Abstract
Context
Air pollution exposure affects autonomic function, heart rate, blood pressure and left ventricular function. While the mechanism for these effects is uncertain, several studies have reported that air pollution exposure modifies activity of the carotid body, the major organ that senses changes in arterial oxygen and carbon dioxide levels, and elicits downstream changes in autonomic control and cardiac function.
Objective
We hypothesized that exposure to acrolein, an unsaturated aldehyde and mucosal irritant found in cigarette smoke and diesel exhaust, would activate the carotid body chemoreceptor response and lead to secondary cardiovascular responses in rats.
Materials and methods
Spontaneously hypertensive (SH) rats were exposed once for 3 h to 3 ppm acrolein gas or filtered air in whole body plethysmograph chambers. To determine if the carotid body mediated acrolein-induced cardiovascular responses, rats were pretreated with an inhibitor of cystathionine γ-lyase (CSE), an enzyme essential for carotid body signal transduction.
Results
Acrolein exposure induced several cardiovascular effects. Systolic, diastolic and mean arterial blood pressure increased during exposure, while cardiac contractility decreased 1 day after exposure. The cardiovascular effects were associated with decreases in pO2, breathing frequency and expiratory time, and increases in sympathetic tone during exposure followed by parasympathetic dominance after exposure. The CSE inhibitor prevented the cardiovascular effects of acrolein exposure.
Discussion and conclusion
Pretreatment with the CSE inhibitor prevented the cardiovascular effects of acrolein, suggesting that the cardiovascular responses with acrolein may be mediated by carotid body-triggered changes in autonomic tone. (This abstract does not reflect EPA policy.)
Keywords: Acrolein, blood pressure, carotid body, pO2, spontaneously hypertensive rat
Introduction
Multiple lines of evidence demonstrate that exposure to air pollution, including cigarette smoke, increases cardiovascular morbidity and mortality (Brook et al., 2010). Several mechanisms have been postulated that overlap among gaseous and particulate pollutants including vascular oxidative stress, endothelial dysfunction, inflammation and altered autonomic tone (Brook et al., 2010; Srebot et al., 2009). Changes in cardiac autonomic tone, when present, are often the most immediate consequences of air pollution exposure (Brook et al., 2010). We have previously shown that one of the more prominent autonomic reflex arcs involves pulmonary C fibers and is triggered by activation of the transient receptor potential channel A (TRPA; Hazari et al., 2011). Recent evidence suggests that some of the cardiovascular effects of air pollution may be mediated by a relatively unexplored neural reflex involving the carotid body, a major sensory organ located at the bifurcation of the carotid artery that initiates reflex responses to changes in pCO2, pO2, pH and temperature. Exposure to acrolein, an unsaturated aldehyde and mucosal irritant found in cigarette smoke and diesel exhaust, reduces arterial pO2, suggesting that downstream effects of air pollutants may be controlled by carotid chemoreceptor responses (Janssens et al., 1994). Several epidemiological studies have reported small changes in oxygen saturation in human subjects with exposure to air pollution (DeMeo et al., 2004; Gong et al., 2004; Pope et al., 1999). Furthermore, human exposures to ambient levels of the air pollutants tobacco smoke (Adgent, 2006), SO2 and NO2 (Hoppenbrouwers et al., 1981) were linked to abnormal cardiopulmonary responses to hypoxia, indicating modified carotid activity. Despite such associations, a direct link between the carotid body and air pollution-induced adverse cardiovascular effects has not been established.
Activation of the carotid body triggers changes in autonomic tone that cause reflex cardiopulmonary changes to maintain homeostasis. In response to hypercapnia/hypoxia, for example, the carotid body triggers increases in sympathetic tone that in turn cause elevations in blood pressure (BP), ventilation and heart rate (HR) (Liu et al., 2003; Lopez-Barneo et al., 2008; Roux et al., 2000). Such shifts in autonomic tone may, however, be detrimental. For example, low heart rate variability (HRV), indicating increased sympathetic tone, is associated with elevated cardiovascular risk, including increased mortality rate in people with heart disease (Fauchier et al., 2004). Low HRV has also been reported with exposure to PM and ozone (Brook et al., 2010). Parallels between carotid body-mediated effects and cardiovascular responses associated with air pollution exposure suggest a linkage is plausible.
Recently, we found that exposure to 3 ppm acrolein causes apnea (Hazari et al., 2008), increases BP and modifies responses to a hypoxia stress test in rats (Perez et al., 2013). In addition, Wang et al. (2012) demonstrated that cardiac arrhythmias occurring in heart failure mice after intratracheal instillation with 20 mg/kg ambient particulates were associated with altered carotid body sensitivity. These findings suggest that the carotid body might mediate cardiac and vascular effects of air pollution exposure. The purpose of this study was to determine if the cardiovascular effects of acrolein inhalation were mediated by carotid body activation. We hypothesized that: (1) exposure to acrolein will alter arterial blood gas levels in spontaneously hypertensive rats, a strain with exaggerated sensitivity to acrolein (Perez et al., 2013) and (2) inhibition of carotid body sensing will prevent the cardiovascular responses to acrolein exposure. Carotid body sensing was blocked by pretreatment with an inhibitor of the H2S-generating enzyme cystathionine γ-lyase (CSE), which is essential for carotid body signal transduction (Peng et al., 2010). Blood gases, respiratory parameters, HR, blood pressure and HRV were monitored before, during and after exposure. In addition, left ventricular pressure, cardiac contractility and lusitropy were assessed before and after vagotomy 1 day after acrolein exposure.
Materials and methods
Drugs
The cystathionine γ-lyase enzyme (CSE) inhibitor DL-propargylglycine (PAG) was obtained from Sigma (St. Louis, MO; 200 mg/kg) and was administered intraperitoneally (i.p.) immediately before exposures. Dose and drug administration were determined in previous work by Peng et al. (2010). All solutions with drugs were prepared fresh before every experiment. Rats not treated with DL-propargylglycine (PAG) were treated with an equivalent volume of saline administered intraperitoneally immediately before exposures.
Animals
Twelve-week-old male spontaneously hypertensive (SH) and Wistar Kyoto (WKY) rats with normal blood pressure (Charles River, Raleigh, NC) were housed in plastic cages (one per cage), maintained on a 12-h light/dark cycle at approximately 22 °C and 50% relative humidity in our AAALAC approved facility, and held for a minimum of 1 week before exposure. Food (Prolab RMH 3000; PMI Nutrition International, St. Louis, MO) and water were provided ad libitum, and all rats were randomized by weight. SH rats were divided into one of three cohorts: (1) rats implanted with femoral artery catheters to enable serial arterial blood draws to monitor blood gases and analytes before, during and after acrolein exposure in conscious, unrestrained rats, (2) rats implanted with radio-telemeters and studied in whole body plethysmographs to monitor blood pressure, HR and ventilation parameters before, during and after acrolein exposure and (3) rats that underwent left ventricular pressure assessments 1 day after acrolein exposure before and after vagotomy. A cohort of WKY rats was also implanted with femoral artery catheters to assess the impact of acrolein exposure on arterial blood gases. WKY rats were not included in additional assessments due to the absence of cardiovascular responses during acrolein exposure in previous studies (Perez et al., 2013). After all experiments were performed, rats were deeply anesthetized with an intraperitoneal injection of Euthasol (200 mg/kg Na pentobarbital and 25 mg/kg phenytoin; Virbac Animal Health, Fort Worth, TX) and euthanized by exsanguination. The IACUC of the U.S. Environmental Protection Agency (US EPA) approved all protocols.
Telemeter and catheter implantation
SH rats were implanted with radiotelemetry transmitters to monitor HR, blood pressure (BP) and heart rhythm (Model TL11M2-C50-PXT; Data Science International, Inc., St. Paul, MN) as previously described (Perez et al., 2013). Animals were allowed approximately 2 weeks to recover prior to exposures. A separate cohort of SH (n = 20) and WKY (n = 5) rats were implanted with femoral artery catheters in accordance with methods specified by the vendor (Charles River Laboratories, 2005). Animals were shipped to the US EPA within 1 week of surgery. The catheters were flushed with saline, locked with heparin and plugged immediately upon arrival and every 2 days thereafter until exposure began.
Acrolein exposure
Rats implanted with telemeters were acclimated to exposure chambers daily for 1 h beginning 2 days before exposure. All rats were assigned to one of four treatments groups (n = 6/group): air exposure with saline pretreatment; acrolein exposure with saline pretreatment; air exposure with PAG pretreatment; and acrolein exposure with PAG pretreatment. On the exposure day, rats were pretreated via intraperitoneal injection of saline or PAG and then allowed to acclimate to the chambers for 30 min. Baseline data were recorded for the next 30 min. All exposures took place in whole body plethysmography chambers (WBP; Model PLY3213, Buxco Electronics, Inc, Wilmington, NC), which continuously and non-invasively monitor ventilatory parameters in conscious animals. SH rats were exposed to filtered air or 3 ppm acrolein for 3 h. Acrolein gas was metered from a 1000-ppm cylinder into a glass mixing chamber where the gas was mixed with dry filtered dilution air to achieve a final concentration of 3 ppm of acrolein with a total flow of 6 L/min. The actual chamber concentration was measured by injection of a batch sample every 10 min into an HP5890 gas chromatograph (GMI Inc., Ramsey, MN) equipped with manual injection, a flame ionization detector and a DB-VRX capillary column. All control rats were exposed to dry filtered dilution air only. The plethysmograph pressure was monitored using Biosystems XA software (Buxco Electronics, Inc, Wilmington, NC). Using respiratory-induced fluctuations in ambient pressure, respiratory parameters including tidal volume, breathing frequency, inspiratory time and expiratory time were calculated and recorded on a breath-by-breath basis and averaged over 10 s intervals. HR, systolic and diastolic BP, ECG waveforms and ventilatory data were collected during the exposure, and animals were returned to their home cages after exposure. The concentration of acrolein (3 ppm) used in this study is representative of concentrations in high-combustion areas (Hazari et al., 2008). Acrolein concentrations ranging from 10 to 140 μg/cigarette have been found in mainstream smoke and concentrations ranging from 100 to 1700 μg/cigarette have been found in side-stream smoke (Jones, 1999). In addition, Sakaguchi et al. (2014) found acrolein levels of 47.10 μg/cigarette after urine analysis of Japanese males who smoke conventional cigarettes.
Arterial blood gas analysis during acrolein exposure
Changes in blood gas parameters during acrolein exposure were determined in a separate cohort of rats. Rats implanted with femoral artery catheters were acclimated to whole body plethysmography chambers (WBP; Model PLY3213, Buxco Electronics, Inc, Wilmington, NC), 1 h on the 2 days prior to exposure. On the day of exposure, the rats received an intraperitoneal injection of either saline or PAG. The exteriorized jugular vein catheter was extended with a 1-mL PE50 catheter attached to a 23-gauge adapter traversing a hole in the wall of the plethysmograph and allowing blood draws outside of the sealed exposure chamber. Rats were acclimated to the exposure chambers for 30 min and then were exposed to either filtered air or acrolein. For all exposure groups, a baseline 250-μL blood sample was taken after acclimation followed by serial 250 μL blood draws at 30 min after baseline, 1 h after baseline and 1 h 15 min after baseline. For each blood draw, the pin was removed from the tip of the catheter, and the heparin was allowed to exit the catheter. When only blood remained, a 23-gauge adapter attached to a 1-mL syringe was placed on the end of the catheter and used to draw a 250-μL sample. The blood sample was immediately read on an OPTI CCA-TS Blood Analyzer (OPTI Medical Systems, Inc., Roswell, GA) using the OPTI Cassette E-Ca which measures blood pH, PCO2, PO2, Na+, K+, Ca2+, total hemoglobin (tHb), oxygen saturation (SaO2) and hematocrit (Hct). The catheter was flushed with saline and locked with heparin after each blood draw.
Ventilatory monitoring during exposure
All exposures were performed in whole-body plethysmography chambers (Buxco Electronics, Sharon, CT). The plethysmography methodology permitted continuous monitoring of breathing frequency, tidal volume, minute volume, inspiratory time and expiratory time as described in Perez et al. (2013).
Cardiovascular monitoring during exposure
Radiotelemetry methodology (Data Sciences International, Inc., St. Paul, MN) enabled constant monitoring of ECG data in unrestrained, un-anesthetized rats from implantation until euthanasia as described in Perez et al. (2013).
Heart rate variability analysis
ECGAuto software (EMKA Technologies, Falls Church, VA) was used for automated analysis of heart rate variability (HRV). Arrhythmias, artifacts and ECG waveforms lacking distinguishable R waves for more than 30 s were identified and excluded. The analysis of HRV generated HR and time-domain measures, including mean time between adjacent QRS complex peaks (the RR interval), a standard deviation of the RR interval (SDNN), SDNN normalized for the effects of HR [SDNN/(RR interval × 100)] and the square root of the mean of squared differences of adjacent RR intervals (RMSSD). The SDNN represents overall HRV, whereas RMSSD represents parasympathetic influence over HR. The analysis of HRV also calculated frequency-domain parameters, particularly low-frequency (LF) and high-frequency (HF), and the ratio of these two frequency-domains (LF/HF). LF is generally believed to represent a mixture of sympathetic and parasympathetic tone, whereas HF indicates cardiac parasympathetic (vagal) tone, and LF/HF serves as an index of sympathovagal balance.
Left ventricular pressure 24 hours after exposure
A third cohort of rats exposed to filtered air or acrolein with or without PAG pre-treatment underwent left ventricular pressure (LVP) assessments to measure cardiac function 24 h after exposure. Rats were anesthetized with urethane (1.5 mg/kg i.p., Sigma) and prepared for LVP measurement by right carotid arterial catheterization with a 2-French transducer (SPR-320, Millar Instruments, Houston, TX). The LV probe was connected via a Pressure Control Unit (Model 2000, Millar Instruments) to a receiver (Powerlab 4/30, ADInstruments, Dunedin, New Zealand) and a computer acquiring data at 1000 Hz. The probe was advanced into the LV for a 5-min baseline. To assess the role of parasympathetic tone in the response to acrolein, rats that were pretreated with saline alone underwent bilateral vagotomy by ligating both vagus nerves. Acquisition software (LabChart Pro version 7.3.2, AD Instruments) generated pressure at end diastole and end systole (EDP and ESP) and the maximum and minimum pressure slopes (dP/dtmax and dP/dtmin, respectively) per beat, indicative of contractility and relaxation rate (lusitropy), respectively.
Statistics
For the statistical analyses of the Buxco, ECG and HRV data in this study, we used SAS version 9.2 software (SAS Institute Inc, Cary, NC). PROC MIXED of SAS was used because of its greater flexibility for the modeling of repeated measures data when compared to PROC GLM. It is also suitable for analysis of large, unbalanced data with missing data at random. A linear mixed model with restricted maximum-likelihood estimation analysis, least squares means and repeated measures ANOVA was used to determine which TIME × TRT interactions were statistically significant between baseline and exposure. Multiple comparison adjustment for the p values and confidence limits for the differences between the least squares means was done using adjust = Tukey HSD (honest significant difference) test. All blood gas data were analyzed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) with a one-way analysis of variance (ANOVA) model examining the main effects of each model.
Results
Effects of acrolein on arterial blood gases
For all acrolein exposures, the average chamber concentration of acrolein was 2.9 ± 0.1 ppm.
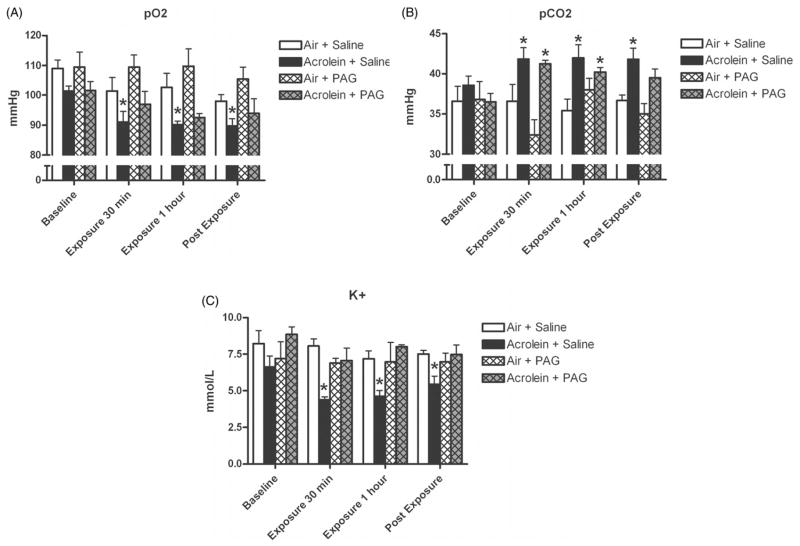
In rats exposed to acrolein and pretreated with saline, pO2 decreased from baseline levels of 101.4 ± 1.7 to 91.0 ± 3.6 mmHg 30 min into exposure, to 90.2 ± 1.2 mmHg 1 h into exposure, and to 89.8 mmHg 15 min after exposure ended (p<0.05; Figure 1A). pCO2 increased from baseline levels of 38.6 ± 1.2 to 41.9 ± 1.4 mmHg 30 min into exposure, to 42.0 ± 1.6 mmHg 1 h into exposure, and remained high at 41.8 ± 1.4 mmHg 15 min after exposure ended (p<0.05; Figure 1B). Rats exposed to acrolein and pretreated with saline had significant decreases in K+ during and after exposure. Baseline K+ levels of 6.6 ± 0.7 mmol/L decreased to 4.4 ± 0.2 30 min into exposure, to 4.6 ± 0.4 1 h into exposure, and increased slightly to 5.5 ± 0.5 mmol/L after exposure ended (p<0.05; Figure 1C). There were no effects of PAG pretreatment on acrolein-induced changes in pO2, pCO2, or K+.
Figure 1.
Acrolein exposure causes significant decreases in arterial blood oxygen and potassium, and significant increases in arterial carbon dioxide. SH rats implanted with femoral artery catheters were injected with saline or PAG carotid body inhibitor, and exposed to 3 ppm acrolein or air control. Panels A, B and C refer to pO2, pCO2 and K+, respectively, measured at baseline, 30 min into acrolein exposure, 1 h into acrolein exposure and 15 min post-exposure. Means and standard errors are reported. Significant differences from baseline values (p<0.05) are denoted with an asterisk.
SaO2, Na+ and pH were not affected by acrolein exposure or PAG pretreatment (data not shown). WKY rats exposed to air or acrolein had no changes in any blood parameters measured (Table 1).
Table 1.
Arterial blood parameters during acrolein exposure in WKY rats.
| Exposure | pO2 (mmHg) | pCO2 (mmHg) | SaO2 (%) | pH | K+ (mmol/L) | Na+ (mmol/L) |
|---|---|---|---|---|---|---|
| Baseline | 109.0 ± 2.7 | 36.6 ± 1.9 | 94.8 ± 0.7 | 7.3 ± 0.1 | 7.7 ± 1.1 | 149.8 ± 3.0 |
| 30 min | 101.4 ± 4.5 | 36.7 ± 2.1 | 94.4 ± 0.2 | 7.3 ± 0.1 | 7.4 ± 0.8 | 147.2 ± 2.0 |
| 1 h | 102.6 ± 4.7 | 35.4 ± 1.5 | 93.4 ± 0.7 | 7.3 ± 0.1 | 6.7 ± 0.7 | 152.2 ± 1.5 |
| Post-exposure | 98.0 ± 2.2 | 36.7 ± 0.7 | 93.4 ± 0.4 | 7.4 ± 0.1 | 5.7 ± 0.5 | 143.6 ± 0.5 |
Means and standard errors are reported.
Effects of acrolein on ventilatory parameters
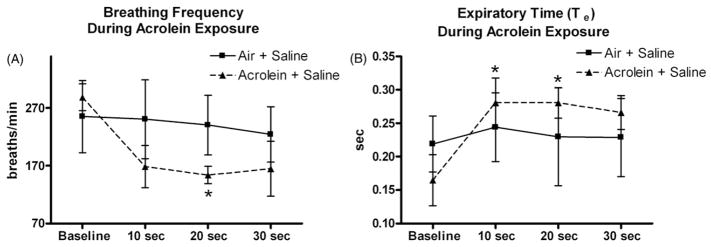
Breathing frequency decreased in rats exposed to acrolein in the first 30 s of exposure. Breathing frequency decreased from 288 ± 23 breaths/min at baseline to 153 ± 15 breaths/min 20 s into acrolein exposure (p<0.05; Figure 2A). In addition, acrolein exposed rats had significant increases in expiratory time (Te), a measure of apnea, within the first 20 s of exposure. Te increased from baseline levels of 0.2 ± 0.03 s to 0.3 ± 0.03 s at 10 s into acrolein exposure and 0.3 ± 0.02 s 20 s into acrolein exposure (p<0.05; Figure 2B). Te returned to normal levels after the first 20 s of exposure. Acrolein exposure caused a decrease in minute ventilation, although it was not statistically significant. PAG treatment in control and acrolein exposed rats had no effect on this parameter (data not shown). There were no significant changes in minute volume, tidal volume or inspiratory time in acrolein exposed rats treated with saline or PAG (data not shown).
Figure 2.
Acrolein exposure causes transient apnea immediately after the beginning of exposure. Panels A and B refer to breathing frequency and expiratory time during the first 30 s of acrolein exposure, respectively. Means and standard errors are reported. Significant differences from baseline values (p<0.05) are denoted with an asterisk.
Effects of acrolein on cardiovascular responses
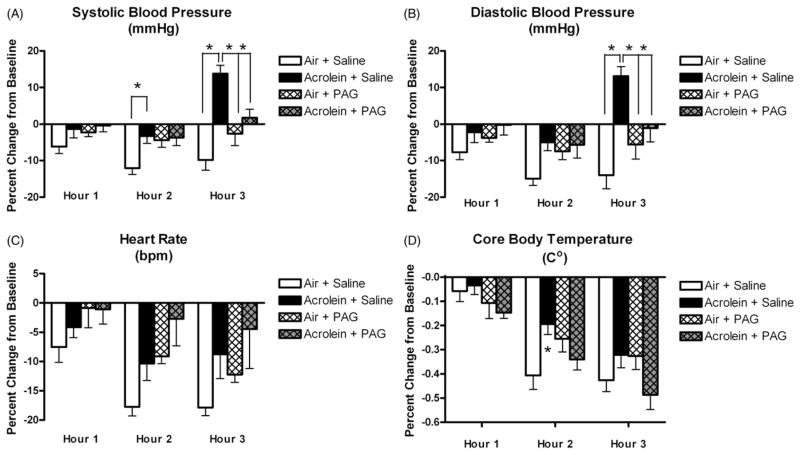
Control rats exposed to air and pretreated with saline had gradual decreases in systolic blood pressure, diastolic blood pressure, HR and temperature during exposure (Figure 3), a common and expected response as the animals acclimate to the chamber. Rats were acclimated to exposure chambers for 1 h once per day beginning 2 days before exposure. On exposure day, rats were allowed to acclimate for 30 min before the 30-min baseline was recorded. Even with multiple acclimation periods, it is normal for blood pressure and HR to continue to decrease even 3 h after the beginning of exposure in control rats exposed to filtered air.
Figure 3.
Carotid body inhibition attenuates acrolein-induced increases in blood pressure and core body temperature. Panels A, B, C and D refer to systolic blood pressure, diastolic blood pressure, heart rate and core body temperature, respectively, at each hour of exposure. Means and standard errors are reported. Significant differences between exposure groups (p<0.05) are denoted with an asterisk.
At hour 2, rats exposed to acrolein and pretreated with saline had a smaller decrease in systolic blood pressure than corresponding air-exposed and saline pretreated rats (3.3 ± 2.0% versus 12.0 ± 2.0% decrease from respective baselines; p<0.05; Figure 3A). Pretreatment with PAG prevented the systolic blood pressure response to acrolein during hour 2 of exposure. At hour 3, rats exposed to acrolein and pretreated with saline had a 14.0 ± 2.2% increase from baseline compared to rats exposed to air and pretreated with saline that had a 10.0 ± 3.0% decrease from baseline (p<0.05). The systolic blood pressure response to acrolein during exposure hour 3 was also blocked with pretreatment with PAG.
At hour 3, diastolic blood pressure in rats exposed to acrolein and pretreated with saline had a 13.1 ± 3.0% increase from baseline compared to rats exposed to air and pretreated with saline who had a 14.0 ± 4.0% decrease from baseline (p<0.05; Figure 3B). The diastolic blood pressure response to acrolein during exposure hour 3 was blocked with pretreatment with PAG.
At hour 2, rats exposed to acrolein and pretreated with saline had a smaller decrease in mean arterial blood pressure (MAP) than corresponding air-exposed and saline pretreated rats (4.0 ± 1.3% versus 13.1 ± 2.0% decrease from respective baselines; p<0.05; data not shown). The MAP response to acrolein during exposure hour 2 was blocked with pretreatment with PAG. At hour 3, rats exposed to acrolein and pretreated with saline had a 13.4 ± 2.5% increase from baseline compared to rats exposed to air and pretreated with saline that had an 11.3 ± 3.4% decrease from baseline (p<0.05; data not shown). The MAP response to acrolein during exposure hour 3 was also blocked with pretreatment with PAG. Raw values for mean arterial, systolic and diastolic blood pressure are reported in Table 2.
Table 2.
Raw values for mean arterial, systolic and diastolic blood pressure in SH rats.
| Air + Saline | Acrolein + Saline | Air + PAG | Acrolein + PAG | |
|---|---|---|---|---|
| Mean arterial blood pressure (mmHg) | ||||
| Baseline | 159.2 ± 5.7 | 151.9 ± 3.4 | 164.0 ± 5.4 | 156.7 ± 7.6 |
| Exposure hour 1 | 148.5 ± 7.9 | 148.8 ± 3.0 | 159.6 ± 4.2 | 156.4 ± 5.9 |
| Exposure hour 2 | 138.0 ± 4.3 | 145.7 ± 2.5 | 155.0 ± 5.1 | 150.6 ± 8.6 |
| Exposure hour 3 | 140.4 ± 2.3 | 171.9 ± 1.5 | 157.6 ± 5.2 | 158.4 ± 9.0 |
| Systolic blood pressure (mmHg) | ||||
| Baseline | 187.5 ± 5.6 | 179.1 ± 3.6 | 193.3 ± 6.0 | 188.4 ± 7.7 |
| Exposure hour 1 | 176.2 ± 8.3 | 176.5 ± 3.0 | 188.8 ± 4.4 | 187.3 ± 5.6 |
| Exposure hour 2 | 164.8 ± 5.0 | 173.0 ± 2.0 | 184.6 ± 5.5 | 181.4 ± 8.0 |
| Exposure hour 3 | 168.5 ± 2.2 | 203.6 ± 1.9 | 187.9 ± 5.8 | 191.7 ± 8.9 |
| Diastolic blood pressure (mmHg) | ||||
| Baseline | 132.2 ± 5.0 | 125.2 ± 2.8 | 135.5 ± 5.0 | 127.2 ± 7.4 |
| Exposure hour 1 | 122.4 ± 6.8 | 122.3 ± 3.2 | 130.3 ± 3.9 | 126.3 ± 5.6 |
| Exposure hour 2 | 112.2 ± 3.1 | 118.9 ± 3.1 | 125.4 ± 4.8 | 120.3 ± 8.8 |
| Exposure hour 3 | 113.1 ± 2.4 | 141.4 ± 2.1 | 127.6 ± 4.6 | 126.0 ± 8.9 |
Means and standard errors are reported.
HR did not change during exposure regardless of exposure group or PAG pretreatment (Figure 3C).
Core body temperature (Tco) decreased from baseline levels of 38.5 ± 0.1 °C to 37.6 ± 0.2 °C at hour 2 (Figure 3D) in rats exposed to acrolein and pretreated with saline compared to rats exposed to and pretreated with saline that had decreases from 38.6 ± 0.2 °C at baseline to 37.3 ± 0.2 °C (p<0.05). The Tco response to acrolein during exposure hour 2 was also blocked with pretreatment with PAG.
PAG treatment alone did not affect MAP, systolic blood pressure, diastolic blood pressure, HR or Tco.
Effects of acrolein on HR variability
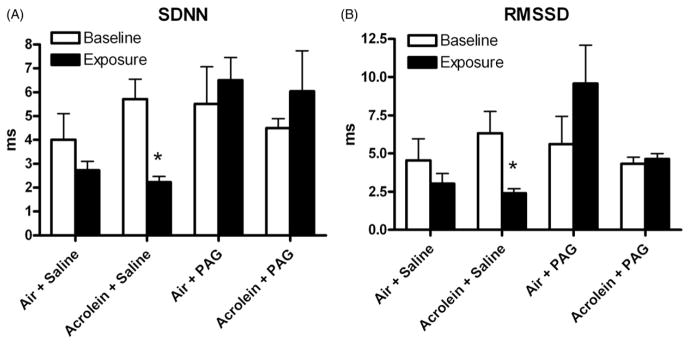
Rats exposed to acrolein and pretreated with saline had a significant decrease in the SDNN component of HRV during exposure (averaged over the entire exposure period; Figure 4A). SDNN decreased from baseline values of 5.7 ± 0.8 ms to 2.2 ± 0.2 ms during exposure (p<0.05; Figure 4A). Pretreatment with PAG prevented this acrolein-induced decrease in SDNN during exposure.
Figure 4.
Carotid body inhibition attenuates acrolein-induced increases in SDNN and RMSSD. Panels A and B refer to averaged baseline and exposure values for SDNN and RMSSD, respectively, for all groups. Means and standard errors are reported. Significant differences between baseline and exposure (p<0.05) are denoted with an asterisk.
Rats exposed to acrolein and pretreated with saline had a significant decrease in the RMSSD component of HRV during exposure (averaged over the entire exposure period; Figure 4B). RMSSD decreased from baseline values of 6.3 ± 1.4 ms to 2.4 ± 0.3 ms during exposure (p<0.05). Pretreatment with PAG prevented this acrolein-induced decrease in RMSSD during exposure.
Rats exposed to acrolein and pretreated with saline had a significant increase in the LF component of HRV during exposure (averaged over the entire exposure period; data not shown). LF increased from baseline values of 0.4 ± 0.1 ms to 1.0 ± 0.1 ms during exposure (p<0.05). Pretreatment with PAG prevented this acrolein-induced increase in LF during exposure. There were no significant changes in the HF or LF/HF components of HRV during acrolein exposure (data not shown).
Effects of acrolein on left ventricular pressure
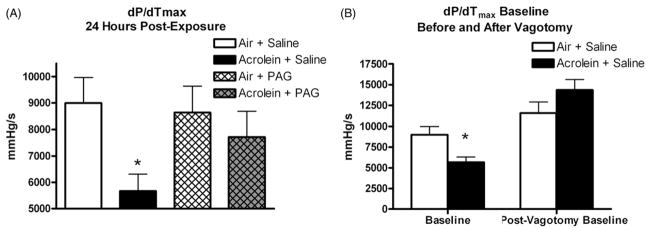
Rats exposed to acrolein and pretreated with saline had a significant decrease in dP/dtmax, a measure of cardiac contractility, 24 h after exposure compared to the control air exposure pretreated with saline. Rats exposed to acrolein and pretreated with saline had dP/dtmax levels of 5,672.6 ± 630.6 mmHg/s compared to rats exposed to air and pretreated with saline (8992.4 ± 971.4 mmHg/s) (p<0.05; Figure 5A). This decrease was prevented with PAG pretreatment (Figure 5A). The acrolein-induced decrease in dP/dtmax was also reversed with bilateral vagotomy (Figure 5B).
Figure 5.
Carotid body inhibition reverses acrolein-induced decreases in dP/dtmax, a measure of contractility, 24 h post-acrolein exposure. Bilateral vagotomy reverses the acrolein-induced decrease in dP/dt. Panel A refers to baseline values of dP/dtmax 24 h post-exposure. Panel B refers to pre- and post-vagotomy values of dP/dtmax 24 h post-exposure. Means and standard errors are reported. Significant differences between groups (p<0.05) are denoted with an asterisk.
Discussion and conclusions
The current study demonstrates four key findings: (1) acrolein exposure-induced apnea is associated with decreases in arterial pO2 and increases in pCO2, (2) arterial blood gas changes were evident in acrolein-exposed SH rats, but not in normotensive WKY rats, (3) acrolein-induced cardiovascular responses were associated with an autonomic pattern characterized by an early increase in sympathetic tone followed by parasympathetic dominance and (4) acrolein-induced cardiovascular responses that included changes in blood pressure, core body temperature, HR variability and cardiac contractility were prevented by pharmacologic blockade of the carotid body, the hypoxia- and hypercapnia-sensing organ.
Acrolein exposure decreased pO2 in hypertensive rats, consistent with the apneic response observed. Acrolein, like other environmental pollutants, stimulates airway irritant sensory nerves (Lee et al., 1992; Oortgiesen et al., 2000), especially chemosensitive bronchopulmonary C-fibers, in part by activation of transient receptor potential vanilloid 1 cation channels (TRPV1) present on the peripheral terminals of these fibers (Hazari et al., 2008). Upon activation of these fibers, a chemoreflex is elicited which is characterized by apnea and bronchospasm (Coleridge & Coleridge, 1994), which in part may explain the responses observed. We found decreases in breathing frequency in SH rats immediately after the start of acrolein as well as increases in expiratory time, a marker of apnea, consistent with our previous findings (Hazari et al., 2008). While this may explain the initial drop in pO2, the apneic response was transient, lasting only 30 s. Thus, apnea alone likely does not explain the decrease in pO2 that was detected up to an hour into exposure and that persisted for 30 min after a 1-h exposure to acrolein. Although we did not assess pulmonary histopathology or measure proinflamatory biomarkers, inflammation and tissue edema that are common at higher acrolein concentrations might have played a role. Janssens et al. (1994) reported decreases in arterial pO2 after acrolein smoke exposure in sheep and attributed the responses to acrolein-induced airway resistance and edema. Acrolein exposure also increases lung lymph flow and extracellular lung water coupled with airway damage and pulmonary edema (Hales et al., 1992). While acrolein exposure decreased pO2 in SH rats, no such change was evident in similarly exposed WKY rats and may be related to underlying differences in sensitivity to air pollution that relate to hypertension or other characteristics unique to the SH strain. Previous studies have shown that the SH rat responds with greater airway inflammation, edema and injury after air pollution exposure than WKY rats (Farraj et al., 2009). In addition, SH rats have larger carotid bodies (Habeck et al., 1985) and are more sensitive to the effects of hypoxia as evidenced by increased carotid sinus nerve activity and intracellular Ca2+ changes (Weil et al., 1998).
Although there were significant reductions in pO2 with acrolein exposure in SH rats, there were no significant changes in arterial blood oxygen saturation (i.e. SaO2). pO2 is a measure of dissolved oxygen in plasma, while SaO2 measures the percentage of hemoglobin sites bound with O2. The discrepancy in pO2 and SaO2 is due to the fact that SaO2 is well buffered from rapid decreases due to the sigmoidal oxy-hemoglobin dissociation curve (Urbano & Mohsenin, 2006). The carotid body responds to changes in pO2, not SaO2, making pO2 the relevant indicator of a pollutant’s effect on the carotid body. Decreases in arterial pO2 were also coupled with increases in pCO2, potentially owing to changes in gas exchange since minute ventilation did not change significantly. Importantly, the body is significantly more sensitive to systemic changes in pCO2, and the magnitude of the change in pCO2 with acrolein exposure in this study has been demonstrated to be sufficient to trigger activation of the carotid body (Iturriaga et al., 2005). Increases in pCO2 as small as 5 mmHg activate the carotid body (Whipp, 1994), suggesting that the carotid body may have been impacted to a greater extent by acrolein-induced increases in pCO2. Thus, while direct assessments of carotid body nerve activity were not made in this study, the small but significant decrease in pO2 and the larger increase in pCO2 suggest that carotid body activation took place. In addition, acrolein exposure reduced serum K+ concentration in hypertensive rats, although baseline concentrations were high in all exposure groups. Stress has been shown to rapidly increase K+ levels (Struthers et al., 1988), and potential stress induced by the chamber and blood draws in conscious and unrestrained animals may explain high-baseline K+ levels.
Acrolein-induced blood pressure and temperature changes may be related to early decreases in arterial pO2 and increases in pCO2, as has been demonstrated in other studies. For example, acute exposure of humans to low O2 tension at high altitude increases systemic arterial blood pressure and precipitates acute mountain sickness (Rhodes et al., 2011). Also, cats exposed to short-term anoxia had decreased pO2 and increased pCO2 coupled with increases in arterial blood pressure (Yang et al., 1992). In addition, acrolein exposure-induced decreases in pO2 in sheep were accompanied by increases in pulmonary arterial pressure and pulmonary vascular resistance (Janssens et al., 1994). Acrolein exposure caused an increase in the LF component of HRV, an indicator of both sympathetic and parasympathetic autonomic nervous system activity (Rowan et al., 2007) and a decrease in SDNN and RMSSD, indicators of parasympathetic tone. Furthermore, acrolein exposure resulted in an immediate increase in body temperature and a decrease in cardiac contractility 1 day after exposure compared to control animals. Although the reason for this finding is uncertain, sympathetic activation has been shown to increase body temperature by stimulating vasoconstriction (Constanzo, 2006). Exposure to acrolein in mice has been shown to cause inflammation and dilated cardiomyopathy characterized by contractile dysfunction (decreased dP/dtmax) and impaired relaxation (Ismahil et al., 2001). Although the early response to acrolein exposure was characterized by an increase in sympathetic tone, the decrease in contractility 1 day after acrolein exposure was mediated by vagal input, as evidenced by reversal with bilateral vagotomy. This shift in autonomic tone is consistent with vagal rebound, often following stressful encounters, including psychological stress (Mezzacappa et al., 2001) that drive a sympathetic response.
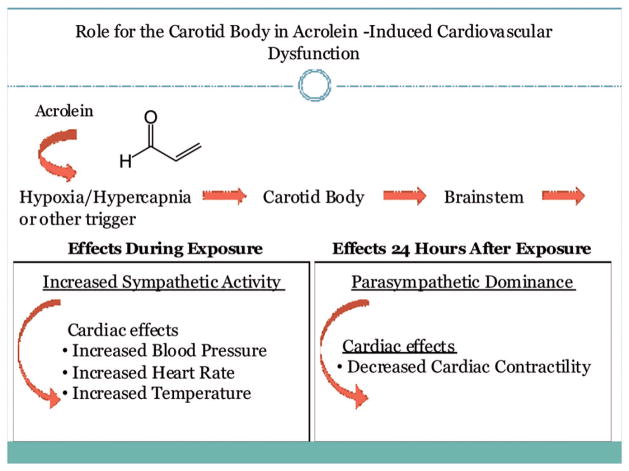
Pharmacological inhibition of carotid body signal transduction prevented acrolein-induced increases in blood pressure, body temperature and reductions in HR variability during exposure and the reduction in cardiac contractility 1 day after exposure. These results are in parallel with the alterations in arterial blood gases and the associated apneic responses, and are in combination suggest a significant role for the carotid body. These findings are also consistent with the recent work by Wang et al. (2012), who demonstrated that cardiac arrhythmias in particulate-exposed heart failure mice were in part due to altered sensitivity of the carotid body. Furthermore, exposures to the air pollutants tobacco smoke (Adgent, 2006), SO2 and NO2 (Hoppenbrouwers et al., 1981) were linked to abnormal cardiopulmonary sensitivity responses to hypoxia. Although cardiovascular responses of acrolein are likely also influenced by irritant and inflammatory mechanisms, the findings suggest a model whereby exposure to air pollution initiates a pathologic sequelae triggered by alterations in arterial blood gases. These alterations in turn activate carotid body-initiated autonomic reflexes that then alter cardiovascular function (Figure 6).
Figure 6.
The carotid body plays a role in mediating acrolein-induced cardiovascular dysfunction.
Although the acrolein concentration used in the present study was comparable to levels found in side-stream tobacco smoke and high-combustion areas, it would be important to examine effects at lower concentrations that are near levels found in ambient air. In addition, these studies should be performed with other environmentally relevant air pollutants including ambient particulate matter, ozone and diesel exhaust both in acute and chronic exposure settings. Moreover, this study was limited by the absence of direct measurement of carotid body nerve activity. Although Peng et al. (2010) showed that carotid body nerve activity was significantly inhibited by PAG at the concentration used in the present study, similar measurements would have directly confirmed the efficacy of PAG.
The present findings nevertheless demonstrate for the first time that acrolein exposure may mediate changes in blood pressure and cardiac function through carotid body chemo-receptor signaling. Exposure to this well-characterized pulmonary irritant known to elicit apnea also affected blood gas concentrations and stimulated chemoreceptor activation with downstream physiological heart and vascular effects, likely mediated by reflex changes in autonomic tone. Taken together, these findings shed new light on the unexplored role of carotid signaling during air pollution exposure and reveal the complex interplay between chemoreceptor signaling, the autonomic nervous system and the regulation of heart rhythm, vascular control and blood pressure.
Acknowledgments
The authors thank the following colleagues at the United States Environmental Protection Agency (Research Triangle Park, NC): Dr. Wayne E. Cascio, Dr. Ian Gilmour and Dr. David Herr for reviewing the manuscript before submission.
Footnotes
Declaration of interest
This work was supported by the EPA/UNC Toxicology Training Agreement [CR-83515201-0 to C.M.P. and A.P.C.]. The funding sources had no involvement in this work, and all authors report no declaration of interest.
This paper has been reviewed and approved for release by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency. Approval does not signify that the contents necessarily reflect the views and policies of the U.S. EPA nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Adgent MA. Environmental tobacco smoke and sudden infant death syndrome: a review. Birth Defects Res B Dev Reprod Toxicol. 2006;77:69–85. doi: 10.1002/bdrb.20068. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope C, III, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Charles River Laboratories. Charles River Surgical Capabilities Reference Paper. 2005;13 #1. [Google Scholar]
- Coleridge HM, Coleridge JC. Neural regulation of bronchial blood flow. Respir Physiol. 1994;98:1–13. doi: 10.1016/0034-5687(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Constanzo LS. BRS physiology. 4. Chapter 3. Lippincott Williams and Wilkins; 2006. Cardiovascular physiology; pp. 90–107. [Google Scholar]
- DeMeo DL, Zanobetti A, Litonjua AA, et al. Ambient air pollution and oxygen saturation. Am J Respir Crit Care Med. 2004;170:383–7. doi: 10.1164/rccm.200402-244OC. [DOI] [PubMed] [Google Scholar]
- Farraj AK, Haykal-Coates N, Winsett DW, et al. Increased non-conducted P-wave arrhythmias after a single oil fly ash inhalation exposure in hypertensive rats. Environ Health Perspect. 2009;117:709–15. doi: 10.1289/ehp.0800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchier L, Babuty D, Melin A, et al. Heart rate variability in severe right or left heart failure: the role of pulmonary hypertension and resistances. Eur J Heart Fail. 2004;6:181–5. doi: 10.1016/j.ejheart.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Gong H, Linn WS, Terrell SL, et al. Exposures of elderly volunteers with and without chronic obstructive pulmonary disease (COPD) to concentrated ambient fine particulate pollution. Inhal Toxicol. 2004;16:731–44. doi: 10.1080/08958370490499906. [DOI] [PubMed] [Google Scholar]
- Habeck JO, Huckstorf C, Honig A. Influence of age on the carotid bodies of spontaneously hypertensive (SHR) and normotensive rats. II. Alterations of the vascular wall. Exp Pathol. 1985;27:79–89. doi: 10.1016/s0232-1513(85)80044-x. [DOI] [PubMed] [Google Scholar]
- Hales CA, Musto SW, Janssens S, et al. Smoke aldehyde component influences pulmonary edema. J Appl Physiol. 1992;72:555–61. doi: 10.1152/jappl.1992.72.2.555. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Rowan WH, Winsett DW, et al. Potentiation of pulmonary reflex response to capsaicin 24h following whole-body acrolein exposure is mediated by TRPV1. Respir Physiol Neurobiol. 2008;160:160–71. doi: 10.1016/j.resp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, et al. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011;119:951–7. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppenbrouwers T, Calub M, Arakawa K, Hodgman JE. Seasonal relationship of sudden infant death syndrome and environmental pollutants. Am J Epidemiol. 1981;113:623–35. doi: 10.1093/oxfordjournals.aje.a113141. [DOI] [PubMed] [Google Scholar]
- Ismahil MA, Hamid T, Haberzettl P, et al. Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2001;301:2050–60. doi: 10.1152/ajpheart.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga R, Rey S, Del Rio R. Cardiovascular and ventilatory acclimatization induced by chronic intermittent hypoxia: a role for the carotid body in the pathophysiology of sleep apnea. Biol Res. 2005;38:335–40. doi: 10.4067/s0716-97602005000400004. [DOI] [PubMed] [Google Scholar]
- Janssens SP, Musto SW, Hutchison WG, et al. Cyclooxygenase and lipoxygenase inhibition by BW-755C reduces acrolein smoke-induced acute lung injury. J Appl Physiol. 1994;77:888–95. doi: 10.1152/jappl.1994.77.2.888. [DOI] [PubMed] [Google Scholar]
- Jones AP. Indoor air quality and health. Atmos Environ. 1999;33:4535–64. [Google Scholar]
- Lee BP, Morton RF, Lee LY. Acute effects of acrolein on breathing: role of vagal bronchopulmonary afferents. J Appl Physiol. 1992;72:1050–6. doi: 10.1152/jappl.1992.72.3.1050. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kim J, Cinotte J, et al. Carotid body denervation effect on cytochrome oxidase activity in pre-Botzinger complex of developing rats. J Appl Physiol. 2003;94:1115–21. doi: 10.1152/japplphysiol.00765.2002. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, Ortega-Sáenz P, Pardal R, et al. Carotid body oxygen sensing. Eur Respir J. 2008;32:1386–98. doi: 10.1183/09031936.00056408. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Kelsey RM, Katkin ES, Sloan RP. Vagal rebound and recovery from psychological stress. Psychosom Med. 2001;63:650–7. doi: 10.1097/00006842-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Oortgiesen M, Veronesi B, Eichenbaum G, et al. Residual oil fly ash and charged polymers activate epithelial cells and nociceptive sensory neurons. Am J Physiol Lung Cell Mol Physiol. 2000;278:683–95. doi: 10.1152/ajplung.2000.278.4.L683. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, et al. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA. 2010;107:10719–24. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CM, Ledbetter AD, Hazari MS, et al. Hypoxia stress test reveals exaggerated cardiovascular effects in hypertensive rats after exposure to the air pollutant acrolein. Toxicol Sci. 2013;132:467–77. doi: 10.1093/toxsci/kft008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, Dockery DW, Kanner RE, et al. Oxygen saturation, pulse rate, and particulate air pollution: a daily time-series panel study. Am J Respir Crit Care Med. 1999;159:365–72. doi: 10.1164/ajrccm.159.2.9702103. [DOI] [PubMed] [Google Scholar]
- Rhodes HL, Chesterman K, Chan CW, et al. Systemic blood pressure, arterial stiffness and pulse waveform analysis at altitude. J R Army Med Corps. 2011;157:110–13. doi: 10.1136/jramc-157-01-18. [DOI] [PubMed] [Google Scholar]
- Roux JC, Peyronnet J, Pascual O, Dalmaz Y. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. J Physiol. 2000;522:493–501. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan WH, III, Campen MJ, Wichers LB, Watkinson WP. Heart rate variability in rodents: uses and caveats in toxicological studies. Cardiovasc Toxicol. 2007;7:28–51. doi: 10.1007/s12012-007-0004-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi C, Kakehi A, Minami N, et al. Exposure evaluation of adult male Japanese smokers switched to a heated cigarette in a controlled clinical setting. Regul Toxicol Pharmacol. 2014;69:338–47. doi: 10.1016/j.yrtph.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Srebot V, Gianicolo EA, Rainaldi G, et al. Ozone and cardiovascular injury. Cardiovasc Ultrasound. 2009;7:30–8. doi: 10.1186/1476-7120-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struthers AD, Quigley C, Brown MJ. Rapid changes in plasma potassium during a game of squash. Clin Sci (Lond) 1988;74:397–401. doi: 10.1042/cs0740397. [DOI] [PubMed] [Google Scholar]
- Urbano F, Mohsenin V. Chronic obstructive pulmonary disease and sleep: the interaction. Panminerva Med. 2006;48:223–30. [PubMed] [Google Scholar]
- Wang T, Lang GD, Moreno-Vinasco L, et al. Particulate matter induces cardiac arrhythmias via dysregulation of carotid body sensitivity and cardiac sodium channels. Am J Respir Cell Mol Biol. 2012;46:524–31. doi: 10.1165/rcmb.2011-0213OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil JV, Stevens T, Pickett CK, et al. Strain-associated differences in hypoxic chemosensitivity of the carotid body in rats. Am J Physiol. 1998;274:767–74. doi: 10.1152/ajplung.1998.274.5.L767. [DOI] [PubMed] [Google Scholar]
- Whipp BJ. Carotid bodies and breathing in humans. Thorax. 1994;49:1081–4. doi: 10.1136/thx.49.11.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Ho CW, Lin RH, et al. Reduction of blood PO2 decrease and PCO2 increase during asphyxia by paramedian reticular nucleus in cats. Brain Res Bull. 1992;29:573–9. doi: 10.1016/0361-9230(92)90125-h. [DOI] [PubMed] [Google Scholar]