (22). The finding of increased 7-KC alone in SLOS patients can therefore be easily explained by conversion of excessive 7-DHC into 7-KC (23).
FH is a rare autosomal dominant genetic disorder that leads to a massive increase of blood cholesterol in most cases due to mutations in the LDL receptor gene (24). In FH, the LDL particles cannot cross the cellular membrane, resulting in the accumulation of cholesterol in the blood compartment with relatively low free cholesterol concentrations in the intracellular compartment. Our finding of normal oxysterol levels in FH is consistent with this mechanism.
Although the processes that regulate cholesterol metabolism within cellular and subcellular compartment cells still remains to be fully elucidated, data obtained in the present study may still help to clarify some of the mechanisms that underlie the regulation of cellular cholesterol machinery.
Our findings confirm that plasma oxysterols are reliable and sensitive biomarkers of NP-C disease, allowing a faster diagnosis than with the more invasive “Filipin test.” However, a high concentration of plasma oxysterols is not specific for NP-C disease as we found increased levels also in other CMRDs. A careful clinical evaluation is needed to distinguish NP-C disease from other CMRDs that present an increase of both plasma oxysterols.
Acknowledgments
The authors acknowledge the technical and clinical support of Dr. Enrico Silvio Bertini, Director of the Unit for Neuromuscular and Neurodegenerative Diseases, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy, for the analysis of enzymatic activity and referring some patients.
Footnotes
Abbreviations:
- 7-KC
- 7-ketocholesterol
- CMRD
- cholesterol metabolism-related disorder
- C-triol
- cholestan-3β,5α,6β-triol
- DMAB
- dimethylaminobutyrate
- FH
- congenital familial hypercholesterolemia
- LAL
- lysosomal acid lipase
- NP-B
- Niemann-Pick type B
- NP-C
- Niemann-Pick type C
- SITO
- sitosterolemia
- SLOS
- Smith-Lemli-Opitz syndrome
- t-bil
- serum total bilirubin
- t-chol
- total cholesterol
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Björkhem I. 2002. Do oxysterols control cholesterol homeostasis? J. Clin. Invest. 110: 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy R. C., and Johnson K. M.. 2008. Cholesterol, reactive oxygen species, and the formation of biologically active mediators. J. Biol. Chem. 283: 15521–15525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaya J., Szuchman A., Tavori H., and Aluf Y.. 2011. Oxysterols formation as a reflection of biochemical pathways: summary of in vitro and in vivo studies. Chem. Phys. Lipids. 164: 438–442. [DOI] [PubMed] [Google Scholar]
- 4.Porter F. D., Scherrer D. E., Lanier M. H., Langmade S. J., Molugu V., Gale S. E., Olzeski D., Sidhu R., Dietzen D. J., Fu R., et al. 2010. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med. 2: 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanier M. T., and Millat G.. 2003. Niemann-Pick disease type C. Clin. Genet. 64: 269–281. [DOI] [PubMed] [Google Scholar]
- 6.Boenzi S., Deodato F., Taurisano R., Martinelli D., Verrigni D., Carrozzo R., Bertini E., Pastore A., Dionisi-Vici C., and Johnson D. W.. 2014. A new simple and rapid LC-ESI-MS/MS method for quantification of plasma oxysterols as dimethylaminobutyrate esters. Its successful use for the diagnosis of Niemann-Pick type C disease. Clin. Chim. Acta. 437: 93–100. [DOI] [PubMed] [Google Scholar]
- 7.Otaegui-Arrazola A., Menéndez-Carreño M., Ansorena D., and Astiasarán I.. 2010. Oxysterols: a world to explore. Food Chem. Toxicol. 48: 3289–3303. [DOI] [PubMed] [Google Scholar]
- 8.Smith W. L., and Murphy R. C.. 2008. Oxidized lipids formed non-enzymatically by reactive oxygen species. J. Biol. Chem. 283: 15513–15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zampieri S., Mellon S. H., Butters T. D., Nevyjel M., Covey D. F., Bembi B., and Dardis A.. 2009. Oxidative stress in NP-C1 deficient cells: protective effect of allopregnanolone. J. Cell. Mol. Med. 13: 3786–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy J. V., Ganley I. G., and Pfeffer S. R.. 2006. Clues to neuro-degeneration in Niemann-Pick type C disease from global gene expression profiling. PLoS One. 1: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vázquez M. C., del Pozo T., Robledo F. A., Carrasco G., Pavez L., Olivares F., González M., and Zanlungo S.. 2011. Alteration of gene expression profile in Niemann-Pick type C mice correlates with tissue damage and oxidative stress. PLoS One. 6: e28777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu R., Yanjanin N. M., Bianconi S., Pavan W. J., and Porter F. D.. 2010. Oxidative stress in Niemann-Pick disease, type C. Mol. Genet. Metab. 101: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanier M. T. 2015. Complex lipid trafficking in Niemann Pick disease type C. J. Inherit. Metab. Dis. 38: 187–199. [DOI] [PubMed] [Google Scholar]
- 14.Klinke G., Rohrbach M., Giugliani R., Burda P., Baumgartner M. R., Tran C., Gautschi M., Mathis D., and Hersberger M.. 2015. LC-MS/MS based assay and reference intervals in children and adolescents for oxysterols elevated in Niemann-Pick diseases. Clin. Biochem. 48: 596–602. [DOI] [PubMed] [Google Scholar]
- 15.Lin N., Zhang H., Qiu W., Ye J., Han L., Wang Y., and Gu X.. 2014. Determination of 7-ketocholesterol in plasma by LC-MS for rapid diagnosis of acid SMase-deficient Niemann-Pick disease. J. Lipid Res. 55: 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Björkhem I., Diczfalusy U., Lövgren-Sandblom A., Starck L., Jonsson M., Tallman K., Schirmer H., Ousager L. B., Crick P. J., Wang Y., et al. 2014. On the formation of 7-ketocholesterol from 7-dehydrocholesterol in patients with CTX and SLO. J. Lipid Res. 55: 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pajares S., Arias A., García-Villoria J., Macías-Vidal J., Ros E., de Las Heras J., Girós M., Coll M. J., and Ribes A.. 2015. Cholestane-3β,5α,6β-triol: high levels in Niemann-Pick type C, cerebrotendinous xanthomatosis, and lysosomal acid lipase deficiency. J. Lipid Res. 56: 1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oninla V. O., Breiden B., Babalola J. O., and Sandhoff K.. 2014. Acid sphingomyelinase activity is regulated by membrane lipids and facilitates cholesterol transfer by NPC2. J. Lipid Res. 55: 2606–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C. Y., Ruel I., Denis M., Genest J., and Kiss R. S.. 2013. Cholesterol trapping in Niemann-Pick disease type B fibroblasts can be relieved by expressing the phosphotyrosine binding domain of GULP. J. Clin. Lipidol. 7: 153–164. [DOI] [PubMed] [Google Scholar]
- 20.Fouchier S. W., and Defesche J. C.. 2013. Lysosomal acid lipase A and the hypercholesterolaemic phenotype. Curr. Opin. Lipidol. 24: 332–338. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg H. N., Le N. A., Short M. P., Ramakrishnan R., and Desnick R. J.. 1987. Suppression of apolipoprotein B production during treatment of cholesteryl ester storage disease with lovastatin. Implications for regulation of apolipoprotein B synthesis. J. Clin. Invest. 80: 1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBarber A. E., Eroglu Y., Merkens L. S., Pappu A. S., and Steiner R. D.. 2011. Smith-Lemli-Opitz syndrome. Expert Rev. Mol. Med. 13: e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinkyo R., Xu L., Tallman K. A., Cheng Q., Porter N. A., and Guengerich F. P.. 2011. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J. Biol. Chem. 286: 33021–33028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahed A. C., and Nemer G. M.. 2011. Familial hypercholesterolemia: the lipids or the genes? Nutr. Metab. (Lond.). 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]