Abstract
The human retina is well-known to have unique lipid profiles enriched in long-chain polyunsaturated fatty acids (LC-PUFAs) and very long-chain polyunsaturated fatty acids (VLC-PUFAs) that appear to promote normal retinal structure and function, but the influence of diet on retinal lipid profiles in health and disease remains controversial. In this study, we examined two independent cohorts of donor eyes and related their retinal lipid profiles with systemic biomarkers of lipid intake. We found that serum and red blood cell lipids, and to a lesser extent orbital fat, are indeed excellent biomarkers of retinal lipid content and n-3/n-6 ratios in both the LC-PUFA and VLC-PUFA series. Eyes from age-related macular degeneration (AMD) donors have significantly decreased levels of VLC-PUFAs and low n-3/n-6 ratios. These results are consistent with the protective role of dietary n-3 LC-PUFAs against AMD and emphasize the importance of monitoring systemic biomarkers of lipid intake when undertaking clinical trials of lipid supplements for prevention and treatment of retinal disease.
Keywords: eye, retina, diet and dietary lipids, omega-3 fatty acids, nutrition, mass spectrometry, elongation of very long-chain fatty acids elongase 4, age-related macular degeneration
The vertebrate retina has long been known to contain unusual lipid profiles that appear to be essential for normal visual function. The photoreceptor cell rod outer segments have extraordinarily high levels of n-3 long-chain polyunsaturated fatty acids (LC-PUFAs) such as DHA (22:6n-3) (1) that far exceed concentrations encountered in the serum and other tissues. More recently, a new class of nondietary polyunsaturated fatty acids with chain lengths greater than 24 carbons, the very long-chain polyunsaturated acids (VLC-PUFAs), were identified in the vertebrate retina and just a few other tissues (2, 3). Enhanced membrane fluidity contributed by LC-PUFAs and VLC-PUFAs is thought to be essential for the maintenance of the highly curved membrane disks of the photoreceptor outer segments, but their high degree of unsaturation renders these lipids susceptible to oxidative damage (4, 5).
Retinal n-3 and n-6 LC-PUFAs cannot be synthesized de novo in vertebrates and must be consumed either intact or from a select group of precursors such as α-linolenic acid (18:3n-3, typically from vegetable sources), EPA (20:5n-3, commonly from marine sources), linoleic acid (18:2n-6), and arachidonic acid (AA, 20:4n-6). The influence of dietary intake on retinal levels and visual health remains controversial, however, especially in humans (6–8). Dietary intake of n-3 LC-PUFAs (EPA + DHA) has the capacity to lower inflammation-based processes (9, 10) mediated by resolvins (11) and neuroprotectins (12), while inflammation is promoted by n-6 LC-PUFAs such as AA (13). A balance between dietary n-3 and n-6 LC-PUFAs is essential for optimal function of cell membranes, enzyme activity, gene expression, and cell-to-cell communication. Multiple epidemiological studies have indicated that diets rich in n-3 LC-PUFAs are associated with lower risk of age-related macular degeneration (AMD) (14–16), but supplementation with 1,000 mg of fish oil for 3–5 years failed to have any impact on progression to advanced AMD in either the Nutritional AMD Treatment 2 (17, 18) or the Age-Related Eye Disease Study 2 (AREDS2) randomized, placebo-controlled clinical trials (19).
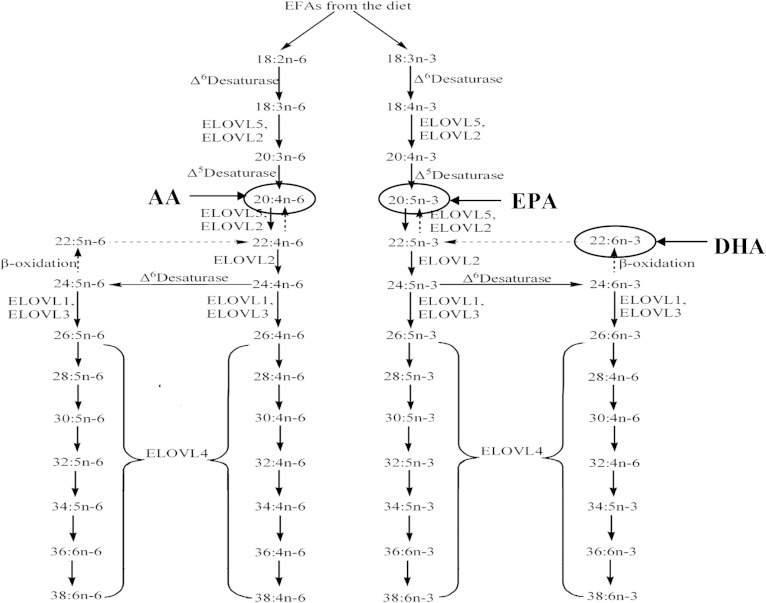
The n-3 and n-6 VLC-PUFAs are not normally consumed in the human diet, and EPA and AA appear to be their major precursors, respectively, in a process mediated by the action of the elongation of very long-chain fatty acids elongase 4 (ELOVL4) enzyme (Fig. 1) (20). Autosomal dominant mutations in this enzyme lead to a rare form of Stargardt macular dystrophy (STGD), STGD3 (21, 22), but the mechanisms underlying this degeneration (VLC-PUFA deficiency vs. mutant protein mislocalization) remain controversial (23–25). Our study of dietary biomarkers of lipid consumption has shown that members of a Utah family with STGD3 who consume large amounts of fish have a milder phenotype than those who rarely consume fish (26), and an open-label clinical intervention trial with fish oil supplementation is in progress for this family (ClinicalTrials.gov, #NCT00420602).
Fig. 1.
Biosynthetic pathways of VLC-PUFA synthesis from dietary precursors.
In order to clarify the roles of LC-PUFAs and VLC-PUFAs in retinal health and disease, we previously undertook a study of human donor eyes to determine whether abnormalities in lipid profiles are present in AMD eyes. We found that DHA and many VLC-PUFA levels are significantly lower in AMD eyes relative to age-matched controls and that n-3/n-6 ratios are significantly lower for both LC-PUFAs and VLC-PUFAs (27). Because ELOVL4 genetic variants are not associated with AMD risk (28), these findings suggested that a retinal deficiency of LC-PUFAs and VLC-PUFAs influenced by diet and/or a dietary imbalance of n-3/n-6 LC-PUFA ratios may be involved in AMD pathology, but our study was limited by the low number of AMD eyes (n = 8) and lack of availability of biomarkers of dietary lipid intake. We now report follow-up studies showing that dietary intake of LC-PUFA and VLC-PUFA precursors clearly influence retinal lipid profiles in retina, and they confirm that AMD eyes have numerous abnormalities in these profiles.
MATERIALS AND METHODS
Sample collection
The studies reported here were conducted on two distinct collections of human donor eye tissues. All experimental procedures including tissue procurement were conducted according to the tenets of the Declaration of Helsinki. In the first-phase study, human donor eyes with no history of eye disease were obtained from the Utah Lions Eye Bank. The time between donor death and enucleation was <4 h. Dissections of donor eyes were carried out 6–24 h after donor death under dim light in cold temperature (4°C) to minimize lipid oxidation and autolysis. Eyes with large drusen, severe macular atrophy, macular hemorrhage, or any grossly visible chorioretinal pathologic abnormalities were excluded. After the cornea, iris, ciliary body, and lens were removed, a 4 mm peripheral retina (PR) region in the nasal mid-PR was punched with a trephine because previous studies from our laboratory have already indicated that lipid profiles of PR do not differ substantially from lipid profiles of macular tissue (29, 30). All punched human ocular tissues were stored in tubes filled with argon gas and kept at −80°C. Serum, red blood cells (RBCs), and orbital fat samples were also collected from these donors and stored at −80°C.
The second-phase study utilized samples from the Utah Center for Translational Medicine Donor Eye Repository, a large collection of rigorously characterized ocular tissues from AMD and control donors collected in a manner similar to the collection described above. Demographic information of the subjects and their AMD grades using a modified Rotterdam scale (31) are provided in Table 1. Midperipheral 6 mm retinal punches were analyzed from age-matched AMD and control eyes along with serum samples. RBCs and orbital fat were not available from these donors.
TABLE 1.
Demographic information of phase II study samples
| Donor Number | Age | Gender | Gradea | ELOVL4 (rs3812153) | AdipoR1 (rs10753929) |
| Control subjects | |||||
| 0166-98 | 83 | F | 0 | TT | CC |
| 0171-98 | 74 | F | 0 | TT | CT |
| 0020-98 | 76 | F | 0 | TT | CC |
| 0248-98 | 76 | M | 0 | TT | CC |
| 0010-02 | 82 | M | 0 | TT | CT |
| 0151-02 | 83 | F | 0 | TT | CC |
| 0148-99 | 74 | F | 0 | TT | CC |
| 0171-99 | 84 | F | 0 | TT | CC |
| 0317-99 | 79 | M | 0 | TT | CT |
| 0059-00 | 88 | M | 0 | TT | CC |
| 0088-00 | 78 | M | 0 | TT | CC |
| 0312-00 | 74 | M | 0 | TT | CT |
| 0508-01 | 83 | F | 0 | TT | CC |
| 0101-11 | 76 | F | 0 | TT | CT |
| 0206-11 | 86 | M | 0 | TT | CC |
| 0815-11 | 77 | M | 0 | CT | CT |
| 0742-11 | 83 | F | 0 | TT | CC |
| 0549-11 | 87 | F | 0 | TT | CC |
| 0307-13 | 82 | M | 0 | CT | CC |
| 1522-12 | 86 | M | 0 | TT | CC |
| 0487-12 | 81 | F | 0 | TT | CC |
| AMD subjects | |||||
| 0459-99 | 83 | M | 2 | TT | CC |
| 0002-00 | 85 | M | 2 | CT | CC |
| 0373-01 | 80 | F | 2 | TT | CC |
| 0457-01 | 85 | M | 2 | TT | CC |
| 0498-01 | 74 | F | 4B | CT | CC |
| 0149-02 | 73 | M | 2 | TT | CT |
| 0034-02 | 87 | F | 2 | TT | CC |
| 1278-11 | 84 | F | 2 | TT | CC |
| 0564-11 | 82 | F | 4B | CT | CC |
| 0596-11 | 85 | F | 4C | TT | CC |
| 0754-13 | 89 | F | 1B | CT | CC |
| 0896-11 | 91 | M | 3 | TT | CC |
| 0919-12 | 77 | M | 1B | TT | CC |
| 0258-11 | 83 | F | 2 | TT | CC |
| 0020-12 | 85 | F | 4A | TT | CC |
CC, the lower risk allele of AdipoR1; CT, the higher risk allele of AdipoR1 and ELOVL4; TT, the lower risk allele of ELOVL4.
AMD grading based on a modified Rotterdam scale (31) [0, no signs of maculopathy; 1A, distinct drusen (≤63 µm); 1B, pigmentary irregularities only; 2, soft drusen (>63 µm and ≤125 µm); 3, soft drusen (>125 µm) with or without pigmentary irregularities; 4A, geographic atrophy; 4B, choroidal neovascular membrane; 4C, both 4A and 4B].
Chemicals
All chemical reagents, such as methanol, hydrochloric acid, isopropanol, n-hexane, n-nonane, and diethyl ether, were of GC/MS grade and purchased from Fisher Scientific (Pittsburgh, PA). All standards including the internal standards such as tridecanoic acid (13:0), hentriacontanoic acid (34:0), and all fatty acids methyl esters (FAMEs) such as methyl eicosanoate, methyl linolenate, methyl melissate, and Supelco-37 (a commercial mixture of FAMEs) were purchased from Sigma-Aldrich (St. Louis, MO) and Matreya (Pleasant Gap, PA). Silica gel, glass-encased, solid-phase extraction cartridges (500 mg/6 ml) were purchased from Sorbent Technology (Atlanta, GA). The internal standards, tridecanoic acid and hentriacontanoic acid, were dissolved in nonane at concentrations of 1.0 mg/ml and 0.023 mg/ml, respectively.
Lipid extraction and purification of lipids with solid-phase extraction
Serum, RBCs, orbital adipose tissue, and retina punches were extracted using the procedure previously adopted in our laboratory (30). The samples and internal standards (50 µg of tridecanoic acid and 1.15 µg of hentriacontanoic acid) were added in 2 ml stainless steel vials and then homogenized with 0.7 ml silica beads and 1 ml hexane-isopropanol (3:2 v/v) by a Mini Bead Beater-16 (BioSpec Products Inc., Bartlesville, OK) and a Sonic Dismembrator Model 100 (Fisher Scientific). The homogenized samples were bath sonicated for 5 min in an ice water bath. After centrifugation at 10,000 rpm for 5 min, the extracted solution supernatant was transferred to a clean vial and then dried under a stream of nitrogen. The dried film was dissolved in 200 µl hexane, and 2 ml of 4% HCl in methanol was added. The tubes were flushed with argon and incubated at 80°C for 4 h to form FAMEs (30) and then allowed to cool. The FAME mixture was extracted three times with 1 ml distilled water and 2 ml hexane. The hexane layers were combined and dried under nitrogen gas.
Silica gel, glass-encased, solid-phase extraction cartridges were subsequently used to clean the FAME extracts. The cartridge was activated with 6 ml of hexane before loading samples. The crude FAME extract was dissolved in 200 µl of hexane and loaded onto the activated cartridge. The cartridge was washed with 6 ml hexane, and the eluate was discarded. FAMEs were eluted from the cartridge with 5 ml hexane-ether (4:1), and the eluate was evaporated under nitrogen gas. The dry film was dissolved in 200 µl of hexane and centrifuged for 3 min at 14,000 rpm to remove particles prior to GC/MS analysis. One microliter of sample was injected into the GC/MS instrument for LC-PUFA analysis. For VLC-PUFA analysis, the sample was dried with nitrogen again and redissolved in 20 μl of n-nonane, and 5 µl samples were injected into the GC/MS instrument.
GC/MS instrumentation and chromatographic conditions
The Thermo Trace GC-DSQ II system (ThermoFisher Scientific, Waltham, MA) consists of an automatic sample injector (AS 3000), gas chromatograph, single quadrupole mass detector, and an analytical workstation. The chromatographic separation was carried out with an Rxi-5MS-coated 5% diphenyl/95% dimethyl polysiloxane capillary column (30 m × 0.25 mm inner diameter, 0.25 µm film thickness) (Restek, Bellefonte, PA). Two methods (A and B) were used for detection and quantitation of LC-PUFAs and VLC-PUFAs, respectively.
For LC-PUFA analyses, the following MS conditions were used (method A): 1 µl from a 200 µl sample was injected into the GC/MS using a splitless mode, the septum purge was on, and the injector temperature was set at 200°C. The column temperature was programmed as follows: initial temperature 60°C, 5 degrees/min to 170°C, 1 degree/min to 180°C, 2 degrees/min to 240°C, 4 degrees/min to 290°C, and a hold at 290°C for 5 min. Transfer line temperature was 290°C. Helium was used as the carrier gas at a flow rate of 1.0 ml/min. MS conditions were as follows: electron ionization mode with full scan and selected ion monitoring (SIM, m/z 79, 108, and 150) because m/z 79, 108, and 150 are typical ions of PUFAs, and n-3 and n-6 PUFAs can be distinguished by comparing the ratio of ions of m/z 108 and 150; ion source temperature, 200°C; multiplier voltage, 1,182 V; and detector delay, 10 min. For peak identification, the data were obtained by collecting the full-scan mass spectra within the scan range of 50–650 amu, and these peaks were identified by comparing their mass spectra with those in the standard solution and the National Institute of Standards and Technology library. For the quantification of LC-PUFAs, the data were obtained by SIM. Authentic reference compounds were used to calculate the mol percentage of each peak.
VLC-PUFAs analyses (method B) were conducted as described previously (30). Bovine retina VLC-PUFAs were extracted and used as VLC-PUFA standards to establish retention times because commercial standards are not available, and identification of each VLC-PUFA in retinal samples was achieved as described in prior work from our laboratory (27). For the quantification of VLC-PUFAs, we used MS conditions similar to the LC-PUFA method but with a larger injection volume of 5 µl from a 20 µl sample that had been concentrated from a 200 µl original volume. The column temperature program was similar to the LC-PUFA method but held at 290°C for 35 min. MS conditions were similar to the LC-PUFA method, but the detector delay time was 20 min.
The low amounts of VLC-PUFAs that are present in the mammalian retina elute very late from the GC/MS, and standards are not available commercially, which means that their quantitation can be particularly challenging. To achieve this, two separate GC/MS runs linked by common C-24 PUFAs were necessary. With method A, the complete set of long-chain fatty acids (LC-FAs) up to 22 carbons in length and two C-24 FAs (24:1n-9 and 24:0) can be quantified under full scan mode, and when the chromatogram is reanalyzed under selective ion mode (m/z 79, 108, and 150), we can identify and quantify all n-3 and n-6 LC-PUFAs, and even the C-24 VLC-PUFAs become detectable and can be quantified by comparing their mole percentages relative to the C-22 PUFAs. The C-24 VLC-PUFAs can then be used as the common link between method A and method B because they are present in both GC/MS chromatograms. All the VLC-PUFAs could be subsequently quantified relative to the total LC-FAs determined by method A after correcting for the effects of carbon chain length and the degree of unsaturation on the response of the mass spectrometer (30).
Genotyping
Genotyping was done using the TaqMan platform (Applied Biosystems, Grand Island, NY). Amplification and genotype assignments were conducted using the 7900HT and SDS 2.4 software. The SNPs, rs381253 and rs10753929, used in this study were selected based on their association with ELOVL4 and adiponectin receptor 1 (AdipoR1) as reported in earlier literature (32, 33).
Statistical analyses
Statistical analyses were performed using ANOVA, linear regressions, Chi-square tests, and t-tests on Prism software (GraphPad Software Inc., La Jolla, CA). Data are represented as the mean ± SD. Significance is indicated by P value measurements, with P < 0.05 considered significant.
RESULTS
Phase I study
Forty-four normal patient samples were collected (average age ± SD = 71.4 ± 13.4 years), and their serum, RBCs, orbital fat, and retinal punches were analyzed using GC/MS techniques. We studied the correlations between the retinal lipid profile and those of serum, RBCs, and fat, which are validated biomarkers of short-term (weeks), medium-term (months), and long-term (years) dietary fat intake (26, 34–36) (Table 2). Individual key LC-PUFAs (EPA, DHA, and AA) in serum, RBCs, and fat generally showed significant positive correlations with corresponding levels in retinal lipids. We observed strong positive correlations between EPA levels in serum, RBCs, and fat with EPA levels in retina (all P < 0.001), while retinal DHA levels correlate relatively weakly only with DHA levels of RBCs (P < 0.05). The levels of the major n-6 fatty acid in retina, AA, were also in positive correlation with AA levels in serum and RBCs (P < 0.001). Likewise, serum and RBC total LC-PUFAs positively correlated with retinal total LC-PUFAs (P < 0.001), but fat had a weaker negative correlation (P < 0.05) (Table 2).
TABLE 2.
Correlations of LC-PUFA levels in serum, RBCs, and fat with retina lipidsa
| Systemic Lipids | Serum | RBCs | Fat | Retinal Lipids |
| EPA | 0.78*** | 0.60*** | 0.55*** | EPA |
| DHA | 0.08 | 0.29* | −0.17 | DHA |
| AA | 0.62*** | 0.60*** | −0.26 | AA |
| Total LC-PUFAs | 0.44*** | 0.51*** | −0.30* | Total LC PUFAs |
| n-3 LC-PUFAsb | 0.16 | 0.26** | −0.20 | n-3 LC-PUFAs |
| n-6 LC-PUFAsc | 0.50*** | 0.37*** | 0.39*** | n-6 LC-PUFAs |
P values: *** P < 0.001; ** P < 0.01; * P < 0.05. Values are based on regression analysis.
n-3 LC-PUFAs: 18:3n-3, 18:4n-3, 20:4n-3, 20:5n-3, 22:6n-3, 22:5n-3.
n-6 VLC-PUFAs: 18:2n-6, 18:3n-6, 20:3n-6, 20:4n-6, 22:4n-6.
Next, we examined various correlations with retinal VLC-PUFAs (Table 3). RBC total LC-PUFAs significantly correlated with retinal total VLC-PUFAs (P < 0.01), but serum and fat did not. However, not all LC-PUFAs are considered to be major precursors of retinal VLC-PUFAs (Fig. 1), so we focused subsequent analyses on the following VLC-PUFA precursors: 18:3n-3, 18:4n-3, 20:4n-3, 20:5n-3, and 22:5n-3 for n-3 VLC-PUFAs and 18:2 n-6, 18:3n-6, 20:3n-6, 20:4n-6, and 22:4n-6 for n-6 VLC-PUFAs. Most notably, this subanalysis specifically excludes DHA (22:6n-3), which is a common systemic lipid but is not considered a significant precursor for VLC-PUFAs (37). This improved correlations substantially. Total VLC-PUFA precursor levels in serum and RBCs were positively associated with total retinal VLC-PUFA levels in the retina (P < 0.01 and P < 0.001, respectively) (Table 3). When we separately analyzed the n-3 and n-6 series, many of these positive correlations persisted (P < 0.001 for RBC n-3 and n-6 lipids and for serum n-6 lipids).
TABLE 3.
Correlations of LC-PUFA levels in serum, RBCs, and fat with retinal VLC-PUFAsa
| Systemic Lipids | Serum | RBCs | Fat | Retinal Lipids |
| Total LC-PUFAs | 0.07 | 0.43** | 0.26 | Total VLC-PUFAs |
| Total VLC-PUFA precursors | 0.30** | 0.51*** | 0.02 | Total VLC-PUFAs |
| n-3 VLC-PUFA precursorsb | 0.11 | 0.53*** | 0.19* | n-3 VLC-PUFAs |
| n-6 VLC-PUFA precursorsc | 0.42*** | 0.56*** | 0.24 | n-6 VLC-PUFAs |
P values: *** P < 0.001; ** P < 0.01; * P < 0.05. Values are based on regression analysis.
n-3 VLC-PUFA precursors: 18:3n-3, 18:4n-3, 20:4n-3, 20:5n-3, 22:5n-3.
n-6 VLC-PUFA precursors: 18:2n-6, 18:3n-6, 20:3n-6, 20:4n-6, 22:4n-6.
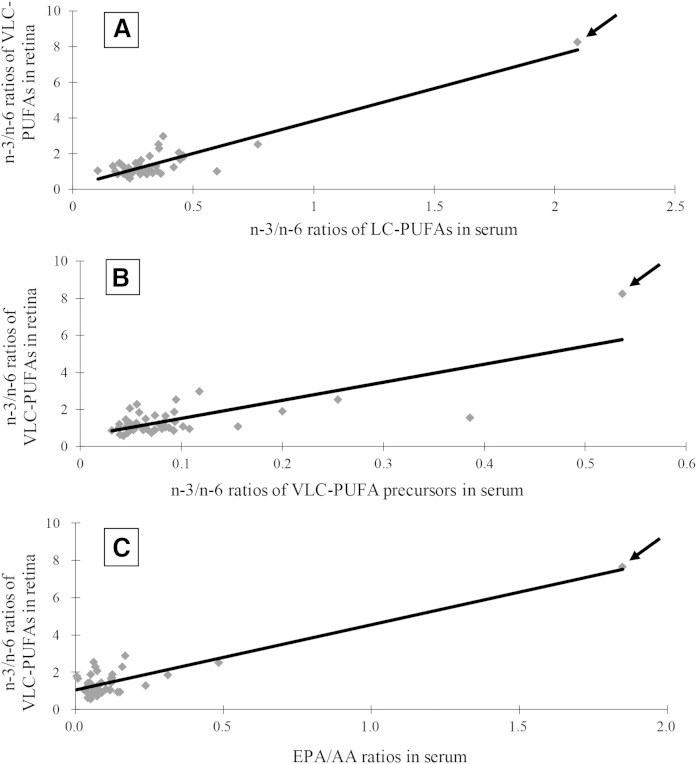
Most organisms do not interconvert lipids between the n-3 and n-6 series, so the n-3/n-6 ratios of various lipids are important to ascertain, especially because n-6 series lipids are generally pro-inflammatory, while the n-3 lipids are not. We found many strong and consistent correlations between serum, RBCs, and fat n-3/n-6 ratios in systemic LC-PUFAs and VLC-PUFA precursors with retinal LC-PUFAs, VLC-PUFAs, and their precursors (Table 4 and Fig. 2A, B). We also noted that systemic EPA/AA ratios correlated well with retina EPA/AA ratios (P < 0.001 for serum, RBCs, and fat), but DHA/AA ratios did not correlate significantly. In fact, the simple EPA/AA ratios in serum, RBCs, or fat were highly predictive biomarkers of n-3/n-6 ratios of retinal VLC-PUFAs (Table 4 and Fig. 2C).
TABLE 4.
Correlations of LC-PUFA ratios in serum, RBCs, and fat with retinal LC-PUFA and VLC-PUFA ratiosa
| Systemic Lipid Ratios | Serum | RBCs | Fat | Retinal Lipid Ratios |
| n-3/n-6 LC-PUFAs | 0.28 | 0.36** | 0.21 | n-3/n-6 LC-PUFAs |
| n-3/n-6 VLC-PUFA precursorsb,c | 0.72*** | 0.62*** | 0.45** | n-3/n-6 VLC-PUFA precursors |
| n-3/n-6 LC-PUFAs | 0.91*** | 0.69*** | 0.49*** | n-3/n-6 VLC-PUFAs |
| n-3/n-6 VLC-PUFA precursors | 0.76*** | 0.65*** | 0.40*** | n-3/n-6 VLC-PUFAs |
| EPA/AA | 0.92*** | 0.91*** | 0.65*** | EPA/AA |
| DHA/AA | 0.12 | 0.09 | 0.16 | DHA/AA |
| EPA/AA | 0.22 | 0.19 | 0.41* | n-3/n-6 LC-PUFAs |
| EPA/AA | 0.92*** | 0.92*** | 0.72*** | n-3/n-6 VLC-PUFAs |
| DHA/AA | 0.86** | 0.27 | 0.29* | n-3/n-6 VLC-PUFAs |
P values: *** P < 0.001; ** P < 0.01; * P < 0.05. Values are based on regression analysis.
n-3 VLC-PUFA precursors: 18:3n-3, 18:4n-3, 20:4n-3, 20:5n-3, 22:5n-3.
n-6 VLC-PUFA precursors: 18:2n-6, 18:3n-6, 20:3n-6, 20:4n-6, 22:4n-6.
Fig. 2.
Correlation of n-3/n-6 LC-PUFA ratios in serum with the n-3/n-6 ratios of VLC-PUFAs in retina (r = 0.91; P < 0.001) (A). Correlation of n-3/n-6 of VLC-PUFA precursor ratios in serum with the n-3/n-6 ratios of VLC-PUFAs in retina (r = 0.76; P < 0.001) (B). Correlation of EPA/AA ratios in serum with the n-3/n-6 ratios of VLC-PUFAs in retina (r = 0.92; P < 0.001) (C). The arrows correspond to data points from an outlier donor who consumed 7 g of fish oil daily for 18 months prior to death.
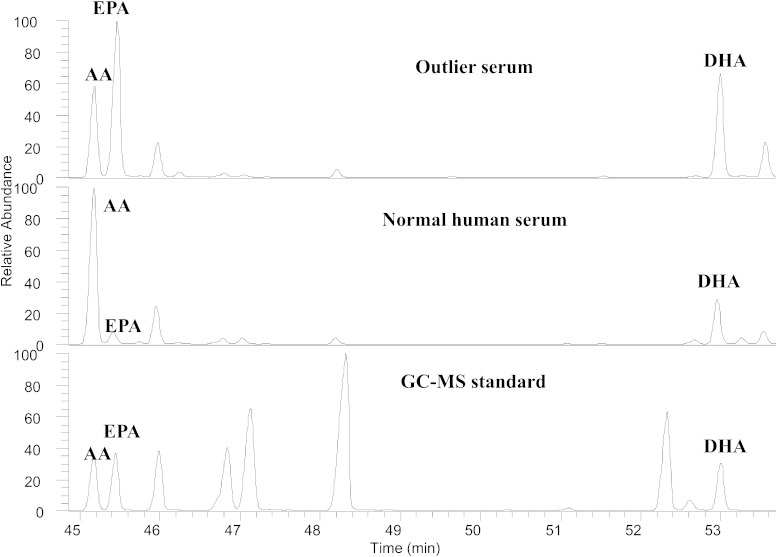
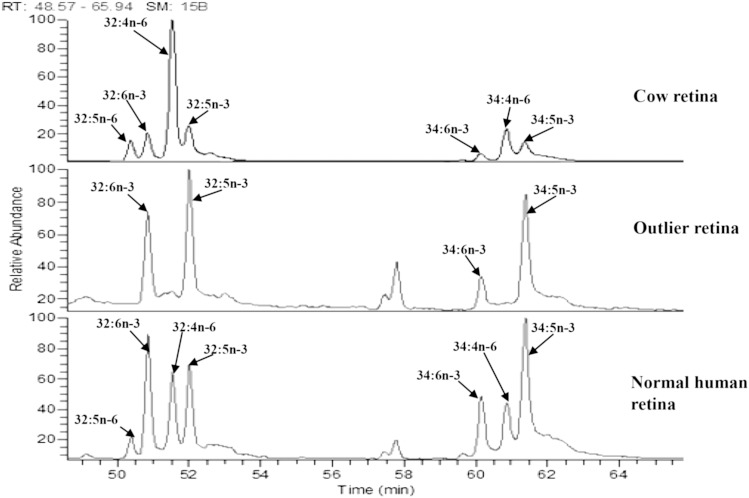
When data from individual participants were examined, one of the 44 subjects was noted to be an outlier with unusually high levels of EPA and DHA in serum, RBCs, and fat (Fig. 3). Likewise, this donor’s n-3/n-6 ratios were much higher than the others (Fig. 2). When we contacted the subject’s family, we learned that he had been consuming a high dose of fish oil (7 g/day) for at least 18 months prior to death. Excluding his data from statistical analysis did not change the statistical significance of any of our findings reported in Tables 2–4, and his unusual systemic lipid levels and n-3/n-6 ratios corresponded well with elevated n-3 retinal VLC-PUFA levels and very high n-3/n-6 VLC-PUFA ratios (Fig. 2, Fig. 3, and Fig. 4).
Fig. 3.
GC/MS chromatograms of LC-PUFAs in the outlier’s serum, normal human serum, and GC/MS standard.
Fig. 4.
GC/MS chromatograms of VLC-PUFAs (C-32 and C-34) in cow retina (GC standard), the outlier’s retina, and a normal human retina.
Phase II study
In the second phase of this project, we examined an independently collected cohort of AMD (n = 15) and control (n = 21) subjects who had donated eyes and serum (Table 1). We sampled normal appearing PR for these analyses in order to rule out macular fibrosis, atrophy, or other advanced AMD pathology as an explanation for the differences between AMD and control eyes. This time, serum LC-PUFAs and VLC-PUFA precursors were significantly associated with retina VLC-PUFA levels for controls, while the associations had similar trends in AMD donors, but they were not significant. Serum LC-PUFAs and serum VLC-PUFA precursors were significantly correlated with retinal VLC-PUFAs for controls (P < 0.05), and a similar trend was seen for the AMD subjects, but it did not reach statistical significance (Table 5). Serum n-3/n-6 LC-PUFA ratios significantly correlated with retinal n-3/n-6 VLC-PUFA ratios for AMD and control eyes (P values ranging from 0.01 to 0.006), and similar trends were also seen when comparing serum n-3/n-6 VLC-PUFA precursor ratios versus retinal n-3/n-6 VLC-PUFA ratios, but statistical significance was reached only when AMD and control data were combined (P = 0.04). Serum EPA/AA ratios were in significant positive correlation with the retinal n-3/n-6 VLC-PUFA ratios in controls but not AMD subjects.
TABLE 5.
Correlations of serum fatty acid profiles with retinal VLC-PUFA profiles in AMD and age-matched control subjectsa
| Control (n = 21) | AMD (n = 15) | Total (n = 36) | |
| Serum LC-PUFAs versus retinal VLC-PUFAs | 0.45 (P = 0.03) | 0.44 (P = 0.09) | 0.26 (P = 0.1) |
| Serum VLC PUFA precursors versus retinal VLC-PUFAs | 0.54 (P = 0.01) | 0.49 (P = 0.07) | 0.28 (P = 0.1) |
| Serum n-3/n-6 LC-PUFA ratios versus retinal n-3/n-6 VLC-PUFA ratios | 0.59 (P = 0.007) | 0.67 (P = 0.016) | 0.47 (P = 0.003) |
| Serum n-3/n-6 VLC-PUFA precursor ratios versus retinal n-3/n-6 VLC-PUFA ratios | 0.39 (P = 0.02) | 0.46 (P = 0.1) | 0.34 (P = 0.0016) |
| Serum EPA/AA ratios versus retinal n-3/n-6 VLC-PUFA ratios | 0.63 (P = 0.0001) | 0.13 (P = 0.30) | 0.56 (P = 0.0002) |
Values are based on regression analysis.
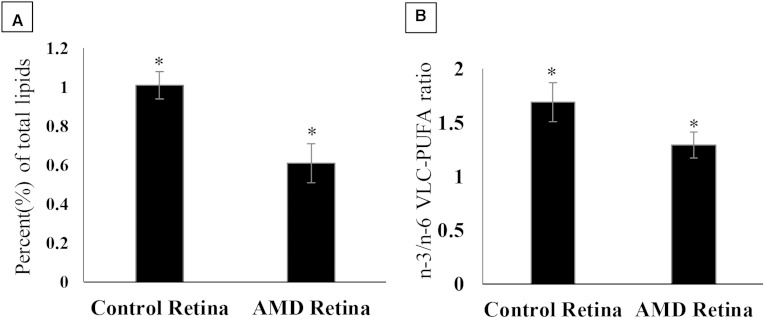
Retinal VLC-PUFA levels in AMD eyes (0.64% ± 0.10) were significantly lower than those found in age-matched control eyes (1.11% ± 0.21) (Fig. 5A). We also observed that n-3/n-6 VLC-PUFA ratios were significantly lower in AMD eyes (1.29 ± 0.12), as compared with those found in age-matched control eyes (1.69 ± 0.18) (Fig. 5B).
Fig. 5.
Comparison of retinal VLC-PUFA levels (A) and n-3/n-6 VLC-PUFA ratios (B) in age-matched control eyes versus AMD eyes. (P values were determined by Student’s t-test, * P < 0.05.)
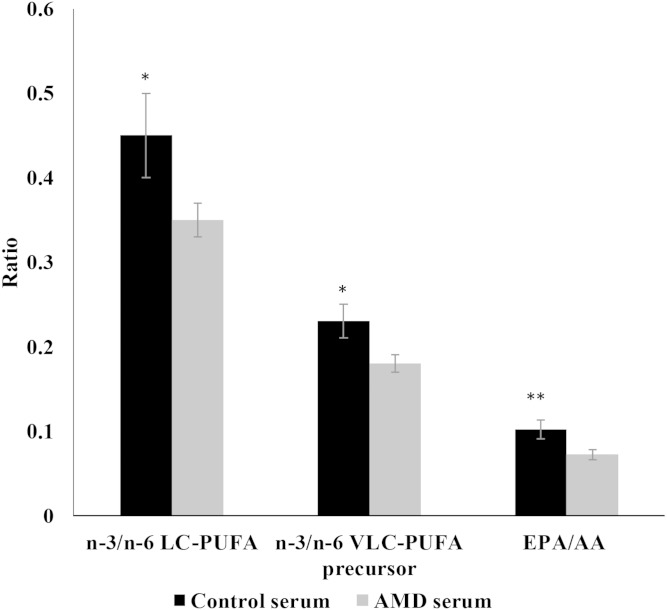
Serum n-3/n-6 LC-PUFA and VLC-PUFA precursor ratios were significantly lower (P < 0.05) in AMD patients (0.35 ± 0.02, 0.18 ± 0.01) when compared with those of age-matched controls (0.45 ± 0.04, 0.23 ± 0.02) (Fig. 6). Likewise, serum EPA/AA ratios in AMD subjects (0.072 ± 0.006) were lower than age-matched controls (0.102 ± 0.011), but this trend was not statistically significant (P = 0.09).
Fig. 6.
Comparative differences in serum lipid profiles of age-matched controls versus AMD subjects. (P values were determined by Student’s t-test; * P < 0.05, ** P = 0.09.)
DNA was also available for these phase II subjects, and we examined whether variants in ELOVL4 and AdipoR1 [a regulator of retinal VLC-PUFA levels (38)] have any influence on AMD risk or lipid profiles in this cohort, but we found no significant relationships, nor did we find any statistically significant influences for AMD grade (Table 1; all P values for comparisons were >0.05).
DISCUSSION
Considering the extraordinarily high concentrations of LC-PUFAs in retinal cell membranes and the largely unique presence of VLC-PUFAs in the human retina, there is a surprising amount of controversy regarding the clinical significance of their roles in retinal health and disease. Epidemiological studies generally support the recommendation that consumption of foods rich in n-3 LC-PUFAs is associated with a lower risk of AMD (14–16), but clinical intervention studies with n-3 LC-PUFA supplements have been either negative or equivocal (17–19). Even when there are genetic mutations in the VLC-PUFA synthesis pathway at the fatty acid elongation step at ELOVL4, which are clearly associated with an early onset form of macular dystrophy known as STGD3 (21, 22), the mechanistic basis (protein aggregation vs. VLC-PUFA deficiency) remains controversial (23–25). Part of the problem lies with the challenges of linking dietary lipid consumption patterns with actual levels in the tissue of interest, the human retina. Dietary surveys are imprecise and cumbersome tools, and nutritional researchers have therefore developed and validated biomarkers of short-term (weeks), medium-term (months), and long-term (years) dietary intake, but until now, there has been relatively little linkage between these biomarkers and retinal tissue levels in adult humans (7, 8).
In the first phase of this study, we prospectively collected serum, RBCs, orbital fat, and retinal tissue from donors with no known eye diseases to ascertain whether these systemic biomarkers of dietary lipid consumption truly represent what is going on at the tissue level. We used a high-sensitivity GC/MS analytical protocol developed in our laboratory (30) that requires minimal amounts of valuable retinal tissue; typically, a single 4–6 mm punch is sufficient. Our GC/MS method readily allows for the distinction between n-3 and n-6 LC-PUFAs and VLC-PUFAs, which is in contrast to LC/MS methods (8, 39), which do not permit distinction between these two important classes of lipids, one of which is generally anti-inflammatory and associated epidemiologically with decreased AMD risk (n-3 series) and one of which is proinflammatory and associated with increased AMD risk (n-6 series) (40, 41).
Our results depicted in Table 2 demonstrate that serum and RBC lipid biomarkers are highly predictive of retinal lipid composition for total LC-PUFAs, EPA, and AA. Orbital fat is associated only with retinal EPA levels. On the other hand, retinal DHA levels have much lower associations with systemic lipid biomarkers, consistent with a more active and regulated uptake of DHA into the human retina relative to other LC-PUFAs. We examined whether LC-PUFA biomarkers correlate with retinal VLC-PUFAs, and we found that only RBCs were significantly associated (Table 3). When we excluded systemic DHA, which is not a major precursor of VLC-PUFAs, we could improve the correlations for RBCs and serum substantially, especially with regard to the n-3 series. Finally, we looked at n-3/n-6 ratios systemically and in the retina and found strong and consistent correlations for all three biomarkers as long as DHA was excluded (Table 4). In fact, just a simple EPA/AA ratio could accurately reflect the n-3/n-6 ratio of the VLC-PUFAs in the retina.
The hypothesis that diet can profoundly influence retinal lipid composition was supported in dramatic fashion by an outlier in our group of 44 donors. As shown in Figs. 2–4, his n-3/n-6 and EPA/AA ratios were several times higher systemically and in the VLC-PUFAs of the retina relative to all of the other donors. Although dietary histories were not part of this study, it is clear that his daily consumption of 7 g per day of fish oil is expected to be more than 10 times higher than what anyone would consume from diet alone and is 7 times higher than the dose tested in AREDS2. The outlier serum showed an increase of EPA over AA (Fig. 3), thereby increasing the serum n-3/n-6 LC-PUFA ratio to 6 times higher than normal human serum. Simultaneously, the n-3/n-6 VLC-PUFA ratio in retina of the outlier patient was also elevated in comparison with normal human retina by 6 times, consistent with a strong effect of diet on n-3/n-6 VLC-PUFA ratios in retina.
In the second-phase study, we tested control and AMD samples from the Utah Center for Translational Medicine Donor Eye Repository, an independent cohort of donors with rigorously characterized clinical histories. As we have seen before, the AMD donors have significantly lower retinal n-3 VLC-PUFAs (50%), VLC-PUFA levels (40%), and n-3/n-6 VLC-PUFA ratios than age-matched controls (Fig. 5), and serum LC-PUFA, VLC-PUFA precursor, EPA/AA, and n-3/n-6 LC-PUFA ratios were likewise lower relative to age-matched controls (Fig. 6). Table 5 shows that for control donors, serum n-3/n-6 ratios of LC-PUFA, VLC-PUFA precursors, and EPA/AA generally predicted retinal VLC-PUFA ratios, and for AMD donors similar trends were seen.
Table 3 indicates that RBC lipids were better biomarkers than serum for retinal VLC-PUFAs in the phase I study, but unfortunately, only serum was available for the phase II study. We are therefore uncertain whether the significant associations of systemic lipid status with retinal VLC-PUFAs in controls versus the nonsignificant associations in AMD subjects seen in Table 5 are simply reflections of serum biomarker variability or if there are true differences in retina lipid uptake and metabolism between normal and AMD eyes. We did explore the possibility that variants in ELOVL4 (the key enzyme in VLC-PUFA synthesis) (20) or AdipoR1 (a regulator of VLC-PUFA synthesis associated with AMD risk) (38) could explain the difference between AMD and control eyes, but we found no evidence for their involvement (Table 1). In agreement with our results, others could not attain a statistically significant correlation between ELOVL4 gene and macular degeneration (28, 42). The association of AdipoR1 gene expression with AMD risk in our cohort was insignificant (P > 0.1), which is in contrast with a recently published Finnish population study (33). In fact, the risk allele of AdipoR1 (CT) was found in only 1 of our 15 AMD subjects.
Few other studies have examined relationships between biomarkers of systemic lipid status and adult human retinal lipid composition. In 2008, a research group from France reported a negative correlation of retinal DHA with orbital fat DHA (8), which is in agreement with our present results. A follow-up study by this same group examined RBCs and retinal lipids from nine elderly donors and found that GC with flame ionization detection yielded significant correlations for only AA and n-3/n-6 ratios (7). Additional positive and negative correlations between RBC lipids with retinal LC-PUFAs and VLC-PUFAs were discernable when they used LC/ESI/MS, but this analytical technique is unable to distinguish between n-3 and n-6 fatty acids.
The results reported here have several important implications. First, they confirm our prior findings that VLC-PUFA levels and n-3/n-6 ratios are lower in AMD eyes (27). This suggests that further studies to understand the physiology of these unusual retinal lipids are warranted. Knockdown of VLC-PUFA levels in mice produces abnormalities only in extreme conditions (i.e., 90% knockdown) (24), but it must be noted that humans have much lower basal levels of retinal VLC-PUFAs than mice (30), so the lower levels that we observed in AMD eyes may indeed induce physiological and structural disruption in the photoreceptors. Second, our results emphasize the importance of assessing n-3/n-6 ratios systemically and in the retina when studying the potential role of dietary and supplemental lipids in modifying AMD risk, as systemic n-3/n-6 ratios show tight and consistent correlations with retinal health and are readily measured with GC/MS. Third, these data demonstrate that biomarkers of systemic lipid status accurately reflect lipid status in the retina, especially with regard to VLC-PUFAs.
Future observational and interventional projects studying nutritional benefits of n-3 fatty acids should include easily obtained biomarkers of short-term and medium-term lipid intake (serum and RBCs, respectively) to account for variability of background diet, intestinal absorption efficiency, and participant compliance. Diet plays an important role in altering the levels and ratios of n-3/n-6 LC-PUFAs in serum, which in turn influence the n-3/n-6 LC-PUFA and VLC-PUFA ratios and levels in retina with possible beneficial effects on macular physiology and protection against degeneration. Our results clarify the biological mechanisms underlying epidemiological studies that have shown that diets rich in n-3 fatty acids are protective against AMD.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- AdipoR1
- adiponectin receptor 1
- AMD
- age-related macular degeneration
- ELOVL4
- elongation of very long-chain fatty acids elongase 4
- FAME
- fatty acid methyl ester
- LC-PUFA
- long-chain polyunsaturated fatty acid
- PR
- peripheral retina
- RBC
- red blood cell
- STGD3
- Stargardt macular dystrophy 3
- VLC-PUFA
- very long-chain polyunsaturated fatty acid
This work was supported by grants from the Macula Vision Research Foundation (New York, NY), the Beckman Initiative for Macular Research (Irvine, CA), and the National Eye Institute, National Institutes of Health (EY-14800). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The invaluable assistance of Chris Pappas, Lisa Nichols, Norma Miller, Kelly Nelson, Yumna K. Subhani, and the donor eye acquisition staff members of the Utah Lions Eye Bank and the Utah Center for Translational Medicine Donor Eye Repository is gratefully acknowledged.
REFERENCES
- 1.Fliesler A. J., and Anderson R. E.. 1983. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 22: 79–131. [DOI] [PubMed] [Google Scholar]
- 2.Agbaga M. P., Mandal M. N., and Anderson R. E.. 2010. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J. Lipid Res. 51: 1624–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aveldaño M. I. 1987. A novel group of very long chain polyenoic fatty acids in dipolyunsaturated phosphatidylcholines from vertebrate retina. J. Biol. Chem. 262: 1172–1179. [PubMed] [Google Scholar]
- 4.Suh M., Wierzbicki A. A., Lien E. L., and Clandinin M. T.. 2000. Dietary 20:4n-6 and 22:6n-3 modulates the profile of long- and very-long-chain fatty acids, rhodopsin content, and kinetics in developing photoreceptor cells. Pediatr. Res. 48: 524–530. [DOI] [PubMed] [Google Scholar]
- 5.McMahon A., and Kedzierski W.. 2010. Polyunsaturated very-long-chain C28–C36 fatty acids and retinal physiology. Br. J. Ophthalmol. 94: 1127–1132. [DOI] [PubMed] [Google Scholar]
- 6.Makrides M., Neumann M. A., Byard R. W., Simmer K., and Gibson R. A.. 1994. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr. 60: 189–194. [DOI] [PubMed] [Google Scholar]
- 7.Bretillon L., Thuret G., Grégoire S., Acar N., Joffre C., Bron A. M., Gain P., and Creuzot-Garcher C. P.. 2008. Lipid and fatty acid profile of the retina, retinal pigment epithelium/choroid, and the lacrimal gland, and associations with adipose tissue fatty acids in human subjects. Exp. Eye Res. 87: 521–528. [DOI] [PubMed] [Google Scholar]
- 8.Acar N., Berdeaux O., Gregoire S., Cabaret S., Martine L., Gain P., Thuret G., Creuzot-Garcher C. P., Bron A. M., and Bretillon L.. 2012. Lipid composition of the human eye: are red blood cells a good mirror of retinal and optic nerve fatty acids? PLoS One. 7: e35102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H., Khor T. O., Saw C. L. L., Lin W., Wu T., Huang Y., and Kong A-N. T.. 2010. Role of Nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid. Mol. Pharm. 7: 2185–2193. [DOI] [PubMed] [Google Scholar]
- 10.SanGiovanni J. P., and Chew E. Y.. 2005. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 24: 87–138. [DOI] [PubMed] [Google Scholar]
- 11.Weiss G. A., Troxler H., Klinke G., Rogler D., Braegger C., and Hersberger M.. 2013. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 12: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazan N. G. 2009. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J. Lipid Res. 50 (Suppl.): S400–S405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwyer J. H., Allayee H., Dwyer K. M., Fan J., Wu H., Mar R., Lusis A. J., and Mehrabian M.. 2004. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N. Engl. J. Med. 350: 29–37. [DOI] [PubMed] [Google Scholar]
- 14.Cho E., Hung S., Willett W. C., Spiegelman D., Rimm E. B., Seddon J. M., Colditz G. A., and Hankinson S. E.. 2001. Prospective study of dietary fat and the risk of age-related macular degeneration. Am. J. Clin. Nutr. 73: 209–218. [DOI] [PubMed] [Google Scholar]
- 15.Seddon J. M., Rosner B., Sperduto R. D., Yannuzzi L., Haller J. A., Blair N. P., and Willett W.. 2001. Dietary fat and risk for advanced age-related macular degeneration. Arch. Ophthalmol. 119: 1191–1199. [DOI] [PubMed] [Google Scholar]
- 16.SanGiovanni J. P., Chew E. Y., Clemons T. E., Davis M. D., Ferris F. L. III, Gensler G. R., Kurinij N., Lindblad A. S., Milton R. C., Seddon J. M., et al. 2007. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch. Ophthalmol. 125: 671–679. [DOI] [PubMed] [Google Scholar]
- 17.Souied E. H., Delcourt C., Querques G., Bassols A., Merle B., Zourdani A., Smith T., Benlian P., and Nutritional A. M. D. T. S. G.. 2013. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology. 120: 1619–1631. [DOI] [PubMed] [Google Scholar]
- 18.Merle B. M. J., Benlian P., Puche N., Bassols A., Delcourt C., Souied E. H.; Nutritional AMD Treatment 2 Study Group. 2014. Circulating omega-3 fatty acids and neovascular age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 55: 2010–2019. [DOI] [PubMed] [Google Scholar]
- 19.The Age-Related Eye Disease Study 2 (AREDS2) Research Group. 2013. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the age-related eye disease study 2 (AREDS2) randomized clinical trial. J. Am. Med. Assoc. 309: 2005–2015. [DOI] [PubMed] [Google Scholar]
- 20.Agbaga M-P., Logan S., Brush R. S., and Anderson R. E.. 2014. Biosynthesis of very long-chain polyunsaturated fatty acids in hepatocytes expressing ELOVL4. In Retinal degenerative diseases. J. D. Ash, C. Grimm, J. G. Hollyfield, R. E. Anderson, M. M. LaVail, C. B. Rickman, editors. Springer, New York. 631–636. [DOI] [PubMed] [Google Scholar]
- 21.Zhang K., Kniazeva M., Han M., Li W., Yu Z., Yang Z., Li Y., Metzker M. L., Allikmets R., Zack D. J., et al. 2001. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat. Genet. 27: 89–93. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein P. S., Tammur J., Singh N., Hutchinson A., Dixon M., Pappas C. M., Zabriskie N. A., Zhang K., Petrukhin K., Leppert M., et al. 2001. Diverse macular dystrophy phenotype caused by a novel complex mutation in the ELOVL4 gene. Invest. Ophthalmol. Vis. Sci. 42: 3331–3336. [PubMed] [Google Scholar]
- 23.Agbaga M-P., Tam B. M., Wong J. S., Yang L. L., Anderson R. E., and Moritz O. L.. 2014. Mutant ELOVL4 that causes autosomal dominant Stargardt-3 macular dystrophy is misrouted to rod outer segment disks. Invest. Ophthalmol. Vis. Sci. 55: 3669–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barabas P., Liu A., Xing W., Chen C. K., Tong Z., Watt C. B., Jones B. W., Bernstein P. S., and Krizaj D.. 2013. Role of ELOVL4 and very long-chain polyunsaturated fatty acids in mouse models of Stargardt type 3 retinal degeneration. Proc. Natl. Acad. Sci. USA. 110: 5181–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harkewicz R., Du H., Tong Z., Alkuraya H., Bedell M., Sun W., Wang X., Hsu Y. H., Esteve-Rudd J., Hughes G., et al. 2012. Essential role of ELOVL4 protein in very long chain fatty acid synthesis and retinal function. J. Biol. Chem. 287: 11469–11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubbard A. F., Askew E. W., Singh N., Leppert M., and Bernstein P. S.. 2006. Association of adipose and red blood cell lipids with severity of dominant Stargardt macular dystrophy (STGD3) secondary to an ELOVL4 mutation. Arch. Ophthalmol. 124: 257–263. [DOI] [PubMed] [Google Scholar]
- 27.Liu A., Chang J., Lin Y., Shen Z., and Bernstein P. S.. 2010. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J. Lipid Res. 51: 3217–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeAngelis M. M., Ji F., Kim I. K., Adams S., Capone A. Jr., Ott J., Miller J. W., and Dryja T. P.. 2007. Cigarette smoking, CFH, APOE, ELOVL4, and risk of neovascular age-related macular degeneration. Arch. Ophthalmol. 125: 49–54. [DOI] [PubMed] [Google Scholar]
- 29.Liu A., Lin Y., Terry R., Nelson K., and Bernstein P. S.. 2011. Role of long-chain and very-long-chain polyunsaturated fatty acids in macular degenerations and dystrophies. Clin. Lipidol. 6: 593–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu A., Terry R., Lin Y., Nelson K., and Bernstein P. S.. 2013. Comprehensive and sensitive quantification of long-chain and very long-chain polyunsaturated fatty acids in small samples of human and mouse retina. J. Chromatogr. A. 1307: 191–200. [DOI] [PubMed] [Google Scholar]
- 31.van Leeuwen R., Klaver C. C., Vingerling J. R., Hofman A., and de Jong P. T.. 2003. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch. Ophthalmol. 121: 519–526. [DOI] [PubMed] [Google Scholar]
- 32.Yi J., Li S., Jia X., Xiao X., Wang P., Guo X., and Zhang Q.. 2012. Evaluation of the ELOVL4, PRPH2 and ABCA4 genes in patients with Stargardt macular degeneration. Mol. Med. Rep. 6: 1045–1049. [DOI] [PubMed] [Google Scholar]
- 33.Kaarniranta K., Paananen J., Nevalainen T., Sorri I., Seitsonen S., Immonen I., Salminen A., Pulkkinen L., and Uusitupa M.. 2012. Adiponectin receptor 1 gene (ADIPOR1) variant is associated with advanced age-related macular degeneration in Finnish population. Neurosci. Lett. 513: 233–237. [DOI] [PubMed] [Google Scholar]
- 34.Sarkkinen E. S., Agren J. J., Ahola I., Ovaskainen M. L., and Uusitupa M. I.. 1994. Fatty acid composition of serum cholesterol esters, and erythrocyte and platelet membranes as indicators of long-term adherence to fat-modified diets. Am. J. Clin. Nutr. 59: 364–370. [DOI] [PubMed] [Google Scholar]
- 35.Baylin A., Kabagambe E. K., Siles X., and Campos H.. 2002. Adipose tissue biomarkers of fatty acid intake. Am. J. Clin. Nutr. 76: 750–757. [DOI] [PubMed] [Google Scholar]
- 36.Baylin A., and Campos H.. 2006. The use of fatty acid biomarkers to reflect dietary intake. Curr. Opin. Lipidol. 17: 22–27. [DOI] [PubMed] [Google Scholar]
- 37.Suh M., and Clandinin M. T.. 2005. 20:5n-3 but not 22:6n-3 is a preferred substrate for synthesis of n-3 very-long- chain fatty acids (C24–C36) in retina. Curr. Eye Res. 30: 959–968. [DOI] [PubMed] [Google Scholar]
- 38.Rice D. S., Calandria J. M., Gordon W. C., Jun B., Zhou Y., Gelfman C. M., Li S., Jin M., Knott E. J., Chang B., et al. 2015. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat. Commun. 6: 6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berdeaux O., Juaneda P., Martine L., Cabaret S., Bretillon L., and Acar N.. 2010. Identification and quantification of phosphatidylcholines containing very-long-chain polyunsaturated fatty acid in bovine and human retina using liquid chromatography/tandem mass spectrometry. J. Chromatogr. A. 1217: 7738–7748. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz G., and Ecker J.. 2008. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 47: 147–155. [DOI] [PubMed] [Google Scholar]
- 41.Patterson E., Wall R., Fitzgerald G. F., Ross R. P., and Stanton C.. 2012. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012: 539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayyagari R., Zhang K., Hutchinson A., Yu Z., Swaroop A., Kakuk L. E., Seddon J. M., Bernstein P. S., Lewis R. A., Tammur J., et al. 2001. Evaluation of the ELOVL4 gene in patients with age-related macular degeneration. Ophthalmic Genet. 22: 233–239. [DOI] [PubMed] [Google Scholar]