Abstract
Abnormal glucose tolerance during pregnancy is associated with perinatal complications. We used continuous glucose monitoring (CGM) in pregnant women with glucose intolerance to achieve better glycemic control and to evaluate the maternal glucose fluctuations. We also used CGM in women without glucose intolerance (the control cases). Furthermore, the standard deviation (SD) and mean amplitude of glycemic excursions (MAGE) were calculated for each case. For the control cases, the glucose levels were tightly controlled within a very narrow range; however, the SD and MAGE values in pregnant women with glucose intolerance were relativity high, suggesting postprandial hyperglycemia. Our results demonstrate that pregnant women with glucose intolerance exhibited greater glucose fluctuations compared with the control cases. The use of CGM may help to improve our understanding of glycemic patterns and may have beneficial effects on perinatal glycemic control, such as the detection of postprandial hyperglycemia in pregnant women.
Keywords: continuous glucose monitoring, gestational diabetes mellitus, overt diabetes in pregnancy, pregestational diabetes, mean amplitude of glucose excursions
Introduction
During pregnancy, insulin resistance and hyperinsulinemia can be caused by the increased secretion of placental hormones, such as human placental lactogen and tumor necrosis factor α, which are secreted from trophoblastic cells.1 Studies have shown that the glycemic profiles in healthy and childbearing-age women are tightly controlled within a very narrow range.2–4 Furthermore, the glycemic profiles in pregnant women without abnormal glucose tolerance also show a similar tendency.5,6 In contrast, because of excessive insulin resistance, postprandial hyperglycemia can occur in pregnant women with abnormal glucose tolerance.5,7,8 Maternal postprandial hyperglycemia is already known to be significantly correlated with infant birth weight, leading to a risk of shoulder dystocia at delivery.9,10 The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study confirmed continuous linear associations between increasing maternal blood glucose levels and the risks of adverse events, such as large-for-gestational-age (LGA) infants, neonatal hypoglycemia, and primary cesarean sections.11 Consequently, careful monitoring and early improvement of maternal blood glucose levels are needed. Using a continuous glucose monitoring (CGM) during pregnancy may be useful for evaluating glucose variability, since the detection of postprandial hyperglycemia using self-monitoring blood glucose (SMBG) only may be insufficient. Furthermore, CGM may be effective as an educational tool, enabling the glycemic profile to be visualized in detail.
The use of CGM has already been reported as an effective means of evaluating glycemic fluctuations in pregnant women with pregestational diabetes. However, studies examining the effectiveness of CGM and glucose variability in women with abnormal glucose tolerance during pregnancy, including those with gestational diabetes mellitus (GDM), remain scarce.
In this observational case series, we used CGM and SMBG to monitor the glucose levels in pregnant women with abnormal glucose tolerance, and we report the glucose fluctuations observed during pregnancy and the pregnancy outcomes.
Patients
In this study, 17 pregnant women who visited the National Center for Global Health and Medicine, Tokyo, Japan, between July 1, 2012, and December 24, 2013, were consecutively enrolled, and they were subsequently diagnosed as having an abnormal glucose tolerance by one of the authors (MN). All the women underwent CGM as part of ordinary clinical care. The diagnoses were made based on the results of a 75-g oral glucose tolerance test (OGTT) at 13–34 weeks of gestation or on the findings of laboratory tests. Abnormal glucose tolerance was considered to include conditions such as GDM, overt diabetes in pregnancy, and pregestational diabetes. GDM was diagnosed using a 75-g OGTT if a woman had any of the following venous plasma glucose values: ≥92 mg/dL (5.1 mmol/L) after an overnight fast, ≥180 mg/dL (10.0 mmol/L) at one hour, or ≥153 mg/dL (8.5 mmol/L) at two hours.12 Overt diabetes in pregnancy was diagnosed as diabetes mellitus that was detected for the first time during pregnancy through a blood test based on a fasting venous plasma glucose level ≥126 mg/dL (7.0 mmol/L), a random plasma glucose level ≥200 mg/dL (11.1 mmol/L), an HbA1c level ≥6.5%, or a diagnosis of overt diabetic reti-nopathy. Pregestational diabetes was defined as diabetes that was diagnosed prior to pregnancy. Soon after the diagnosis of abnormal glucose tolerance during pregnancy, the women were asked to initiate SMBG and diet therapy under the guidance of dieticians. In addition, they were asked to wear a CGM.
A CGM was also worn by a nonpregnant woman and a pregnant woman with normal glucose tolerance who served as control cases. The pregnant woman with normal glucose tolerance was evaluated using a 50-g OGTT at 25 weeks and 3 days, and her HbA1c level was 5.0%.
This study was conducted in accordance with ethical principles of the Declaration of Helsinki, and all the study participants provided informed consent for this observational analysis. CGM was conducted as a part of clinical practice. Since the data in this case series were obtained by a retrospective chart search of patients treated in an ordinary clinical settings without specific protocol defined, we did not seek a review result from the institutional review board.
Self-Monitoring Blood Glucose
We used OneTouch Ultra™ (Johnson and Johnson) for SMBG. We instructed pregnant women to check their blood glucose levels four to six times a day before and after meals.
Continuous Glucose Monitoring
For CGM, both the iPro™2 and the CGMS® System Gold™ (Medtronic MiniMed) were used. These systems are composed of a disposable subcutaneous glucose-sensing device and an electrode impregnated with glucose oxidase. The interstitial glucose levels in the subcutaneous tissue are measured electrochemically every 10 seconds, and an average value is stored every five minutes, providing up to 288 measurements per day. The interstitial glucose level, as measured using CGM, is strongly correlated with the venous plasma glucose level (r = 0.91–0.92) and is used as an indicator of the actual glycemic profiles.13,14 The CGM data were recorded for 72–168 continuous hours and were downloaded to a personal computer using the software provided by the manufacturer.
Treatment
Patients with abnormal glucose tolerance during pregnancy were initially assigned an appropriate caloric intake value based on their standard body weight and gestational age. For the first trimester, the caloric requirements were calculated using the following formula: standard weight (kg) × 25 0 50 kcal. For the second and third trimesters, 250 kcal/day and 450 kcal/day were added to this value, respectively.15 If the women were overweight, the daily caloric intake was calculated as follows: standard weight × 30 kcal throughout the pregnancy. Women were instructed to increase their caloric intakes if ketone bodies were detected in a morning urine sample.
If postprandial hyperglycemia persisted after the adjustment of caloric intake, pregnant women were told to begin eating five to six meals per day to divide the caloric intake. Insulin therapy with rapid insulin analogs, such as insulin lispro or insulin aspart, was administered to women whose postprandial glucose levels increased above 140 mg/dL. Furthermore, as measured using CGM, women whose postprandial glucose level was above 140 mg/dL began to receive insulin therapy, even if the postprandial glucose level measured using SMBG was below 140 mg/dL.
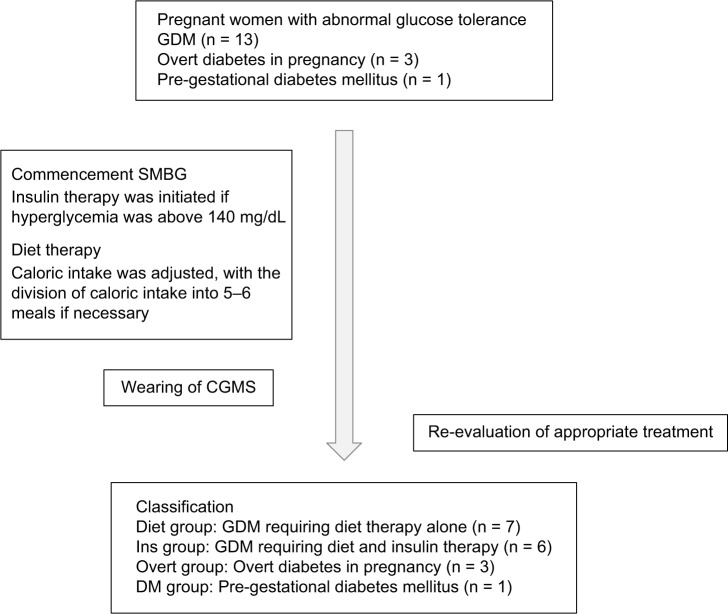
Women with overt diabetes in pregnancy were also initially treated with nutrition therapy. If the maternal postprandial hyperglycemia persisted, insulin injections were administered before each meal. If fasting hyperglycemia occurred, an intermediate-acting insulin analog, such as neutral protamine Hagedorn (NPH), or a long-acting insulin analog, such as insulin detemir, was administered (Fig. 1).
Figure 1.
Diagram of the present case series.
Analysis of CGM Data
We retrospectively calculated the standard deviation (SD) and the mean amplitude of glycemic excursions (MAGE, calculated under the criterion that both segments of the glycemic excursion exceed the value of one SD)16 using CGM data for all 19 women, including the control cases. The SD shows how much variation is there from the average, while the MAGE summarizes glycemic variability by identifying glucose peaks and troughs with amplitudes >1 SD of the mean. The SD values were calculated using Excel®, version 14.5.7. The MAGE values were calculated by measuring the arithmetic mean of the glycemic difference between consecutive peaks and nadirs, provided that the difference was greater than the SD around the mean glucose values.
Results
Classification
The 17 pregnant women with abnormal glucose tolerance who underwent CGM were classified into four groups (Table 1). Seven women were treated with only diet therapy (Diet group), six women required insulin therapy in addition to nutrition therapy (Ins group), three women were diagnosed as having overt diabetes in pregnancy (Overt group), and one patient with prepregnancy diabetes mellitus required basal-bolus insulin therapy (DM group).
Table 1.
Characteristics of 17 pregnant women at the time of diagnosis.
| CASE | GROUP | INSULIN THERAPY | AGE AT DIAGNOSIS | COMORBIDITY | GESTATIONAL WEEK AT DIAGNOSIS | ABNORMAL VALUE POINT ON 75-g OGTT | HbA1c (%) |
|---|---|---|---|---|---|---|---|
| 1 | Diet | − | 44 | 24 w, 0 d | 1 hour | 5.4 | |
| 2 | Diet | − | 38 | 30 w, 4 d | 1 hour | 5.2 | |
| 3 | Diet | − | 42 | Administration of ritodrine | 28 w, 0 d | 2 hours | 5.0 |
| 4 | Diet | − | 40 | Administration of ritodrine | 26 w, 1 d | 1 hour | 5.1 |
| 5 | Diet | − | 34 | 21 w, 2 d | 2 hours | 5.1 | |
| 6 | Diet | − | 41 | 31 w, 6 d | 2 hours | 5.4 | |
| 7 | Diet | − | 39 | 25 w, 2 d | 1 hour | 5.5 | |
| 8 | Ins | + | 35 | 30 w, 0 d | 2 hours | 5.2 | |
| 9 | Ins | + | 39 | 31 w, 6 d | 0, 1 and 2 hours | 5.4 | |
| 10 | Ins | + | 36 | 27 w, 0 d | 1 and 2 hours | 5.2 | |
| 11 | Ins | + | 42 | HIV infection, Depression, Obesity | 17 w, 1 d | 1 hour | 4.8 |
| 12 | Ins | + | 37 | Pregnancy-induced hypertension, Obesity | 30 w, 6 d | 2 hours | 5.5 |
| 13 | Ins | + | 30 | Monochorionic diamniotic twins | 25 w, 0 d | 1 hour | 5.7 |
| 14* | Overt | + | 35 | 30 w, 3 d | 1 and 2 hours | 6.8 | |
| 15 | Overt | + | 31 | Hypertension, Obesity | 13 w, 1 d | 1 and 2 hours | 7.2 |
| 16 | Overt | − | 38 | Ulcerative colitis, Obesity | 25 w, 4 d | 0 and 1 hour | 6.7 |
| 17 | DM | + | 37 | Obesity | 9 w, 0 d | Not administered | 9.8 |
Notes:
After two years, this woman visited a doctor because of fatigue and was diagnosed as having insulin-dependent diabetes mellitus. Anti-glutamic acid decarboxylase antibody was positive, and insulin secretion was impaired.
CGM and fetal outcomes
The glycemic SD and MAGE obtained using CGM and the perinatal complications at delivery are shown for both the patients with abnormal glucose tolerance and the two controls in Table 2. The typical CGM patterns, methods of treatment, and infant outcomes are described in the following sections for each group.
Table 2.
Glycemic control values (median, SD, MAGE) and neonatal complications.
| CASE | GROUP | MODE OF DELIVERY | BIRTH WEIGHT (g) | PERINATAL COMPLICATIONS | GESTATIONAL AGE AT CGM | MEDIAN | SD | MAGE |
|---|---|---|---|---|---|---|---|---|
| Control 1 | N/A | N/A | 85.0 | 12.1 | 29.3 | |||
| Control 2 | NVD | 2582 (AFD) | 36 w, 5 d | 79.0 | 13.0 | 34.3 | ||
| 1 | Diet | NVD | 3220 (AFD) | 34 w, 2 d | 94.0 | 16.7 | 33.0 | |
| 2 | Diet | NVD | 2970 (AFD) | Neonatal hypoglycemia | 32 w, 3 d | 99.0 | 28.7 | 53.0 |
| 3 | Diet | pC/S | 3030 (AFD) | 30 w, 2 d | 96.5 | 14.5 | 30.0 | |
| 4 | Diet | pC/S | 2980 (AFD) | Neonatal hypoglycemia, asphyxia | 33 w, 4 d | 107.0 | 20.4 | 49.2 |
| 5 | Diet | pC/S | 2400 (SGA) | Neonatal hypoglycemia | 23 w, 5 d | 80.5 | 22.2 | 53.2 |
| 6 | Diet | NVD | 3575 (AFD) | Neonatal hypoglycemia | 33 w, 3 d | 93.0 | 10.9 | 24.0 |
| 7 | Diet | NVD | 2980 (AFD) | 29 w, 2 d | 89.0 | 25.9 | 52.5 | |
| 8 | Ins | NVD | 3150 (AFD) | Neonatal hypoglycemia | 35 w, 1 d | 95.0 | 24.9 | 31.2 |
| 9 | Ins | pC/S | 3120 (AFD) | Hyperbilirubinemia | 33 w, 2 d | 115.0 | 23.1 | 75.6 |
| 10 | Ins | FD | 2610 (LFG) | Asphyxia, subgaleal hematoma | 31 w, 1 d | 82.0 | 31.3 | 77.5 |
| 11 | Ins | pC/S | 2231 (SGA) | Low birth weight, asphyxia, microphthalmia | 25 w, 3 d | 102.0 | 25.5 | 53.7 |
| 12 | Ins | eC/S | 2447 (AFD) | 32 w, 1 d | 107.0* | 19.8* | 54.3* | |
| 13 | Ins | pC/S | 2520 (AFD)/2220 (AFD) | Neonatal hypoglycemia/low birth weight | 28 w, 2 d | 105.5 | 21.2 | 47.2 |
| 14 | Overt | NVD | 2790 (AFD) | 31 w, 2 d | 140.5* | 44.2* | 84.0* | |
| 15 | Overt | NVD | 3065 (AFD) | 14 w, 1 d | 100.0* | 44.2* | 98.7* | |
| 16 | Overt | pC/S | 3150 (AFD) | 34 w, 5 d | 93.0 | 22.0 | 43.4 | |
| 17 | DM | NVD | 2355 (SGA) | Tetralogy of Fallot | 35 w, 4 d | 125.5* | 19.8* | 62.3* |
Notes: Control 1 was a nonpregnant woman with normal glucose tolerance, and control 2 was a pregnant woman with normal glucose tolerance.
Values after the initiation of insulin injections.
Abbreviations: N/A, not applicable; MAGE, mean amplitude of glycemic excursions; SD, standard deviation; NVD, normal vaginal delivery; pC/S, planned cesarean section; eC/S, emergency cesarean section; FD, forceps delivery; AFD, appropriate-for-date infant; SGA, small-for-gestational age infant; LFG, light-for-gestational age infant.
Control group
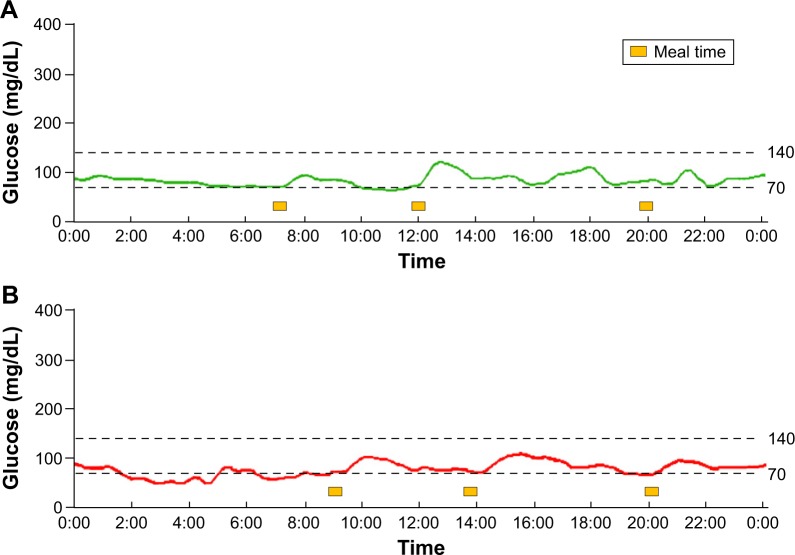
The results of the nonpregnant woman and the pregnant woman with normal glucose tolerance are shown in Figure 2A and B, respectively. Their glucose levels fell within a very narrow range, and the CGM figures were similar.
Figure 2.
(A) CGM data for a nonpregnant woman with normal glucose tolerance. All the glucose levels were within a narrow range. (B) CGM data for a pregnant woman with normal glucose tolerance at 36 weeks and 5 days. The glucose levels showed an almost flat curve and were within a narrower range than those of the nonpregnant woman with normal glucose tolerance. All the glucose values, including those obtained during the postprandial period, were below 120 mg/dL, as measured using CGM.
Diet group
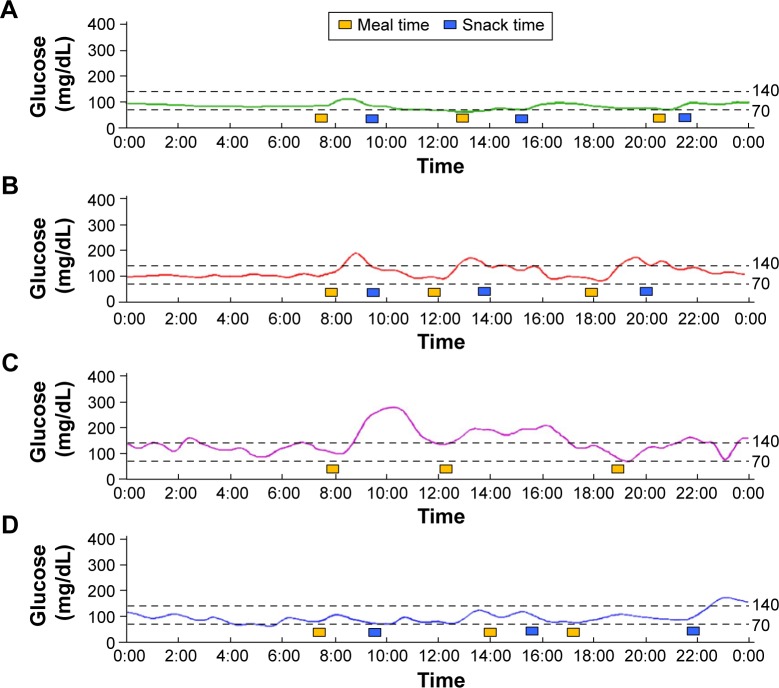
Typical results are shown in Figure 3A (Case 1 in Table 1) after the initiation of nutrition therapy, including the division of caloric intake into five to six meals. The glucose levels were almost flat and fell within a narrow range. Because CGM also did not reveal postprandial hyperglycemia, the patient was treated with diet therapy alone and continued to perform SMBG until delivery. The woman had a normal vaginal delivery at 40 weeks and 6 days of pregnancy. After delivery, the infant presented with transient neonatal hypoglycemia that improved immediately upon glucose administration.
Figure 3.
(A) CGM data for a woman in the Diet group at 34 weeks and 3 days of pregnancy. SMBG monitoring did not reveal preprandial or postprandial hyperglycemia. CGM also showed that the glucose level remained below 140 mg/dL. This case was only treated with diet therapy, including the division of caloric intake into five to six meals. The controlled glycemic level remained within a narrow range until delivery. (B) CGM data for a woman in the Ins group at 33 weeks and 2 days of pregnancy. The CGM data were recorded after caloric restriction and the division of caloric intake into five to six meals. Postprandial hyperglycemia occasionally occurred at one hour after meals; therefore, the woman began receiving insulin injections immediately before meals. (C) CGM data for a woman in the Overt group at 31 weeks and 2 days of pregnancy. The woman had already exhibited postprandial hyperglycemia during SMBG; therefore, caloric restriction and insulin injections had been immediately initiated. The CGM data were recorded after insulin injection and diet therapy. (D) CGM data for a women in the Overt group at 34 weeks and 5 days of pregnancy. The woman had a high HbA1c level (6.7%) when she became pregnant, but her HbA1c level had decreased during her pregnancy. Therefore, she initially received dietary therapy alone. This CGM figure was recorded after dietary therapy, which included the division of caloric intake into five to six meals. Postprandial hyperglycemia was occasionally observed, but most of her glucose levels were below 140 mg/dL. This case continued to be treated with dietary therapy alone.
The SD and MAGE values of the Diet group were lower than those of the other groups of women with abnormal glucose tolerance. Nutrition therapy helped to control accelerated starvation, to attenuate fasting hypoglycemia level, and to ensure only a moderate rise in postprandial glucose levels; this helped to minimize the difference between the pre-prandial and postprandial glucose levels.
Ins group
Typical results are shown in Figure 3B (Case 9 in Table 1). Postprandial hyperglycemia was noted after each meal, even after the initiation of nutrition therapy; therefore, insulin injections were initiated immediately before meals. We frequently adjusted the insulin dose so that the maternal blood glucose level would not exceed 140 mg/dL at any time. Because she had previously undergone a cesarean section, this patient underwent a planned cesarean section at 38 weeks of pregnancy. The neonate was an appropriate-for-date infant, but phototherapy was administrated for hyperbilirubinemia.
In the Ins group, the median maternal glucose level measured using CGM was higher than that in the Diet group. Similarly, the SD and MAGE values were also higher.
Overt group
The CGM profile of a patient in the Overt group is shown in Figure 3C (Case 14 in Table 1). The woman was treated not only with nutrition therapy but also with insulin injections because of postprandial hyperglycemia. The woman had to adjust her insulin dose continuously. Eventually, she was treated with rapid insulin without the use of long-acting insulin injections until delivery.
The CGM profile of another patient in the Overt group is shown in Figure 3D (Case 16 in Table 1). This woman was diagnosed as having overt diabetes in pregnancy but had normal HbA1c levels during the period of observation; she continued to receive only SMBG and nutrition therapy until delivery. The woman had a normal vaginal delivery without perinatal complications.
Among the Overt group, two women had already initiated insulin injections, so the SD and MAGE values for these two patients were used as references. Among the three patients, two women had normal vaginal deliveries and one woman had a planned cesarean section. None of the women had perinatal complications.
DM group
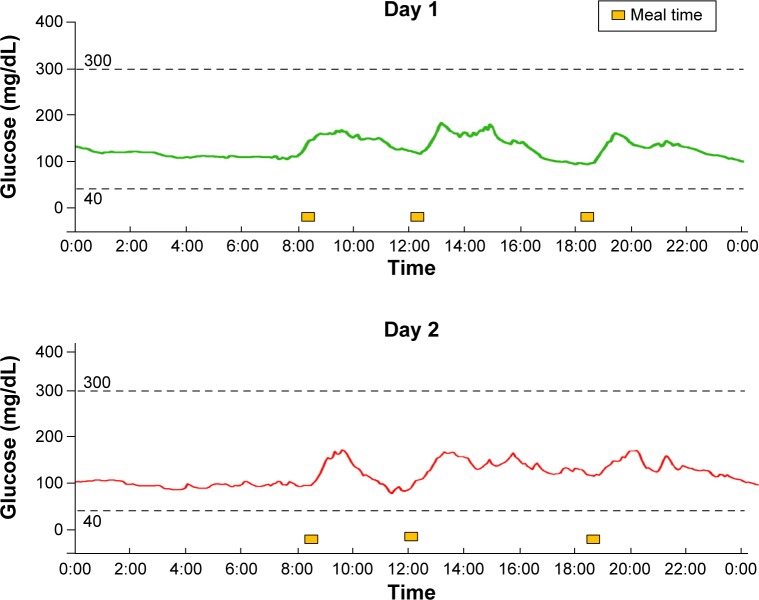
One woman had stopped receiving insulin therapy after her third pregnancy for economic reasons. Her glutamic acid decarboxylase antibody level was negative, and she continued to have the capacity to secrete insulin; therefore, she was regarded as having type 2 diabetes mellitus. She restarted insulin injections using a rapid-acting insulin and an intermediate-acting insulin because of hyperglycemia (Case 17 in Table 1). To adjust her insulin dose, she was asked to wear a CGM. The CGM profile is shown in Figure 4. Since the woman had already initiated insulin therapy, the SD and MAGE values were used as references and were not significantly different from those in the other groups. However, her median glucose level was higher than those in the other groups.
Figure 4.
CGM data for a pregnant woman in the DM group. The HbA1c level of the woman was 9.8%. Soon after the detection of pregnancy, she began receiving insulin injections. The data were recorded at 36 weeks and 1 day and at 36 weeks and 2 days. Because of the adjustment to the insulin dose, excessive hyperglycemia was not observed.
She had a normal vaginal delivery at 38 weeks and 1 day of gestation. The neonate was a small-for-gestational-age infant; after a careful investigation, the infant was diagnosed as having tetralogy of Fallot.
Postpartum
All the women in the Diet, Ins, and Overt groups, but not the mother with preexisting diabetes mellitus, underwent a second 75-g OGTT to confirm whether they had an abnormal glucose tolerance at 12–16 weeks after delivery. The test results showed that none of the women, including those who needed insulin therapy during their pregnancy, exhibited a postpartum abnormal glucose tolerance.
Discussion
In this study, we present the results of CGM and SMBG in pregnant women with abnormal glucose tolerance. Most of the patients had normal deliveries without any serious perinatal complications, except for one woman who had preexisting diabetes mellitus and whose neonate was exposed to maternal hyperglycemia during the organogenesis period.
The goal for the management of abnormal glucose tolerance during pregnancy is to achieve tight glycemic control during the first trimester so as to prevent major malformations and pregnancy loss. For women with preexisting diabetes mellitus, planned pregnancies are very important, because poor glycemic control during the period of organogenesis is known to cause fetal malformations.17 During the second and third trimesters, strict glycemic control prevents excess fetal growth and minimizes metabolic abnormalities at birth, such as neonatal hypoglycemia.18
Women with GDM are known to have higher rates of LGA infants and neonatal hypoglycemia.19 Regarding neonatal hypoglycemia, a previous study has suggested that chronic maternal hyperglycemia can lead to fetal hyperinsulinemia and can cause hypoglycemia at birth.20 Therefore, for a neonate whose mother has GDM, early intervention is needed.
Regarding the target glucose levels during pregnancy, 70–100 mg/dL after fasting and <120 mg/dL at two hours after meal intake are recommended, and the maintenance of a glucose level <140 mg/dL at one hour after meals is reportedly necessary to reduce perinatal complications.10
Moreover, a previous study has suggested that the production of ketone bodies during gestation may affect infants’ neurogenic function.21 Therefore, women were asked to consume an appropriate caloric intake and not to follow a strict diet so as to prevent any increase in ketone bodies because of starvation.
As expected, the SD and MAGE values of the control cases were relatively low; high SD and MAGE values tend to reflect postprandial hyperglycemia, and women who required insulin therapy developed postprandial hyperglycemia despite nutrition therapy. However, after the initiation of nutrition therapy, the SD and MAGE values decreased for all the pregnant women for whom these values could be calculated. Accordingly, nutrition therapy for pregnant women with abnormal glucose tolerance was considered to be a much more effective way of improving glycemic control, regardless of insulin therapy.
In this case series, three cases among the Overt group did not have perinatal complications. Previous studies have shown that maternal hyperglycemia during the period of organogenesis causes fetal malformations, and the rate of deformity is correlated with the maternal HbA1c level.18 In this study, neonatal malformations might not have occurred in the offspring of the women with overt diabetes mellitus because of the relatively low level of maternal HbA1c.
In this study, we analyzed the glycemic variability in women not only with GDM but also with overt diabetes in pregnancy and prepregnancy diabetes. A major limitation of this study was its small sample size, because only 17 participants were included. Further studies involving larger numbers of women are needed to confirm the present findings.
Regarding the pregnant women with pregestational diabetes, a previous study showed that a group of pregnant women with diabetes who used both SMBG and CGM had lower HbA1c levels and a reduced risk of macrosomia compared with a group of pregnant women who used only SMBG for glycemic control.22 However, another large study suggested that the use of real-time CGM in women with pregestational diabetes did not improve pregnancy outcomes.23 This previous study differs from this investigation in that it included type 1 diabetic patients. A subsequent multicenter study showed that many women developed LGA infants, and LGA was associated with higher mean glucose levels during the second and third trimesters and higher maternal glucose levels during the evening.24 In these large studies, the use of CGM for pregnant women with pregestational diabetes did not further improve pregnancy outcome in terms of reducing perinatal complications; however, the use of CGM enabled the insulin dose to be adjusted and better glycemic control to be achieved.
Moreover, a previous study has shown that women with a history of GDM have a higher risk (odds ratio, 7.4) of developing type 2 diabetes in the future compared with women without a history of GDM.25 Therefore, follow-up monitoring of glucose tolerance is needed in mothers, even after delivery. A previous study showed that energy expenditure might increase during lactation.26 Because women were evaluated for glucose tolerance during lactation, the improvement of insulin resistance might have been only temporary.27,28 Thus, women should be encouraged to undergo frequent examinations throughout the postpartum period.
Conclusion
In conclusion, compared with nonpregnant and pregnant woman with normal glucose tolerance, pregnant women with abnormal glucose tolerance tend to exhibit greater glucose fluctuations mainly because of postprandial hyperglycemia. For pregnant women with abnormal glucose tolerance, the range of glucose variability tended to be at least slightly higher than that of woman with normal glucose tolerance. Reducing glucose fluctuations is extremely important so that they approximate those of pregnant women with normal glucose tolerance. Furthermore, the use of CGM may have beneficial effects on perinatal glycemic control, such as the detection of postprandial hyperglycemia, in pregnant women.
Footnotes
ACADEMIC EDITOR: Yasuo Ito, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1095 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Mitsuhiko Noda has received speaker honoraria from Sanofi, Mitsubishi Tanabe Pharma, Daiichi Sankyo, Eli Lilly Japan, MSD, Sanwa Kagaku Kenkyusho, Ono Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, Astellas, Kowa Pharmaceutical Co. Ltd, Taisho Toyama Pharmaceutical Co. Ltd, Kissei Pharmaceutical Co. Ltd, Meiji Seika Pharma Co. Ltd, Kyowa Hakko Kirin Co. Ltd, ABBVie Inc. and Johnson & Johnson K.K. and has received research grants from Takeda Pharmaceutical Co. Ltd, Daiichi Sankyo, Mitsubishi Tanabe Pharma and Kyowa Hakko Kirin Co. Ltd. Other authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: MT, MK, and MN. Analyzed the data: MT. Wrote the first draft of the manuscript: MT. Contributed to the writing of the manuscript: MK and MN. Agreed with the manuscript results and conclusions: MT, MK, MY, TY, and MN. Jointly developed the structure and arguments for the paper: MT, MY, and MN. Made critical revisions and approved the final version: MT, MK, and MN. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Norbert F. Banting lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1006–1022. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Li H, Ran X, et al. Reference values for continuous glucose monitoring in Chinese subjects. Diabetes Care. 2009;32(7):1188–1193. doi: 10.2337/dc09-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Fox LA, Beck RW. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care. 2010;33(6):1297–1299. doi: 10.2337/dc09-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsujino D, Nishimura R, Taki K, Miyashita Y, Morimoto A, Tajima N. Daily glucose profiles in Japanese people with normal glucose tolerance as assessed by continuous glucose monitoring. Diabetes Technol Ther. 2009;11(7):457–460. doi: 10.1089/dia.2008.0083. [DOI] [PubMed] [Google Scholar]
- 5.Yariv Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Lager O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191:576–581. doi: 10.1016/j.ajog.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez TL, Friedman JE, Van Pelt RE, Barbour LA. Patterns of glycemia in normal pregnancy: should the current therapeutic targets be changed? Diabetes Care. 2011;34(7):1660–1668. doi: 10.2337/dc11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kestilä KK, Ekblad UU, Rönnemaa T. Continuous glucose monitoring versus self-monitoring of blood glucose in the treatment of gestational diabetes mellitus. Diabetes Res Clin Pract. 2007;77:174–179. doi: 10.1016/j.diabres.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Siegmund T, Rad NT, Ritterath C, Siebert G, Henrich W, Buhling KJ. Longitudinal changes in the continuous glucose profile measured by the CGMS® in healthy pregnant women and determination of cut-off values. Eur J Obstet Gynecol Reprod Biol. 2008;139:46–52. doi: 10.1016/j.ejogrb.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Willman SP, Leveno KJ, Guzick DS, Williams ML, Whalley PJ. Glucose threshold for macrosomia in pregnancy complicated by diabetes. Am J Obstet Gynecol. 1986;154(2):470–475. doi: 10.1016/0002-9378(86)90692-7. [DOI] [PubMed] [Google Scholar]
- 10.de Veciana M, Major CA, Morgan MA, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237–1241. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 11.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 12.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Investig. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross TM, Bode BW, Einhorn D, et al. Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol Ther. 2000;2:49–56. doi: 10.1089/152091500316737. [DOI] [PubMed] [Google Scholar]
- 14.Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31:1160–1164. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health, Labour and Welfare of Japan The Dietary Reference Intakes for Japanese. 2010 ed. 2015. [Accessed December 15, 2015]. Available at: http://www.mhlw.go.jp/bunya/kenkou/sessyu-kijun.html.
- 16.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 17.Miller E, Hare JW, Cloherty JP, et al. Evaluated maternal hemoglobin A1c in early pregnancy and major congenital anomalies in infants of diabetic mothers. N Engl J Med. 1981;304:1331–1334. doi: 10.1056/NEJM198105283042204. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez TL, Barbour LA. A standard approach to continuous glucose monitor data in pregnancy for the study of fetal growth and infant outcomes. Diabetes Technol Ther. 2013;15(2):172–179. doi: 10.1089/dia.2012.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheffield JS, Butler-Koster EL, Casey BM, Mclntire DD, Leveno KJ. Maternal diabetes mellitus and infant malformations. Obstet Gynecol. 2002;100:925–930. doi: 10.1016/s0029-7844(02)02242-1. [DOI] [PubMed] [Google Scholar]
- 20.Metzger BE, Persson B, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcome study: neonatal glycemia. Pediatrics. 2010;126:1545–1552. doi: 10.1542/peds.2009-2257. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo T, Metzger BE, Burns WJ, Burns K. Correlations between antepartum maternal metabolism and intelligence of offspring. N Engl J Med. 1991;325:911–916. doi: 10.1056/NEJM199109263251303. [DOI] [PubMed] [Google Scholar]
- 22.Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomized clinical trial. BMJ. 2008;337:a1680. doi: 10.1136/bmj.a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes. Diabetes Care. 2013;36:1877–1883. doi: 10.2337/dc12-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law GR, Ellison GT, Secher AL, et al. Analysis of continuous glucose monitoring in pregnant women with diabetes: distinct temporal patterns of glucose associated with large-for-gestational-age infants. Diabetes Care. 2015;38:1319–1325. doi: 10.2337/dc15-0070. [DOI] [PubMed] [Google Scholar]
- 25.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta- analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 26.Butte NF, Hopkinson JM, Mehta N, Moon JK, Smith EO. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am J Clin Nutr. 1999;69:299–307. doi: 10.1093/ajcn/69.2.299. [DOI] [PubMed] [Google Scholar]
- 27.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 28.Gunderson EP, Jacobs DR, Jr, Chiang V, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus: a 20-year prospective study in CARDIA. Diabetes. 2010;59:495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]