Abstract
The endocannabinoids and their main receptor, cannabinoid type-1 (CB1), suppress intracellular cyclic AMP levels and have emerged as key players in the control of energy metabolism. CB1 agonists and blockers have been reported to influence the thermogenic function of white and brown adipose tissue (WAT and BAT), affecting body weight through the inhibition and stimulation of energy expenditure, respectively. The purpose of the current study was to investigate the regulation of the endocannabinoid system in WAT and BAT following exposure to either cold or specific agonism of β3-adrenoceptors using CL316,243 (CL), conditions known to cause BAT activation and WAT browning. To address this question, we performed quantitative PCR-based mRNA profiling of genes important for endocannabinoid synthesis, degradation, and signaling, and determined endocannabinoid levels by LC-MS in WAT and BAT of control, cold-exposed, and CL-treated wild-type mice as well as primary brown adipocytes. Treatment with CL and exposure to cold caused an upregulation of endocannabinoid levels and biosynthetic enzymes in WAT. Acute β3-adrenoceptor activation increased endocannabinoids and a subset of genes of biosynthesis in BAT and primary brown adipocytes. We suggest that the cold-mediated increase in endocannabinoid tone is part of autocrine negative feed-back mechanisms controlling β3-adrenoceptor-induced BAT activation and WAT browning.
Keywords: cannabinoids, protein kinases/protein kinase A, gene expression, phospholipids
The recent discovery of active brown adipose tissue (BAT) in adult humans (1–3) is one of the most intriguing findings, as it raises hope for the treatment of obesity and related chronic metabolic diseases. The natural function of BAT is to combust energy from high-caloric nutrients to defend the body against cold environments (4). The ability to burn energy-dense triglycerides as fuels for heat production could enable BAT to diminish hypertrophic white adipose tissue (WAT) depots, a prerequisite for the prevention of metabolic lifestyle diseases (5, 6). In humans, BAT activity, determined by positron emission tomography-computed tomography (PET-CT), is positively correlated with BAT mass (1–3), BAT activation status (2), and environmental factors such as low temperatures (7). Repeated cold exposure leads to increased BAT activity (8, 9), a condition that is associated with a self-reported decrease in sensitivity to cold. The thermogenic process is dependent on the presence of uncoupling protein 1 (UCP1), a protein located in the inner membrane of mitochondria that is able to separate electron transport in the respiratory chain from the production of energy in the form of ATP. The heat generated by this exothermic reaction is transported via the blood circulation system to maintain body temperature (10). Low BAT activity in humans correlates with ageing and obesity (2, 11), suggesting a causal link between decreased BAT activity, weight gain, and the development of metabolic diseases. In this context, channeling fatty acids and triglycerides into BAT could attenuate deleterious effects that saturated fatty acids cause by ectopic lipid accumulation in the liver or heart. In fact, up to 90% of energy for heat production is derived from fatty acids that are delivered by triglyceride-rich lipoproteins (4, 10, 12). These latter are hepatic VLDLs and intestinal chylomicrons that are both processed by endothelium-bound lipoprotein lipase to allocate fatty acids to BAT and energy storing WAT, respectively.
In response to cold exposure, both brown and white adipocytes are activated via sympathetic neurons that release noradrenalin (10). Catecholamine release causes the activation of β3-adrenoceptor signaling stimulating lipolysis of triglycerides stored in lipid droplets, a process mediated by the enzymatic activity of adipose tissue triglyceride lipase (ATGL) and hormone sensitive lipase (HSL) (13). In brown adipocytes, fatty acids are transferred to mitochondria for β-oxidation and UCP1-dependent heat production. In white adipocytes, lipid droplet-derived fatty acids are released into the circulation for hepatic VLDL production to maintain energy supply for cold-activated brown adipocytes. Thus, short term activation of β3-adrenoceptors stimulates intracellular lipolysis orchestrating the systemic energy homeostasis. It is not surprising that both WAT and BAT undergo adaptive and dynamic changes in response to sustained β3-adrenoceptor activation or cold exposure. In this context one of the most intensively studied cell types implicated in cold-induced tissue remodeling in WAT (browning) is the so-called beige adipocyte (14). Prolonged cold exposure or pharmacological treatment using β3-adrenoceptor agonists, such as CL316,243 (CL), stimulate the development of these inducible brown-like adipocytes (14–17). Brown and beige adipocytes are characterized by a large number of mitochondria and numerous small lipid droplets and both cell types are functionally active with regard to adaptive thermogenesis (18).
The endocannabinoid system and, in particular, the G protein-coupled receptor known as cannabinoid type-1 (CB1) and its endogenous agonists, N-arachidonoylethanolamine (AEA, anandamide) and 2-arachidonoylglycerol (2-AG), have emerged as major players in the control of metabolism at both the central and peripheral level (19). Importantly, malfunctioning of this system due to enhanced tissue levels of endocannabinoids and subsequent overactivation of CB1 receptors in the hypothalamus, visceral WAT, liver, muscle, and pancreas, accompanies, and is probably one of the causes of, fat accumulation and insulin resistance in animal models of obesity. High plasmatic levels of endocannabinoids have been associated with increased cardiometabolic risk, visceral WAT accumulation, and type 2 diabetes in obese patients (19). Genetic impairment of CB1 receptors selectively in neurons of the brain and sympathetic nervous system (SNS) enhances energy expenditure by the BAT (20), and very recent evidence suggests an inhibitory role of prejunctional CB1 on brown adipocyte glucose utilization (21), thermogenesis, and lipid droplet formation (22). Furthermore, CB1 blockade in Sim1-expressing neurons of the paraventricular nucleus of the hypothalamus enhances energy expenditure during high-fat diet feeding (23). While mice overexpressing neuropeptide Y on noradrenergic neurons of the brain and SNS exhibit age-dependent elevation of endocannabinoid levels in many target organs, including the WAT (24), genetic knockout of CB1 in Sim1-expressing neurons is accompanied by increased mRNA expression of the β3-adrenoceptor and UCP1 in the BAT of high-fat diet-fed mice (23). These data point to a strong and SNS-mediated association between the endocannabinoid system and the control of BAT and WAT function. While there are several examples of reports [summarized in (19)] pointing to an autocrine role of endocannabinoids and CB1 receptors on white adipocytes, also facilitating, among others, their transformation into beige adipocytes (25, 26), evidence for a similar role in brown adipocytes is still scant. Through the use of a peripherally restricted CB1 antagonist in vivo, and of T37i brown adipocyte-like cells in vitro, Boon et al. (22) very recently suggested that some of the above effects of CB1 blockade in the BAT, and in particular the stimulation of uncoupled respiration, may be exerted postjunctionally and directly on brown adipocytes, most likely by enhancing cyclic AMP/PKA signaling induced by β3-adrenoceptor activation. However, the question of whether exposure to cold, via sympathetic stimulation of β3-adrenoceptors in brown adipocytes, enhances endocannabinoid levels in BAT has not been tested so far. In the present study, we addressed this hypothesis by examining the effect of cold exposure and pharmacological β3-adrenoceptor activation in mice using the selective agonist, CL, on: a) the levels of AEA, 2-AG, and nonendocannabinoid N-acylethanolamines (NAEs); and b) mRNA expression levels of genes encoding for CB1 receptors and endocannabinoid metabolic enzymes (Fig. 1). In order to analyze the effects on BAT activity, as well as browning of subcutaneous WAT, these studies were conducted under conditions of acute and chronic cold and β3-adrenergic stimulation.
Fig. 1.
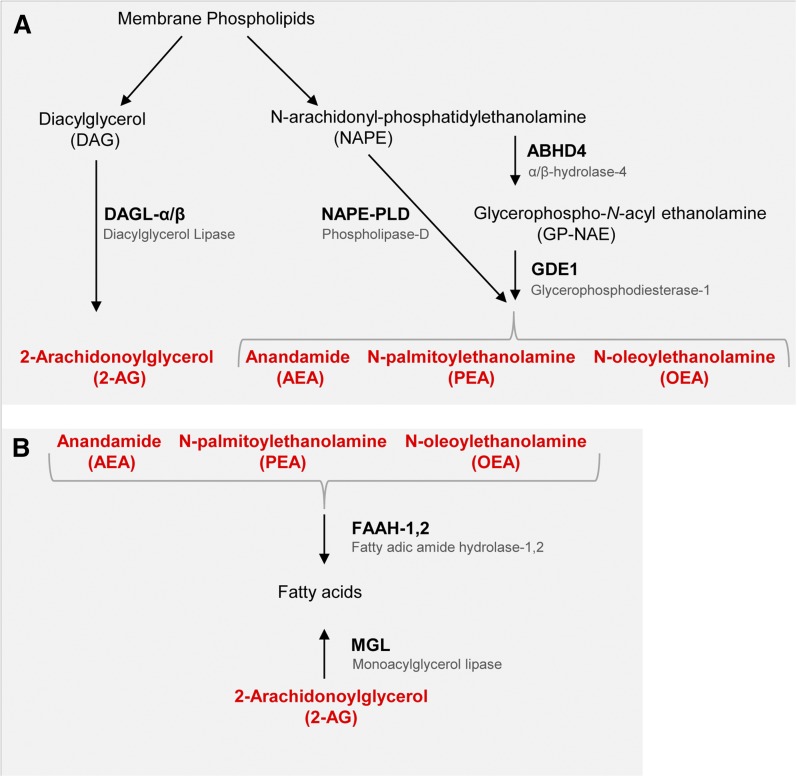
Schematic diagram of enzymes involved in endocannabinoid synthesis and degradation. A: From membrane phospholipids, 2-AG is produced via DAGLα/β, while AEA, PEA, and OEA synthesis is enabled either directly via NAPE-PLD or by ABHD4 and GDE1. B: The 2-AG is degraded via MGL, whereas AEA, PEA, and OEA are hydrolyzed by FAAH.
METHODS
Experimental animals and diets
All animal experiments were approved by the Animal Welfare Officers of University Medical Center Hamburg-Eppendorf and Behörde für Gesundheit und Verbraucherschutz Hamburg. Mice were bred and housed in the animal facility of University Medical Center Hamburg-Eppendorf at 22–24°C with a day and night cycle of 12 h. Male age-matched (12–14 weeks) C57BL/6J wild-type mice, housed in single cages and fed a standard chow diet (Lasvendi) with ad libitum access to food and water, were used for the experiments. Cold exposure was performed by housing the mice at 6°C for 1 day or 7 days. The β3-adrenoceptor agonist, CL (0.2 mg CL/ml in 0.9% NaCl (w/v); Tocris) was administered by subcutaneous injection (1 μg per gram body weight per day) either for: a) 7 days without treatment on the day of necropsy (chronic CL); b) only once, 4 h before necropsy (acute CL); or c) chronically for 7 days with an additional injection on day 8 before necropsy (chronic+acute CL). Mock-treated control mice and acute CL mice received corresponding 0.9% NaCl (w/v) injections throughout the treatment period. All tissue and blood collections were performed after 4 h fasting. Mice were anesthetized with a lethal dose (15 μl per gram mouse body weight) of a mix containing ketamine (25 mg/ml)/xylazine (0.2%) in 0.9% NaCl. Blood was withdrawn transcardially and animals were perfused with 5 ml ice-cold PBS containing 10 U/ml heparin. Organs were harvested, immediately frozen, and stored at −80°C. For RNA analysis samples were conserved in TRIzol® reagent (Invitrogen).
Gene expression analysis
Total RNA was isolated from inguinal subcutaneous WAT and subscapular BAT using NucleoSpin RNA II kit (Macherey and Nagel). Synthesis of cDNA was performed using SuperScript® III reverse transcriptase (Invitrogen). Quantitative real-time PCR reactions for indicated genes were conducted on a 7900HT sequence detection system (Applied Biosystems) using TaqMan Assay-on-Demand primer sets [Ucp1, Mm00494069_m1; Ppargc1a, Mm00447183_m1; Dio2, Mm00515664_m1; Elovl3, Mm00468164_m1; Cnr1, Mm01212171_s1; Cnr2, Mm02620087_s1; α/β domain containing-4 (Abhd4), Mm00506368_m1; glycerophosphodiester phosphodiesterase-1 (Gde1), Mm00450997_m1; N-acyl phosphatidylethanolamine phospholipase D (Napepld), Mm00724596_m1; fatty acid amide hydrolase (Faah), Mm00515684_m1; diacylglycerol lipase-α (Dagla), Mm00813830_m1; Daglb, Mm00523381_m1; monoglyceride lipase (Mgll), Mm00449274_m1] supplied by Applied Biosystems and selected to recognize RefSeq sequences and a maximum of GenBank expressed sequence tags. Gene of interest cycle thresholds (Cts) were normalized to TATA-box binding protein (Tbp, Mm00446973_m1) house keeper levels by the ΔΔCt method and displayed as normalized copy numbers relative to experimental control groups (fold).
Extraction, purification, and quantitative analysis of endocannabinoids from adipose tissue
Lipids were extracted from inguinal subcutaneous WAT and interscapular BAT of mice, and endocannabinoid purified from lipid extracts, as previously described (27). Measurement of endocannabinoids (AEA and 2-AG), as well as of nonendocannabinoid NAEs, N-palmitoylethanolamine (PEA), and N-oleoylethanolamine (OEA), was carried out by isotope dilution LC-atmospheric pressure chemical ionization-MS using deuterated standards, as previously described (27).
Culture of primary brown adipocytes
For the preparation of primary brown adipocytes, 9-week-old C57BL6/J mice were anesthetized, interscapular BAT was removed and digested in isolation buffer [123 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 5 mM glucose, 100 mM HEPES (pH 7.4)] containing collagenase II (Biochrom). The stromavascular fraction was isolated by filtration of the cell suspension through 100 μm and 40 μm nylon mesh and plated out. The cells (including preadipocytes) were cultured for 10 days and differentiated through addition of 20 nM insulin (Sigma), 1 nM tri-iodothyronine-sodium (Sigma), 0.5 mM 3-isobutyl-1-methylxanthine (Sigma), 1 μM dexamethasone (Sigma). The resulting primary brown adipocytes were treated with and without 1 μM of CL in DMEM containing 0.1% fatty acid-free BSA (GE Healthcare) for 4 h. Supernatants were harvested and nonesterified fatty acids were determined using standard colorimetric assays (Wako). RNA analysis of brown adipocytes was performed as described for the tissue samples.
RESULTS
Effect of cold and β3-adrenoceptor activation on the expression of thermogenesis markers and endocannabinoid receptors in BAT and WAT
In order to study the endocannabinoid system in cold-activated BAT and WAT, and to differentiate short-term from long-term effects, the following treatments were chosen: cold exposure was applied to mice for either 1 day or for 7 days. To another set of mice, the β3-adrenergic agonist, CL, was administered either once, 4 h before necropsy (acute CL) for seven consecutive days and also on day 8 before necropsy (chronic+acute CL) or for 7 days, but not before necropsy (chronic CL).
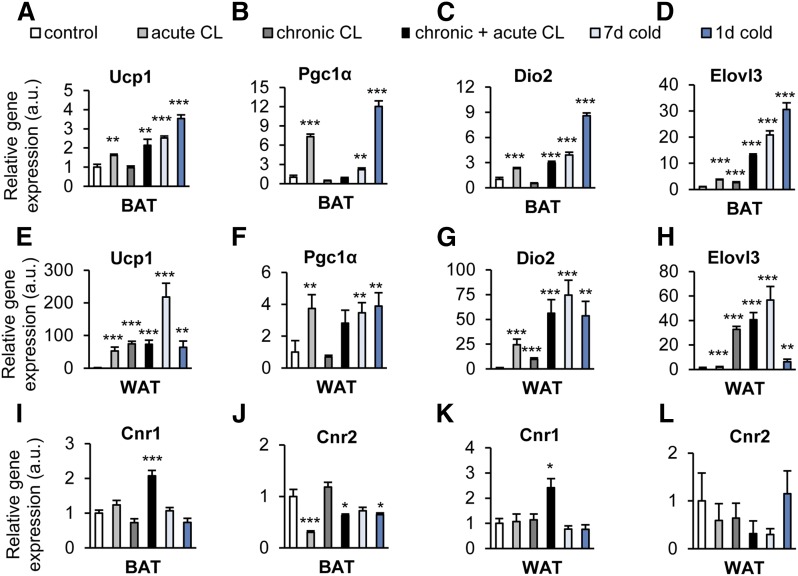
Stimulation of β3-adrenoceptors with CL and exposure to cold are known to markedly enhance the expression of characteristic markers of uncoupled respiration and thermogenesis via specific transcription factors in WAT and BAT (14, 15), revealing different degrees of WAT browning and BAT activation, respectively (Fig. 2). In both tissues, activation of the respective signaling pathways induced mRNA levels of Ucp1 (Fig. 2A, E), which encodes the heat generating protein, UCP1, that is located in the inner mitochondrial membrane of thermogenic adipocytes. In comparison to its expression in BAT, the basal Ucp1 mRNA levels are much lower in WAT, leading to a much higher relative induction of Ucp1 mRNA after CL treatment or cold exposure (10). The increased expression of the PPAR coactivator 1α (Ppargc1a, Fig. 2B, F) indicates an upregulation of genes important for brown and beige adipocyte differentiation and their functional maintenance (28). The respective transcriptional networks also rely on enhanced activation of thyroid hormone receptors that is mediated by the induction of type II iodothyronine deiodinase (Dio2; Fig. 2C, G) mRNA (29), which encodes an enzyme converting inactive tetra-iodothyronine to active tri-iodothyronine. In addition, we observed a profound induction of Elovl3 (Fig. 2D, H), a fatty acid elongase specifically expressed in brown adipocytes during increased fatty acid oxidation (30).
Fig. 2.
Treatment with the β3-adrenergic receptor agonist, CL, and cold exposure activate thermogenesis in BAT, induce browning in WAT, and alter endocannabinoid receptor expression in both adipose tissues. In order to activate BAT and/or browning of WAT, C57BL6/J wild-type mice housed at ambient temperature (22–24°C) received a single injection of CL (acute CL), 7 days of CL treatment (chronic CL), or the combination of both (chronic+acute CL), as described in the Methods. To stimulate BAT and/or WAT browning by cold, C57BL6/J wild-type mice were housed for 1 day (1d cold) or 7 days (7d cold) at 6°C. Expression of brown adipocyte activation marker genes relative to mock-injected controls was determined in BAT (A–D) and in subcutaneous WAT (E–H). I–L: Relative expression of endocannabinoid receptors Cnr1 and Cnr2 in BAT and WAT. Values are mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test.
In both tissues, chronic+acute CL treatment, but not any of the other treatments, significantly stimulated the expression of Cnr1, the gene encoding for the CB1 endocannabinoid receptor (Fig. 2I, K). Finally, in BAT, but not in WAT, acute CL, as well as acute+chronic CL treatment, and 1 day exposure to cold, reduced the expression of Cnr2, the cannabinoid type-2 (CB2) receptor (Fig. 2J, L), which was, however, based on total copy numbers less expressed than CB1 in both adipose tissues (data not shown).
The endocannabinoid system is induced in BAT by acute β3-adrenergic stimulation
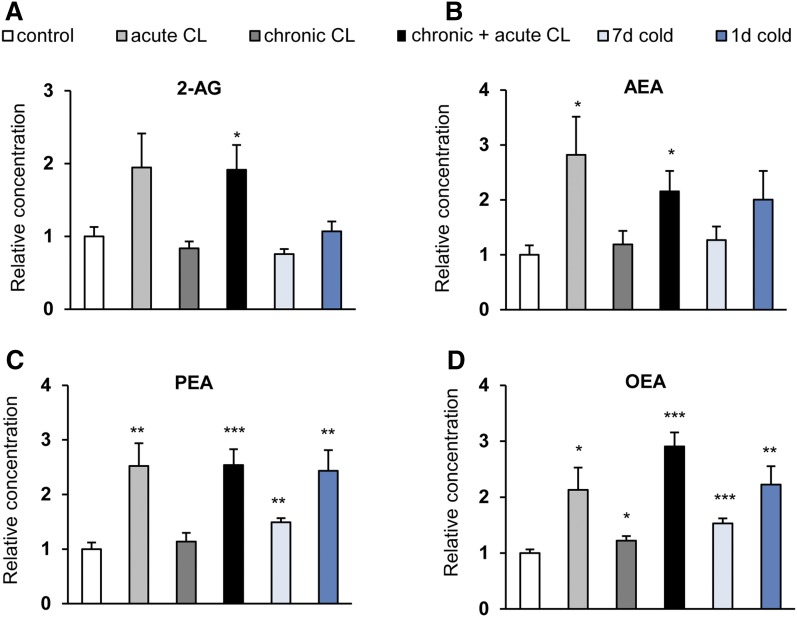
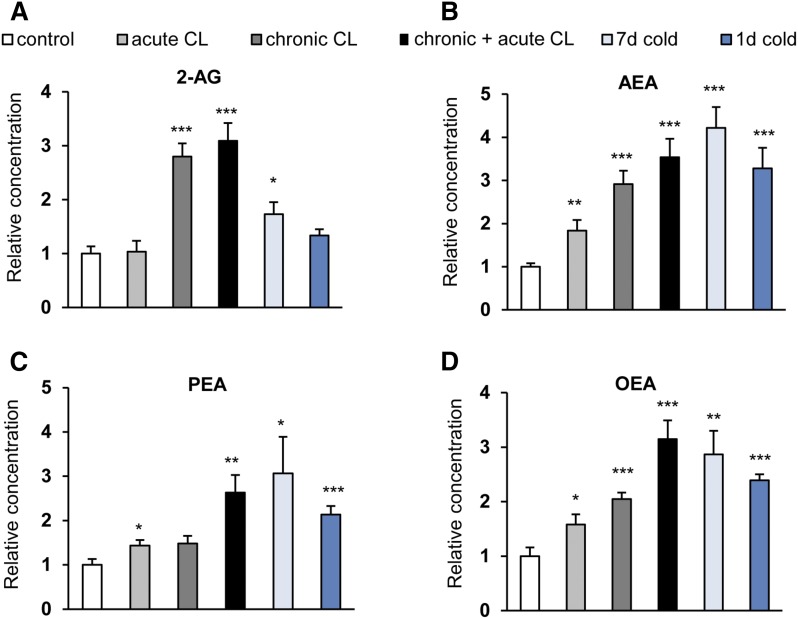
Both acute and chronic+acute CL treatment of the mice, but not chronic CL treatment per se, elevated the levels of the endocannabinoids, 2-AG and AEA, in BAT. The effect was statistically significant for AEA in both cases, however, for 2-AG only with chronic+acute CL treatment. Cold exposure did not cause the same effect, although a trend was observed for AEA with a 1 day exposure (Fig. 3A, B). Interestingly, acute and chronic+acute CL administration, as well as short-term cold exposure, caused a strong and significant elevation in the levels of the AEA congeners, PEA and OEA, which was also present after 1 day and 7 day cold exposure (Fig. 3C, D, Table 1). Thus, short-term β3-adrenergic stimulation leads to increased levels of both CB1 agonists and nonendocannabinoid NAEs in BAT.
Fig. 3.
Acute β3-adrenergic stimulation increases endocannabinoid levels in BAT. Mice were treated as described in the legend of Fig. 2. The concentrations of 2-AG (A), AEA (B), PEA (C), and OEA (D) were measured in BAT. Data are calculated as picomoles per milligram wet weight and presented relative to mock-injected controls. Values are mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test.
TABLE 1.
Absolute values for endocannabinoids in WAT and BAT
| AEA (pmol/mg) | 2-AG (pmol/mg) | PEA (pmol/mg) | OEA (pmol/mg) | |
| BAT | ||||
| Control | 0.25 ± 0.04 | 0.93 ± 0.12 | 1.31 ± 0.16 | 1.95 ± 0.12 |
| Acute CL | 0.70 ± 0.17a | 1.80 ± 0.43 | 3.31 ± 0.55a | 4.16 ± 0.78a |
| Chronic CL | 0.30 ± 0.06 | 0.78 ± 0.09 | 1.49 ± 0.21 | 2.39 ± 0.15a |
| Chronic and acute CL | 0.54 ± 0.09a | 1.78 ± 0.31a | 3.33 ± 0.38c | 5.68 ± 0.49c |
| 1 day cold | 0.50 ± 0.13 | 0.99 ± 0.06 | 3.19 ± 0.50b | 4.35 ± 0.64b |
| 7 day cold | 0.32 ± 0.06 | 0.70 ± 0.13 | 1.96 ± 0.10b | 2.99 ± 0.17c |
| WAT | ||||
| Control | 0.03 ± 0.003 | 0.30 ± 0.04 | 0.75 ± 0.10 | 0.67 ± 0.11 |
| Acute CL | 0.06 ± 0.007b | 0.31 ± 0.06 | 1.08 ± 0.10a | 1.06 ± 0.13a |
| Chronic CL | 0.09 ± 0.010c | 0.85 ± 0.07c | 1.12 ± 0.13 | 1.37 ± 0.08c |
| Chronic and acute CL | 0.11 ± 0.0013c | 0.94 ± 0.10c | 1.99 ± 0.30a | 2.11 ± 0.23c |
| 1 day cold | 0.10 ± 0.0015c | 0.40 ± 0.04 | 1.61 ± 0.15c | 1.60 ± 0.08c |
| 7 day cold | 0.13 ± 0.0015c | 0.52 ± 0.07a | 2.31 ± 0.62a | 1.92 ± 0.29b |
Mice were treated as described in the legend of Fig. 2. The concentrations of 2-AG, AEA, PEA, and OEA were measured in BAT and WAT. Data are calculated as picomoles per milligram wet weight. Values are mean ± SEM.
P < 0.05, Student’s t-test.
P < 0.01, Student’s t-test.
P < 0.001, Student’s t-test.
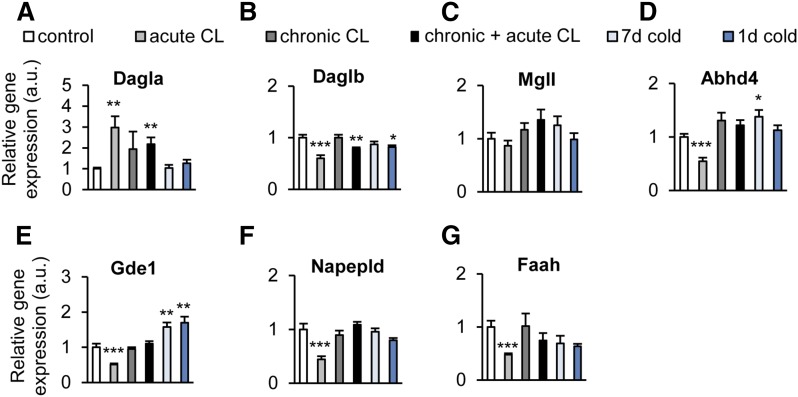
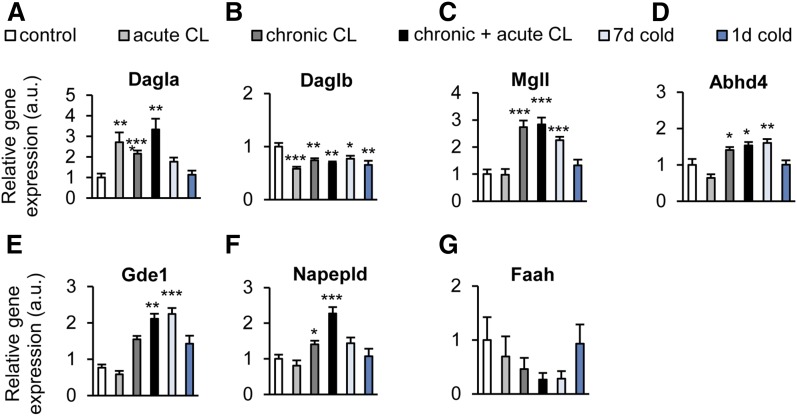
The mRNA expression of the 2-AG biosynthetic enzyme, Dagla, was significantly elevated by acute and chronic+acute CL, corresponding to the 2-AG concentrations observed in BAT (Fig. 4A). In contrast, the isoform, Daglb, was downregulated by short-term cold exposure, whereas no effect was observed with any of the treatments on the expression of the gene encoding for the 2-AG degrading enzyme, Mgll (Fig. 4A–C). Surprisingly, the mRNA levels of the NAE biosynthetic enzymes, Abhd4, Gde1, Napepld, and the degrading enzyme, Faah, did not mirror the AEA levels. Instead, they were all reduced by acute CL, whereas cold exposure elevated the mRNA levels of Abhd4 and Gde1 (Fig. 4D–G).
Fig. 4.
Cold exposure and β3-adrenergic stimulation alter endocannabinoid system gene expression in BAT. C57BL6/J wild-type mice were treated as described in the legend of Fig. 2. A–C: Relative expression of 2-AG synthesis (Daglα/β) and degradation (Mgll) genes in BAT. D–G: Relative expression of AEA synthesis (Abhad4, Gde1, Napepld) and degradation (Faah) genes in BAT. Values are mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test.
WAT β3-adrenergic stimulation and browning are accompanied by upregulation of the endocannabinoid system
Next, we asked whether β3-adrenergic stimulation or cold exposure has an effect on endocannabinoids in WAT. The 2-AG levels were significantly elevated after chronic, but not acute, CL treatment and, to a smaller extent, after prolonged cold exposure (Fig. 5A, Table 1). AEA concentrations in WAT were significantly enhanced by all treatments, the strongest effects being observed with prolonged cold exposure and chronic+acute CL treatment (Fig. 5B). As observed in BAT, the levels of OEA and PEA were enhanced by the treatments in a manner very similar to AEA, although the effect of chronic CL did not reach statistical significance for PEA (Fig. 5C, D).
Fig. 5.
Cold exposure and β3-adrenergic stimulation increase endocannabinoid levels in WAT. C57BL6/J wild-type mice were treated as described in the legend of Fig. 2. The concentrations of 2-AG (A), AEA (B), PEA (C), and OEA (D) were measured in WAT. Data are calculated as picomoles per milligram wet weight and presented relative to mock-injected controls. Values are mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test.
All treatments, except for acute cold exposure, produced a statistically significant upregulation of Dagla (Fig. 6A), whereas these treatments (including acute cold exposure) produced an opposite downregulatory effect on Daglb (Fig. 6B). All chronic treatments enhanced the mRNA levels of Mgll (Fig. 6C). The mRNAs for NAE biosynthetic enzymes appeared to be regulated by the treatments in a similar manner, with chronic CL and, hence, chronic+acute CL producing statistically significant upregulation of Abhd4, Gde1, and Napepld, and long-term cold exposure causing significant upregulation only of Abhd4 and Gde1 (Fig. 6D–F). Faah expression was not altered by any treatment (Fig. 6G). Maximum induction of Mgll, Abhd4, and Gde1 required chronic CL or cold stimulation, suggesting that beige, rather than white, adipocytes regulate the endocannabinoid system in brownish WAT. Taken together, in WAT, both endocannabinoids were increased by β3-adrenergic and cold stimulation, and this was linked to an upregulation of biosynthetic enzymes of both 2-AG and NAEs.
Fig. 6.
Cold exposure and β3-adrenergic stimulation change endocannabinoid system gene expression in WAT. C57BL6/J wild-type mice were treated as described in the legend of Fig. 2. A–C: Relative expression of 2-AG synthesis (Dagla, Daglb) and degradation (Mgll) genes in WAT. D–G. Relative expression of AEA synthesis (Abhad4, Gde1, Napepld) and degradation (Faah) genes in WAT. Values are mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test.
Acute β3-adrenoceptor activation induces the expression of Cnr1 and endocannabinoid metabolic enzymes in primary brown adipocytes
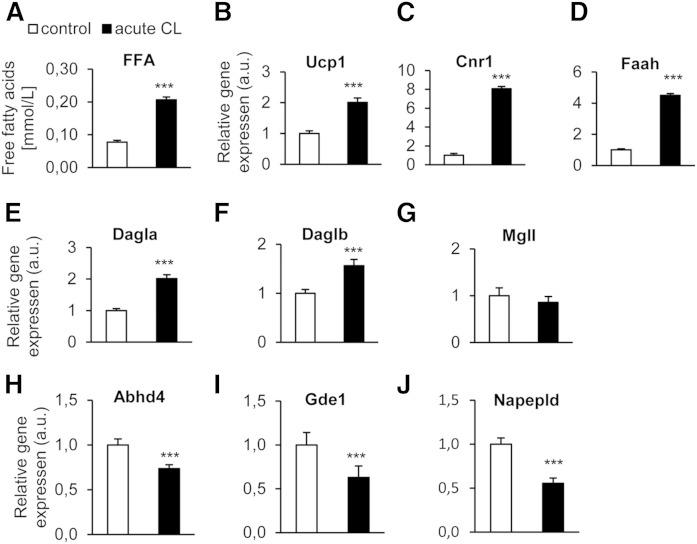
Next, we asked whether the acute regulation of Cnr1 and endocannabinoid synthetic enzymes observed in BAT could be reproduced in brown adipocytes in vitro. Acute CL stimulation of primary brown adipocytes caused elevated lipolysis and thermogenic activation, as shown by upregulation of free fatty acids in the supernatant and Ucp1 mRNA (Fig. 7A, B). Furthermore, it led to strong and statistically significant mRNA upregulation of Cnr1, as well as of Faah, Dagla, and Daglb, with no effect on Mgll (Fig. 7C–G). Abhd4, Gde1, and Napepld were significantly downregulated. These data show that most (Cnr1, Dagla, Mgll, Abhd4, Gde1, and Napepld), but not all (the exceptions being Daglb and Faah), gene expression effects observed in activated BAT in vivo could be mimicked in brown adipocytes by acute CL treatment.
Fig. 7.
Acute β3-adrenergic treatment of primary brown adipocytes significantly increases Cnr1 and 2-AG synthesis gene expression. Adipocytes were treated with saline or 1 μM CL for 4 h. A: Concentrations of unesterified fatty acids (FFA) were determined in cell supernatants. B–J. Relative mRNA expression of Ucp1, Cnr1, and endocannabinoid synthetic and degrading enzymes. Results are presented as means ± SEM from triplicates of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test.
DISCUSSION
In the present study, we have shown for the first time that activation of BAT and browning of WAT in mice cause upregulation of endocannabinoid levels and, presumably, endocannabinoid signaling via CB1 receptors in these tissues. Given that CB1 receptor stimulation leads to a decrease of cyclic AMP signaling, we hypothesize that the upregulation of endocannabinoids by thermogenic stimuli reported here constitutes a local autocrine negative feed-back control by endocannabinoids over β3-adrenoceptor-induced BAT activation and WAT browning, which could account, in part, for the previously demonstrated enhancement of thermogenesis and energy expenditure by CB1 receptor inverse agonists, both in animal models of obesity (21, 22) and in obese humans (31).
In the BAT, cold was ineffective at elevating endocannabinoid signaling in a statistically significant manner, and treatment of mice with CL had to be acute in order to achieve a statistically significant elevation of 2-AG and/or AEA (Fig. 3), and it had to be chronic to observe enhancement of Cnr1 expression (Fig. 2). It is possible that chronic β-adrenergic stimulation under cold exposure leads to the desensitization of those intracellular signals required to elevate endocannabinoid levels following an acute stimulation; whereas, instead, the effect on the expression of CB1 receptors requires prolonged stimulation of other β3-adrenoceptor-coupled intracellular signals. The reasons for these differences might also lie in the differential regulation of the expression of AEA and 2-AG metabolic enzymes in the two tissues, measured here by quantifying the levels of the mRNAs encoding for the corresponding proteins (Fig. 1). In BAT, the effect (or lack thereof) of CL on AEA levels cannot be accounted for by effects on the mRNA expression of enzymes encoding for AEA biosynthesis from N-arachidonoyl-phosphatidyl-ethanolamines and inactivation to arachidonic acid (Fig. 4). It is possible, therefore, that CL causes elevation of AEA levels by affecting the availability of its biosynthetic precursors, the N-arachidonoyl-phosphatidyl-ethanolamines, which are produced by enzymes that have not yet been molecularly characterized. In the case of 2-AG, instead, while Mgll was unchanged, Dagla was upregulated by acute and chronic+acute treatment with CL (Fig. 4), thus mirroring the effects of the drug on 2-AG levels and suggesting that the latter effects might be a consequence of the former. Daglb was downregulated by chronic+acute treatment with CL, suggesting that this enzyme does not play a role in 2-AG biosynthesis in the BAT of β3-adrenoceptor-treated animals. Importantly, when we acutely stimulated β3-adrenoceptors in mouse primary brown adipocytes, we found the same changes of the mRNA expression of AEA biosynthetic enzymes and Dagla and Mgll (Fig. 7) as those observed in the BAT in vivo (Fig. 4), suggesting cell-autonomous regulation triggered by β-adrenergic signaling. Conversely, Faah and Daglb were regulated in an opposite manner. This finding indicates that other cell types/tissue might also contribute to the acute effects of CL observed in vivo. However, it must be emphasized that the overall impact on the brown adipocyte endocannabinoid system of β3-adrenoceptor stimulation, i.e., a strong activation, is likely to be similar both in vitro and in vivo, particularly because in isolated brown adipocytes such treatment also induced upregulation of Cnr1 mRNA expression. Unfortunately, we could not assess the levels of endocannabinoids in primary brown adipocytes because of the lack of sufficient cell mass to overcome the detection limits of our LC-MS method.
In WAT, both acute and prolonged treatments with either CL or cold appeared to produce significant elevation of at least one of the two endocannabinoids (Fig. 5), although, again, only chronic treatment enhanced Cnr1 mRNA expression (Fig. 2). The expression of at least one of the possible biosynthetic enzymes for AEA was stimulated to varying degrees by both CL and cold (Fig. 6), thus potentially explaining why the levels of this endocannabinoid were increased by these treatments. Likewise, and, in this case, similar to what observed in the BAT, Dagla, opposite to Daglb, was upregulated by the same treatments that led to elevation of 2-AG levels (Fig. 6), pointing once again to the former enzyme as the one potentially responsible for increased 2-AG biosynthesis in browning WAT. In summary, β3-adrenergic receptor stimulation by either a synthetic agonist or cold differently affects the expression of endocannabinoid metabolic enzymes. This observation might be a consequence of the different effects of these stimuli in activated BAT and browning WAT. Differences between AEA and 2-AG regulation might reflect the fact that these two endocannabinoids also have additional targets apart from CB1 and CB2 receptors (32).
Slight differences in the effects of β3-adrenoceptor stimulation on the expression of AEA and NAE metabolic enzymes might also explain why, both in the BAT and WAT, the levels of PEA and OEA were more often elevated in a statistically significant manner than those of AEA. For example, in the BAT, cold elevated the levels of these two NAEs, possibly due to its stimulatory action on the mRNA levels of Abhd4 and Gde1 (which, therefore, might contribute to the biosynthesis of PEA and OEA, but not AEA, in this tissue). It is important to remember that OEA and PEA do not directly activate CB1 and CB2 receptors, and were reported to act on other targets of potential importance for the regulation of adipose tissue metabolism, i.e., PPARα, TRPV1 (33), and GPR55 (in the case of PEA) or GPR119 (in the case of OEA) (34, 35). Therefore, the role of these two mediators in the control of BAT activation and WAT browning will have to be fully assessed when the function of their proposed receptors in this context is further clarified. Interestingly, conditional knockout of Napepld in the WAT was recently shown to reduce PEA and OEA, but not AEA, levels and to downregulate Ucp1 expression in this tissue, suggesting a possible stimulatory role of either or both of these compounds on WAT browning (36).
The other major target for AEA and, particularly, 2-AG, i.e., the CB2 receptor, appeared to be regulated by CL and cold exposure in the BAT in a manner opposite to CB1 receptors and/or endocannabinoid levels. Unlike CB1, the role of CB2 in the WAT and, particularly, the BAT has hardly been investigated, and hence future studies will have to address this issue through the use of selective CB2 agonists and antagonists, as well as CB2-null mice.
In conclusion, we have provided here, for the first time, data on the regulation of the endocannabinoid system by stimuli leading to BAT activation and WAT browning, and have hypothesized a role for the endocannabinoids, when signaling at CB1 receptors, as negative feedback autocrine modulators of β3-adrenoceptor-induced UCP1 upregulation and, hence, of thermogenesis. Given the wealth of data indicating that CB1 blockers induce thermogenesis and energy expenditure in obesity, our findings provide the first hint of what could be the possible role of CB1 receptors and their endogenous agonists in a more physiological type of acute and chronic perturbation of energy homeostasis, such as that induced by exposure to cold and subsequent β3-adrenoceptor activation. Future studies should be carried out in primary brown adipocytes as well, as in BAT explants, with selective pharmacological tools or BAT-specific cannabinoid receptor knockout to conclusively assess whether brown adipocyte-derived endocannabinoids also negatively control thermogenesis via CB1-mediated autocrine and postjunctional, and not only “retrograde” and prejunctional, actions and to explore the role of CB2 receptors in this setting.
Acknowledgments
The authors thank S. Ehret for expert technical assistance.
Footnotes
Abbreviations:
- Abhd4
- α/β domain containing-4
- AEA
- N-arachidonoylethanolamine (anandamide)
- 2-AG
- 2-arachidonoylglycerol
- BAT
- brown adipose tissue
- CB1
- cannabinoid type-1
- CB2
- cannabinoid type-2
- CL
- CL316,243
- Dagla
- diacylglycerol lipase-α
- Faah
- fatty acid amide hydrolase
- Gde1
- glycerophosphodiester phosphodiesterase-1
- Mgll
- monoglyceride lipase
- NAE
- N-acylethanolamine
- Napepld
- N-acyl phosphatidylethanolamine phospholipase D
- OEA
- N-oleoylethanolamine
- PEA
- N-palmitoylethanolamine
- SNS
- sympathetic nervous system
- UCP1
- uncoupling protein 1
- WAT
- white adipose tissue
This work was supported by the Graduiertenkolleg der Deutschen Forschungsgemeinschaft 1459 (L.M.K.) and by a grant from the Fondation Leducq-Triglyceride Metabolism in Obesity and Cardiovascular Disease (12CVD04) (J.H.).
REFERENCES
- 1.Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., et al. 2009. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., and Teule G. J.. 2009. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360: 1500–1508. [DOI] [PubMed] [Google Scholar]
- 3.Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerback S., et al. 2009. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360: 1518–1525. [DOI] [PubMed] [Google Scholar]
- 4.Bartelt A., and Heeren J.. 2012. The holy grail of metabolic disease: brown adipose tissue. Curr. Opin. Lipidol. 23: 190–195. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson J., Lindstrom J., and Tuomilehto J.. 2001. Potential for the prevention of type 2 diabetes. Br. Med. Bull. 60: 183–199. [DOI] [PubMed] [Google Scholar]
- 6.Knowler W. C., Barrett-Connor E., Fowler S. E., Hamman R. F., Lachin J. M., Walker E. A., and Nathan D. M.; Diabetes Prevention Program Research Group. 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouellet V., Routhier-Labadie A., Bellemare W., Lakhal-Chaieb L., Turcotte E., Carpentier A. C., and Richard D.. 2011. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J. Clin. Endocrinol. Metab. 96: 192–199. [DOI] [PubMed] [Google Scholar]
- 8.Yoneshiro T., Aita S., Matsushita M., Kayahara T., Kameya T., Kawai Y., Iwanaga T., and Saito M.. 2013. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123: 3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Lans A. A., Hoeks J., Brans B., Vijgen G. H., Visser M. G., Vosselman M. J., Hansen J., Jorgensen J. A., Wu J., Mottaghy F. M., et al. 2013. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123: 3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon B., and Nedergaard J.. 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84: 277–359. [DOI] [PubMed] [Google Scholar]
- 11.Yoneshiro T., Aita S., Matsushita M., Okamatsu-Ogura Y., Kameya T., Kawai Y., Miyagawa M., Tsujisaki M., and Saito M.. 2011. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring). 19: 1755–1760. [DOI] [PubMed] [Google Scholar]
- 12.Bartelt A., Bruns O. T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M. G., Tromsdorf U. I., Weller H., Waurisch C., et al. 2011. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17: 200–205. [DOI] [PubMed] [Google Scholar]
- 13.Young S. G., and Zechner R.. 2013. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 27: 459–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J., Cohen P., and Spiegelman B. M.. 2013. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27: 234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartelt A., and Heeren J.. 2014. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10: 24–36. [DOI] [PubMed] [Google Scholar]
- 16.Lowell B. B., and Spiegelman B. M.. 2000. Towards a molecular understanding of adaptive thermogenesis. Nature. 404: 652–660. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J., Cannon B., and Nedergaard J.. 1998. Thermogenesis is beta3- but not beta1-adrenergically mediated in rat brown fat cells, even after cold acclimation. Am. J. Physiol. 275: R2002–R2011. [DOI] [PubMed] [Google Scholar]
- 18.Shabalina I. G., Petrovic N., de Jong J. M., Kalinovich A. V., Cannon B., and Nedergaard J.. 2013. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Reports. 5: 1196–1203. [DOI] [PubMed] [Google Scholar]
- 19.Silvestri C., and Di Marzo V.. 2013. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 17: 475–490. [DOI] [PubMed] [Google Scholar]
- 20.Quarta C., Bellocchio L., Mancini G., Mazza R., Cervino C., Braulke L. J., Fekete C., Latorre R., Nanni C., Bucci M., et al. 2010. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 11: 273–285. [DOI] [PubMed] [Google Scholar]
- 21.Bajzer M., Olivieri M., Haas M. K., Pfluger P. T., Magrisso I. J., Foster M. T., Tschop M. H., Krawczewski-Carhuatanta K. A., Cota D., and Obici S.. 2011. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia. 54: 3121–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boon M. R., Kooijman S., van Dam A. D., Pelgrom L. R., Berbee J. F., Visseren C. A., van Aggele R. C., van den Hoek A. M., Sips H. C., Lombes M., et al. 2014. Peripheral cannabinoid 1 receptor blockade activates brown adipose tissue and diminishes dyslipidemia and obesity. FASEB J. 28: 5361–5375. [DOI] [PubMed] [Google Scholar]
- 23.Cardinal P., Bellocchio L., Guzman-Quevedo O., Andre C., Clark S., Elie M., Leste-Lasserre T., Gonzales D., Cannich A., Marsicano G., et al. 2015. Cannabinoid type 1 (CB1) receptors on Sim1-expressing neurons regulate energy expenditure in male mice. Endocrinology. 156: 411–418. [DOI] [PubMed] [Google Scholar]
- 24.Vähätalo L. H., Ruohonen S. T., Mäkelä S., Ailanen L., Penttinen A. M., Stormi T., Kauko T., Piscitelli F., Silvestri C., Savontaus E., et al. 2015. Role of the endocannabinoid system in obesity induced by neuropeptide Y overexpression in noradrenergic neurons. Nutr. Diabetes. 5: e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perwitz N., Wenzel J., Wagner I., Buning J., Drenckhan M., Zarse K., Ristow M., Lilienthal W., Lehnert H., and Klein J.. 2010. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes. Metab. 12: 158–166. [DOI] [PubMed] [Google Scholar]
- 26.Wagner I. V., Perwitz N., Drenckhan M., Lehnert H., and Klein J.. 2011. Cannabinoid type 1 receptor mediates depot-specific effects on differentiation, inflammation and oxidative metabolism in inguinal and epididymal white adipocytes. Nutr. Diabetes. 1: e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartelt A., Orlando P., Mele C., Ligresti A., Toedter K., Scheja L., Heeren J., and Di Marzo V.. 2011. Altered endocannabinoid signalling after a high-fat diet in Apoe (-/-) mice: relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia. 54: 2900–2910. [DOI] [PubMed] [Google Scholar]
- 28.Puigserver P., Wu Z., Park C. W., Graves R., Wright M., and Spiegelman B. M.. 1998. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 92: 829–839. [DOI] [PubMed] [Google Scholar]
- 29.de Jesus L. A., Carvalho S. D., Ribeiro M. O., Schneider M., Kim S. W., Harney J. W., Larsen P. R., and Bianco A. C.. 2001. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J. Clin. Invest. 108: 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakobsson A., Jörgensen J. A., and Jacobsson A.. 2005. Differential regulation of fatty acid elongation enzymes in brown adipocytes implies a unique role for Elovl3 during increased fatty acid oxidation. Am. J. Physiol. Endocrinol. Metab. 289: E517–E526. [DOI] [PubMed] [Google Scholar]
- 31.Addy C., Wright H., Van Laere K., Gantz I., Erondu N., Musser B. J., Lu K., Yuan J., Sanabria-Bohorquez S. M., Stoch A., et al. 2008. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 7: 68–78. [DOI] [PubMed] [Google Scholar]
- 32.Di Marzo V., and De Petrocellis L.. 2012. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. Lond. B Biol. Sci. 367: 3216–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matias I., Gonthier M. P., Petrosino S., Docimo L., Capasso R., Hoareau L., Monteleone P., Roche R., Izzo A. A., and Di Marzo V.. 2007. Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells. Br. J. Pharmacol. 152: 676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godlewski G., Offertaler L., Wagner J. A., and Kunos G.. 2009. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 89: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Marzo V., and Piscitelli F.. 2015. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 12: 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geurts L., Everard A., Van Hul M., Essaghir A., Duparc T., Matamoros S., Plovier H., Castel J., Denis R. G., Bergiers M., et al. 2015. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat. Commun. 6: 6495. [DOI] [PMC free article] [PubMed] [Google Scholar]