Abstract
Exposure to adverse life events during pregnancy has been linked to increased risk of schizophrenia spectrum disorders (SSD) in offspring. Nevertheless, much of the previous work inferred maternal stress from severe life events rather than directly assessing maternal reports of stress. The present study aimed to examine maternal reports of stress during pregnancy and risk for offspring SSD. Participants were 95 SSD cases and 206 controls who were offspring from a large birth cohort study that followed pregnant women from 1959–1966. During pregnancy interviews, women were asked if anything worrisome had occurred recently. Interviews were qualitatively coded for stress-related themes, including reports of daily life stress, by two independent raters. None of the maternal psychosocial stress themes were significantly associated with increased odds of offspring SSD in analyses of the full sample. However, results indicated a significant daily life stress by infant sex interaction. Maternal daily life stress during pregnancy was associated with significantly increased odds of SSD among male offspring. Findings suggest sex-specific fetal sensitivity to maternal reported daily life stress during pregnancy on risk for SSD, with males appearing to be more vulnerable to the influences of maternal stress during pregnancy.
Keywords: psychosis, sex differences, prenatal stress
1. Introduction
A growing body of literature suggests an association between prenatal maternal stress and a variety of adverse offspring outcomes, including neurodevelopmental disorders like schizophrenia and autism spectrum disorders (Class et al., 2014; Walder et al., 2014). A number of stressful life events during pregnancy, including exposure to war, earthquake, flood, and death of a spouse or relative, have been associated with offspring schizophrenia (Huttunen and Niskanen, 1978; Selten et al., 1999; Watson et al., 1999; Malaspina et al., 2008). Similarly, unwantedness of pregnancy has been linked to offspring schizophrenia spectrum disorders (SSD) (Myhrman et al., 1996; Herman et al., 2006; McNeil et al., 2009). These findings support a link between maternal stress and offspring SSD; however, much of the previous work presumed a uniform level of stress based on adverse events experienced by a population (van Os and Selten, 1998; Selten et al., 1999), or examined offspring SSD in relation to relatively rare traumatic events, such as the death of a close relative, during pregnancy (Khashan et al., 2008). Additionally, results have been inconsistent, as two recent studies found no significant association between prenatal bereavement stress and offspring SSD (Abel et al., 2014; Class et al., 2014).
Studies have also found that unwanted pregnancy (i.e., a negative or ambivalent attitude towards the pregnancy/reproduction, a potential prenatal stressor) is associated with increased risk of psychosis in offspring (Myhrman et al., 1996; Herman et al., 2006; McNeil et al., 2009). These studies made methodological improvements by prospectively measuring maternal psychological experiences during pregnancy; however, they were still limited by not including direct assessments of perceived stress among the mothers. However, one study assessed both unwanted pregnancy and stress throughout pregnancy, and found that controlling for general pregnancy stress strengthened the relationship between unwanted pregnancy and offspring SSD; however, findings were limited to offspring of pregnant women with psychosis and may not generalize (McNeil et al., 2009). Further, factors other than stress processes could be related to unwantedness of a pregnancy (e.g., health-risk behaviors). Methodological improvements were also made in longitudinal birth cohort studies that linked stressful life events during pregnancy to offspring psychotic experiences, although it is unclear whether these findings extend to diagnosable psychotic disorders, such as schizophrenia (Betts et al., 2014; Dorrington et al., 2014).
Although there is now a large body of work linking prenatal maternal stress to offspring schizophrenia, few studies have specifically examined sex differences despite evidence that males and females differ in their vulnerability to the adverse influences of prenatal stress (Mueller and Bale, 2008; Sandman et al., 2013) and in the onset and course of schizophrenia (Castle and Murray, 1991; Leung and Chue, 2000; Walker et al., 2002). Compared to females, male fetuses appear to be at particular risk for early mortality and morbidity following prenatal stress exposure (Sandman et al., 2013). Similarly, a growing body of evidence suggests that males may be more vulnerable to prenatal gonadal hormone disruptions than females, contributing to sexual dimorphisms in behavioral sequelae and risk for psychopathology (Walder et al., 2006). Some, but not all (reviewed in (Cannon et al., 2002; Goldstein and Walder, 2006)), studies have found that males with schizophrenia are more likely to have a history of pre- or perinatal events (Hultman et al., 1999) or obstetric complications (Foerster et al., 1991; Matsumoto et al., 2001) than their female counterparts, suggesting a more “neurodevelopmental” form of the disorder (Castle and Murray, 1991). Taken together, these studies underscore the importance of understanding sexually differentiated predictors associated with risk for psychosis (Walder et al., 2013).
The present study used prospectively collected maternal reports during pregnancy to examine whether prenatal maternal psychosocial stress is related to offspring SSD. Based on studies linking stress during pregnancy to increased risk of offspring SSD and to birth outcomes associated with SSD (Myhrman et al., 1996; Dole et al., 2003; Slykerman et al., 2005; Diego et al., 2006; Khashan et al., 2008), we examined the following stress themes: traumatic life events (TLEs), daily life stress, negative affect, and pregnancy-specific anxiety. We expected that TLEs would be associated with offspring SSD, based on research linking severe life events during pregnancy to offspring SSD (van Os and Selten, 1998; Selten et al., 1999; Khashan et al., 2008). We also predicted that daily life stress and negative affect during pregnancy would be associated with increased risk of offspring SSD, given findings linking these constructs to offspring SSD (Norman and Malla, 1993; Malaspina et al., 2008) and birth outcomes associated with SSD (Cannon et al., 2002). We explored pregnancy-specific anxiety based on known associations between pregnancy-specific anxiety and birth outcomes associated with SSD (Rini et al., 1999), but these analyses were exploratory based on lack of evidence supporting a direct relationship between pregnancy-specific anxiety and offspring SSD. Finally, we examined interactions between fetal sex and maternal stress on risk for offspring SSD, given known sex differences in SSD and in the influence of prenatal stress and stress hormones on fetal development (Leung and Chue, 2000; Mueller and Bale, 2008). In keeping with the previously discussed studies suggesting that males with schizophrenia more often have a history of pre- and perinatal adversity relative to their female counterparts (reviewed in (Goldstein and Walder, 2006; Abel et al., 2010)), we predicted that male fetuses would be particularly sensitive to prenatal stress (Ellman et al., 2008; Mueller and Bale, 2008), such that prenatal maternal stress would increase risk of SSD among males.
2. Methods
2. 1 Participants
Participants provided written informed consent and the study was approved by the Institutional Review Boards of the New York State Psychiatric Institute, Kaiser Foundation Research Institute, and Temple University. Participants were derived from the Prenatal Determinants of Schizophrenia (PDS)-I and PDS-II studies, which were subsamples of the Child Health and Development Study (CHDS) (Susser et al., 2000). The CHDS prospectively enrolled nearly all pregnant women receiving prenatal care from the Kaiser Foundation Health Plan (KFHP) clinics in Alameda County, California from 1959–1966 (live births N = 19,044).
PDS-I and II were designed to examine the relationship between pre-/perinatal factors and SSD. PDS-I used KFHP’s computerized registries to ascertain potential cases of SSD. PDS-II extended PDS-I by ascertaining new SSD cases from two sources: 1) SSD cases among KFHP members with onset following the end of PDS-I ascertainment (1998–2005); 2) SSD cases with onset after leaving KFHP.
2.2 Case Ascertainment
A “case” was defined as an individual diagnosed with SSD (schizophrenia, schizoaffective disorder, schizotypal personality disorder, other nonaffective psychoses). PDS-I case ascertainment methods have been described elsewhere (Susser et al., 2000). PDS-I included 71 cases of SSD: 43 schizophrenia, 17 schizoaffective, 5 schizotypal personality disorder, 1 delusional disorder, 5 nonaffective psychosis.
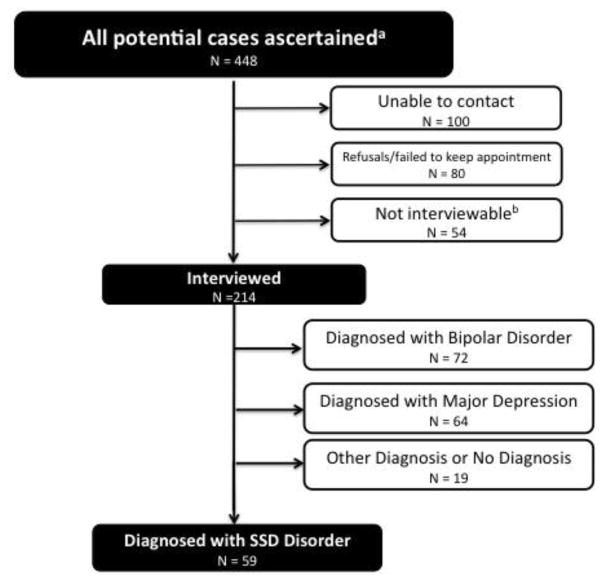
For PDS-II (see Figure 1 for flow chart and (Parboosing et al., 2013) for detailed description of methods), potential cases were ascertained using KFHP electronic records, Alameda County Behavioral Health Care Services (ACBHCS) electronic records, and a mailing to CHDS mothers and children. Individuals were ascertained through KFHP records for those who received treatment during KFHP membership. Individuals who left KFHP before receiving treatment and remained in Alameda County were ascertained from ACBHCS treatment records. Individuals ascertained through the KFHP and ACBHCS were screened for ICD-9 diagnoses 295–298. Remaining cohort members were mailed a questionnaire. If a questionnaire respondent endorsed “mental health problems” in an eligible cohort member, a KFHP study interviewer contacted the individual and administered the Family Interview for Genetic Studies (FIGS) to screen for psychotic illness. In total, 448 potential cases were identified. Two hundred fourteen of the 448 potential cases were interviewed using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1994). Fifty-nine individuals were diagnosed with SSD (20 schizophrenia, 17 schizoaffective-bipolar subtype, 15 schizoaffective-depressive subtype, 7 psychotic disorder not otherwise specified). Three diagnosticians and a primary interviewer assigned a consensus diagnosis. In total there were 130 cases from PDS-I and PDS-II, 95 of whom had available interview data.
Figure 1.
PDS-II Flow Chart
aFrom database linkages with Kaiser Permanente Medical Care Plan (KPMCP), Alameda County Behavioral Health Care Services (ABHCS) (see Methods for further description of database diagnoses) and subjects screening positive following mailed questionnaire on mental health to CHDS mothers and offspring. Additional information on ascertainment methods can be found in a recent publication that used the PDS II cohort to examine bipolar disorder (Parboosing et al., 2013).
bIncludes deceased, incarcerated, no permission from physician, too ill (psychosis, severe mental disability) Note: PDS I has been described elsewhere (see Susser et al., 2000).
2.3 Matched Controls
Eligible controls excluded siblings of cases, and only one person was selected as a control from families with multiple siblings in CHDS. PDS-I control matching has been described elsewhere (Susser et al., 2000). For PDS-II, each case was matched to up to 8 controls based on ascertainment method: cases ascertained from KFHP were matched to controls who were KFHP members at the time of case ascertainment; cases ascertained from ACBHCS and screenings of the mailed questionnaire were matched to controls who were residents of Alameda County (and not KFHP members). PDS-II controls were screened to not be positive for bipolar/psychotic disorders or part of PDS-I and were matched to cases on date of birth and sex. Matching procedures resulted in 754 potential controls from PDS-II. For the present study, two controls were selected at random from matched sets resulting in 260 controls; however, only 206 had available interview data.
2.4 Maternal Interviews
Interviews during pregnancy were conducted on 77% of the CHDS study cohort; some additional interviews were lost over the 50 years of follow-up (while all subjects were interviewed, at the time of this study interview data was available for 95 of 130 cases and 206 of 260 controls). Mean gestational age at time of interview was 15.85 weeks (early second trimester).
2.5 Measures of Psychosocial Stress
Maternal psychosocial stress during pregnancy was measured by extracting stressful life events and stress-related themes from responses to the question, “What kinds of things have been worrying you recently?” Mothers responded in an open-ended narrative, frequently providing detailed information on a variety of stressors (e.g., financial, relationship) and stressful life events.
Two independent raters followed a detailed coding manual, developed by the authors based on well-established definitions and/or measures of the constructs of interest (for details, see below) to extract stress-related themes. To minimize bias, inter-rater reliability was established after coders analyzed 50 interviews chosen at random. Reliability was evaluated using intraclass correlations (ICCs), which are the most appropriate statistic for continuous data (Shrout and Fleiss, 1979). ICCs ranged from 0.7 to 1.0 (mean ICC=0.90; substantial to almost perfect reliability). ICCs of 0.7 or above on the aforementioned test cases were required before a coder was allowed to analyze study interviews.
Raters, blind to offspring case-control status, coded interviews for the following themes: TLEs, daily life stress, negative affect, and pregnancy-specific anxiety. These themes were chosen because these constructs occurring during pregnancy have previously been associated with risk of offspring SSD or obstetric factors associated with SSD (Norman and Malla, 1993; van Os and Selten, 1998; Rini et al., 1999; Selten et al., 1999; Cannon et al., 2002; Khashan et al., 2008; Malaspina et al., 2008). Stress-related themes were operationally defined and coded based on well-established definitions of the constructs (e.g., (Cohen et al., 1997; Serido et al., 2004; Khashan et al., 2008)) and/or using validated measures of the construct as a guide (e.g., (Cohen et al., 1983; Rini et al., 1999)). TLEs were coded if there was actual or threatened death or serious injury, threat to physical integrity of self/others, and/or loss or diagnosis with cancer, acute myocardial infarction, or cerebrovascular accident of a close relative (Khashan et al., 2008). Daily life stress was coded if the mother reported discrete, observable events that required an adjustment in identity or routines (Cohen et al., 1997) or relatively minor events that disrupt daily life (Serido et al., 2004). Examples of daily life stress include marriage difficulties, moving, chronic (not life-threatening) illness of family member, and financial stress. Using the Perceived Stress Scale as a model, negative affect was coded if the mother appraised situations in her life as overwhelming or difficult (e.g., “emotional strain” or feeling “upset”) (Cohen et al., 1983). Pregnancy-specific anxiety was coded if the mother reported worries or concerns about the pregnancy (e.g., fear of losing baby) (Rini et al., 1999). Coding documented the frequency with which women reported stress themes; however, stress themes were reduced to dichotomous (present/absent) variables due to limited variability in frequency data (data available upon request). Stress themes were coded using ATLAS.ti 6 (ATLAS.ti Scientific Software Development GmbH, Berlin, Germany).
2.6 Statistical Analysis
Given that pre- and perinatal factors can directly or indirectly influence offspring development (Siegel, 1982) and that many of these variables have been associated with risk for schizophrenia and/or obstetric complications associated with schizophrenia (Cannon et al., 2002; Laurens et al., 2015), infant sex, maternal race, maternal age, maternal psychiatric status during pregnancy, gestational age (GA) at birth, GA at time of interview, and birth weight (BW) were examined as potential covariates. We determined which variables to include as covariates by exploring whether the aforementioned pre- and perinatal factors were associated with the main independent (maternal stress-related themes) and dependent variables (offspring SSD). Maternal psychiatric diagnoses were made by a physician and extracted from medical records using ICD coding. Potential demographic differences in PDS-I and II participants were examined and differences were controlled for (see Table 2). Maternal education was controlled for in analyses, as it has been strongly correlated with other measures of socioeconomic status (SES) (e.g., income, employment) in the PDS study and is often used to control for potential contributions of postnatal adversity to risk of developmental sequelae (Schlotz and Phillips, 2009). Additionally, maternal education had less missing data than the other variables related to SES and fewer problems with interpretation (e.g., the income variable is categorical, has a lot of missing data, and is based on incomes from the 1960’s). Moreover, there were significant differences in maternal education between cases and controls (see Table 1) and significant differences in maternal education between cases in the PDS-I and II studies (see Table 2); therefore controlling for this variable also allowed us to control for potential biases in ascertainment between the two arms of the PDS study. Although we coded TLEs, we lacked sufficient power to conduct planned statistical analyses due to the low frequency of TLEs in our sample (see Table 1). Separate logistic regression analyses (for each remaining stress theme) were conducted to test whether maternal stress during pregnancy was associated with increased odds of offspring SSD. Separate logistic regression analyses were also conducted to examine whether the interaction between maternal psychosocial stress and infant sex was associated with increased odds of offspring SSD. If a model had a significant sex by stress interaction, logistic regressions were conducted stratifying by sex to examine sex-specific contributions of maternal stress during pregnancy to offspring SSD, given that the odds ratios associated with interaction terms are not interpretable. Statistical analyses were conducted using SAS version 9.3 software (SAS, Inc., Cary, NC). All tests were two-tailed with p < 0.05 indicating significance.
Table 2.
Demographic characteristics of PDS-I and PDS-II (Means, standard deviations, frequencies, and percentages).
| Characteristic | PDS-I (n = 155) | PDS-II (n = 146) | Analysis | ||
|---|---|---|---|---|---|
| N | % | N | % | p-value | |
| Female | 52 | 33.55 | 61 | 41.78 | 0.14 |
| Maternal Raceb | |||||
| Caucasian | 85 | 57.43 | 77 | 53.47 | 0.69 |
| African American | 53 | 35.81 | 54 | 37.50 | |
| Asian | 10 | 6.76 | 13 | 9.03 | |
| Traumatic Life Events | 13 | 8.39 | 9 | 6.16 | 0.46 |
| Daily Life Stress | 55 | 35.48 | 61 | 41.78 | 0.26 |
| Negative Affect | 33 | 21.29 | 23 | 15.75 | 0.21 |
| Pregnancy Specific Anxiety | 22 | 14.19 | 25 | 17.12 | 0.48 |
| Mean | SD | Mean | SD | p-value | |
| Birth Weight (Grams) | 3328.84 | 524.55 | 3321.61 | 580.19 | 0.91 |
| Gestational Age at Interview (Weeks) | 16.52 | 6.70 | 15.12 | 6.80 | 0.07 |
| Gestational Age at Birth (Weeks) | 40.13 | 2.36 | 39.94 | 2.42 | 0.50 |
| Maternal Age | 28.11 | 6.10 | 29.27 | 8.41 | 0.17 |
| Maternal Educationa | 2.98 | 1.41 | 2.82 | 1.76 | < 0.01 |
Maternal education values are based on a rating scale in which 2 = high school graduate – no other special school; and 3 = high school graduate – plus special school like trade school, not college
Missing data for 7 subjects from PDS-I and 2 subjects from PDS-II; Percentages for PDS-I calculated using n = 148; Percentages for PDS-II calculated using n = 144
Table 1.
Demographics characteristics and endorsement of stress-related themes (Means, standard deviations, frequencies, and percentages).
| Characteristic | Cases (n = 95) | Controls (n = 206) | Analysis | ||
|---|---|---|---|---|---|
| N | % | N | % | p-value | |
| Female | 33 | 34.74 | 80 | 38.83 | 0.49 |
| Maternal Raceb | |||||
| Caucasian | 41 | 44.56 | 121 | 60.5 | < 0.01 |
| African American | 48 | 52.17 | 59 | 29.5 | |
| Asian | 3 | 3.26 | 20 | 10 | |
| Traumatic Life Events | 4 | 4.21 | 18 | 8.74 | 0.16 |
| Daily Life Stress | 40 | 44.10 | 76 | 36.89 | 0.39 |
| Negative Affect | 21 | 22.10 | 35 | 16.99 | 0.29 |
| Pregnancy Specific Anxiety | 14 | 14.74 | 33 | 16.02 | 0.78 |
| Mean | SD | Mean | SD | p-value | |
| Birth Weight (Grams) | 3325.30 | 584.99 | 3325.345 | 536.56 | 0.99 |
| Gestational Age at Interview (Weeks) | 16.10 | 6.37 | 15.74 | 6.96 | 0.67 |
| Gestational Age at Birth (Weeks) | 40.19 | 2.35 | 39.974 | 2.40 | 0.47 |
| Maternal Age | 28.82 | 9.44 | 28.60 | 6.13 | 0.81 |
| Maternal Educationa | 2.73 | 1.72 | 2.98 | 1.52 | 0.02 |
Maternal education values are based on a rating scale in which 2 = high school graduate – no other special school; and 3 = high school graduate – plus special school like trade school, not college
Missing data for 3 cases and 6 controls; Percentages calculated based on 92 cases and 200 controls
3. Results
Cases and controls did not differ on infant sex, maternal age, BW, GA, or GA at the time of maternal interviews; therefore these variables were not controlled for in analyses (see Table 1). Cases and controls differed on maternal race; however, there were no significant differences in endorsement of stress themes by race (all ps > 0.05). There were no significant differences between PDS-I and II subjects on maternal race, maternal age, BW, GA, infant sex, or any of the stress-related themes (see Table 2). There were significant differences in maternal education between PDS-I and II subjects and between cases and controls, with women in PDS-I and mothers of controls having higher levels of education (see Table 2). GA and GA at time of interview were not significantly related to SSD or any of the stress-related themes. There were no cases of SSD among pregnant women in the sample based on CHDS medical records (Parboosing et al., 2013). The stress-related themes appeared to be largely independent constructs and were only weakly correlated (rtet = 0.01–0.47; data available upon request).
No maternal psychosocial stress themes were associated with statistically significant increased odds of offspring SSD (see Table 3); however, the daily life stress by infant sex interaction model was significant (p = 0.009; p = 0.008 after controlling for maternal education). After controlling for maternal education, maternal daily life stress during pregnancy was significantly associated with increased odds of SSD among male offspring (ncases = 62, ncontrols = 126; OR = 1.995, p = 0.032). Results for male offspring were consistent in unadjusted analyses (ncases = 62, ncontrols = 126; OR = 2.091, p = 0.027). Results for female offspring failed to reach significance in adjusted and unadjusted analyses (ncases = 33, ncontrols = 80; OR = 0.437, p = 0.071; OR = 0.505, p = 0.121).
Table 3.
Stress Themes and Odds Ratios for Risk of SSD in Offspring
| Stress Theme |
All Offspring, unadjusteda | All Offspring, adjusted for maternal educationa |
Male Offspring, unadjustedb | Male Offspring, adjusted for maternal educationb |
Female Offspring, unadjustedc |
Female Offspring, adjusted for maternal educationc |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Perceived Negative Affect | 1.387 | .756 – 2.541 | 0.290 | 1.356 | .735 – 2.499 | 0.330 | 1.075 | .495 – 2.334 | 0.855 | 1.082 | .496 – 2.362 | 0.843 | 2.125 | .769 – 5.672 | 0.132 | 1.967 | .725 – 5.335 | 0.184 |
| Pregnancy-Specific Anxiety | 0.906 | .460 – 1.786 | 0.776 | 0.956 | .482 – 1.896 | 0.897 | 1.254 | .584 – 2.696 | 0.562 | 1.293 | .598 – 2.797 | 0.513 | 0.196 | .024 – 1.584 | 0.126 | 0.219 | .027 – .787 | 0.156 |
| Daily Life Stress | 1.244 | .758 – 2.043 | 0.388 | 1.159 | .700 – 1.920 | 0.566 | 2.091 | 1.119 – 3.907 | 0.021 | 1.995 | 1.061 – 3.750 | 0.032 | 0.505 | 0.213 – 1.197 | 0.121 | 0.393 | .178 – 1.073 | 0.071 |
All offspring analyses included 95 cases and 206 controls.
Male offspring analyses included 62 cases and 126 controls.
Female offspring analyses included 33 cases and 80 controls.
4. Discussion
This is the first study, to our knowledge, to prospectively examine open-ended maternal reports of psychosocial stress and stressful life events (including more routine, daily life stress) during pregnancy in relation to offspring SSD in a large, unselected cohort-based sample. Analyses of the full sample found no significant associations between maternal psychosocial stress and offspring SSD. However, among male offspring, exposure to maternal daily life stress during pregnancy was associated with a two-fold increase in the odds of developing SSD in adulthood. Results suggest that collapsing across infant sex obscured findings in initial analyses and that maternal stress during pregnancy may have sex-specific influences on fetal development resulting in increased vulnerability for SSD among males. Whereas previous research linked SSD to relatively rare traumatic events during pregnancy (Huttunen and Niskanen, 1978; Khashan et al., 2008), this study suggests that prenatal exposure to routine stressors (e.g., moving, time-limited financial or relationship challenges) that occur fairly commonly in the population can have a lasting influence on offspring development.
Although we expected that exposure to maternal psychosocial stress during pregnancy would be associated with increased risk of SSD in the overall sample, there are a few possible explanations for our sex-specific findings. Specifically, our findings are consistent with a growing body of literature suggesting that prenatal stress differentially influences male and female fetuses (Ellman et al., 2008), such that males are more vulnerable to the deleterious influences of prenatal maternal stress and are at increased risk for mortality and morbidity following exposure (Mueller and Bale, 2008; Sandman et al., 2013). Relatedly, fetal exposure to increases in second trimester maternal cortisol has been associated with decreased infant maturation among males but not females, an obstetric complication that has been associated with risk of schizophrenia (Ellman et al., 2008; Fineberg et al., 2013). Furthermore, the activity of placental 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2), the cortisol-inactivating enzyme, seems to be sex-linked, as placentae from male fetuses exhibit decreased 11β-HSD2 activity following betamethasone exposure (Stark et al., 2009). This is especially important, as 11β-HSD2 activity seems to be responsible for only 10–20% of maternal cortisol crossing the placenta in weeks 13 to 35 (reviewed in (Ellman et al., 2008)). Human fetal plasma testosterone, which is higher in male versus female fetuses during earlier gestation, has been significantly correlated with fetal plasma cortisol (Gitau et al., 2005), suggesting that differential sensitivity of male fetuses to stress hormones might be partially attributable to higher baseline concentrations of fetal cortisol and testosterone (Beck-Peccoz et al., 1991; Gitau et al., 2005). Similarly, the human placenta exhibits sexually dimorphic characteristics (e.g., non-random X inactivation), which in adverse environments may confer an advantage for females over males (Reik and Lewis, 2005; Clifton, 2010). Although we believe that our results reflect the differential influences of maternal stress during pregnancy on fetal development by sex, it is possible that we may have been slightly underpowered to detect effects among female offspring, as 62% of the sample was male (see Table 1). Potential support for this idea comes from our finding that maternal perceived negative affect during pregnancy was associated with a similarly large yet non-significant increase in the risk of SSD among female offspring (see Table 3). Future research should examine whether these sex-specific findings remain in a larger, more gender balanced sample.
Although we detected significant results for the association between daily life stress and offspring SSD, we did not detect significance for other stress-related themes. Cohort effects may have influenced our ability to detect results with negative affect, as “stress” was an uncommonly used term in the 1950s/60s and women may have been reticent to make spontaneous appraisals of stress. Analyses for pregnancy-specific anxiety were exploratory and our results suggest that pregnancy-specific anxiety might not contribute to risk of offspring SSD. We were underpowered in our ability to conduct analyses for TLEs; though it is probable that, consistent with previous studies, more severe life events would also increase risk for SSD. Our results extend previous findings by suggesting that the link between maternal stress during pregnancy and risk of SSD may not be restricted to rare, severe TLEs. Another methodological limitation that may have contributed to our ability to detect significance is that some variables associated with the impact of stress exposure (Cohen et al., 1997), including exact timing, duration, and severity (beyond traumatic versus non-traumatic life event) of stress exposure, could not be determined. Previous studies suggest that early second trimester (corresponding to the preponderance of our interviews) is a key period for contributions of maternal stress on alterations of fetal development (Ellman et al., 2008). However, the study design did not allow for assessment of factors like the exact timing of stress exposure relative to the time at which women completed the pregnancy interview. Previous research has found that timing of stress exposure is related to women’s emotional reactivity to stress (Glynn et al., 2001) and the influence of stress on offspring development (Davis and Sandman, 2012). Additionally, although the majority (60%) of our prenatal interviews were conducted during the second trimester, the range of mean gestational age at time of interview was wide (2 – 37 weeks), which is a limitation. Finally, the maternal interviews used in the CHDS studies were designed to assess numerous aspects of pregnancy rather than maternal psychosocial stress specifically and may have been less sensitive than a measure designed for that specific purpose.
Our study has a number of strengths, including prospectively collected maternal stress data and rigorous diagnostic case assessment. Additionally, other studies have analyzed stress-related themes from open-ended narratives, providing support for the feasibility of our methods (Duggan et al., 2008). There are also limitations of using qualitative measures of stress. Although coders used a manual and exhibited very high inter-rater reliability, there is the potential for subjectivity to be introduced. However, coders were blind to case-control status; therefore, there is no reason to believe that subjectivity related to coding would preferentially favor cases over controls. One advantage of our daily stress variable is that it captures many of the common, mild-to-moderately stressful events of daily living experienced by the general population. A possible disadvantage of this inclusiveness is that the variable is fairly heterogeneous. Future studies should seek to parse apart which aspects of maternal psychosocial stress are most associated with offspring development.
Although none of the women in our sample had a known diagnosis of SSD during pregnancy, it is likely that vulnerabilities associated with SSD contributed to our finding, as the majority of offspring who were exposed to maternal daily stress during pregnancy did not develop a SSD. Additionally, although we included maternal education in our analyses to control for exposure to postnatal adversity, we were not able to assess stress during childhood, which is a limitation of this study as well as many other studies of maternal stress and SSD. Another limitation is that some interview files from PDS-I were lost over time as a result of: 1) files being physically moved to different locations (e.g., storage warehouses); 2) files being pulled for examination/data analysis and being misplaced. However, there is no reason to believe that loss of files led to an over- or underrepresentation of women who experienced stress during pregnancy, as stress during pregnancy has not been examined in previous studies. Additionally, although all of the lost files were from PDS-I (PDS-II is a more recent study, corresponding to a newer and improved files system for the CHDS, as well as less time for PDS-II investigators to pull relevant files), there were no significant differences between PDS-I and PDS-II on any of the study variables, indicating that the lost files did not bias the results. Finally, there is the possibility of Type I error, given that multiple tests were conducted; however, our results are consistent with previous prenatal sex-difference findings and the magnitude of the odds ratios were moderately large. Further, this is the first study to examine the long-term effects of daily stress during pregnancy on risk of offspring schizophrenia in a longitudinal, prospective, population-based design, which is a major methodological advantage.
The current study has the potential to further our understanding of the relationship between maternal psychosocial stress during pregnancy and risk of SSD in offspring. These findings are particularly important because much of the literature has examined SSD in relation to TLEs during pregnancy, which occur at a lower frequency than daily life stress, potentially reducing generalizability and public health implications. Our findings are also interesting to consider in the context of research suggesting that daily life stress can increase risk of psychosis (Tessner et al., 2011) and that behavioral sensitization (i.e., a process by which exposure to psychosocial stress increases behavioral and biological responses to subsequent stress exposure) may explain how psychosocial stress increases risk for psychosis (Van Winkel et al., 2008). Despite a moderately large odds ratio in the current study (2.091), practically this means that approximately 2% of male offspring of mothers who experience stress during pregnancy will develop schizophrenia (i.e. odds ratio x the base rate of schizophrenia in the population); therefore, future studies should examine additional factors that may make the fetus particularly vulnerable to maternal stress, such as genetic factors, biomarkers (e.g., cortisol), and placental alterations. Additionally, although the current study used a case/control matched design specifically to assess SSD, future studies should examine whether the current results extend to other disorders and shared behavioral phenotypes. Further research into the developmental period between prenatal stress and offspring psychopathology is also needed, as postnatal influences likely contribute to the impact of prenatal maternal stress on offspring development. The results from this study have the potential to influence early intervention and prevention strategies, as it is becoming increasingly clear that maternal experiences during pregnancy have the potential to alter the developmental trajectory of offspring.
Highlights.
Women were interviewed during pregnancy as part of a large birth cohort study.
Interviews were coded for stress-related themes by independent raters.
No stress themes were significantly associated with offspring SSD in full sample.
There was a significant interaction between daily life stress and infant sex.
Daily life stress during pregnancy linked to increased odds of SSD in male progeny.
Acknowledgments
This study was supported by funding to LME (R01 MH096478, Temple University start-up award, schizophrenia research fellowship-5T32 MH018870-20), AMF (National Science Foundation Graduate Research Fellowship awarded to AMF-DGE-1144462), CAS (R01 MH069819), and ASB (5R01-MH073080, 5K02-MH65422) from the National Institute of Mental Health and grants N01HD13334 and N01HD63258 from The Eunice Kennedy Shriver Institute of Child Health and Human Development. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Footnotes
Conflict of Interest
All authors have declared that they have no conflicts of interest and/or financial disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. International Review of Psychiatry. 2010;22:417–428. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- Abel KM, Heuvelman HP, Jorgensen L, Magnusson C, Wicks S, Susser E, Hallkvist J, Dalman C. Severe bereavement stress during the prenatal and childhood periods and risk of psychosis in later life: population based cohort study. BMJ. 2014:348. doi: 10.1136/bmj.f7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Peccoz P, Padmanabhan V, Baggiani AM, Cortelazzi D, Buscaglia M, Medri G, Marconi AM, Pardi G, Beitins IZ. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-s. The Journal of Clinical Endocrinology & Metabolism. 1991;73:525–532. doi: 10.1210/jcem-73-3-525. [DOI] [PubMed] [Google Scholar]
- Betts KS, Williams GM, Najman JM, Scott J, Alati R. Maternal prenatal infection, early susceptibility to illness and adult psychotic experiences: A birth cohort study. Schizophrenia Research. 2014;156:161–167. doi: 10.1016/j.schres.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. American Journal of Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Castle DJ, Murray RM. The neurodevelopmental basis of sex differences in schizophrenia. Psychological medicine. 1991;21:565–575. doi: 10.1017/s0033291700022194. [DOI] [PubMed] [Google Scholar]
- Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, Hultman CM, Långström N, Lichtenstein P, D’Onofrio BM. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychological medicine. 2014;44:71–84. doi: 10.1017/S0033291713000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–9. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Gordon LU. Measuring Stress: A Guide for Health and Social Scientists. Oxford University Press; US, New York: 1997. Measuring stress: A guide for health and social scientists; p. 256. [Google Scholar]
- Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–33. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego MA, Jones NA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Garcia A. Maternal psychological distress, prenatal cortisol, and fetal weight. Psychosomatic medicine. 2006;68:747–53. doi: 10.1097/01.psy.0000238212.21598.7b. [DOI] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal Stress and Preterm Birth. American Journal of Epidemiology. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- Dorrington S, Zammit S, Asher L, Evans J, Heron J, Lewis G. Perinatal maternal life events and psychotic experiences in children at twelve years in a birth cohort study. Schizophrenia Research. 2014;152:158–163. doi: 10.1016/j.schres.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan CH, Albright KJ, Lequerica A. Using the ICF to code and analyse women’s disability narratives. Disability and rehabilitation. 2008;30:978–90. doi: 10.1080/09638280701797549. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Developmental psychobiology. 2008;50:232–41. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM, Buka S, Yolken R, Cannon TD. Decreased Birth Weight in Psychosis: Influence of Prenatal Exposure to Serologically Determined Influenza and Hypoxia. Schizophrenia Bulletin. 2013;39:1037–1044. doi: 10.1093/schbul/sbs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for Axis I DSM-IV disorders. Biometrics Research; New York: 1994. [Google Scholar]
- Foerster A, Lewis SW, Owen MJ, Murray RM. Low birth weight and a family history of schizophrenia predicts poor premorbid functioning in psychosis. Schizophrenia Research. 1991;5:13–20. doi: 10.1016/0920-9964(91)90049-w. [DOI] [PubMed] [Google Scholar]
- Gitau R, Adams D, Fisk NM, Glover V. Fetal plasma testosterone correlates positively with cortisol. Archives of disease in childhood. Fetal and neonatal edition. 2005;90:F166–9. doi: 10.1136/adc.2004.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. American journal of obstetrics and gynecology. 2001;184:637–42. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Walder DJ. The Early Course of Schizophrenia. Oxford University Press; Oxford: 2006. Sex differences in schizophrenia: The case for developmental origins and etiological implications. [Google Scholar]
- Herman DB, Brown AS, Opler MG, Desai M, Malaspina D, Bresnahan M, Schaefer CA, Susser ES. Does unwantedness of pregnancy predict schizophrenia in the offspring? Findings from a prospective birth cohort study. Social psychiatry and psychiatric epidemiology. 2006;41:605–10. doi: 10.1007/s00127-006-0078-7. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sparen P, Takei N, Murray RM, Cnattingius S. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case-control study. BMJ. 1999;318:421–426. doi: 10.1136/bmj.318.7181.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen MO, Niskanen P. Prenatal loss of father and psychiatric disorders. Archives of general psychiatry. 1978;35:429–31. doi: 10.1001/archpsyc.1978.01770280039004. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Archives of general psychiatry. 2008;65:146–52. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Luo L, Matheson SL, Carr VJ, Raudino A, Harris F, Green MJ. Common or distinct pathways to psychosis? A systematic review of evidence from prospective studies for developmental risk factors and antecedents of the schizophrenia spectrum disorders and affective psychoses. BMC Psychiatry. 2015;15:205. doi: 10.1186/s12888-015-0562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta psychiatrica Scandinavica Supplementum. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health psychology. 2008;27:604–15. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, Friedlander Y, Harlap S. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC psychiatry. 2008;8:71. doi: 10.1186/1471-244X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Takei N, Saito F, Kachi K, Mori N. The association between obstetric complications and childhood-onset schizophrenia: a replication study. Psychological medicine. 2001;31:907–914. doi: 10.1017/s0033291701003944. [DOI] [PubMed] [Google Scholar]
- McNeil TF, Schubert EW, Cantor-Graae E, Brossner M, Schubert P, Henriksson KM. Unwanted pregnancy as a risk factor for offspring schizophrenia-spectrum and affective disorders in adulthood: a prospective high-risk study. Psychological medicine. 2009;39:957–65. doi: 10.1017/S0033291708004479. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhrman A, Rantakallio P, Isohanni M, Jones P, Partanen U. Unwantedness of a pregnancy and schizophrenia in the child. The British Journal of Psychiatry. 1996;169:637–640. doi: 10.1192/bjp.169.5.637. [DOI] [PubMed] [Google Scholar]
- Norman RM, Malla AK. Stressful life events and schizophrenia. I: A review of the research. The British Journal of Psychiatry. 1993;162:161–166. doi: 10.1192/bjp.162.2.161. [DOI] [PubMed] [Google Scholar]
- Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry. 2013;70:677–85. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- Reik W, Lewis A. Co-evolution of X-chromosome inactivation and imprinting in mammals. Nature reviews Genetics. 2005;6:403–10. doi: 10.1038/nrg1602. [DOI] [PubMed] [Google Scholar]
- Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 1999;18:333–45. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. Journal of psychosomatic research. 2013;75:327–35. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W, Phillips DIW. Fetal origins of mental health: evidence and mechanisms. Brain, behavior, and immunity. 2009;23:905–16. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Selten JP, van der Graaf Y, van Duursen R, Gispen-de Wied CC, Kahn RS. Psychotic illness after prenatal exposure to the 1953 Dutch Flood Disaster. Schizophrenia research. 1999;35:243–5. doi: 10.1016/s0920-9964(98)00143-1. [DOI] [PubMed] [Google Scholar]
- Serido J, Almeida DM, Wethington E. Chronic stressors and daily hassles: Unique and interactive relationships with psychological distress. Journal of Health and Social Behavior. 2004;45:17–33. doi: 10.1177/002214650404500102. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychological bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Siegel LS. Reproductive, Perinatal, and Environmental Factors as Predictors of the Cognitive and Language Development of Preterm and Full-Term Infants. Child development. 1982;53:963–973. [PubMed] [Google Scholar]
- Slykerman RF, Thompson JMD, Pryor JE, Becroft DMO, Robinson E, Clark PM, Wild CJ, Mitchell EA. Maternal stress, social support and preschool children’s intelligence. Early Human Development. 2005;81:815–21. doi: 10.1016/j.earlhumdev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Stark MJ, Wright IMR, Clifton VL. Sex-specific alterations in placental 11β-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2009;297:510–514. doi: 10.1152/ajpregu.00175.2009. [DOI] [PubMed] [Google Scholar]
- Susser ES, Schaefer CA, Brown AS, Begg MD, Wyatt RJ. The design of the prenatal determinants of schizophrenia study. Schizophrenia Bulletin. 2000;26:257–73. doi: 10.1093/oxfordjournals.schbul.a033451. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Mittal V, Walker EF. Longitudinal study of stressful life events and daily stressors among adolescents at high risk for psychotic disorders. Schizophrenia Bulletin. 2011;37:432–441. doi: 10.1093/schbul/sbp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. The British Journal of Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- Van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophrenia Bulletin. 2008;34:1095–1105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder DJ, Andersson TLC, McMillan AL, Breedlove SM, Walker EF. Sex differences in digit ratio (2D:4D) are disrupted in adolescents with schizotypal personality disorder: Altered prenatal gonadal hormone levels as a risk factor. Schizophrenia Research. 2006;86:118–122. doi: 10.1016/j.schres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Holtzman CW, Addington J, Cadenhead K, Tsuang M, Cornblatt B, Cannon TD, McGlashan TH, Woods SW, Perkins DO, Seidman LJ, Heinssen R, Walker EF. Sexual dimorphisms and prediction of conversion in the NAPLS psychosis prodrome. Schizophrenia Research. 2013;144:43–50. doi: 10.1016/j.schres.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder DJ, Laplante DP, Sousa-Pires A, Veru F, Brunet A, King S. Prenatal maternal stress predicts autism traits in 61/2 year-old children: Project Ice Storm. Psychiatry Research. 2014;219:353–360. doi: 10.1016/j.psychres.2014.04.034. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Lewine R, Loewy R. Sex differences in the origins and premorbid development of schizophrenia. In: Lewis-Hall F, Williams TS, Panetta JA, Herrera JM, editors. Psychiatric Illness in Women: Emerging Treatments and Research. American Psychiatric Publishing, Inc; Arlington, VA: 2002. pp. 193–214. [Google Scholar]
- Watson JB, Mednick SA, Huttunen M, Wang X. Prenatal teratogens and the development of adult mental illness. Development and psychopathology. 1999;11:457–66. doi: 10.1017/s0954579499002151. [DOI] [PubMed] [Google Scholar]