Abstract
Background
Autism is a syndrome of unknown cause, marked by abnormal development of social behavior. Attempts to link pathological features of the amygdala, which plays a key role in emotional processing, to autism have shown little consensus.
Objective
To evaluate amygdala volume in individuals with autism spectrum disorders and its relationship to laboratory measures of social behavior to examine whether variations in amygdala structure relate to symptom severity.
Design
We conducted 2 cross-sectional studies of amygdala volume, measured blind to diagnosis on high-resolution, anatomical magnetic resonance images. Participants were 54 males aged 8 to 25 years, including 23 with autism and 5 with Asperger syndrome or pervasive developmental disorder not otherwise specified, recruited and evaluated at an academic center for developmental disabilities and 26 age- and sex-matched community volunteers. The Autism Diagnostic Interview–Revised was used to confirm diagnoses and to validate relationships with laboratory measures of social function.
Main Outcome Measures
Amygdala volume, judgment of facial expressions, and eye tracking.
Results
In study 1, individuals with autism who had small amygdalae were slowest to distinguish emotional from neutral expressions (P=.02) and showed least fixation of eye regions (P=.04). These same individuals were most socially impaired in early childhood, as reported on the Autism Diagnostic Interview–Revised(P<.04).Study 2 showed smaller amygdalae in individuals with autism than in control subjects (P=.03) and group differences in the relation between amygdala volume and age. Study 2 also replicated findings of more gaze avoidance and childhood impairment in participants with autism with the smallest amygdalae. Across the combined sample, severity of social deficits interacted with age to predict different patterns of amygdala development in autism (P=.047).
Conclusions
These findings best support a model of amygdala hyperactivity that could explain most volumetric findings in autism. Further psychophysiological and histopathological studies are indicated to confirm these findings.
Autism forms the severe end of a spectrum of developmental disorders defined by impairment in 3 core domains: reciprocal social interaction, communication, and repetitive or restricted behaviors.1,2 Although investigations into underlying brain anatomical features are inconsistent, autism spectrum disorders are believed to have a biological basis and are highly heritable3 and, therefore, offer a unique opportunity to discover the genetic and neural underpinnings of reciprocal social behaviors.
A candidate region for focal neuropathological features, reported to have small, densely packed neurons in individuals with autism, is the medial temporal lobe.4 While several other structures also show histopathological features, Baron-Cohen et al5 outlined a theoretical basis to connect autistic social deficits to specific pathological features of the amygdala. Imaging studies6-8 have reported differences in amygdala activation to faces in individuals with autism, and amygdala lesions have been shown to impair perception of emotional expressions and higher-order social behavior (eg, understanding of social norms)9-11; this fueled speculation that autistic behavior reflects impaired perception of social stimuli because of loss of function in the amygdala. Amaral et al12 challenged this model, noting that pure amygdala lesions principally affect fear processes, sparing most social behaviors. Rather than conceptualizing the deficit as akin to an amygdala lesion, an alternative framework is to view the deficit as arising from amygdala hyperexcitability. Evidence of exaggerated sympathetic arousal, particularly to social engagement, was reported in approximately 70% of a group of children with autism13 and was interpreted as reflecting amygdala hyperexcitability. Furthermore, amygdala hyperactivation specifically when viewing the eyes of facial stimuli was reported by the first study, to our knowledge, of concomitant eye tracking and functional imaging in autism.8 Some deficits in autism may, therefore, be secondary to avoidance of social stimuli because of exaggerated amygdala responsiveness and social fear.
Despite extensive theory, amygdala pathological characteristics have not been linked to autistic social impairments. Group analyses have shown increased,14,15 decreased,6,16,17 and normal amygdala volumes.18 Schumann et al19 report an increase in amygdala volume with age in control subjects but not in individuals with autism, leading to enlarged amygdalae in children with autism but normal volumes in teenagers with autism. Age effects cannot reconcile all reported results, however, so we predicted that some differences would relate to degree of behavioral impairment.
A major obstacle to these investigations is the broad variability in behavioral presentation in autism, which also evolves with age.20 Rigorous laboratory measures of autistic behavior using tasks involving judgment of standardized facial expressions and tasks assessing “holistic” face processing indicate that individuals with autism underuse eye regions,21-23 a finding supported by quantitative eye-tracking studies.8,24,25 Herein, we attempt to use the heterogeneity indexed by these quantitative measures of face processing to investigate the neuropathological features of autism.
We examined relations between amygdala volumes and quantitative measures of face processing and gaze fixation; to our knowledge, we report the first relationship between amygdala structure and current and past measures of social impairment in autism.
METHODS
PARTICIPANTS
All participants gave voluntary consent or assent in accordance with the University of Wisconsin Medical School institutional review board. Behavioral and functional imaging data from this sample were previously described.8 Participants were 28 males with autism spectrum disorders, aged 8 to 25 years (Table 1), recruited from the Waisman Center, University Center for Excellence in Developmental Disabilities, and by advertisement in autism-related newsletters. Controls were 26 age-matched males with no known psychiatric disorders recruited by word of mouth and advertisement in local newspapers.
Table 1.
Participant Age, IQ, and Diagnostic Measures
| Variable | Control Group | Autism Group |
|---|---|---|
| Study 1 | ||
| No. of subjects | 12 | 12 |
| Age, y | ||
| Range | 13-23 | 10-24 |
| Mean ± SD | 17.0 ± 2.9 | 16.8 ± 4.5 |
| ADI-R score* | ||
| QIRS | NA | 25.4 ± 3.0 |
| NVC | NA | 11.6 ± 2.1 |
| VC | NA | 17.9 ± 2.7 |
| RB | NA | 4.6 ± 3.0 |
|
| ||
| Study 2 | ||
| No. of subjects | 14 | 16† |
| Age, y | ||
| Range | 8-21 | 8-25 |
| Mean ± SD | 13.7 ± 3.9 | 14.3 ± 4.7 |
| IQ* | ||
| Full scale | 122 ± 13 | 97 ± 26‡ |
| Verbal | 119 ± 14 | 91 ± 27‡ |
| Performance | 122 ± 13 | 102 ± 12‡ |
| ADI-R score* | ||
| QIRS | NA | 20.7 ± 7.4§ |
| NVC | NA | 8.6 ± 3.5∥ |
| VC | NA | 14.5 ± 4.5 |
| RB | NA | 6.6 ± 2.7 |
Abbreviations: ADI-R, Autism Diagnostic Interview–Revised; NA, data not applicable; NVC, nonverbal communication; QIRS, qualitative impairment in reciprocal social behavior; RB, repetitive behavior; VC, verbal communication;
Data are given as mean ± SD.
Five of these subjects had Asperger syndrome or pervasive developmental disorder not otherwise specified.
Significantly different (P<.01 for all) from the control group.
There was a trend toward lower scores than in study 1 (P=.06).
Significantly different (P=.02) from study 1.
Diagnoses were confirmed with the Autism Diagnostic Interview–Revised (ADI-R)26 by trained experimenters (K.M.D. and B.M.N.) who achieved greater than 90% reliability with raters from 2 other institutions. For study 1, caretakers for 2 individuals were unavailable for interviews; diagnoses for these individuals were derived without the ADI-R from previous clinical assessment by specialists in developmental disorders. Another caretaker was unable to recall behavior from the diagnostic age range of 4 to 5 years, when behavior is thought to be most abnormal, but adequately described relevant behavior at older ages that well surpassed thresholds for the diagnosis of autism; these scores were excluded from analysis for consistency. All participants met the criteria for autism in social reciprocity, verbal and nonverbal communication, and repetitive behavior. Individuals with comorbid disorders of known cause (eg, fragile X and fetal alcohol syndrome) were excluded. One individual in the autism group had a history of epilepsy. All findings were similar with or without this individual included, and so we retained this individual in our analyses.
Participants for study 2 were recruited by similar means. Only males with clinical diagnoses of autism, Asperger syndrome (AS), or pervasive developmental disorder not otherwise specified (PDD-NOS) were enrolled. Autism diagnoses were verified by ADI-R, while individuals with AS or PDD-NOS (n=5) met diagnostic thresholds in 3 domains (n=3), 2 domains (n=1), or 1 domain (n=1) of the algorithm. Three caretakers were unavailable for interviews; as previously described, diagnoses were made without the ADI-R by clinical specialists. The Wide Range Intelligence Test27 was used to evaluate IQ. One participant did not demonstrate an understanding of the test instructions; no IQ was obtained. Individuals with comorbid disorders of known cause were excluded, except for one individual with epilepsy; all findings were similar with exclusion of this participant. Control subjects were age-matched male volunteers with no known psychiatric disorders recruited as described for study 1.
BEHAVIORAL TASKS AND EYE TRACKING
Experimental paradigms and eye-tracking procedures for both studies were previously described.8 Briefly, study 1 involved evaluation of 40 standardized images of posed facial expressions (8 each of happy, angry, and sad and 16 neutral), and participants distinguished neutral from emotional expressions by pressing a button. Study 2 involved 20 images of naturalistic faces from digital photographs, including images of friends and family and of strangers matched on general appearance. Participants were instructed to differentiate between familiar and unfamiliar faces. We were unable to teach the task from study 1 to 2 functionally nonverbal participants; however, eye-tracking and magnetic resonance imaging data were acquired from these individuals. Eye-tracking data were not obtained from 1 control subject in study 1 and 1 control subject and 3 individuals with autism in study 2 because of equipment malfunction or excessive movement or blinks during the task.
IMAGE ACQUISITION
Magnetic resonance images for both studies were acquired with a 3-T scanner equipped with high-speed gradients and a whole-head transmit-receive quadrature birdcage head coil (Signa model; GE Medical Systems, Waukesha, Wis). Study 1 anatomical volumes were high-resolution, 3-dimensional, T1-weighted, spoiled-grass images acquired with the following parameters: echo time, 8.0 milliseconds; repetition time, 21.0 milliseconds; field of view, 240×240 mm; flip angle, 30°; number of excitations, 1; matrix, 256×256; 124 axial sections; and section thickness, 1.1 to 1.2 mm.
Study 2 anatomical images included a high-resolution, 3-dimensional, inversion recovery–prepared, fast spin-echo image with the following parameters: echo time, 1.8 milliseconds; repetition time, 8.9 milliseconds; field of view, 240×240 mm; flip angle, 10°; number of excitations, 1; matrix, 256×256; 124 axial sections; and section thickness, 1.1 to 1.2 mm. An additional T2-weighted image was collected with the following parameters: echo time, 92.0 milliseconds; repetition time, 7500.0 milliseconds; field of view, 240×240 mm; flip angle, 90°; number of excitations, 1; matrix, 256×256; 68 axial sections; section thickness, 1.7 mm; gap between sections, 0.3 mm. The T2-weighted images were included in a multispectral segmentation/bias correction algorithm (FSL; available at: http://www.fmrib.ox.ac.uk/fsl/) to smooth inhomogeneities in the inversion recovery–prepared images.
By using in-house software that permits simultaneous visualization and region-of-interest definition in the 3 cardinal planes (Spamalize; available at: http://brainimaging.waisman.wisc.edu/~oakes/spam/spam_frames.htm), images were first re-oriented to the “pathological plane”28 for optimal comparison with anatomical atlases.29-33 Contrast was matched by alignment of white and gray matter peaks on intensity histograms. All region-of-interest analyses were done blind to participant diagnosis.
AMYGDALA DELINEATION
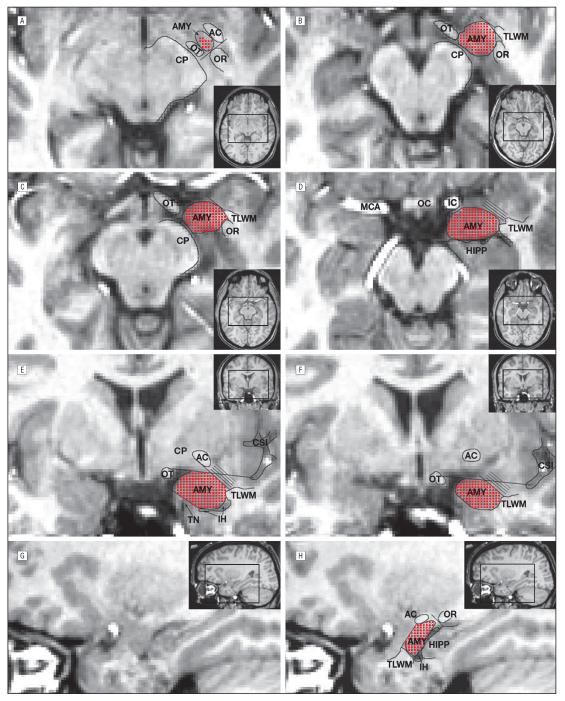
Tracing started in the most superior plane in which gray matter was present lateral to the optic tract, posteromedial to the anterior commissure, and anteromedial to the optic radiations (Figure 1A). Working inferiorly, a tangent to the anteromedial extent of the optic tract (Figure 1A) defined the posterolateral border. Special effort was made to include the semilunar gyrus immediately anterolateral to the cerebral peduncle and posterolateral to the optic tract (Figure 1B). Initial separation of the medial amygdala from the hippocampus was by linear extension of the posterior amygdala–cerebrospinal fluid border (Figure 1D), but more precise separation was reserved for coronal sections. An arc extending anteriorly and medially from the temporal lobe white matter and following its curvature formed the anterolateral border (Figure 1B and C). More inferiorly, the anterolateral border was approximated, but exclusion of other mesial temporal structures was achieved in the coronal view. Superiorly, the anterior border extended as far as the middle cerebral artery (Figure 1D [left]). More inferiorly, the medial and anterior amygdala was separated from the entorhinal cortex; rarely, these borders were indistinguishable and a semicircle was substituted, as previously described.34
Figure 1.
Amygdala (AMY) tracing prescription: unwarped (native space) images oriented to the pathological plane show amygdala tracing landmarks in axial (A-D), coronal (E and F), and sagittal (G and H) sections. The inset pictures show areas of focus on full-brain sections (black box). Red points indicate inclusion in the final region of interest. The parallel lines in part D denote anterolateral areas removed in the coronal plane (E and F). AC indicates anterior commissure; CP, cerebral peduncle; CSI, circular sulcus of the insula; HIPP, hippocampus; IC, internal carotid artery; IH, inferior horn of the lateral ventricle; MCA, middle cerebral artery; OC optic chiasm; OR, optic radiations; OT, optic tract; TLWM, temporal lobe white matter; and TN, tentorial notch.
Regions were then refined through plane-by-plane comparison with ex vivo atlas sections.32 Tracing started at the most posterior coronal section in which gray matter was present as the lateral roof of the inferior horn. While white matter formed the superolateral border, a tangent to the optic tract defined the superomedial extent. Moving anteriorly, the close approximation of the anterior commissure to the amygdala was exploited to exclude the caudate: regions of interest never extended between the superomedial extent of the temporal lobe white matter and the more lateral of (1) the medial edge of the anterior commissure (Figure 1E) or (2) the lateral extent of the collateral sulcus (Figure 1F). This step may exclude some superolateral amygdala but enhances precision. The inferior boundary was extended medially to within 1 to 2 mm of the tentorial notch and beyond to form the inferomedial border (Figure 1E).
Working medially, separation from the hippocampus, optic radiations, caudate/putamen, and entorhinal cortex was confirmed in the sagittal view (Figure 1G and H). Regions were refined until surfaces were smooth to ensure agreement in all planes. Working anterior to posterior, the superior border was then trimmed in the coronal plane, as previously described35 (Figure 1E and F).
BRAIN VOLUME MEASUREMENT AND AMYGDALA VOLUME RELIABILITY
Whole brain regions of interest were defined using an automated, threshold-based, connected pixel search and then hand edited to ensure removal of skull, eye regions, brainstem, and cerebellum.
Images from 5 randomly selected study 1 participants (10 amygdalae) were retraced for an intrarater intraclass correlation of 0.95. Two raters (B.M.N. and K.M.D.) used the same technique to retrace images from 5 different randomly selected participants for an interrater intraclass correlation of 0.97. Because image acquisition differed in study 2, reliability was reevaluated: 2 raters (B.M.N. and M.T.L.) traced images from 5 randomly selected individuals to yield an intraclass correlation of 0.95; analysis of spatial reliability (intersection/union) averaged 0.84. These values for volumetric and spatial reliability meet or exceed the range of recently published reliability estimates.17,19
STATISTICAL ANALYSES
All statistical analyses were performed with statistical software (Statistica; StatSoft, Tulsa, Okla). The normality of comparison measures and covariates was confirmed by the Kolmogorov-Smirnov test. To control for multiple comparisons, variables of interest for each data set were combined in a mixed-model analysis of covariance. All correlations were carried out after correction for age, brain volume, and IQ (study 2 only) by multiple linear regression of whole sample amygdala volume data.
RESULTS
STUDY 1
Group Analyses of Amygdala Volume
We first assessed amygdala volumes in 12 individuals with autism and 12 controls aged 10 to 24 years (described in Table 1). Measures of IQ were obtained from few controls and were, therefore, excluded from analysis.
The mean±SD volumes for the full sample (N=24) of left and right sides of the amygdalae were 1874±187 mm3 and 1874±166 mm3, respectively. Group means were evaluated by mixed analysis of covariance, covarying age and brain volume; to formally evaluate laterality, hemisphere was included as a within-subject factor. Mean±SD volumes of 1853±130 mm3 for the control group and 1895±212 mm3 for the autism group did not differ (F1,20=0.1, P=.74). There were no effects of hemisphere, age, or brain volume. Subsequent results are reported as age- and brain volume–corrected mean amygdala volumes.
Amygdala Volume Predicts Task Performance in the Autism Group
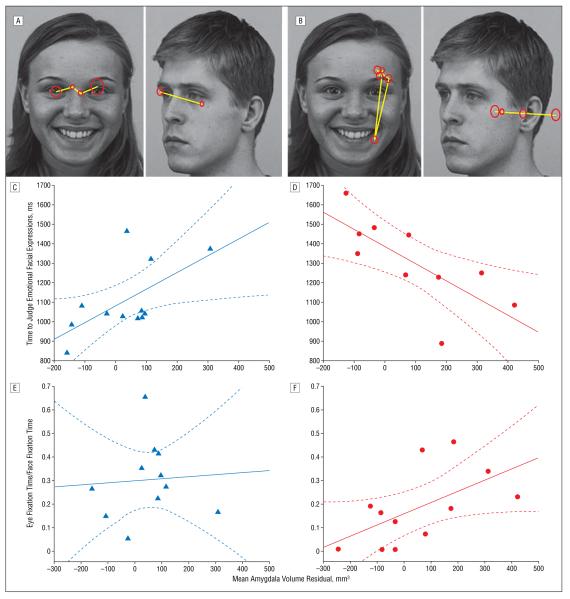
During the experimental session, participants distinguished emotional expressions from neutral expressions (Figure 2A and B). As previously described,8 control subjects performed the task with minimal errors, while participants in the autism group were less accurate. Controls, but not individuals with autism, showed faster judgment of emotional expressions than neutral ones; there were no effects of emotionality on accuracy for either group.
Figure 2.
A small amygdala volume predicts face-processing abnormalities in individuals with autism. Example task stimuli show gray scale images from a standardized picture set depicting emotional and neutral facial expressions. The overlay depicts representative visual scanning (yellow lines) and fixations (red circles; the diameter reflects duration) from a typically developing individual (A) and an individual with autism (B). Behavioral performance is plotted against residual variance in mean amygdala volume after correction for age and brain volume in a control individual (C) and in an individual with autism (D). In C, judgment times for emotional stimuli are positively correlated with the mean amygdala volume (r=0.61, P=.02) in the control group, but this is not significant after removal of an outlier at 300 mm3 and 1375 milliseconds (r=0.46, P=.16). In D, judgment times are slower for emotional stimuli in the autism group but are strongly correlated with amygdala volume (r=−0.73, P=.02). This correlation differs significantly from the control correlation (without outlier: z=2.8, P=.002). Eye fixation time as a fraction of total face fixation time per trial is unrelated to amygdala volume in controls (r=0.07, P=.80) (E), but positively correlates with amygdala volume in the autism group (r=0.58, P=.049) (F) such that individuals with the least eye fixation have small amygdalae. In C-F, the solid line indicates the line of best fit; the broken lines, the 95% confidence interval.
Amygdala volume did not correlate with task accuracy in either group. In controls, amygdala volume did not correlate with judgment times for neutral (Table 2) or emotional stimuli (without outlier: r=0.46, P=.16) (Figure 2C). In individuals with autism, amygdala volume was uncorrelated with judgment time for neutral stimuli (Table 2), but small amygdalae significantly predicted slow judgment time for emotional expressions (r=−0.73, P=.02) (Figure 2D).
Table 2.
Study 1 Amygdala-Behavior Relationships by Hemisphere*
| Control Group† |
Autism Group‡ |
|||
|---|---|---|---|---|
| Behavior | Left Hemisphere |
Right Hemisphere |
Left Hemisphere |
Right Hemisphere |
| Face processing | ||||
| Accuracy | 0.22 | 0.14 | 0.43 (.21) | 0.54 (.11) |
| Judgment times | ||||
| Neutral | 0.26 | 0.32 | −0.04 | 0.03 |
| Emotional | 0.62 (.03)§ | 0.55 (.07) | −0.73 (.02)§∥¶ | −0.72 (.02)§∥ |
| Eye fixation | ||||
| Face | −0.07 | −0.15 | 0.56 (.06) | 0.56 (.06) |
| Eye | −0.05 | 0.22 | 0.46 (.14) | 0.47 (.12) |
| Eye/face | 0.02 | 0.15 | 0.55 (.06) | 0.60 (.04)§ |
| ADI-R algorithm | ||||
| QIRS | NA | NA | −0.66 (.05) | −0.71 (.03)§ |
| NVC (sections B1 + B4) | NA | NA | −0.61 (.08) | −0.68 (.04)§ |
| VC (sections B2 + B3)# | NA | NA | −0.45 (.26) | −0.46 (.26) |
| RB | NA | NA | 0.39 (.29) | 0.48 (.19) |
Abbreviations: See Table 1.
Data are given as r value (P value). If no P value is given, P >.30.
n = 12 for face-processing data, and n = 11 for eye fixation data.
n = 10 for face-processing data, n = 12 for eye fixation data, and n = 9 for ADI-R data.
P<.05.
Significantly different from the control group.
Significantly different from the judgment time for neutral facial expressions.
Excludes items contributing to NVC score.
Small Amygdala Volume Predicts Decreased Eye Fixation
Previous results suggest that poor judgment of facial expressions might reflect decreased eye fixation8,10; we, therefore, evaluated eye tracking for controls (Figure 2A) and individuals with autism (Figure 2B).8 The mean time fixating faces did not correlate with amygdala volume for either group (Table 2), although the autism group showed a trend linking small amygdalae to decreased face fixation (P=.06). Eye fixation time was, therefore, evaluated as raw values and as a fraction of total face fixation (eye fixation fraction) to specifically evaluate fixation of eye regions relative to other face regions. In controls, amygdala volume was uncorrelated with both eye fixation measures (Table 2 and Figure 2E). The autism group, however, showed a positive correlation between mean amygdala volume and eye fixation fraction (Figure 2F): individuals with small amygdalae showed the least fixation of eyes relative to other facial regions.
Small Amygdala Volume Predicts More Childhood Social Impairment
While rigorously controlled and quantitated, this task tests a single area of social behavior. To assess the generalizability of these findings, we examined the relationship between amygdala volume and diagnostic algorithm scores from the ADI-R. Based on face-processing results, we hypothesized that individuals with small amygdalae would show the most pervasive social impairments.
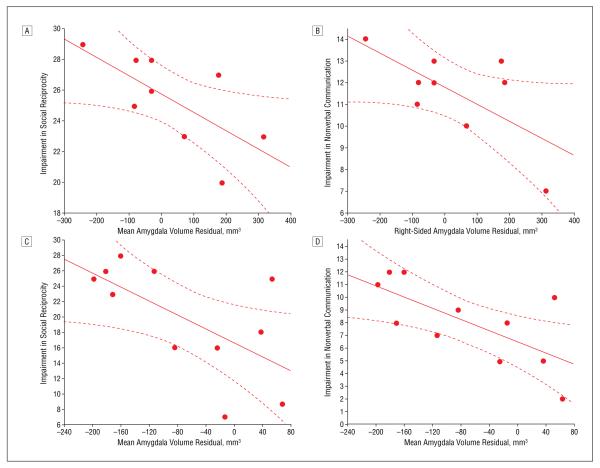
The data reveal that individuals with smaller amygdalae exhibited a more significant level of impairment in social reciprocity derived from the ADI-R (Figure 3A and Table 2). To avoid content overlap, social reciprocity scores were recalculated without the item assessing eye contact: identical correlations emerged. In addition, a similar correlation between right-sided amygdala volume and impairment in nonverbal communication reached significance (Figure 3B). Because the diagnostic algorithm verbal communication subscale includes nonverbal items, we performed a comparison with only verbal items. Amygdala volume was unrelated to childhood impairments in verbal communication and presence of repetitive behaviors (Table 2).
Figure 3.
More extensive childhood social deficits are found in individuals with autism who have small amygdalae, including impairments in social reciprocity (A and C) and nonverbal communication (B and D). The maximum score in A and C is 30; and in B and D, 14. In A and B, age- and brain volume–corrected amygdala volume is plotted against algorithm scores from the Autism Diagnostic Interview–Revised for individuals with autism in study 1; higher scores indicate more abnormal behaviors. Scores on the social reciprocity subscale are negatively correlated with mean amygdala volume (r=−0.69, P=.04) (A), and impairment in nonverbal communication is negatively correlated with right-sided amygdala volume (r=−0.68, P=.04) (B). In C and D, age- and brain volume–corrected amygdala volume with additional correction for full-scale IQ is depicted for individuals with autism spectrum disorders from study 2. Significant correlations with impairment in social reciprocity (r=−0.63, P=.04) (C) and nonverbal communication (r=−0.69, P=.02) (D) in the same direction and of similar magnitudes to those of study 1 suggest that these effects are not mediated by IQ. The solid line indicates the line of best fit; the broken lines, the 95% confidence interval.
STUDY 2
Processing of Naturalistic Facial Stimuli
Study 2 aimed to replicate these results using naturalistic stimuli in a better-characterized sample of 16 males with autism (n=11) or AS or PDD-NOS (n=5) and 14 control males (Table 1). Participants viewed images of their family and friends and of other participants’ family and friends. As previously reported, individuals with autism judged familiarity of faces less accurately than controls, but did not differ in judgment time.8
Small Amygdalae in the Autism Group
The mean±SD amygdala volumes for study 2 (N=30) were 1844±164 mm3 and 1840±171 mm3 for left and right sides of the amygdalae, respectively, and were statistically similar to study 1. Group differences in amygdala volume were assessed as before, but with full-scale IQ as an additional covariate. There were no significant differences in amygdala volume, brain volume, age, or IQ between individuals with diagnoses of autism and those with AS or PDD-NOS (P>.20 for all), so individuals were combined into a single autism group. Both left- and right-sided amygdalae were significantly larger in controls (mean±SD in the left hemisphere, 1921±173 mm3; and mean±SD in the right hemisphere, 1921±186 mm3) than in the autism group (mean±SD in the left hemisphere, 1778 ± 125 mm3; and mean ± SD in the right hemisphere, 1770±123 mm3) for raw values and after correction for age, brain volume, and IQ (F1,23=5.4, P=.03). Brain volume significantly contributed to amygdala volume (F1,23=8.9, P=.01), but there were no effects of hemisphere. There were no significant contributions of IQ to amygdala volume for either group (control group: r=−0.44, P=.11; and autism group: r=−0.37, P=.19).
Age-Dependent Differences in Amygdala Volume
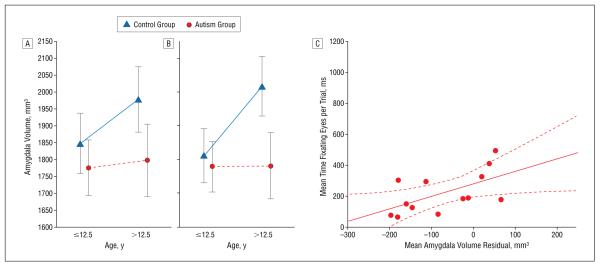
In light of previous findings,19 we split the sample into those younger than 12.5 years (n=16) and those older than 12.5 years (n=14). This factor was entered into a mixed analysis of covariance along with diagnostic group, hemisphere, brain volume, and IQ. A trend toward differential age-volume relationships emerged formeanamygdala volume (F1,22=4.0, P=.06); hemisphere contributed significantly to this effect (hemisphere × group × age group interaction: F1,22=8.6, P=.01) (Figure 4A and B).
Figure 4.
Amygdala volume was abnormally low in older but not younger individuals with autism. Data are given as mean±95% confidence interval; age, brain volume, and IQ were included as covariates in the analysis. In C, small amygdala volume correlated with the least eye fixation. The solid line indicates the line of best fit; the broken lines, the 95% confidence interval.
Relationship With Eye Fixation and Childhood Behavior
We again compared amygdala volumes, additionally covarying IQ, with eye-tracking measures. Amygdala volume was unrelated to eye and face fixation in the control group (Table 3). In the autism group, the mean amygdala volume predicted raw eye fixation time (r=0.59, P=.03) (Figure 4C), a finding driven by right-sided amygdala volume (Table 3). As with posed faces, individuals with autism who had small amygdalae exhibited the least eye fixation.
Table 3.
Study 2 Amygdala-Behavior Relationships by Hemisphere*
| Control Group† |
Autism Group‡ |
|||
|---|---|---|---|---|
| Behavior | Left Hemisphere |
Right Hemisphere |
Left Hemisphere |
Right Hemisphere |
| Eye fixation | ||||
| Face | 0.02 | −0.18 | 0.10 | 0.24 |
| Eye | −0.18 | −0.25 | 0.49 (.09) | 0.61 (.03)§∥ |
| Eye/face | −0.32 (.28) | −0.14 | 0.51 (.13)§ | 0.45 (.09) |
| ADI-R algorithm | ||||
| QIRS | NA | NA | −0.69 (.02) | −0.62 (.04)∥ |
| NVC (sections B1 + B4) | NA | NA | −0.71 (.01) | −0.68 (.02)∥ |
| VC (sections B2 + B3) | NA | NA | −0.14 | −0.08 |
| RB | NA | NA | 0.18 | 0.04 |
Abbreviations: See Table 1.
Data are given as r value (P value). If no P value is given, P>.30.
n = 13 for eye fixation data.
n = 13 for eye fixation data, and n = 11 for ADI-R data.
Significantly different from the control group.
P<.05.
Amygdala volumes were again related to ADI-R algorithm measures. Left- and right-sided amygdala volumes correlate with childhood impairment in social reciprocity (Figure 3C) and nonverbal communication (Figure 3D). As in study 1, amygdala volume was unrelated to verbal communication and repetitive behavior (Table 3). This replicates the study 1 finding that individuals with small amygdalae exhibited the most nonverbal social impairment in childhood.
COMBINED SAMPLE: AMYGDALA VOLUME REFLECTS SEVERITY AND DURATION OF IMPAIRMENT
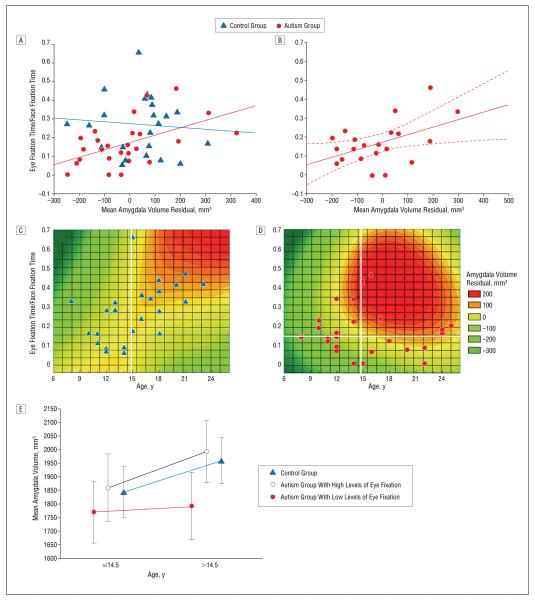
Based on previous findings of amygdala hyperactivity to eye fixation,8 we evaluated the hypothesis that amygdala size reflects a combination of duration and severity of hypersensitivity to social engagement. We combined both studies for adequate statistical power (N=49) and chose eye fixation fraction as an objective indicator of nonverbal social behavior.
Across the combined control group (n=24), amygdala volume was uncorrelated with eye fixation fraction (r=−0.08) (Figure 5A). In the combined autism group (n=25), amygdala volume significantly correlated with eye fixation fraction, denoting that individuals with autism who have smaller amygdalae spend less time fixating eye regions (r=0.52) (Figure 5A). This relationship holds when all individuals with available IQ data are combined (n=21) and IQ is covaried (r=0.48) (Figure 5B).
Figure 5.
Decreased amygdala volume in autism is a product of age and degree of nonverbal social impairment. In A, age- and brain volume–corrected amygdala volumes were combined across studies and plotted against eye fixation fraction for each group. The 2 measures are unrelated in the combined control group (r=−0.08, P=.70) but are significantly correlated in the combined autism group (r=0.52, P=.01); these correlations are significantly different (z=2.2, P=.01). In B, the relationship remains significant when IQ is included as a covariate (r=0.48, P=.03). Eye-fixation fraction was used as an indicator of nonverbal social functioning to examine its relationship with age-related differences in amygdala volume. In C, although eye fixation does not predict amygdala volume in control individuals, a spline-interpolated contour plot of amygdala volume (corrected for brain volume) with respect to age and eye fixation fraction shows that amygdala volume and eye fixation increase with age. In D, a similar plot for the autism group shows that individuals with high levels of eye fixation do show an age-related increase in amygdala volume, but those with low levels of eye fixation have similar amygdala volumes throughout this age range. For visualization, the sample was split by median age (vertical white lines in C and D), and the autism group was further divided by eye fixation (horizontal white line in D). In E, the mean±95% confidence interval–corrected amygdala volume for the 3 populations is shown for younger and older participants.
A mixed analysis of covariance was constructed as previously described, with an additional group × eye fixation × age interaction to formally test our hypothesis. There were significant effects of group (F1,44=4.2, P=.046), age (F1,44=9.3, P=.004), and brain volume (F1,44=7.5, P=.009); a nonsignificant trend emerged for eye fixation fraction (F1,44= 3.6, P=.06). The group × age × eye fixation interaction was significant (F1,44=4.2, P=.047), and is represented by contour plots of amygdala volume (Figure 5C and D). The control plot shows, with few deviations, a steady increase in amygdala volume and eye fixation fraction with age (Figure 5C). The autism group shows an earlier more pronounced increase in amygdala volume in individuals with normal eye fixation, but little difference in amygdala volume across this age range in those with low levels of eye fixation (Figure 5D). A plot of mean amygdala volumes after a median split by age and eye fixation fraction (Figure 5E) further illustrates this finding: individuals with autism who exhibit low levels of eye fixation show little increase in amygdala volume with age, while individuals with autism who show high levels of eye fixation are indistinguishable from controls and display an age-related increase in amygdala volume.
COMMENT
These results36 provide the first evidence for a link between objective measures of social impairment and amygdala structure in autism. Amygdala volume not only predicts current deficits in processing facial emotions but also reflects early childhood impairment in nonverbal social behaviors estimated from retrospective diagnostic measures. This relatively time-independent degree of impairment interacts with age to predict abnormally small amygdala volumes by late adolescence in the most affected individuals. These results are consistent with a model of hyperactivity-induced changes that could reconcile most extant studies of amygdala volume in autism.
The neural and endocrine adaptation to chronic stress, termed allostasis, has been described with respect to medial temporal lobe structures.36 McEwen drew on animal models of “chronic immobilization stress,” which potentiates fear conditioning and can increase dendritic arborization of amygdalar primary neurons.37 He likened this to humans after a single episode of major depression, another condition associated with amygdala hyperactivity, who showed enlarged amygdalae compared with controls and individuals with recurrent depression.38 He further suggested that increased load might give way to eventual shrinkage, citing reports of reduced amygdala volume in long-term recurrent depression39 and further supported by findings of highest activation in depressed individuals with the smallest amygdalae.40 This initial hypertrophy and subsequent atrophy due to amygdala hyperactivity might be occurring at an early age in autism.
An allostatic load model suggests that the degree of hyperactivity will influence the time course of amygdala development. In those most severely affected, amygdala hypertrophy might be initiated within the first years of life, during symptom onset. Those with the least hypersensitivity might show a slower delayed overgrowth. By adulthood, however, chronic hyperactivity might lead to excitotoxic changes and amygdalar anergy or atrophy in most individuals with autism.
In a large sample of 3- to 4-year-old subjects, boys who developed autism showed larger amygdala volumes than typically developing individuals and the less affected individuals with PDD-NOS15; only individuals with more severe social impairments manifest overgrowth at this age. This overgrowth remains evident in a sample of 7.5- to 12.5-year-old boys with autism.19 Although we did not find overgrowth in boys aged 8 to 12.5 years, this might simply reflect a difference in overall impairment between the 2 samples. This is particularly important because all but 2 participants in our sample are older than 10 years and, thus, are closer to the age range at which possible stasis or shrinkage in more affected individuals allows typical amygdala growth to even out group differences. A differential age–amygdala volume relationship resulted in normal amygdala volumes in a sample of males aged 12.5 to 18 years.19 Our findings of an age × severity interaction leave open the possibility of normal amygdala growth or overgrowth during this age range in less affected individuals, while those with more impairment show abnormally small amygdalae into adulthood. Without proper characterization of social impairment, this could lead to decreased, normal, or possibly enlarged amygdala volumes in different samples of adolescents with autism spectrum disorders.
We found individuals with autism spectrum disorders older than 12.5 years (mean, 19 years) to have abnormally small amygdalae, particularly in older adolescents and adults. This replicates findings from studies of 14 adolescents and adults who were a mean age of 20.5 years,16 7 adults who were a mean age of 29.5 years,6 and 15 adults who were a mean age of 30 years.17 There is, thus, an emergent consensus that amygdala volume is decreased at older ages in individuals with autism.
A possible challenge to our model is a report14 of enlarged amygdalae in 10 adolescents and adults with “high-functioning autism,” aged 16 to 40 years. The diagnoses were not confirmed by ADI-R, however, leaving open the possibility that some individuals had only mildly impaired nonverbal social behavior; mildly increased demand might have been sufficiently met by amygdala overgrowth to preclude excitotoxic atrophy. Another discrepant finding comes from a study18 of 10 adults with autism and 7 adults with AS (mean age, 28 years), with neither group significantly differing from controls but individuals with autism being distinguished from those with AS by smaller left-sided amygdalae. This might reflect differences in social function between groups, however, because a negative correlation (across the combined sample) between left-sided amygdala volume and impairment in nonverbal communication on the ADI-R is reported. Taken together, these results suggest that individuals with autism spectrum disorders with the mildest deficits might have an amygdala overgrowth that is sufficient to achieve a new equilibrium without damage from allostatic overload.
An important implication of this model is that non-autistic family members, known to show mild social and communicative deficits,41-43 should show proportionate amygdala differences. To our knowledge, only one study17 has measured amygdala volume in parents of individuals with autism, and found no significant differences from controls. Because social behavior was not characterized, this null result might simply reflect a weaker expression of autistic traits in parents without multiple affected children and in females.44 Studies with large samples of first-degree relatives, well matched on demographic characteristics to comparison groups, are necessary to test this corollary.
While consistent with mean amygdala volumes from multiple samples, this model will not likely apply to all individuals with autism spectrum disorders. The study13 that reported elevated electrodermal activity in most children with autism (70%) also described a subset of individuals with near-absent responses (11%). Similarly, a conjoint functional magnetic resonance imaging and eye-tracking study8 detected heightened amygdala responses to faces in only 78% of individuals with autism. There are likely a few individuals with autism who are simply oblivious to social information, akin to some amygdala lesion results.
Most important, amygdala volume does not determine all autistic behavior. In this study, amygdala volume did not correlate with verbal or repetitive behaviors and predicted, at most, 53% of the variance in nonverbal social impairment. Abnormalities of hippocampus, cerebellum, superior temporal cortex, and prefrontal cortex and the white matter connecting these regions are all likely involved in autism.45,46
Great caution must be used when inferring developmental patterns from cross-sectional studies: only longitudinal studies could validate a model of amygdala development in autism. An allostatic load model does, however, have implications for cross-sectional studies that distinguish it from a model of amygdala hypofunction. Young children with immature hypoactive amygdalae, particularly those who engage social stimuli least, would likely show little overgrowth; under a model of hyperactivity, young children with the most severe behavioral impairments would show the greatest overgrowth.
While postmortem findings of small neurons were originally described as immature looking,47 such changes could arise from excitotoxicity, which in severe cases might produce cell loss and gliosis, as in the hippocampus in models of chronic stress and epilepsy.36,48 In support of this, preliminary data using stereologic techniques indicate a decreased cell number in the amygdalae of adults with autism.49 In contrast, a hypoactive amygdala may exhibit atrophic neurons but is unlikely to experience cell loss. Quantitative postmortem studies of the amygdala in adults with autism, including assessment of cell number, dendritic arborization, and astrocyte markers, might better elucidate the cellular changes that underlie differences in amygdala volume.
Our study was limited to males aged 8 years to early adulthood and, therefore, does not address relationships between amygdala volume and autistic behavior in younger children or in females. Although more categorical, Autism Diagnostic Observation Schedule50 data might also be used in the future to complement our measurements of face-processing impairment. These limitations and the current findings underscore the need for longitudinal studies of large samples characterized on both range and severity of autistic behaviors to better elucidate the neuropathological features of autism.
Acknowledgment
We thank all the participants and families who volunteered their time for this study; William Irwin, MS, for his invaluable critique of the volumetric technique; James Keidel, BS, for constructive comments on the manuscript; and Michael Anderle, BS, and Ron Fisher, BS, for their patience and technical expertise in magnetic resonance imaging collection.
Funding/Support: This study was supported by Studies to Advance Autism Research and Treatment grant U54MH066398 Project IV from the National Institute of Mental Health, Bethesda, Md (Dr Davidson); a Distinguished Investigator Award from the National Alliance for Research on Schizophrenia and Affective Disorders, Great Neck, NY (Dr Davidson); core grant P30 HD03352 from the National Institutes of Health, Bethesda; and training grant T32 HD07489 from the National Institutes of Child Health and Human Development, Bethesda.
Footnotes
Financial Disclosure: None reported.
Previous Presentations: Portions of study 1 were presented at the 33rd annual meeting of the Society for Neuroscience; Nov 9, 2003; New Orleans, La; and portions of study 2 were presented at the Fourth International Meeting for Autism Research; May 5, 2005; Boston, Mass.
REFERENCES
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 2.World Health Organization . International Statistical Classification of Diseases and Related Health Problems, 1989 Revision. World Health Organization; Geneva, Switzerland: 1992. [Google Scholar]
- 3.Muhle R, Trentacoste SV, Rapin I. [Accessed February 15, 2006];The genetics of autism. Pediatrics. 2004 113:e472–e486. doi: 10.1542/peds.113.5.e472. http://pediatrics.aappublications.org/cgi/content/full/113/5/e472. [DOI] [PubMed] [Google Scholar]
- 4.Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- 5.Baron-Cohen S, Ring HA, Bullmore ET, Ashwin S, Wheelwright C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 6.Pierce K, Müller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform “face area” in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 7.Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 8.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- 10.Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 11.Shaw P, Lawrence EJ, Radbourne C, Bramham J, Polkey CE, David AS. The impact of early and late damage to the human amygdala on “theory of mind” reasoning. Brain. 2004;127:1535–1548. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- 12.Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 13.Hirstein W, Iversen P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Proc Biol Sci. 2001;268:1883–1888. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard MA, Cowell PE, Boucher J, Broks P, Mayes A, Farrant A, Roberts N. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11:2931–2935. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- 15.Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 16.Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Bartra PE, Pearlson GD. MRI volumes of amygdala and hippocampus in non–mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 17.Rojas DC, Smith JA, Benkers TL, Camou S, Reite ML, Rogers SJ. Hippocampus and amygdala volumes in parents of children with autistic disorder. Am J Psychiatry. 2004;161:2038–2044. doi: 10.1176/appi.ajp.161.11.2038. [DOI] [PubMed] [Google Scholar]
- 18.Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, Hollander E. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- 19.Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism: the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seltzer MM, Krauss MW, Shattuck PT, Orsmond G, Swe A, Lord C. The symptoms of autism spectrum disorders in adolescence and adulthood. J Autism Dev Disord. 2003;33:565–581. doi: 10.1023/b:jadd.0000005995.02453.0b. [DOI] [PubMed] [Google Scholar]
- 21.Hobson RP, Ouston J, Lee A. Emotion recognition in autism: coordinating faces and voices. Psychol Med. 1988;18:911–923. doi: 10.1017/s0033291700009843. [DOI] [PubMed] [Google Scholar]
- 22.Tantam D, Monaghan L, Nicholson H, Stirling J. Autistic children’s ability to interpret faces: a research note. J Child Psychol Psychiatry. 1989;30:623–630. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 23.Joseph RM, Tanaka J. Holistic and part-based face recognition in children with autism. J Child Psychol Psychiatry. 2003;44:529–542. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- 24.Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- 25.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 26.Lord C, Rutter M, Couteur AL. Autism Diagnostic Interview–Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 27.Glutting J, Adams W, Sheslow D. Wide Range Intelligence Test. Psychological Assessment Resources, Inc; Wilmington, Del: 2000. [Google Scholar]
- 28.Convit A, McHugh P, Wolf OT, de Leon MJ, Bobinski M, De Santi S, Roche A, Tsui W. MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Res. 1999;90:113–123. doi: 10.1016/s0925-4927(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 29.Fix JD. Atlas of the Human Brain and Spinal Cord. Aspen Publishers; Rockville, Md: 1987. [Google Scholar]
- 30.Daniels DL, Haughton VM, Naidich TP. Cranial and Spinal Magnetic Resonance Imaging: An Atlas and Guide. Raven Press; New York, NY: 1987. [Google Scholar]
- 31.Jennes L. Atlas of the Human Brain. Lippincott; Philadelphia, Pa: 1995. [Google Scholar]
- 32.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. Academic Press; San Diego, Calif: 1997. [Google Scholar]
- 33.Duvernoy HM, Bourgouin P. The Human Brain: Surface, Three-Dimensional Sectional Anatomy and MRI. Springer-Verlag NY Inc; New York, NY: 1999. [Google Scholar]
- 34.Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 35.Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 36.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 37.Vyas A, Mitra R, Shankaranarayana Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, Bottlender R, Leinsinger G, Moller HJ. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 39.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 40.Siegle GJ, Konecky RO, Thase ME, Carter C. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals: an fMRI investigation. Ann N Y Acad Sci. 2003;985:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 41.Bolte S, Poustka F. The recognition of facial affect in autistic and schizophrenic subjects and their first-degree relatives. Psychol Med. 2003;33:907–915. doi: 10.1017/s0033291703007438. [DOI] [PubMed] [Google Scholar]
- 42.Bishop DV, Maybery M, Maley A, Wong D, Hill W, Hallmayer J. Using self-report to identify the broad phenotype in parents of children with autistic spectrum disorders: a study using the Autism-Spectrum Quotient. J Child Psychol Psychiatry. 2004;45:1431–1436. doi: 10.1111/j.1469-7610.2004.00849.x. [DOI] [PubMed] [Google Scholar]
- 43.Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, Singh D, Todd RD. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163:294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- 44.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 45.Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, Barale F. Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull. 2003;61:557–569. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Barnea-Goraly N, Kwon H, Menon V, Lotspeich S, Eliez L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 47.Kemper TL, Bauman ML. The contribution of neuropathologic studies to the understanding of autism. Neurol Clin. 1993;11:175–187. [PubMed] [Google Scholar]
- 48.Oprica M, Eriksson C, Schultzberg M. Inflammatory mechanisms associated with brain damage induced by kainic acid with special reference to the interleukin-1 system. J Cell Mol Med. 2003;7:127–140. doi: 10.1111/j.1582-4934.2003.tb00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism Diagnostic Observation Schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]