Abstract
We developed a Drosophila model of T2D in which high sugar (HS) feeding leads to insulin resistance. In this model, adipose TG storage is protective against fatty acid toxicity and diabetes. Initial biochemical and gene expression studies suggested that deficiency in CoA might underlie reduced TG synthesis in animals during chronic HS feeding. Focusing on the Drosophila fat body (FB), which is specialized for TG storage and lipolysis, we undertook a series of experiments to test the hypothesis that CoA could protect against the deleterious effects of caloric overload. Quantitative metabolomics revealed a reduction in substrate availability for CoA synthesis in the face of an HS diet. Further reducing CoA synthetic capacity by expressing FB-specific RNAi targeting pantothenate kinase (PK orfumble) or phosphopantothenoylcysteine synthase (PPCS) exacerbated HS-diet-induced accumulation of FFAs. Dietary supplementation with pantothenic acid (vitamin B5, a precursor of CoA) was able to ameliorate HS-diet-induced FFA accumulation and hyperglycemia while increasing TG synthesis. Taken together, our data support a model where free CoA is required to support fatty acid esterification and to protect against the toxicity of HS diets.
Keywords: triglycerides, fatty acid/metabolism, diabetes, obesity, nutrition, lipotoxicity, coenzyme A
The prevalence of obesity, or excessive TG accumulation in adipose tissue, results from dietary excess and is increasing in many parts of the world. Obesity is a risk factor for several diseases, including cardiovascular disease, T2D, and cancer. A growing consensus exists, however, that obesity is protective against the adverse biochemical effects of caloric excess (1–4). Several studies have shown that obesity and T2D are accompanied by increases in FFAs, ceramides, DAG, and other potentially toxic lipids in various tissues (5–9). These lipid mediators induce cellular stress known as lipid toxicity or “lipotoxicity” (4, 10, 11). Therefore, it is of interest to learn the mechanisms that enable animals to store excess carbons safely as TG in the face of caloric overload.
In Drosophila, a high sugar (HS) diet induces obesity accompanied by hyperglycemia, hyperlipidemia, and insulin resistance (12–16). Interestingly, the ability to convert dietary sugar into stored fat was protective against the effects of caloric overload, as lean mutants were unable to survive on HS diets (14, 17). We showed that carbon flux into esterified fat was blunted after chronic HS feeding, consistent with a limited ability of animals to convert dietary sugar into TG (17). The Drosophila fat body (FB) stores and metabolizes fat during feeding and starvation, and also controls whole-animal glucose disposal. Both larvae and adult flies become hyperglycemic and insulin resistant when their capacity to store fat is exceeded (17, 18). This is consistent with the model of “adipose tissue expandability” proposed as a mechanism of insulin resistance (19, 20). This model holds that the ability to increase the storage of fatty acids as inert TG is protective against metabolic disease. Consistent with this model, increasing adipose volume or fat content is protective, whereas exceeding the maximum capacity of adipose fat storage is deleterious. Based on our expression and metabolomic analyses in HS-fed larvae, we propose that CoA levels tightly regulate the FB’s maximum storage capacity by limiting TG synthesis.
CoA is required in all organisms, functioning as a cofactor for an estimated 4% of enzymes and in more than 100 different reactions (21, 22). CoA is required for fatty acid synthesis by acting as a cosubstrate for fatty acid synthase (23, 24) and CoA is also required in order to esterify fatty acid substituents (25). Beta-oxidation requires two CoAs, one for each fatty acid to enter the mitochondrion and a second CoA for thiolysis after transport (26). CoA also regulates intracellular redox state via its free sulfhydryl group (25) and it regulates ketone biogenesis via acetyl-CoA (27).
In this study, we set out to identify metabolites that could contribute to lipotoxicity in the face of caloric overload. Our studies revealed that CoA levels were reduced, while FFA levels were increased, in HS-fed larvae. Reducing FB levels of pantothenate kinase (fumble, CG5725) or phosphopantothenyl cysteine synthase (PPCS, CG5629), both of which catalyze steps in CoA synthesis from pantothenic acid (PA), led to reduced TG storage and an increase in the severity of HS diet-induced fatty acid accumulation. By contrast, supplementing the HS diet with the CoA precursor, PA, increased TG synthesis and lowered glucose and FFA levels in the presence of caloric overload. Thus, CoA is limiting in the face of a HS diet, and we propose that increasing CoA levels represents a novel therapeutic target for individuals with obesity-associated metabolic disorders.
MATERIALS AND METHODS
Genetics
Wild-type w1118 lines were from the Vienna Drosophila Resource Center. TRiP control, UAS-PKi and UAS-PPCSi (stocks GL00149 and JF03206, respectively) UAS-RNAi lines were from Harvard’s DRSC TRiP collection. UAS-Dcr2; r4-GAL4 was used to express transgenes in the larval FB (28).
Diets
The control (0.15 M sucrose) and HS (0.7 M sucrose) diets were made using a modified Bloomington semi-defined food as described previously (12). D-PA hemicalcium salt was from Sigma (P5155) and was added in a volume of 30 μl to a final concentration of 0.3 to 3 mM, with 3 mM producing optimal phenotypic rescue. Water (30 μl) was added to HS food as a control in these experiments. Wild-type w1118 were used in PA supplementation experiments.
Expression analyses
FBs were isolated from wild-type w1118; UAS-Dcr2; r4-GAL4 or w1118; UAS-Dcr2; cg-GAL4 wandering third-instar larvae and RNA isolated and quantified as described using Illumina Hi-Seq (17). Data from both control lines were pooled to increase power to detect expression differences due to diet. A total of 13 biological FB replicates per diet were analyzed over several lanes. These data were deposited at GEO (GSE76214). For guts, six biological replicates were isolated and RNA extracted. RT was used to make cDNA, which was then analyzed by quantitative (q)PCR. Primers used to detect vanin-like mRNA were 5′-TCCCGAGGATCAGATAAACC-3′ and 5′-ACAGGGTCACCAGAAACTCC-3′. Vanin-like levels were normalized to RpL32 mRNA (CG7939) using 5′-CAGCATACAGGCCCAAGAT-3′ and 5′- GCACTCTGTTGTCGATACCC-3′. Similar results were observed using a primer pair targeting a different region of vanin-like.
PA, carnitine, acyl-carnitine, cysteine, and glycerol determination
FBs were isolated from wild-type w1118; UAS-Dcr2; r4-GAL4 wandering third-instar larvae and immediately placed in PBS on ice, then homogenized before shipping on dry ice to Metabolon. Hemolymph was collected and shipped frozen. All metabolite extraction was done by Metabolon. GC-MS (glycerol, cysteine) and LC-MS (positive ion monitoring mode; pantothenate, carnitine, palmitoyl-carnitine, and oleoyl-carnitine) were used to isolate the peaks representing each analyte, with injection standards used at fixed concentrations to quantify the relative amounts of each metabolite in six biological replicates per diet. Metabolites were identified by automated comparison of the ion features in the extracted samples to a reference library as described previously (29). Metabolite levels were normalized to protein content for FB, and to total volume for hemolymph.
CoA-SH determination
Eighty wild-type (w1118) wandering third-instar larvae were homogenized in 10 mM DTT/water and frozen at −80°C. Homogenates were defrosted, TCA precipitated, and washed six times with ether to remove lipids. Aqueous fractions were dried, resuspended, and run on a Waters HPLC, as described (17).
Metabolic assays
TG, FFA, and glycogen were assayed from groups of six frozen wandering L3 larvae as previously described (17). Hemolymph was isolated from groups of five to twenty wandering larvae and assayed as previously described (17).
RESULTS
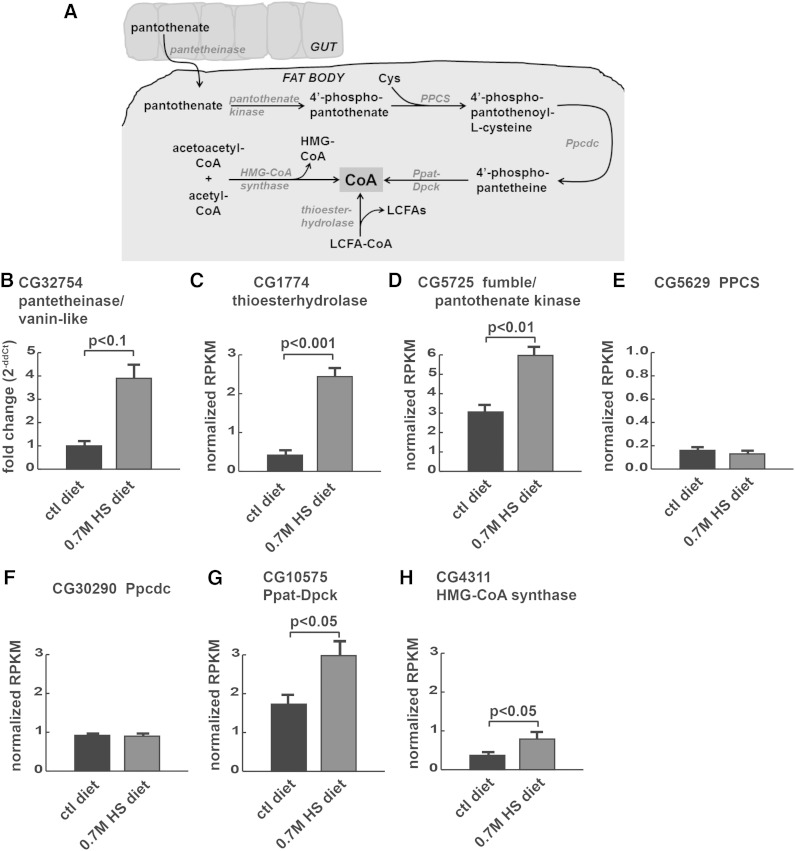
In previous gene expression profiling experiments of HS-fed insulin-resistant larvae, we saw a dramatic (10-fold) increase in whole body expression of vanin-like (CG32754), which is predicted to encode a CoA metabolic enzyme, pantetheinase, that converts pantetheine to pantothenate (also known as vitamin B5) and cysteamine (12). We previously observed changes in fatty acid synthesis and esterification, which require CoA, in HS-fed larvae (17). We hypothesized that the increase in pantetheinase might be evidence of a homeostatic response to defects in CoA availability. FlyAtlas (30) data shows the highest levels of vanin-like expression in the midgut, with expression only in midgut, hindgut, and Malpighian tubule (flyatlas.org). Therefore, we quantified vanin-like expression in isolated guts.
HS feeding increased expression of vanin-like in the midgut by 4-fold (Fig. 1B), suggesting that the gut might contribute to maintenance of systemic CoA levels by producing pantothenate to be used in other tissues when CoA levels are depleted. We also observed a 50% increase in expression of the putative pantothenate transporter, CG10444, in gut RNA-seq, although edgeR did not identify a significant difference (data not shown), consistent with a need for pantothenate or CoA during HS feeding. Once exported from the gut, circulating pantothenate could be converted back into CoA to support fatty acid synthesis and esterification in the FB, which is essential for larval survival on a HS diet (17) (Fig. 1A).
Fig. 1.
HS diets increase expression of CoA metabolic enzymes. A: CoA metabolic pathway: pantothenate can be derived from the diet and trafficked from the gut to the FB for CoA synthesis. CoA can also be derived from several intracellular sources. B–H: Gene expression was quantified using tissue-specific qPCR [(B) guts] or RNA-seq [(C–H) FBs] from wild-type larvae reared on control (ctl) or 0.7M sucrose (HS) diets. Pantetheinase, also known as vanin-like (B), thioesterhydrolase (C), fumble/pantothenate kinase (D), phosphopantothenoylcysteine synthase (PPCS) (E), phosphopantothenoylcysteine decarboxylase (Ppcdc) (F), bifunctional phosphopantetheine adenylyltransferase-dephospho-CoA kinase (Ppat-Dpck) (G), and HMG-CoA synthase (H).
We focused subsequent studies of expression and metabolism in the FB by using RNA-seq and metabolomics to characterize CoA metabolism there. Thioester hydrolase (encoded by CG1774), an enzyme that catalyzes the production of CoA-SH from acyl-CoA rather than pantothenate, was increased 3-fold at the mRNA level in whole animals (12). We observed a corresponding increase in thioester hydrolase expression in FBs upon HS feeding (Fig. 1C). The transcriptional upregulation of both vanin-like and thioester hydrolase in gut and FB, respectively, could occur in response to a deficit in CoA. CoA is required to produce fatty acid substrates for lipogenesis in the FB and for over 100 other reactions (21, 22). Examining the pathway more closely in FB gene expression datasets, we noted upregulation of catalytic steps in CoA synthesis including pantothenate kinase (CG5725, known as fumble in Drosophila; Fig. 1D), although there was no change in expression of genes encoding the enzymes PPCS and Ppcdc (Fig. 1E, F), which encode the intermediate steps in the CoA biosynthesis pathway. We also observed increases in mRNA encoding two other enzymes that produce free CoA (Ppat-Dpck and HMG-CoA synthase, Fig. 1G, H). Therefore, we decided to study this pathway biochemically.
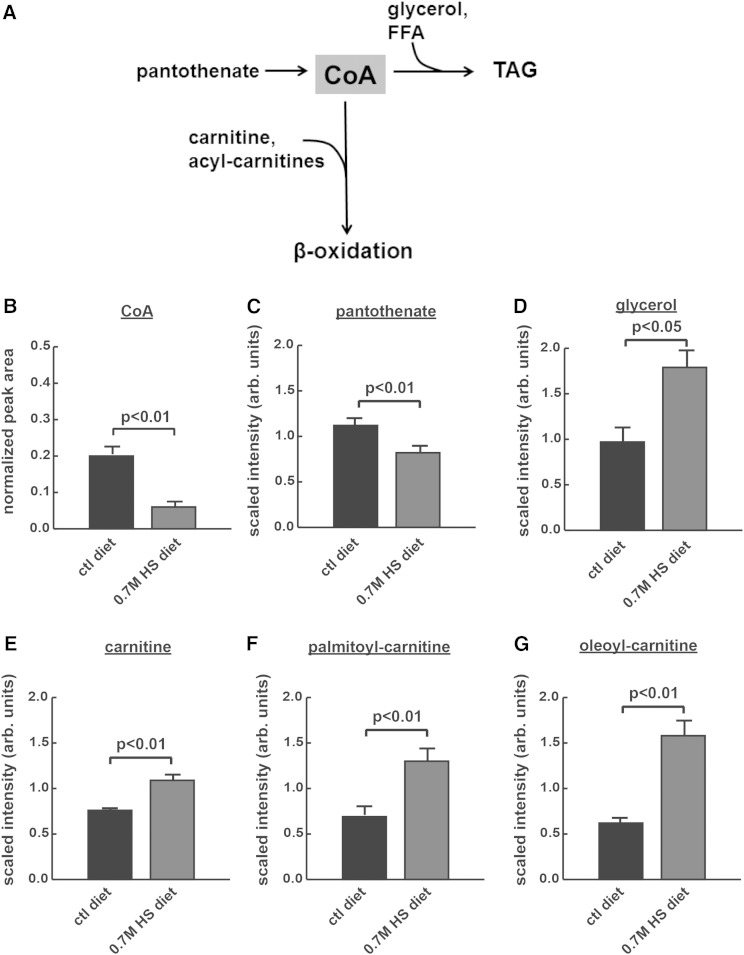
The gene expression results shown in Fig. 1 suggested compensatory regulation of CoA production in HS-reared larvae. Therefore, we hypothesized that CoA might limit fatty acid esterification into TG in the face of a HS diet (Fig. 2A). First, we characterized the levels of free CoA in whole larvae reared on control or HS diets. HS feeding led to a significant decrease in CoA concentrations in whole third-instar larvae (Fig. 2B), consistent with a trend toward decreased CoA shown in our previous work (17). Metabolomic analyses of FBs and hemolymph supported a role for pantothenate in lipogenesis during challenge with HS diets. Pantothenate levels were decreased in the FB, consistent with an increase in shunting of pantothenate toward CoA synthesis via increased pantothenate kinase expression in FBs from larvae fed HS diets (Fig. 2C). At the same time, intermediates that require free CoA to promote lipid metabolism accumulated in both the FB (glycerol and carnitine; Fig. 2D, E, respectively) and hemolymph (palmitoyl-carnitine and oleoyl-carnitine; Fig. 2F, G, respectively). Glycerol requires CoA in order to generate TGs from FFAs, whereas carnitine, palmitoyl-carnitine, and oleoyl-carnitine all require CoA in order to promote fatty acid β-oxidation (Fig. 2A). Our metabolomic analyses were not sensitive enough to detect free CoA or fatty acyl-CoAs in these samples, but support the model nonetheless.
Fig. 2.
CoA may be limiting in HS-reared wild-type larvae. A: CoA and pantothenate (the ionic form of PA, which is required for the synthesis of CoA) were observed in reduced quantities, whereas metabolites involved in the utilization of CoA were observed in increased quantities, in HS-fed larvae. B: Free CoA-SH in whole larvae measured by HPLC; pantothenate (C), glycerol (D), carnitine (E), palmitoyl-carnitine (F), oleoyl-carnitine (G). Parts (C–E) were extracted from isolated FBs and (F, G) were extracted from isolated hemolymph.
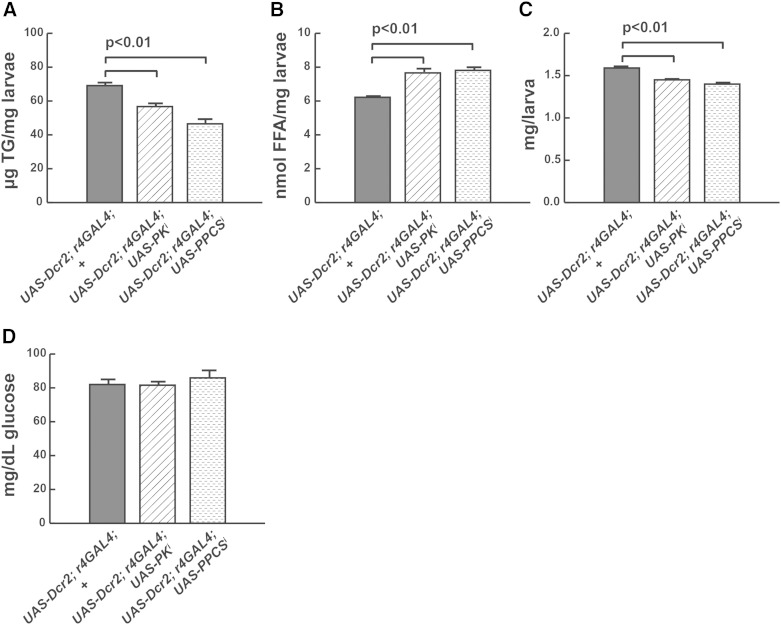
Because steady-state levels of metabolites do not reflect the metabolic flux through that pathway, we took a functional approach to test whether the conversion of pantothenate to CoA was important for tolerance of HS diets. We targeted two enzymes that catalyze CoA synthesis from pantothenate, the rate-limiting pantothenate kinase (PK also known as fumble, CG5725) and phosphopantothenoylcysteine synthase (PPCS, CG5629), which, like fumble, is required for growth of Drosophila S2 cells (31). Because the FB is the primary site of TG synthesis in the fly, we used FB-specific RNAi to reduce CoA biosynthesis from pantothenate by targeting PK/fumble and PPCS in this tissue (Fig. 1A). The r4-GAL4 driver was used in combination with a UAS-Dicer2 transgene to maximize degradation of endogenous mRNA. Knockdown of either gene product led to reduced numbers of larvae on HS diets (not shown), consistent with an essential role for CoA in supporting TG synthesis. Mutant larvae were leaner, with increased FFA levels, when compared with wild-type controls reared on the same HS diet (Fig. 3A, B). These larvae also exhibited reduced size, compared with HS-fed wild-type larvae (Fig. 3C). Surprisingly, no increase in hemolymph glucose concentration was observed in either mutant (Fig. 3D).
Fig. 3.
FB-specific targeting of genes encoding enzymes in CoA synthesis exacerbates metabolic phenotypes resulting from challenge with the HSD. UAS-Dcr2; r4-GAL4 transgenes were used to target fumble (PK, CG5725) or PPCS (CG5629) in the FB using transgenic RNAi. TG (A), FFA (B), weights (C), and hemolymph glucose (D).
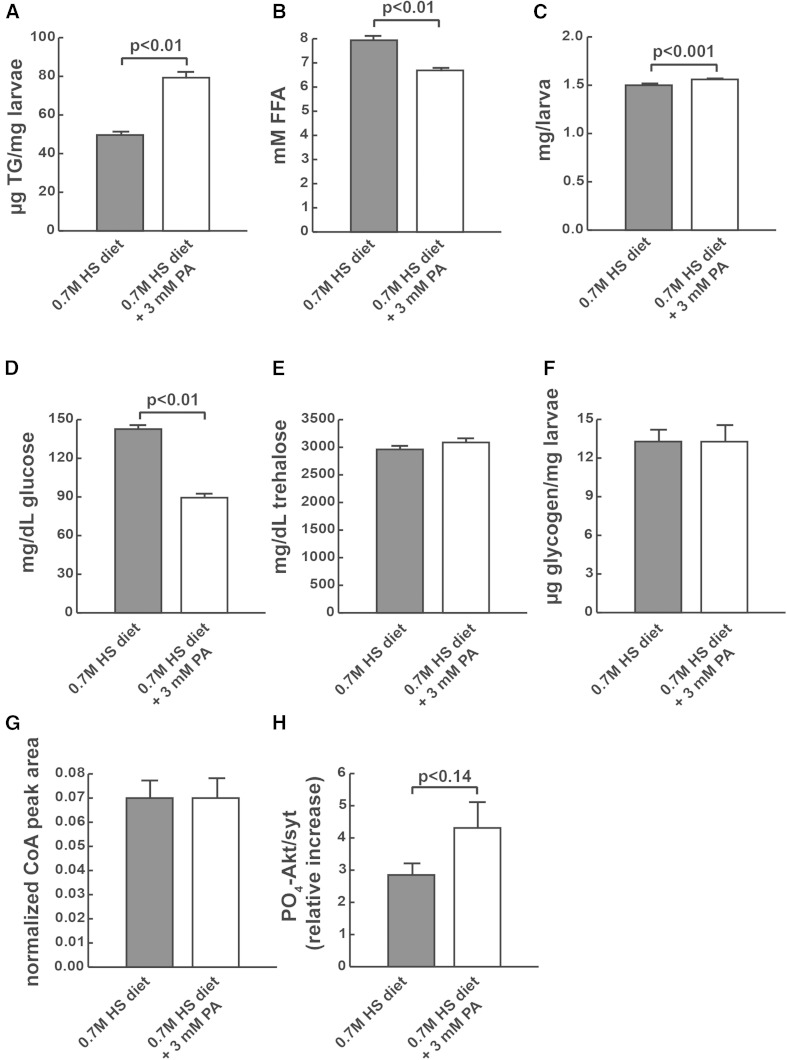
Given that knockdown of the CoA synthetic enzymes, pantothenate kinase and PPCS, exacerbated phenotypes, we concluded that CoA is likely limiting for several processes on HS diets, including TG storage and fatty acid β-oxidation. Therefore, we tested to determine whether we could rescue some of the effects of HS diets by increasing CoA levels. To do this, we supplemented the HS diet with the CoA precursor, PA, to test whether it could increase the ability of larvae to store TG when fed HS diets. PA supplementation significantly increased TG storage in HS-fed larvae (Fig. 4A) and reduced FFA concentrations (Fig. 4B). PA supplementation also increased weight and reduced hemolymph glucose in larvae reared on HS diets, consistent with a potential increase in insulin signaling (Fig. 4C, D). The decrease in hemolymph glucose was likely not due to incorporation into disaccharides or polysaccharides because PA did not affect levels of trehalose or glycogen (Fig. 4E, F). PA supplementation did not affect free CoA-SH levels (Fig. 4G). We also tested whether PA could improve insulin sensitivity in cultured FBs, as measured by exogenous insulin stimulation of Akt phosphorylation at serine 505. No significant improvement in insulin responsiveness was seen when PO4-Akt was measured in insulin-stimulated FBs from wandering larvae reared on HS compared with HS + PA (4H). As an additional dietary approach, we tested whether adding cysteine to pantothenate could improve any HS-induced phenotypes, as cysteine also contributes to CoA synthesis as a substrate for PPCS (Fig. 1A). No improvements were observed relative to pantothenate supplementation, and decreased weights were observed with cysteine alone, in agreement with another recent study showing that flies do not tolerate cysteine supplementation (32) (data not shown). A modest but nonsignificant increase in FB cysteine levels was observed by LC-MS (1.5-fold, P < 0.2), suggesting that cysteine depletion was not a contributing factor to the observed reduction in CoA.
Fig. 4.
PA supplementation of wild-type larvae increases metabolic flux into TG in the face of caloric overload. Whole animal TG (A), whole animal FFA (B), weights (C), hemolymph glucose (D), hemolymph trehalose (E), whole animal glycogen (F), free CoA-SH (G), and FB insulin sensitivity (H).
DISCUSSION
Previous studies have shown a reduced capacity for TG synthesis in obesity that is accompanied by increases in FFAs, ceramides, and DAG, all potential mediators of lipotoxicity. Still, it remains unknown what mechanisms limit the ability of animals to store excess carbons from dietary sugar as TG. We noticed a dramatic upregulation in the expression of CoA synthetic enzymes that prompted us to take a closer look at these steps of the pathway. The CoA pool is known to be limiting for several metabolic processes, including the TCA cycle, ketogenesis, lipogenesis, and mitochondrial fatty acid import and β-oxidation (21, 26, 33). Although we did not probe all of these pathways, our data support a model where CoA is limiting in the face of caloric excess, reducing animal fitness by contributing to metabolic lipotoxicity.
The Drosophila gut may be an important source of pantothenate. The fly gut is known to harbor commensal bacteria that regulate nutritional status and might help to provide pantothenate, as has been demonstrated in mammals (34–36). We observed measurable quantities of this nutrient in isolated guts, although no change in pantetheine or pantothenate levels was observed upon HS feeding (data not shown). Increased gut expression of genes predicted to encode the pantetheine hydrolase vanin-like and the pantothenate transporter, CG10444, may represent an attempt of the gut to compensate for inadequate CoA levels and suggests a concerted systemic effort to provide this nutrient to the FB.
One open question is: what metabolites indicate an increased requirement for pantothenate in peripheral tissues? The carnitine-acyl carnitine system is one way in which free CoA pools are maintained in cells (37, 38). Serum acyl-carnitine concentrations reflect an excess of intracellular acyl groups, increasing when fatty acid oxidation is defective in the presence of increased FFAs (39, 40). It follows that these acyl-carnitines might accumulate when metabolic flux is reduced during insulin resistance. Increased long-chain carnitine esters have been observed in the serum, liver, muscle, and urine of individuals with obesity and T2D (41–46), although reduced levels of long-chain acyl-carnitines have also been associated with metabolic syndrome and T2D (47). Rodent models of obesity and T2D also accumulate acyl-carnitines (48–50). In Drosophila, acyl-carnitines decline with age, along with obesity (51, 52). Perhaps circulating acyl-carnitines signal a demand for CoA to enable proper fatty acid esterification into TG in the FB and adipose. Our data support a model where CoA bioavailability enables metabolic flexibility and channeling of the endocrine fatty acid pool.
Another potential rate-limiting substrate for CoA synthesis in the face of caloric overload is cysteine (Fig. 1A), although our data suggest that cysteine is not limiting in the context of caloric overload. Cysteine supplementation alone slightly reduced fitness on HS diets and did not rescue HS phenotypes (data not shown). Metabolite analysis showed that cysteine levels were slightly elevated in HS-fed FBs compared with controls. Further increasing cysteine levels could adversely affect redox status in the FB, impairing cellular processes and masking any benefit to lipogenesis. It is interesting to note that some studies have shown a benefit for cysteine supplementation in T2D (53, 54). We presume that a number of metabolites have the potential to become rate-limiting under different physiological conditions. Nonetheless, our data support a substrate-limited model where increasing the production of CoA benefits animal health in the face of a HS diet.
PA is available over-the-counter as calcium pantothenate in vitamin B5 supplements. In a recent study, pantothenate supplementation promoted CoA-dependent ketogenesis and improved liver function in an animal model of nonalcoholic fatty liver disease (23). We propose that vitamin B5 represents a potential therapy for insulin resistance resulting from overnutrition. Although pantothenate supplementation would be expected to increase adiposity, our work suggests a significant benefit in terms of metabolic health. PA’s low cost and toxicity profile make it an especially attractive target for future clinical studies.
Acknowledgments
The authors acknowledge David Cotter, Peter Crawford, Mignon Keaton, Rob Mohney, Haowei Song, John Turk, and an anonymous reviewer from a previous manuscript for helpful discussions. They thank Zeke Maier and Washington University’s Genome Technology Access Center for contributing to the RNA-seq data.
Footnotes
Abbreviations:
- FB
- fat body
- HS
- high sugar
- PA
- pantothenic acid
- PK
- pantothenate kinase
- PPCS
- phosphopantothenoylcysteine synthase
This research was funded by National Institutes of Health, Building Interdisciplinary Research Careers in Women’s Health program Grant K12HD001459-12 and National Institutes of Health Grant P60DK020579-34 (L.P.M.), and Children’s Discovery Institute Grant MD-II-2010-41 (T.J.B.).
REFERENCES
- 1.Mittendorfer B. 2011. Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Curr. Opin. Clin. Nutr. Metab. Care. 14: 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi J. K. 2010. Activatin’ human adipose progenitors in obesity. Diabetes. 59: 2354–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan C. Y., and Vidal-Puig A.. 2008. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem. Soc. Trans. 36: 935–940. [DOI] [PubMed] [Google Scholar]
- 4.Virtue S., and Vidal-Puig A.. 2010. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome–an allostatic perspective. Biochim. Biophys. Acta. 1801: 338–349. [DOI] [PubMed] [Google Scholar]
- 5.Foster D. J., Ravikumar P., Bellotto D. J., Unger R. H., and Hsia C. C.. 2010. Fatty diabetic lung: altered alveolar structure and surfactant protein expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 298: L392–L403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gealekman O., Gurav K., Chouinard M., Straubhaar J., Thompson M., Malkani S., Hartigan C., and Corvera S.. 2014. Control of adipose tissue expandability in response to high fat diet by the insulin-like growth factor-binding protein-4. J. Biol. Chem. 289: 18327–18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodson D. J., Mitchell R. K., Bellomo E. A., Sun G., Vinet L., Meda P., Li D., Li W. H., Bugliani M., Marchetti P., et al. 2013. Lipotoxicity disrupts incretin-regulated human beta cell connectivity. J. Clin. Invest. 123: 4182–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina-Gomez G., Gray S. L., Yetukuri L., Shimomura K., Virtue S., Campbell M., Curtis R. K., Jimenez-Linan M., Blount M., Yeo G. S., et al. 2007. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 3: e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prieur X., Mok C. Y., Velagapudi V. R., Nunez V., Fuentes L., Montaner D., Ishikawa K., Camacho A., Barbarroja N., O’Rahilly S., et al. 2011. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 60: 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brookheart R. T., Michel C. I., and Schaffer J. E.. 2009. As a matter of fat. Cell Metab. 10: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unger R. H., and Scherer P. E.. 2010. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol. Metab. 21: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musselman L. P., Fink J. L., Narzinski K., Ramachandran P. V., Hathiramani S. S., Cagan R. L., and Baranski T. J.. 2011. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model. Mech. 4: 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed L. K., Williams S., Springston M., Brown J., Freeman K., DesRoches C. E., Sokolowski M. B., and Gibson G.. 2010. Genotype-by-diet interactions drive metabolic phenotype variation in Drosophila melanogaster. Genetics. 185: 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havula E., Teesalu M., Hyötyläinen T., Seppälä H., Hasygar K., Auvinen P., Orešič M., Sandmann T., and Hietakangas V.. 2013. Mondo/ChREBP-Mlx-regulated transcriptional network is essential for dietary sugar tolerance in Drosophila. PLoS Genet. 9: e1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasco M. Y., and Leopold P.. 2012. High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLoS One. 7: e36583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrido D., Rubin T., Poidevin M., Maroni B., Le Rouzic A., Parvy J-P., and Montagne J.. 2015. Fatty acid synthase cooperates with glyoxalase 1 to protect against sugar toxicity. PLoS Genet. 11: e1004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musselman L. P., Fink J. L., Ramachandran P. V., Patterson B. W., Okunade A. L., Maier E., Brent M. R., Turk J., and Baranski T. J.. 2013. Role of fat body lipogenesis in protection against the effects of caloric overload in Drosophila. J. Biol. Chem. 288: 8028–8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Na J., Musselman L. P., Pendse J., Baranski T. J., Bodmer R., Ocorr K., and Cagan R.. 2013. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 9: e1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taubes G. 2009. Insulin resistance. Prosperity’s plague. Science. 325: 256–260. [DOI] [PubMed] [Google Scholar]
- 20.Virtue S., and Vidal-Puig A.. 2008. It’s not how fat you are, it’s what you do with it that counts. PLoS Biol. 6: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonardi R., Zhang Y. M., Rock C. O., and Jackowski S.. 2005. Coenzyme A: back in action. Prog. Lipid Res. 44: 125–153. [DOI] [PubMed] [Google Scholar]
- 22.Robishaw J. D., and Neely J. R.. 1985. Coenzyme A metabolism. Am. J. Physiol. 248: E1–E9. [DOI] [PubMed] [Google Scholar]
- 23.Soulié J. M., Sheplock G. J., Tian W. X., and Hsu R. Y.. 1984. Transient kinetic studies of fatty acid synthetase. A kinetic self-editing mechanism for the loading of acetyl and malonyl residues and the role of coenzyme A. J. Biol. Chem. 259: 134–140. [PubMed] [Google Scholar]
- 24.Stern A., Sedgwick B., and Smith S.. 1982. The free coenzyme A requirement of animal fatty acid synthetase. Participation in the continuous exchange of acetyl and malonyl moieties between coenzyme a thioester and enzyme. J. Biol. Chem. 257: 799–803. [PubMed] [Google Scholar]
- 25.Sahlin K., Sallstedt E-K., Bishop D., and Tonkonogi M.. 2008. Turning down lipid oxidation during heavy exercise–what is the mechanism? J. Physiol. Pharmacol. 59(Suppl 7): 19–30. [PubMed] [Google Scholar]
- 26.Eaton S. 2002. Control of mitochondrial beta-oxidation flux. Prog. Lipid Res. 41: 197–239. [DOI] [PubMed] [Google Scholar]
- 27.Cotter D. G., Ercal B., Huang X., Leid J. M., d’Avignon D. A., Graham M. J., Dietzen D. J., Brunt E. M., Patti G. J., and Crawford P. A.. 2014. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J. Clin. Invest. 124: 5175–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee G., and Park J. H.. 2004. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 167: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dehaven C. D., Evans A. M., Dai H., and Lawton K. A.. 2010. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminform. 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chintapalli V. R., Wang J., and Dow J. A.. 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720. [DOI] [PubMed] [Google Scholar]
- 31.Rana A., Seinen E., Siudeja K., Muntendam R., Srinivasan B., van der Want J. J., Hayflick S., Reijngoud D. J., Kayser O., and Sibon O. C.. 2010. Pantethine rescues a Drosophila model for pantothenate kinase-associated neurodegeneration. Proc. Natl. Acad. Sci. USA. 107: 6988–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maguire S. E., Rhoades S., Chen W-F., Sengupta A., Yue Z., Lim J. C., Mitchell C. H., Weljie A. M., and Sehgal A.. 2015. Independent effects of γ-aminobutyric acid transaminase (GABAT) on metabolic and sleep homeostasis. J. Biol. Chem. 290: 20407–20416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotter D. G., Schugar R. C., and Crawford P. A.. 2013. Ketone body metabolism and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 304: H1060–H1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erkosar B., Erkosar Combe B., Defaye A., Bozonnet N., Puthier D., Royet J., and Leulier F.. 2014. Drosophila microbiota modulates host metabolic gene expression via IMD/NF-κB signaling. PLoS One. 9: e94729 [Erratum. 2014. PLoS One 9: e104120.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma D., Storelli G., Mitchell M. L., and Leulier F.. 2015. Studying host-microbiota mutualism in Drosophila: harnessing the power of gnotobiotic flies. Biomed. J. 38: 285–293. [DOI] [PubMed] [Google Scholar]
- 36.Stein E. D., and Diamond J. M.. 1989. Do dietary levels of pantothenic acid regulate its intestinal uptake in mice? J. Nutr. 119: 1973–1983. [DOI] [PubMed] [Google Scholar]
- 37.Foster D. W. 2004. The role of the carnitine system in human metabolism. Ann. N. Y. Acad. Sci. 1033: 1–16. [DOI] [PubMed] [Google Scholar]
- 38.Ramsay R. R., and Zammit V. A.. 2004. Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol. Aspects Med. 25: 475–493. [DOI] [PubMed] [Google Scholar]
- 39.Kien C. L., Matthews D. E., Poynter M. E., Bunn J. Y., Fukagawa N. K., Crain K. I., Ebenstein D. B., Tarleton E. K., Stevens R. D., Koves T. R., et al. 2015. Increased palmitate intake: higher acylcarnitine concentrations without impaired progression of β-oxidation. J. Lipid Res. 56: 1795–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lepage N., and Aucoin S.. 2010. Measurement of plasma/serum acylcarnitines using tandem mass spectrometry. Methods Mol. Biol. 603: 9–25. [DOI] [PubMed] [Google Scholar]
- 41.Ferrara C. T., Wang P., Neto E. C., Stevens R. D., Bain J. R., Wenner B. R., Ilkayeva O. R., Keller M. P., Blasiole D. A., Kendziorski C., et al. 2008. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genet. 4: e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floegel A., Wientzek A., Bachlechner U., Jacobs S., Drogan D., Prehn C., Adamski J., Krumsiek J., Schulze M. B., Pischon T., et al. 2014. Linking diet, physical activity, cardiorespiratory fitness and obesity to serum metabolite networks: findings from a population-based study. Int. J. Obes. (Lond). 38: 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koves T. R., Li P., An J., Akimoto T., Slentz D., Ilkayeva O., Dohm G. L., Yan Z., Newgard C. B., and Muoio D. M.. 2005. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem. 280: 33588–33598. [DOI] [PubMed] [Google Scholar]
- 44.Mai M., Tönjes A., Kovacs P., Stumvoll M., Fiedler G. M., and Leichtle A. B.. 2013. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS One. 8: e82459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Möder M., Kiessling A., Löster H., and Brüggemann L.. 2003. The pattern of urinary acylcarnitines determined by electrospray mass spectrometry: a new tool in the diagnosis of diabetes mellitus. Anal. Bioanal. Chem. 375: 200–210. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X., Zhang C., Chen L., Han X., and Ji L.. 2014. Human serum acylcarnitine profiles in different glucose tolerance states. Diabetes Res. Clin. Pract. 104: 376–382. [DOI] [PubMed] [Google Scholar]
- 47.Bene J., Márton M., Mohás M., Bagosi Z., Bujtor Z., Oroszlán T., Gasztonyi B., Wittmann I., and Melegh B.. 2013. Similarities in serum acylcarnitine patterns in type 1 and type 2 diabetes mellitus and in metabolic syndrome. Ann. Nutr. Metab. 62: 80–85. [DOI] [PubMed] [Google Scholar]
- 48.Henagan T. M., Stefanska B., Fang Z., Navard A. M., Ye J., Lenard N. R., and Devarshi P. P.. 2015. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br. J. Pharmacol. 172: 2782–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warren B. E., Lou P-H., Lucchinetti E., Zhang L., Clanachan A. S., Affolter A., Hersberger M., Zaugg M., and Lemieux H.. 2014. Early mitochondrial dysfunction in glycolytic muscle, but not oxidative muscle, of the fructose-fed insulin-resistant rat. Am. J. Physiol. Endocrinol. Metab. 306: E658–E667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wessels B., Ciapaite J., van den Broek N. M. A., Houten S. M., Nicolay K., and Prompers J. J.. 2015. Pioglitazone treatment restores in vivo muscle oxidative capacity in a rat model of diabetes. Diabetes Obes. Metab. 17: 52–60. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman J. M., Soltow Q. A., Li S., Sidik A., Jones D. P., and Promislow D. E. L.. 2014. Effects of age, sex, and genotype on high-sensitivity metabolomic profiles in the fruit fly, Drosophila melanogaster. Aging Cell. 13: 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skorupa D. A., Dervisefendic A., Zwiener J., and Pletcher S. D.. 2008. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 7: 478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain S. K. 2012. L-cysteine supplementation as an adjuvant therapy for type-2 diabetes. Can. J. Physiol. Pharmacol. 90: 1061–1064. [DOI] [PubMed] [Google Scholar]
- 54.McPherson R. A., and Hardy G.. 2011. Clinical and nutritional benefits of cysteine-enriched protein supplements. Curr. Opin. Clin. Nutr. Metab. Care. 14: 562–568. [DOI] [PubMed] [Google Scholar]