Abstract
Background and Aim:
To assess the amount of propofol required for induction based on bispectral index (BIS) after administering epidural anesthesia with ropivacaine alone and ropivacaine with dexmedetomidine in patients undergoing lower extremities and abdominal surgeries.
Subjects and Methods:
A double-blinded randomized clinical trial was carried out in 60 patients over a period of 2 years in a tertiary care hospital. American Society of Anaesthesiologists I or II in age group 18–65 years were included in the study. Group R received epidural anesthesia with ropivacaine alone, and Group D received ropivacaine and dexmedetomidine. General anesthesia was induced with propofol under BIS monitoring after 15 min. Onset of sensory and motor block, time for loss of consciousness and total amount of propofol used during induction to achieve the BIS value < 55 were recorded. Student's t-test and Chi-square test were used to find the significance of study parameters.
Results:
Time of onset of sensory block (Group R 11.30 ± 1.64/Group D 8.27 ± 0.83 min), motor block (Group R 14.16 ± 1.33/Group D 12.63 ± 1.22 min), time for loss of consciousness (Group R 90.57 ± 11.05/Group D 73.67 ± 16.34 s), and total amount of propofol (Group R 129.83 ± 22.38/Group D 92.13 ± 12.93 s) were reduced in Group D which was statistically significant with P < 0.001.
Conclusion:
Epidural ropivacaine with dexmedetomidine significantly reduces the total propofol dose required for induction of anesthesia. Also, it decreases the onset time of sensory and motor block and provides good hemodynamic stability.
Keywords: Bispectral index, dexmedetomidine, epidural anesthesia, general anesthesia, propofol, ropivacaine
INTRODUCTION
The combination of epidural anesthesia with general anesthesia technique is widely used for the management of major abdominal and thoracic surgery for decades. There is a reduced general anesthetics requirement during surgery when the two techniques are combined.[1] Alpha-2 adrenergic receptor agonists like dexmedetomidine have been the focus of interest for their sedative, analgesic, perioperative sympatholytic, and cardiovascular stabilizing effects with reduced anesthetic requirements.[2] Intraoperative uses of dexmedetomidine include its uses as adjunct to general anesthesia, epidural, intrathecal, brachial plexus block, caudal anesthesia, and monitored anesthesia care or as an adjuvant for total intravenous (IV) anesthesia. Dexmedetomidine prolongs the duration of analgesia, motor, and postoperative analgesic requirements. Ropivacaine is a local anesthetic with long duration of action, having similar pharmacology to bupivacaine; however, it has a wider safety margin and was shown to possess less cardiotoxicity in comparison with bupivacaine. In our study, we have evaluated the clinical effects of the combination of epidural ropivacaine and epidural ropivacaine with dexmedetomidine on the requirement of propofol based on the time taken to achieve bispectral (BIS) value of 45–55 during the induction of anesthesia. The study tests the hypothesis that epidurally administered drugs can reduce the general anesthetic requirement.
SUBJECTS AND METHODS
After approval by the Institutional Ethics Committee, informed consent was obtained from each of the 60 patients who were scheduled for elective lower extremities and abdominal surgeries under combined epidural and general anesthesia. This study was carried out from October 2012 to October 2014. All the patients belonged to American Society of Anaesthesiologists I or II and were in the age group of 18–65 years were included in the study. Patients with hypersensitivity to local anesthetics, history of cardiac, pulmonary, liver or renal diseases, lumbar surgeries, body mass index >35 kg/m2, bleeding disorders, uncontrolled hypertension and diabetes mellitus, preexisting peripheral neuropathy, patients on regular medication with central nervous system disorders (benzodiazepines and/or opiates), pregnant women, and infection at the site of epidural catheter insertion were excluded from the study.
Patients satisfying the inclusion criteria were selected during the study period from the operation register on a daily basis and were allocated into two groups of 30 each by sealed envelope method. All the patients received oral ondansetron 8 mg, pantoprazole 40 mg on the morning of surgery, and intramuscular glycopyrrolate 0.2 mg 30 min before surgery as premedication. After securing an IV cannula, the patients were preloaded with 15 ml/kg IV fluids (normal saline/Ringer lactate) over 20 min.
In the theater, the patient monitoring included electrocardiogram, noninvasive blood pressure (NIBP), heart rate (HR), oxygen saturation (SpO2), and BIS index. The baseline HR, NIBP, and SpO2 were recorded. The epidural was performed with an 18-gauge Tuohy needle through L1-L2, L2-L3 or L3-L4 interspaces with patients in the sitting position. Epidural space was identified with loss of resistance technique. Catheter was threaded and fixed at 5 cm in the epidural space.
Group R received 15 ml of 0.5% of ropivacaine 75 mg (Ropin® 0.5% Neon Laboratories Ltd, Mumbai, India)and 1 ml normal saline (total 16 ml).
Group D received 15 ml of 0.5% of ropivacaine 75 mg (Ropin® 0.5% Neon Laboratories Ltd, Mumbai, India) and dexmedetomidine 1 mcg/kg (Dexem™, Themis Medicare Ltd, Haridwar, India) and diluting it with normal saline to a volume of 1 ml (total 16 ml).
Sensory block was assessed by the pin prick method and graded as Grade 0 - sharp pin felt, Grade 1 - analgesia, dull sensation felt, Grade 2 - anesthesia, no sensation felt. Motor block was assessed in both legs using modified Bromage scale, that is, Grade 0 - able to move the hip, knee, and ankle Grade 1 - unable to move the hip, but is able to move the knee and ankle, Grade 2 - unable to move the hip and knee, but is able to move the ankle, Grade 3 - unable to move the hip, knee, and ankle.
After 15 min of epidural catheter insertion, the patient was preoxygenated with 100% oxygen for 3 min. The patients were given fentanyl (2 mcg/kg) and received propofol at a rate of 1 mg/kg/min administered via an infusion pump. Once the BIS value reached 45–55, muscle relaxation was attained using rocuronium (0.6 mg/kg) to facilitate laryngeal mask airway (LMA) ProSeal insertion. The total amount of propofol used during induction to achieve the BIS value <55 was recorded. BIS measurement was started before induction and was recorded every 15 s during induction until the value reached 45–55. End-tidal carbon dioxide concentration (EtCO2) and temperature were also monitored.
Statistical analysis
Sample size calculation: A study carried out by Xiang and Li.[1] in 2007 has found that the time to loss of consciousness with propofol in ropivacaine group to be 111 s (standard deviation = 17). Based on the above finding the sample size for the present study was estimated with a relative precision of 6% and desired confidence level of 95%, it was estimated that 30 patients need to be included in ropivacaine group. However, the present study attempted to make a comparison between ropivacaine and dexmedetomidine; hence another 30 patients were included in the second arm of the study. A total of 60 patients were selected for this study.
Significance was assessed at 5% level of significance. Student's t-test (two-tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups (inter group analysis) on metric parameters. Chi-square/Fisher exact test has been used to find the significance of study parameters on categorical scale between two or more groups. In the above tests, the P < 0.05 was accepted as indicating statistical significance. Data analysis was carried out using Statistical Package for Social Science (SPSS) version 15.0. (SPSS Inc., Chicago, IL, USA).
RESULTS
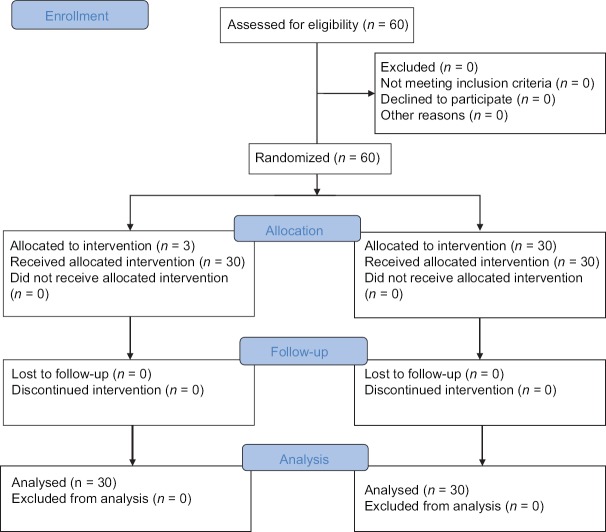
Of the 60 patients initially randomized into the study, no patient was excluded and all patients completed the study [Figure 1]. The demographic data were comparable in both the groups [Table 1]. The onset of sensory blockade, onset of motor blockade, time to loss of consciousness, time to loss of eyelash reflex, and time to reach BIS value of 45–55 were compared in both groups and was less in Group D which was statistically significant with P < 0.001. Addition of epidural dexmedetomidine significantly reduces the total propofol requirement during the induction of anesthesia [Table 2]. The total propofol requirement (mg) in Groups R and D were 129.83 ± 22.38 and 92.13 ± 12.93, respectively which was statistically significant with P < 0.001 [Figure 2]. The dose of propofol/kg body weight in Group R was 2.05 ± 0.29, and that of Group D was 1.49 ± 0.24 which was statistically significant [Figure 3]. The hemodynamic parameters taken into consideration were the HR, blood pressure (systolic and diastolic), SpO2, and EtCO2. The variables were monitored in the baseline, 15 min after epidural injection, at loss of consciousness, before LMA insertion, and 1-min after LMA insertion. It was comparable in both groups and was not statistically significant.
Figure 1.
CONSORT 2010 flow diagram
Table 1.
Demographic profile of patients. Data are mean±SD
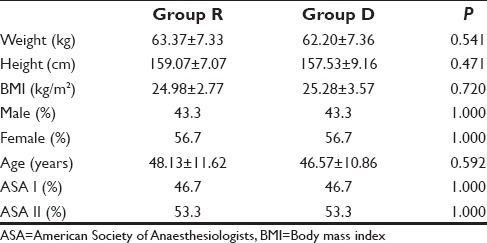
Table 2.
Comparison of Various Study Variables in two Groups. Data are mean±SD
Figure 2.
Total Dose of Propofol used in the two groups
Figure 3.
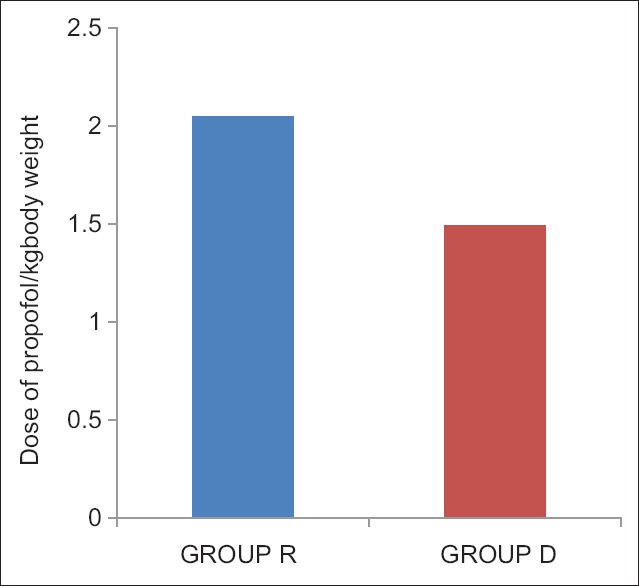
Dose of propofol/kg body weight used in the two groups
DISCUSSION
Epidural anesthesia has been conventionally used in combination with general anesthesia for providing intraoperative and postoperative analgesia. Recent studies have shown that local anesthetic agents have a sparing effect on general anesthesia. In our study, significant reduction in the induction dose of propofol was observed along with faster onset of sensory and motor block in patients who received epidural ropivacaine with dexmedetomidine prior to the induction of general anesthesia.
Neuraxial anesthesia potentiates the sedative effects of midazolam[3] in humans which suggest that neural blockade itself may have sedative properties.[4] Also, there are multiple studies that have shown that there is a significant reduction in the propofol dose required for induction of anesthesia.[1,5] BIS is used to measure the depth of anesthesia. We titrated the BIS value to 45–55 to avoid awareness. In our study, we have standardized the target BIS to be achieved at induction before any noxious stimuli like intubation or surgical stress, in order to accurately determine the induction dose of propofol. The recommended dose of propofol for induction of anesthesia is 1.5–2.5 mg/kg. This was similar to the dose of propofol required for induction of anesthesia in ropivacaine group. We observed a 29% reduction in the induction dose of propofol in the epidural ropivacaine with dexmedetomidine group. The propofol dose required for induction in Group R was 2.05 ± 0.29 mg/dl which was similar to 2.16 ± 0.15 mg/dl according to the study by Xiang and Li.[1] However, in Group D the induction dose of propofol was significantly reduced to 1.49 ± 0.24 mg/dl.
There are multiple mechanisms proposed to explain the interaction between epidural anesthesia and general anesthesia. There may be inhibition of tonic afferent spinal nerve signals to the brain and spinal cord above the level of block.[6] Another theory has proposed that the state of wakefulness is measured by tonic sensory and muscle spindle activity.[7] The reduced afferent input to the brain may reduce the excitatory descending modulation of spinal cord neuron and suppresses motor function. Thus, the combination of decreased inputs from the sensory/motor afferents seen with epidural anesthesia should explain the mechanism of general anesthetic effects and reduced requirements of anesthetics.[8] Local anesthetic agents are sodium channel blockers and cause large transient sodium influx causing membrane depolarization.[9] Another mechanism is local anesthetics and propofol enhances GABA mediated chloride channels which facilitate inhibitory neurotransmission in the neurons.[10] Prolongation of inhibition by positive modulation of postsynaptic GABAA receptor function at GABAergic synapses is thought to be an important component of the depressant effects of volatile anesthetics and multiple chemically distinct IV anesthetics.[11]
Dexmedetomidine also potentiates the sensory and motor block of epidurally administered local anesthetic agents. The probable mechanism may be the intrinsic ability to block conduction in C and A δ fibers by stimulation of alpha-2 receptors will increase the intensity of conduction block of local anesthetic agents. It may cause some degree of local vasoconstriction decreasing the clearance of epidural drugs and lastly any analgesic used either neuraxially or systemically will potentiate the neuraxial block. The onset of sensory (8.27 ± 0.83 min) and motor block (12.63 ± 1.22 min) was significantly reduced in our study which is similar to the findings of Bajwa et al.[12]
Epidural administration of dexmedetomidine is associated with sedation, analgesia, anxiolysis, and sympatholysis.[12,13,14,15] Bajwa et al.[12] showed that 36% of patients had profound sedation. In another study, epidural dexmedetomidine reduced the anesthetic requirements significantly thus preventing awareness.[16] The sedative property of epidural dexmedetomidine may be responsible for the reduction in time to achieve target BIS and reduced propofol requirements in our study.
A larger sample size and inclusion of a control group would have improved the power of our study. In a recent study by Bajwa et al.[17] found that epidural anesthesia with ropivacaine and dexmedetomidine can be safely and effectively used in patients undergoing renal surgeries. However, they have not studied about the effect of dexmedetomidine on general anesthetic requirements.
CONCLUSION
We are the only study that has evaluated the propofol requirement using BIS as an endpoint and have confirmed the reduction in requirement of propofol following administration of epidural dexmedetomidine along with ropivacaine. These findings have impact on clinical practice where combination of epidural with general anesthesia is practiced regularly. The findings of this study will help us to better titrate our anesthetic regimen.
Financial support and sponsorship
M. S. Ramaiah Medical College.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Xiang Y, Li YH. Comparison of 1.5% lidocaine and 0.5% ropivacaine epidural anesthesia combined with propofol general anesthesia guided by bispectral index. J Zhejiang Univ Sci B. 2007;8:428–34. doi: 10.1631/jzus.2007.B0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salgado PF, Sabbag AT, Silva PC, Brienze SL, Dalto HP, Módolo NS, et al. Synergistic effect between dexmedetomidine and 0.75% ropivacaine in epidural anesthesia. Rev Assoc Med Bras. 2008;54:110–5. doi: 10.1590/s0104-42302008000200011. [DOI] [PubMed] [Google Scholar]
- 3.Tverskoy M, Shifrin V, Finger J, Fleyshman G, Kissin I. Effect of epidural bupivacaine block on midazolam hypnotic requirements. Reg Anesth. 1996;21:209–13. [PubMed] [Google Scholar]
- 4.Ben-David B, Vaida S, Gaitini L. The influence of high spinal anesthesia on sensitivity to midazolam sedation. Anesth Analg. 1995;81:525–8. doi: 10.1097/00000539-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Kanata K, Sakura S, Kushizaki H, Nakatani T, Saito Y. Effects of epidural anesthesia with 0.2% and 1% ropivacaine on predicted propofol concentrations and bispectral index values at three clinical end points. J Clin Anesth. 2006;18:409–14. doi: 10.1016/j.jclinane.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson PS, Liu SS, Gras TW. Does epidural anesthesia have general anesthetic effects. A prospective, randomized, double-blind, placebo-controlled trial? Anesthesiology. 1999;91:1687–92. doi: 10.1097/00000542-199912000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Lanier WL, Iaizzo PA, Milde JH, Sharbrough FW. The cerebral and systemic effects of movement in response to a noxious stimulus in lightly anesthetized dogs. Possible modulation of cerebral function by muscle afferents. Anesthesiology. 1994;80:392–401. doi: 10.1097/00000542-199402000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Eappen S, Kissin I. Effect of subarachnoid bupivacaine block on anesthetic requirements for thiopental in rats. Anesthesiology. 1998;88:1036–42. doi: 10.1097/00000542-199804000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Morgan GE, Murray M, Mikhail M, editors. Clinical Anaesthesiology. 4th ed. New York: Lange Medical Books; 2006. Local anesthetics. [Google Scholar]
- 10.Nordmark J, Rydqvist B. Local anaesthetics potentiate GABA-mediated Cl- currents by inhibiting GABA uptake. Neuroreport. 1997;8:465–8. doi: 10.1097/00001756-199701200-00018. [DOI] [PubMed] [Google Scholar]
- 11.Lingamaneni R, Hemmings HC., Jr Differential interaction of anaesthetics and antiepileptic drugs with neuronal Na+ channels, Ca2+ channels, and GABA (A) receptors. Br J Anaesth. 2003;90:199–211. doi: 10.1093/bja/aeg040. [DOI] [PubMed] [Google Scholar]
- 12.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauro VA, Brandão ST. Clonidine and dexmedetomidine through epidural route for post-operative analgesia and sedation in a cholecystectomy. Rev Bras Anestesiol. 2004;4:1–10. doi: 10.1590/s0034-70942004000400003. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel JS, Gordin V. Alpha 2 agonists in regional anesthesia and analgesia. Curr Opin Anaesthesiol. 2001;14:751–3. doi: 10.1097/00001503-200112000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Oriol-lopez SA, Maldonado-Sanchez KA, Hernandez-Bernal CE, Castelazo-Arredondo JA, Moctezuma RL. Epidural dexmedetomidine in regional anaesthesia to reduce anxiety. Rev Mex Anestesiol. 2008;31:271–7. [Google Scholar]
- 16.Elhakim M, Abdelhamid D, Abdelfattach H, Magdy H, Elsayed A, Elshafei M. Effect of epidural dexmedetomidine on intraoperative awareness and post-operative pain after one-lung ventilation. Acta Anaesthesiol Scand. 2010;54:703–9. doi: 10.1111/j.1399-6576.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 17.Bajwa SJ, Kaur J, Singh A. A comparative evaluation of epidural and general anaesthetic technique for renal surgeries: A randomised prospective study. Indian J Anaesth. 2014;58:410–5. doi: 10.4103/0019-5049.138975. [DOI] [PMC free article] [PubMed] [Google Scholar]