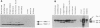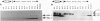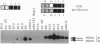Abstract
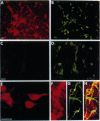
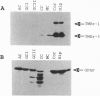
In this study we report on the developmental and regional expression of two brain-specific isoforms of tropomyosin, TMBr-1 and TMBr-3, that are generated from the rat alpha-tropomyosin gene via the use of alternative promoters and alternative RNA splicing. Western blot analysis using an exon-specific peptide polyclonal antibody revealed that the two isoforms are differentially expressed in development with TMBr-3 appearing in the embryonic brain at 16 days of gestation, followed by the expression of TMBr-1 at 20 days after birth. TMBr-3 was detected in all brain regions examined, whereas TMBr-1 was detected predominantly in brain areas that derived from the prosencephalon. Immunocytochemical studies on mixed primary cultures made from rat embryonic midbrain indicate that expression of the brain-specific epitope is restricted to neurons. The developmental pattern and neuronal localization of these forms of tropomyosin suggest that these isoforms have a specialized role in the development and plasticity of the nervous system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Chen M., Keller F., Kandel E. R. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science. 1992 May 1;256(5057):645–649. doi: 10.1126/science.1585177. [DOI] [PubMed] [Google Scholar]
- Barinaga M. The brain remaps its own contours. Science. 1992 Oct 9;258(5080):216–218. doi: 10.1126/science.1411520. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. Specificity of the glial fibrillary acidic protein for astroglia. J Histochem Cytochem. 1977 Jun;25(6):466–469. doi: 10.1177/25.6.69656. [DOI] [PubMed] [Google Scholar]
- Black D. L. Does steric interference between splice sites block the splicing of a short c-src neuron-specific exon in non-neuronal cells? Genes Dev. 1991 Mar;5(3):389–402. doi: 10.1101/gad.5.3.389. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casper D., Mytilineou C., Blum M. EGF enhances the survival of dopamine neurons in rat embryonic mesencephalon primary cell culture. J Neurosci Res. 1991 Oct;30(2):372–381. doi: 10.1002/jnr.490300213. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hoffman P. N. Neuronal and glial cytoskeletons. Curr Opin Neurobiol. 1991 Oct;1(3):346–353. doi: 10.1016/0959-4388(91)90051-8. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C., Had L., Ferraz C., Sri Widada J. S., Liautard J. P., Rabié A. Expression of tropomyosin genes during the development of the rat cerebellum. J Neurochem. 1990 Sep;55(3):899–906. doi: 10.1111/j.1471-4159.1990.tb04576.x. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Galloway P. G., Likavec M. J., Perry G. Tropomyosin isoform expression in normal and neoplastic astrocytes. Lab Invest. 1990 Feb;62(2):163–170. [PubMed] [Google Scholar]
- Galloway P. G., Perry G. Tropomyosin distinguishes Lewy bodies of Parkinson disease from other neurofibrillary pathology. Brain Res. 1991 Feb 15;541(2):347–349. doi: 10.1016/0006-8993(91)91036-z. [DOI] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Kuczmarski E. R., Rosenbaum J. L. Studies on the organization and localization of actin and myosin in neurons. J Cell Biol. 1979 Feb;80(2):356–371. doi: 10.1083/jcb.80.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H. C., Nasim F. H., Grabowski P. J. Control of alternative splicing by the differential binding of U1 small nuclear ribonucleoprotein particle. Science. 1991 Mar 1;251(4997):1045–1050. doi: 10.1126/science.1825520. [DOI] [PubMed] [Google Scholar]
- Lees-Miller J. P., Goodwin L. O., Helfman D. M. Three novel brain tropomyosin isoforms are expressed from the rat alpha-tropomyosin gene through the use of alternative promoters and alternative RNA processing. Mol Cell Biol. 1990 Apr;10(4):1729–1742. doi: 10.1128/mcb.10.4.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller J. P., Helfman D. M. The molecular basis for tropomyosin isoform diversity. Bioessays. 1991 Sep;13(9):429–437. doi: 10.1002/bies.950130902. [DOI] [PubMed] [Google Scholar]
- Nawa H., Kotani H., Nakanishi S. Tissue-specific generation of two preprotachykinin mRNAs from one gene by alternative RNA splicing. Nature. 1984 Dec 20;312(5996):729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- Nona S. N., Trowell S. C., Cronly-Dillon J. R. Postnatal developmental profiles of filamentous actin and of 200 kDa neurofilament polypeptide in the visual cortex of light- and dark-reared rats and their relationship to critical period plasticity. FEBS Lett. 1985 Jul 1;186(1):111–115. doi: 10.1016/0014-5793(85)81350-8. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Riederer B. M., Zagon I. S., Goodman S. R. Brain spectrin(240/235) and brain spectrin(240/235E): differential expression during mouse brain development. J Neurosci. 1987 Mar;7(3):864–874. doi: 10.1523/JNEUROSCI.07-03-00864.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. P. The biochemistry of memory. Essays Biochem. 1991;26:1–12. [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner B. S., Holladay C. R. Distribution of tubulin and actin in neurites and growth cones of differentiating nerve cells. Cell Motil. 1981;1(2):167–178. doi: 10.1002/cm.970010202. [DOI] [PubMed] [Google Scholar]
- Stamm S., Casper D., Dinsmore J., Kaufmann C. A., Brosius J., Helfman D. M. Clathrin light chain B: gene structure and neuron-specific splicing. Nucleic Acids Res. 1992 Oct 11;20(19):5097–5103. doi: 10.1093/nar/20.19.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Weinberger R. P., Henke R. C., Tolhurst O., Jeffrey P. L., Gunning P. Induction of neuron-specific tropomyosin mRNAs by nerve growth factor is dependent on morphological differentiation. J Cell Biol. 1993 Jan;120(1):205–215. doi: 10.1083/jcb.120.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]