Abstract
The development of therapeutics for neurological disorders is constrained by limited access to the central nervous system (CNS). ATP-binding cassette (ABC) transporters, particularly P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), are expressed on the luminal surface of capillaries in the CNS and transport drugs out of the endothelium back into the blood against the concentration gradient. Survival motor neuron (SMN) protein, which is deficient in spinal muscular atrophy (SMA), is a target of the ubiquitin proteasome system. Inhibiting the proteasome in a rodent model of SMA with bortezomib increases SMN protein levels in peripheral tissues but not the CNS, because bortezomib has poor CNS penetrance. We sought to determine if we could inhibit SMN degradation in the CNS of SMA mice with a combination of bortezomib and the ABC transporter inhibitor tariquidar. In cultured cells we show that bortezomib is a substrate of P-gp. Mass spectrometry analysis demonstrated that intraperitoneal co-administration of tariquidar increased the CNS penetrance of bortezomib, and reduced proteasome activity in the brain and spinal cord. This correlated with increased SMN protein levels and improved survival and motor function of SMA mice. These findings show that CNS penetrance of treatment for this neurological disorder can be improved by inhibiting drug efflux at the blood-brain barrier.
Keywords: spinal muscular atrophy (SMA), survival motor neuron (SMN), p-glycoprotein (P-gp), blood brain barrier (BBB), bortezomib, tariquidar, proteasome
Introduction
Pharmacotherapy for neurodegenerative disorders is hampered by the inability of most small molecules to cross the blood-brain barrier (BBB) and the blood spinal cord barrier (BSCB). Three main cell types, the endothelial cells, pericytes, and astrocytes form the BBB and BSCB and protect the central nervous system (CNS) by restricting diffusion of many solutes into the cerebrospinal fluid. This is achieved through a combination of tight junctions formed by the endothelial cells, a lack of pinocytotic activity, and efflux transporters. The predominant efflux transporters, P-glycoprotein (P-gp, ABCB1) and breast cancer resistance protein (BCRP, ABCG2), are members of the ATP-binding cassette (ABC) class. They are expressed on the apical surface of endothelial cell and function to intercept drugs entering the CNS capillary cells and transport them against their concentration gradient back into the blood (Schinkel et al., 1994, Schinkel et al., 1996, Rao et al., 1999, Cherry et al., 2013). The BBB and BSCB are fully formed (Saunders et al., 2012) and P-gp and BCRP transporters are correctly localized well before birth (Daood et al., 2008). While there are some differences between the BBB and BSCB including lower protein levels of tight junction markers and increases to permeability there are no significant differences in P-gp levels (Bartanusz et al 2011).
Spinal muscular atrophy (SMA) is a motor neuron disorder caused by deletions and other mutations of the highly conserved survival of motor neuron-1 gene (SMN1) with retention of the nearly identical paralog, SMN2. A promising approach to treating SMA is to increase levels of SMN protein by reducing its degradation through the ubiquitin proteasome system (Lefebvre et al., 1995, McAndrew et al., 1997, Kwon et al., 2013). We have previously shown that inhibition of the proteasome by the inhibitor bortezomib increases SMN protein levels in cultured cells and in peripheral tissues of SMA mice. Long term bortezomib treatment resulted in an improvement in motor function in SMA mice compared with vehicle treated animals; however survival was unaffected (Kwon et al., 2011). Pre-clinical studies have shown that bortezomib is unable to penetrate into the CNS, impeding its use for treating SMA and other neurological disorders (Nakamura et al., 2007, Rumpold et al., 2007, Lu et al., 2010). To overcome bortezomib’s low biodistribution in the CNS, we hypothesized that pre-treatment with an ABC transporter inhibitor would prevent bortezomib’s efflux at the blood-brain and -spinal cord barriers.
In this study we used overexpress cell lines, genetic mouse models, and systemic administration of an inhibitor to demonstrate that bortezomib is a substrate of P-gp. Using mass spectrometry we were able to detect bortezomib in the CNS of SMA model mice co-administered with a P-gp inhibitor tariquidar. SMN levels were also increased in the CNS of model mice treated with both drugs. Tariquidar and bortezomib treated mice also lived longer and had improved motor function compared with tariquidar-alone, bortezomib-alone, and vehicle treated counterparts. These data suggest that P-gp inhibition increases the CNS bioavailability of a proteasome inhibitor, which is of particular interest in the development of therapeutics for neurological disorders.
Results
Bortezomib is transported by P-gp
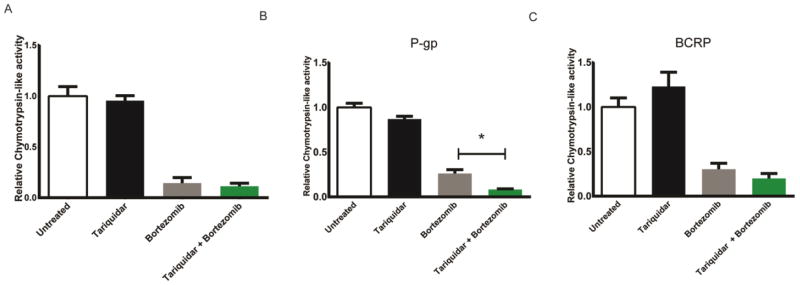
In order to verify that bortezomib is transported by P-gp and not by other transporters (O’Connor et al., 2013) we used MCF-7 and HEK293 cells that express P-gp, BCRP, or no drug efflux transporters and tested bortezomib’s transport. MCF-7 cells do not express high levels of P-gp or BCRP at baseline; however selection with flavopiridol increases BCRP expression (Robey et al., 2001) and selection with etoposide increases P-gp levels (Schneider et al., 1994). We therefore generated MCF-7 cells that expressed P-gp or BRCP using these selection processes. Since we were interested in the basal activity of the proteasome in the treated cells we tested several different cell lysate protein concentrations to find an appropriate concentration at which chymotrypsin-like activity of the endogenous proteasome was measurable (Supplemental Figure 1a). The chymotrypsin-like activity of the endogenous proteasome of 70μg of cell lysate was well above the residual activity seen with no substrate or no lysate negative controls, but less than the maximal amount seen when excess purified proteasome was tested. The chymotrypsin-like activity of the proteasome of the drug naive cells in the presence of bortezomib and tariquidar was similar to bortezomib alone (n=6, p>0.05) (Figure 1a). However, MCF-7 cells that had been selected to express P-gp showed further reduction of chymotrypsin-like activity of the proteasome with the pre-treatment with tariquidar (1 μM, 15 min) before bortezomib treatment (0.5 μM, 30 min) (n=6, p<0.05) (Figure 1b). However, the pre-treatment with tariquidar did not significantly increase the inhibition of the proteasome of the BCRP overexpressing cells when compared with bortezomib alone treatment (n=6, p>0.05) (Figure 1c).
Figure 1.
Bortezomib is transported by P-gp1, but not by BCRP in cultured MCF-7 cells. Cells drug selected for P-gp, BCRP, or no drug treatment were treated with tariquidar (1 μM) or DMSO (1 μL per 1 ml) for 15 min then with bortezomib (0.5 μM) or PBS (1 μL per 1 ml) for 30 min. The cells were lysed and the chymotrypsin-like activity of the proteasome was assessed by examining the cleavage of the fluorogenic peptide Suc-LLVY-AMC (a,b,c) when it was added to the cell lysates. Values represent chymotrypsin-like activity relative to vehicle control ± SEM,*P< 0.05, n= 6.
HEK293 cells do not typically express P-gp (Robey et al., 2011); bortezomib (0.5 μM, 30 min) inhibited the chymotrypsin-like activity of the proteasome (n=6, p<0.01) and there was no additive effect when the cells were pretreated with tariquidar (n=6, p>0.05) (Supplemental Fig. 2a). Cells that stably express P-gp showed increased inhibition to the chymotrypsin-like activity of the proteasome when treated with tariquidar (15min, 1 μM) before bortezomib treatment (0.5 μM, 30 min) (n=6, p<0.05) (Supplemental Fig. 2b). Tariquidar treatment alone does not inhibit the chymotrypsin-like activity of the proteasome in either the MCF-7 cells (Figures 1a,b,c) or the HEK cells (Supplemental Fig. 2c). Together, these data indicated that bortezomib was a substrate of P-gp, but not of BRCP.
Bortezomib levels and proteasome inhibition are enhanced in spinal cord of P-gp1a/b BCRP knockout mice compared with control mice
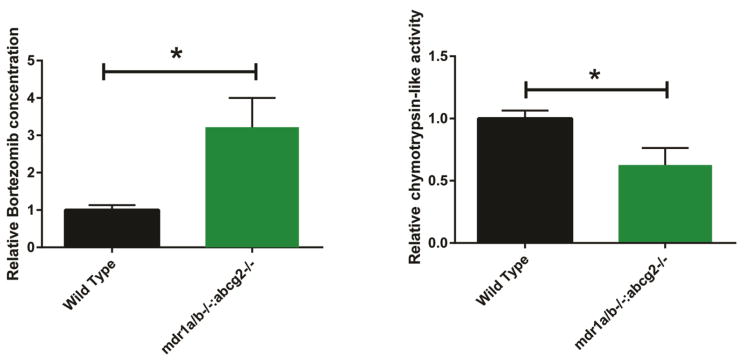
We verified that bortezomib was transported by P-gp in vivo by treating P-gp1a/b/BCRP triple knockout mice (FVB.129P2-Abcb1atm1Bor:Acb1btm1Bor:Abcg2tm1Ahs N7) or the parental strain (FVB) mice with a single dose of bortezomib (Velcade) by intraperitoneal (i.p.) injection (0.15 mg/kg). Mice carry two P-gp genes, unlike humans, and both genes must be knocked down to prevent drug extrusion from the cytoplasmic membrane (Croop et al., 1989). Tariquidar is a known inhibitor of both P-gp and BCRP (Bauer et al., 2013) and these triple knockout mice most closely mimic the anticipated effects of tariquidar treatment.
We measured the levels of bortezomib in the CNS by liquid chromatography-tandem mass spectrometry (LCMS/MS) and observed that bortezomib in the spinal cord was 3.2 fold higher 1 hr post injection in the P-gp1a/b:BCRP knockout mouse spinal cords (257.9 ± 79.1 ng/mg tissue) compared with the spinal cords of the parental strain mice (38.7 ± 0.9 ng/mg tissue) (p<0.01) (Fig. 2a). Additionally, the chymotrypsin-like activity of the proteasome was decreased in the spinal cord lysates 1 hr post-injection of the bortezomib- treated P-gp1a/b/BCRP knockout mice compared with the bortezomib- treated parental strain mice (p<0.05) (Fig. 2b). A single dose of bortezomib (0.15 mg/kg) did not alter the expression of the proteasome subunit responsible for chymotrypsin-like catalytic activity PSMB5 (Supplemental Fig. 3).
Figure 2.
Bortezomib penetrates the BBB of PGP1a/b:BCRP knockout mice, but not the parental strain control mice (FVB). Mice at P12 were injected with bortezomib (0.15 mg/kg) and sacrificed 1 hr later. The spinal cords were removed and bortezomib levels were analyzed with LCMS/MS (a). The chymotrypsin-like activity of the endogenous proteasome in the spinal cords was assessed by analyzing the cleavage of the fluorogenic peptide Suc-LLVY-AMC (b) when it was added to spinal cord lysate. Values represent, respectively, bortezomib concentration or chymotrypsin-like activity relative to wild type control ± SEM,*P<0.01.
Bortezomib levels in SMA mouse CNS increase and proteasome activity is inhibited with co-administration of an ABC transporter inhibitor
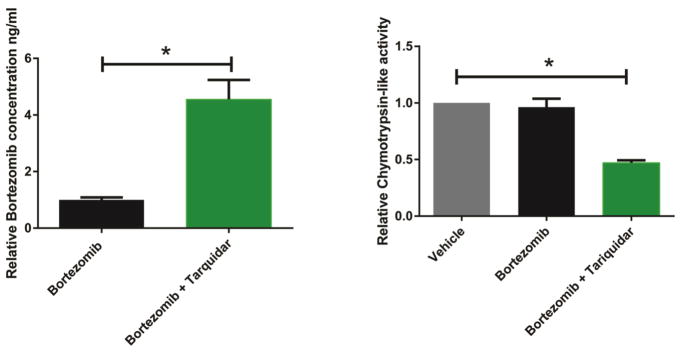
Having shown that bortezomib was a substrate of P-gp in cultured cells and in mice we next sought to determine whether the ABC transporter inhibitor tariquidar could increase the CNS penetrance of bortezomib and slow SMN degradation in SMA model mice. SMA pups (hSMN2:Δ7SMN:smn−/−) were treated by i.p. injections of bortezomib (0.15 mg/kg) starting at post-natal day 4 (P4) and continued every other day until P8. At P8 the dose of bortezomib was decreased to 0.075 mg/kg, due to diarrhea observed in preliminary studies (outlined in Kwon et al., 2011) from extended treatment with the higher dose of bortezomib, and the mice were subsequently treated every other day until P12. In addition, pups were administered (99% pure by high performance liquid chromatography) tariquidar (i.p., 6.5 or 13 mg/kg) 15 min before bortezomib administration. The bortezomib and tariquidar combination treatment paradigm used here did not alter the typical weight gain of control mice compared to tariquidar- only treatment and untreated controls (Supplemental Fig. 4), and no early deaths were seen.
Mice were sacrificed at P12, and levels of bortezomib in the brains of bortezomib-only and bortezomib and tariquidar-treated mice were measured by LCMS/MS. Bortezomib levels in the brain were nearly undetectable in bortezomib-only treated mice (106 ± 28 ng/mg tissue), but increased 4.5 fold when bortezomib was co-administered with tariquidar (455 ± 118 ng/mg tissue) (p<0.01) (Fig. 3a). Similar increases in bortezomib penetrance occurred in spinal cord tissue. However, the small tissue volumes of the spinal cord from late stage SMA disease mice led to higher levels of variability, and a statistically significant difference was not observed in the spinal cord samples (Supplemental Fig. 5). In addition, the chymotrypsin-like activity of the proteasome in the brain was reduced in animals bortezomib and tariquidar- treated compared with bortezomib-only treated animals (p<0.01) (Fig. 3b).
Figure 3.
Bortezomib penetrates the BBB and BSCB when mice are pre-treated with tariquidar. Mice at P4 were given tariquidar (6.5 or 13 mg/kg) or vehicle (100% DMSO, 1 μl/g) 15 min before bortezomib (0.15 mg/kg – 0.075 mg/kg) or vehicle (Ringer’s solution, 1 μl/g) i.p. injection every other day and sacrificed at P12. The brains were analyzed for bortezomib concentration using LCMS/MS (a). The chymotrypsin-like activity of the proteasome in the brains was assessed by examining the cleavage of the fluorogenic peptide Suc-LLVY-AMC (b) when it was added to the brain lysate. Values represent, respectively, bortezomib concentration or chymotrypsin-like activity relative to bortezomib-only treated control ± SEM,*P<0.01.
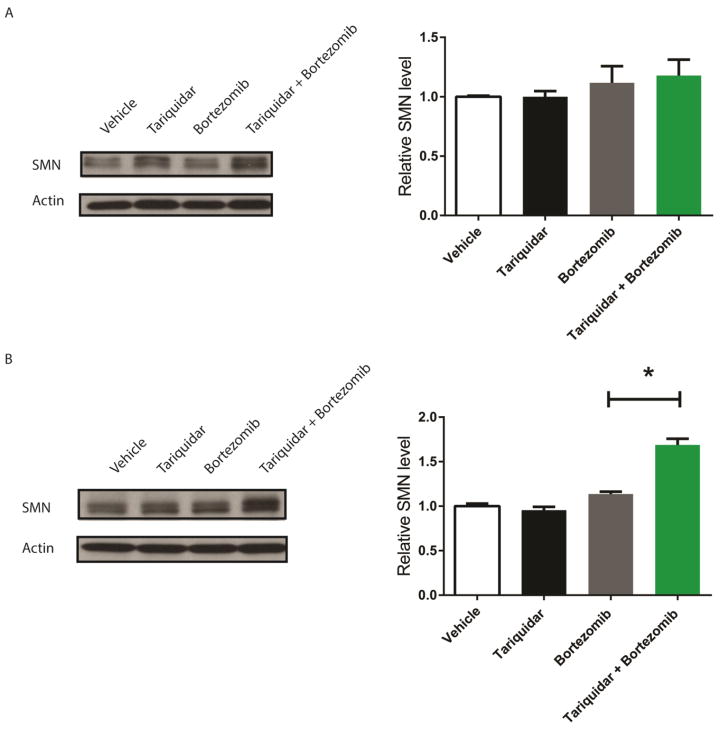
Bortezomib increases SMN protein levels in the SMA mouse CNS when paired with a P-gp inhibitor
Since we observed increased penetrance of bortezomib into the CNS with tariquidar pre-treatment, we investigated whether SMN levels increased as well. We reported previously that levels of SMN increased in peripheral tissue in bortezomib- treated mice, but no change was seen in CNS tissue (Kwon et al 2011). Co-administration of two different doses of tariquidar (6.5 and 13 mg/kg) with bortezomib increased SMN protein levels in the brain, but the increase in SMN protein only reached statistical significance with the 13 mg/kg dose of tariquidar (n=6, p<0.05) (Fig. 4a,b). Similar SMN protein increases were seen in spinal cord tissue although the small tissue volumes from late stage SMA disease mice led to higher levels of variability, and a statistically significant difference was not observed (Supplemental Fig. 6).
Figure 4.
Bortezomib with tariquidar pre-treatment increases SMN protein levels in the CNS. Mice were treated with tariquidar (6.5 mg/kg or 13 mg/kg) or vehicle (100% DMSO, 1 μl/g) 15 min before bortezomib (0.15 mg/kg) or vehicle (Ringer’s solution, 1 μl/g) i.p. injection every other day starting at P4 and sacrificed at P12. 13 mg/kg tariquidar increased SMN protein levels in the brain (b), while the SMN increase with 6.5 mg/kg tariquidar (a) was not significantly different from bortezomib alone. Brain tissue was removed and protein lysates from these tissues were isolated to examine SMN protein levels. The ratio of SMN to actin protein levels was determined by a densitometry analysis. Values represent mean ± SEM. *P<0.05, n=6.
Bortezomib enhances motor function in SMA model mice when paired with a P-gp inhibitor
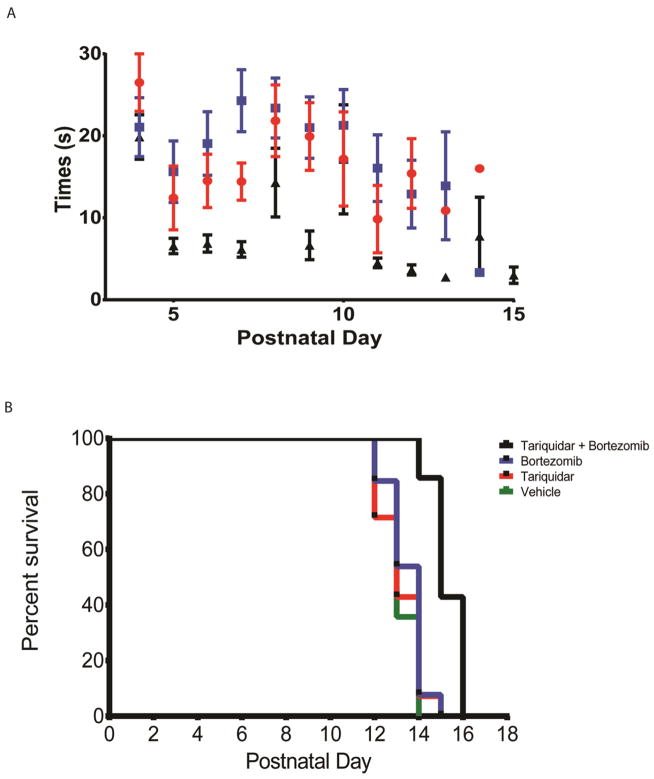
Since there were increases to SMN protein levels in the CNS we asked whether the CNS penetrance would also improve the functional benefit of bortezomib. SMA mice were treated as described above, but with treatment continuing until mice reached pre-specified conditions for euthanasia (30% weight loss or inability to right after 30 sec). The SMA mice begin to show difficulty with righting at day 10 leading to an inability to right within 30 sec by day 14 with the median age of death 13.3 days (Le et al., 2005). We detected an improvement in the righting times of mice treated with bortezomib and tariquidar over bortezomib alone (n=10, p<0.05 starting at day 8) (Fig. 5a). Combining bortezomib with tariquidar pre-treatment further extended survival by 1.5 day compared with bortezomib treatment alone (n=13, p<0.05) (Fig. 5b).
Figure 5.
CNS penetrance of bortezomib with tariquidar pre-treatment improves motor function and increases survival over bortezomib treatment alone. Mice at P4 were treated with tariquidar (13 mg/kg) or vehicle (100% DMSO, 1 μl/g) 15 min before bortezomib (0.15 mg/kg – 0.075 mg/kg) or vehicle (Ringer’s solution, 1 μl/g) i.p. injection every other day. Righting times of SMA mice improved when treated with bortezomib and tariquidar compared to bortezomib treatment alone (a). CNS penetrance of bortezomib confers a survival benefit. Mice at P4 were treated with tariquidar (13 mg/kg) or vehicle (DMSO) 15 min before bortezomib (0.15 mg/kg – 0.075 mg/kg) or vehicle (Ringer’s solution) injection every other day. Mouse survival was increased by one and a half days with bortezomib and tariquidar treatment compared to bortezomib alone. Kaplan Meier survival curve *P<0.05, n=13.
Discussion
The BBB and BSCB restrict the ability of drugs to reach their intended site of action in the CNS and thus present a major challenge to the treatment of neurological disorders. This has prompted interest in developing novel drug delivery strategies to improve therapeutic access to the CNS (Pardridge, 2012). In this study we aimed to show that (i) P-gp impacted the efficacy of bortezomib in our model systems and that (ii) inhibiting P-gp improved the CNS delivery and efficacy of a pharmaceutical agent in a model of motor neuron disease. We showed that proteasome function is inhibited when P-gp was either deleted or inhibited in the presence of bortezomib. Importantly, bortezomib inhibition of the proteasome increased steady-state SMN protein levels in the CNS when P-gp was inhibited. Additionally, motor function and survival were enhanced with SMN stabilization in the CNS.
It has previously been reported that bortezomib is transported by P-gp (Nakamura et al., 2007, Rumpold et al., 2007, Lu et al., 2010, O’Connor et al., 2013), primarily in the context of cancer multidrug resistance (Jung et al., 2004). Interestingly a silent polymorphism in P-gp, MDR1 C3435T, confers increased latency to relapse and higher bortezomib efficacy in human multiple myeloma (Buda et al., 2009, Buda et al., 2010). While BCRP has also been implicated in bortezomib efflux (Gil et al., 2007, Wiberg et al., 2009) it has been stated by others that P-gp is the primary transporter responsible for bortezomib efflux (O’Connor et al., 2013). We used the P-gp and BCRP inhibitor tariquidar to prevent bortezomib efflux (Kannan et al., 2011). While the contribution of other efflux pumps appears unlikely it has also been postulated that the decrease in efficacy of bortezomib in patients is due to other mechanisms such as upregulation of PSMB5, a subunit of the proteasome (Lu et al., 2008, Lu et al., 2009). Using genetic models and pharmacological agents we confirm that bortezomib is indeed a substrate of P-gp, independent of PSMB5 expression.
We showed previously that bortezomib stabilizes SMN protein levels in peripheral tissue (Kwon et al., 2011). We showed here that bortezomib is a potent proteasome inhibitor in the CNS when allowed to cross the BBB. Bortezomib activity in the CNS stabilizes SMN protein levels and improves SMA disease manifestations in mice. It has been established that increasing SMN protein levels through induction of the SMN2 gene or altering splicing improves SMA pathology in mouse models (Foust et al., 2010, Hua et al., 2011, Cherry et al., 2013). Stabilizing SMN protein by blocking protein degradation in peripheral tissue improves motor function of SMN mice, but does not extend survival, reinforcing the critical role of SMN in the CNS. We demonstrated here that bortezomib was normally excluded from the CNS and that increasing bortezomib levels in the CNS had positive effects on SMA pathology in mice. However, the survival benefit of bortezomib was less robust than that seen with other compounds that increased SMN to similar levels. One possibility is that off-target toxicity associated with bortezomib prevented full effects of the SMN stabilization. Indeed, it has been shown in human patients that bortezomib can cause peripheral neuropathy (Argyriou et al., 2008). It has been determined that bortezomib also inhibits HtrA2/Omi, an ATP-dependent serine protease in mitochondria which protects neurons from undergoing apoptosis (Arastu-Kapur et al., 2011), and this likely accounts for the peripheral neuropathy. Importantly, increasing specificity dramatically decreased proteasome inhibitor cytotoxicity in cultured cells (Screen et al., 2010). The next-generation proteasome inhibitors currently undergoing clinical consideration can achieve stronger inhibition of chymotrypsin-like activity of the proteasome in vivo and do not inhibit HtrA2.
Recently, it was shown that the amyotrophic lateral sclerosis (ALS) drug riluzole has increased efficacy in an animal model of familial ALS when co-administered with an ABC transporter inhibitor elacridar (Jablonski et al., 2014). Additionally, co-administration of tariquidar with the anti-seizure drug pentobarbital improves anti-epileptic activity in previously drug resistant rats (Brandt et al., 2006). Any therapy under development intended to be active in the CNS must be able to traverse the BBB and BSCB and not be transported by P-gp. There are no current FDA-approved proteasome inhibitors reported to be CNS permeable. The drug efflux transporters represent a formidable obstacle when developing new therapies for neuromuscular and neurodegenerative disorders. Our results show that pharmacological inhibition of P-gp can enhance CNS penetrance of bortezomib. Inhibition of P-gp by tariquidar has been demonstrated in humans with PET imaging, using IV administration (Bauer et al., 2015, Kreisl et al., 2015). Our study and the study with the ALS model reported by Jablonski et al. demonstrate that co-administration of P-gp inhibitors with CNS treatments can improve their efficacy.
Materials and Methods
Mice
Studies were approved by the National Institute of Neurological Diseases and Stroke Animal Care and Use Committee and done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Transgenic SMA mice on the FVB background (hSMN2/Δ7SMN/mSmn −/− ) were purchased from Jackson Laboratories. The animals were genotyped using polymerase chain reaction on tail DNA using two sets of primers against mouse smn and beta-galactosidase to ensure that the mouse smn gene is knocked out (Kernochan et al., 2005).
Pgp-1a/b and BCRP knock out mice (FVB.129P2-Abcb1atm1Bor Abcb1btm1BorAbcg2tm1Ahs) were a kind gift from Dr. Robert Innis, NIMH. The animals were genotyped using polymerase chain reaction on ear punch tissues to ensure that they lacked both isoforms of P-gp1 and BCRP.
For biochemical studies, the mice were anesthetized with isofluorane and sacrificed by cervical dislocation. Spinal cords, brains, and livers were dissected, flash-frozen in liquid nitrogen, and stored at −80°C.
For survival studies, litters were maintained and kept with the mother until euthanasia with no supplementation. Mice that lost 30% of their body weight and were unable to right themselves after 30 sec were euthanized as mandated by the NINDS ACUC committee.
Drug treatment
Bortezomib was dissolved in sterile Ringer’s solution to a concentration of 0.15 μg/μl or 0.075 μg/ul. Pups at postnatal day 4 (P4) and at day 6 (P6) were administered 1 μl of 0.15 μg/μl bortezomib per gram (for a concentration of 0.15 mg/kg) intraperitoneally. We had found that 0.15 mg/kg was the highest tolerated in neonatal mice (Kwon et al., 2011). From P8 to P12 pups were injected every other day with 1 μl of 0.075 μg/μl bortezomib per gram (for a concentration of 0.075 mg/kg). Control animals received equal volumes of Ringer’s saline. 99% pure by HPLC tariquidar was dissolved in 100% DMSO to a concentration of 6.5 μg/μl or 13 μg/μl. Pups at P4 were administered 1 μl of tariquidar per gram every other day for a total concentration of 6.5 mg/kg or 13 mg/kg in a manner similar to bortezomib. To maximize the dose of bortezomib crossing the BBB, mice were given tariquidar 15 min before bortezomib. Control animals received equal volumes of DMSO.
Motor function analysis
For one-time measures, pups were weighed and tested for their ability to right themselves. Righting time was defined as the average of four trials of the time required for a pup to turn over after being placed on its back (maximum 30 sec) (Cherry et al., 2013).
Protein extraction and quantification
Spinal cords were homogenized and incubated in 500 μl of lysis buffer (0.1% NP-40 and 0.5% sodium deoxycholate) for 15 min on ice, and the collected lysates were sonicated for 3 pulses of 10 sec before another 15 min incubation on ice. Supernatants were collected after 15 min centrifugation at 14000g at 4°C and stored at −80°C. Protein concentrations were determined by the Bradford Protein Assay kit (Bio-Rad) according to the manufacturer’s protocol.
Western blotting
Protein lysates (50 μg) were run and separated on a 10% SDS-PAGE gel electrophoresis gel and transferred to a PVDF membrane. These membranes were then probed with a mouse anti-SMN antibody (BD Transduction Laboratories, diluted 1:1000), and a mouse anti-β-actin antibody (Sigma-Aldrich, diluted 1:10,000) or a mouse anti-B-tubulin (Sigma-Adrich, 1:1000)) and followed by incubation with goat HRP-conjugated anti-mouse secondary antibody. Blots were developed using Clarity ECL (BioRad). All western blot images were collected with Chemidoc (Bio-Rad).
In vitro transport
HEK293 cells stably transfected with either an empty vector or P-gp were cultured in DMEM + 10%FBS and geneticin (Life Technologies, 1:1000). The cells were plated in 6-well plates, 2 × 106 cells per well. The cells were treated with tariquidar (15 min, 1 μM) then treated with bortezomib (30 min, 0.5 μM). The cells were then collected in 500 μl lysis buffer (0.1% NP-40 and 0.5% sodium deoxycholate) for 15 min on ice. Protein concentrations were determined by the Bradford Protein Assay kit (Bio-Rad) according to the manufacturer’s protocol.
Proteasome assay
Chymotrytic-like activity of the proteasome was measured by mixing (70 μg) tissue or cell homogenate with 50 μM fluorogenic peptide Suc-LLVY-AMC (succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin) (Enzo Life Sciences, Farmingdale, NY) in a final volume of 100 μl. The reaction buffer consisted of 50 mM Tris-HCl (pH 7.8), 20 mM KCl, 5 mM MgCl2, and 1 mM dithiothreitol. The mixture was incubated at 37°C for 30 min, and the released fluorogenic AMC was measured at 360-nm excitation and 460-nm emission. Epoxomicin (or MG132)-insensitive activity was subtracted from the final activity calculation. Substrate only or lysate only controls with no residual fluorescence signal and incubation with excess purified proteasome with multifold higher levels of signal were included in every assay.
Liquid chromatography tandem mass spectrometry (LCMS/MS)
Flash frozen spinal cord tissue samples were mixed with PBS (pH 7.2, 1:1 ratio) and homogenized with a pestle-type homogenizer. The homogenate was treated with 2 volumes of acetonitrile to precipitate the proteins, and the supernatant was analyzed by LCMS/MS. Samples were created for calibration and quality control with a working dilution of bortezomib in warfarin at 50 times the final concentration, and this was serially diluted to make the standard samples. These samples were diluted 31-fold into blank tissue extract and processed as above.
The signal was optimized for bortezomib by electrospray negative ionization (ESI) mode. A MS2 targeted SIM scan was used to optimize the precursor ion, and a product ion analysis was used to identify the best fragment for analysis and to optimize the collision energy.
Samples were analyzed with an ABI3000 mass spectrometer coupled with a Shimadzu HPLC and a Sil-HTc chilled autosampler, all controlled by Analyst software (ABI). After separation on a C18 reverse phase HPLC column (Agilent, Waters, or equivalent) mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% formic acid in acetonitrile.
Statistics
All analysis was carried out by investigators who were blinded to the drug conditions or mouse genotype. All statistics were carried out using the GraphPad Prism software package (version 6; GraphPad Software) and compared with either two-tailed Student’s t-test or one-way ANOVA followed by the Newman-Keuls multiple comparison post hoc tests. P ≤ 0.05 was considered significant.
Supplementary Material
(a) Increasing amounts of HEK293 cell lysate were incubated with the Suc-LLVY-AMC fluorogenic compound. Fluorescence (excitation: 360 nm, emission: 460 nm) increased over time with increased concentration of protein lysate. Based on these data we chose to use 70 μg of protein lysate for all subsequent experiments (b) No lysate and no substrate controls have minimal background florescence. Purified proteasome released fluorogenic cleavage products leading to readings that were far in excess of that obtained using 70 μg of protein lysate after 30 minutes, illustrating that our experimental conditions were well within the dynamic range of the assay. We verified that the cleavage activity measured was due to the proteasome using the chymotryptic activity specific proteasome inhibitor MG132.
Bortezomib is transported by P-gp in cultured cells. Cells stably expressing either P-gp or an empty vector were treated with tariquidar (1 μM) or DMSO (1 μl per 1 ml) for 15 min then with bortezomib (0.5 μM) for 30 min. The chymotrypsin-like activity of the proteasome was assessed by examining the cleavage of the fluorogenic peptide Suc-LLVY-AMC (a and b) when added to the cell lysate. Values represent chymotrypsin-like activity relative to vehicle control ± SEM,*P<0.05, n=6.
Tariquidar treatment does not alter proteasome activity. Cell transfected with an empty vector were treated with tariquidar (1 μM) or DMSO (1 μL per 1 ml) for 15 min. The chymotrypsin-like activity of the proteasome in cell lines was analyzed by assessing the cleavage of the fluorogenic peptide Suc-LLVY-AMC (c) when added to cell lysate. Values represent chymotrypsin-like activity relative to vehicle control ± SEM, p>0.05, n=6.
Single dose treatment with bortezomib does not alter proteasome protein expression. Mice at P12 were injected with bortezomib (0.15 mg/kg) and sacrificed 1 h later. PSMB5 protein levels are consistent in the spinal cords between wildtype and (pgp1a/b−/−:bcrp−/−) triple knockout mice. A densitometry analysis was performed on the respective western blots to ascertain relative SMN protein levels. Values represent mean relative to tariquidar-treated control ± SEM. p>0.05, n=6,
Tariquidar plus bortezomib treatment of unaffected animals do not alter the weight gain seen during the first two weeks of life compared to tariquidar only treated mice. Mice at P4 were treated with tariquidar (13 mg/kg) or vehicle (DMSO) 15 min before bortezomib (0.15 mg/kg) or vehicle (Ringer’s solution) injection every other day.
There is a trend to increased bortezomib penetration of the BSCB when mice are pre-treated with tariquidar. Mice at P4 were treated with tariquidar (13 mg/kg) or vehicle (DMSO) 15 min before bortezomib (0.15 mg/kg) or vehicle (Ringer’s solution) i.p. injection every other day and sacrificed at P12. The spinal cords were analyzed for bortezomib using LCMS/MS. p>0.05, n=6.
Bortezomib requires prior treatment with tariquidar to increase SMN protein levels in the CNS. Mice at P4 were treated with tariquidar (13 mg/kg) or vehicle (DMSO, 1 μl/g) 15 min before bortezomib (0.15 mg/kg – 0.075 mg/kg) or vehicle (Ringer’s solution, 1 μ/g) injection every other day and sacrificed at P12. The 13 mg/kg tariquidar pre-treatment result was not significantly different from bortezomib alone. A densitometry analysis was performed on the resulting western blots to ascertain relative SMN protein levels. Values represent mean relative to tariquidar-treated control ± SEM. p>0.05, n=6.
Highlights.
Bortezomib is transported by P-gp in vitro and in vivo
Pre-treatment with a P-gp inhibitor tariquidar is required for bortezomib to stabilize SMN protein levels in the CNS of treated mice
Pre-treatment with tariquidar before bortezomib administration improves righting behavior and survival of SMA model mice
Acknowledgments
We thank Robert Innis and Jeih-San Liow (NIMH, NIH)) for their kind gift of the P-gp1a/b−/−:BCRP−/− mice. We thank Michael Gottesman and Robert Robey (NCI, NIH) for their kind gift of the P-gp and BCRP transfected HEK cells and the drug selected MCF-7 cells. The work was supported by intramural research funds from NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ, Muchamuel T, et al. Nonproteasomal Targets of the Proteasome Inhibitors Bortezomib and Carfilzomib: A Link to Clinical Adverse Events. Clin Cancer Res. 2011 May 1;17(9):2734–43. doi: 10.1158/1078-0432.CCR-10-1950. [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-Induced Peripheral Neuropathy in Multiple Myeloma: A Comprehensive Review of the Literature. Blood. 2008 Sep 1;112(5):1593–9. doi: 10.1182/blood-2008-04-149385. [DOI] [PubMed] [Google Scholar]
- Bauer M, Karch R, Zeitlinger M, Philippe C, Romermann K, Stanek J, Maier-Salamon A, et al. Approaching Complete Inhibition of P-Glycoprotein at the Human Blood-Brain Barrier: An (R)-[11c]Verapamil Pet Study. J Cereb Blood Flow Metab. 2015 May;35(5):743–6. doi: 10.1038/jcbfm.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Karch R, Zeitlinger M, Stanek J, Philippe C, Wadsak W, Mitterhauser M, et al. Interaction of 11c-Tariquidar and 11c-Elacridar with P-Glycoprotein and Breast Cancer Resistance Protein at the Human Blood-Brain Barrier. J Nucl Med. 2013 Aug;54(8):1181–7. doi: 10.2967/jnumed.112.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Bethmann K, Gastens AM, Loscher W. The Multidrug Transporter Hypothesis of Drug Resistance in Epilepsy: Proof-of-Principle in a Rat Model of Temporal Lobe Epilepsy. Neurobiol Dis. 2006 Oct;24(1):202–11. doi: 10.1016/j.nbd.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Buda G, Martino A, Maggini V, Orciuolo E, Galimberti S, Gentile M, Morabito F, et al. Mdr1 C3435t Polymorphism Indicates a Different Outcome in Advanced Multiple Myeloma. Acta Haematol. 2009;122(1):42–5. doi: 10.1159/000235820. [DOI] [PubMed] [Google Scholar]
- Buda G, Ricci D, Huang CC, Favis R, Cohen N, Zhuang SH, Harousseau JL, et al. Polymorphisms in the Multiple Drug Resistance Protein 1 and in P-Glycoprotein 1 Are Associated with Time to Event Outcomes in Patients with Advanced Multiple Myeloma Treated with Bortezomib and Pegylated Liposomal Doxorubicin. Ann Hematol. 2010 Nov;89(11):1133–40. doi: 10.1007/s00277-010-0992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JJ, Osman EY, Evans MC, Choi S, Xing X, Cuny GD, Glicksman MA, Lorson CL, Androphy EJ. Enhancement of Smn Protein Levels in a Mouse Model of Spinal Muscular Atrophy Using Novel Drug-Like Compounds. EMBO Mol Med. 2013 Jul;5(7):1035–50. doi: 10.1002/emmm.201202305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop JM, Raymond M, Haber D, Devault A, Arceci RJ, Gros P, Housman DE. The Three Mouse Multidrug Resistance (Mdr) Genes Are Expressed in a Tissue-Specific Manner in Normal Mouse Tissues. Mol Cell Biol. 1989 Mar;9(3):1346–50. doi: 10.1128/mcb.9.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daood M, Tsai C, Ahdab-Barmada M, Watchko JF. Abc Transporter (P-Gp/Abcb1, Mrp1/Abcc1, Bcrp/Abcg2) Expression in the Developing Human Cns. Neuropediatrics. 2008 Aug;39(4):211–8. doi: 10.1055/s-0028-1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, et al. Rescue of the Spinal Muscular Atrophy Phenotype in a Mouse Model by Early Postnatal Delivery of Smn. Nat Biotechnol. 2010 Mar;28(3):271–4. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gil L, Styczynski J, Dytfeld D, Debski R, Kazmierczak M, Kolodziej B, Rafinska B, et al. Activity of Bortezomib in Adult De Novo and Relapsed Acute Myeloid Leukemia. Anticancer Res. 2007 Nov-Dec;27(6B):4021–5. [PubMed] [Google Scholar]
- Hua Y, Sahashi K, Rigo F, Hung G, Horev G, Bennett CF, Krainer AR. Peripheral Smn Restoration Is Essential for Long-Term Rescue of a Severe Spinal Muscular Atrophy Mouse Model. Nature. 2011 Oct 6;478(7367):123–6. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski MR, Markandaiah SS, Jacob D, Meng NJ, Li K, Gennaro V, Lepore AC, Trotti D, Pasinelli P. Inhibiting Drug Efflux Transporters Improves Efficacy of Als Therapeutics. Ann Clin Transl Neurol. 2014 Dec;1(12):996–1005. doi: 10.1002/acn3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung L, Holle L, Dalton WS. Discovery, Development, and Clinical Applications of Bortezomib. Oncology (Williston Park) 2004 Dec;18(14 Suppl 11):4–13. [PubMed] [Google Scholar]
- Kannan P, Telu S, Shukla S, Ambudkar SV, Pike VW, Halldin C, Gottesman MM, Innis RB, Hall MD. The “Specific” P-Glycoprotein Inhibitor Tariquidar Is Also a Substrate and an Inhibitor for Breast Cancer Resistance Protein (Bcrp/Abcg2) ACS Chem Neurosci. 2011 Feb 16;2(2):82–9. doi: 10.1021/cn100078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernochan LE, Russo ML, Woodling NS, Huynh TN, Avila AM, Fischbeck KH, Sumner CJ. The Role of Histone Acetylation in Smn Gene Expression. Hum Mol Genet. 2005 May 1;14(9):1171–82. doi: 10.1093/hmg/ddi130. [DOI] [PubMed] [Google Scholar]
- Kreisl WC, Bhatia R, Morse CL, Woock AE, Zoghbi SS, Shetty HU, Pike VW, Innis RB. Increased Permeability-Glycoprotein Inhibition at the Human Blood-Brain Barrier Can Be Safely Achieved by Performing Pet During Peak Plasma Concentrations of Tariquidar. J Nucl Med. 2015 Jan;56(1):82–7. doi: 10.2967/jnumed.114.146894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon DY, Dimitriadi M, Terzic B, Cable C, Hart AC, Chitnis A, Fischbeck KH, Burnett BG. The E3 Ubiquitin Ligase Mind Bomb 1 Ubiquitinates and Promotes the Degradation of Survival of Motor Neuron Protein. Mol Biol Cell. 2013 Jun;24(12):1863–71. doi: 10.1091/mbc.E13-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon DY, Motley WW, Fischbeck KH, Burnett BG. Increasing Expression and Decreasing Degradation of Smn Ameliorate the Spinal Muscular Atrophy Phenotype in Mice. Hum Mol Genet. 2011 Sep 15;20(18):3667–77. doi: 10.1093/hmg/ddr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Pham LT, Butchbach ME, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, et al. Smndelta7, the Major Product of the Centromeric Survival Motor Neuron (Smn2) Gene, Extends Survival in Mice with Spinal Muscular Atrophy and Associates with Full-Length Smn. Hum Mol Genet. 2005 Mar 15;14(6):845–57. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, et al. Identification and Characterization of a Spinal Muscular Atrophy-Determining Gene. Cell. 1995 Jan 13;80(1):155–65. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- Lu S, Chen Z, Yang J, Chen L, Gong S, Zhou H, Guo L, Wang J. Overexpression of the Psmb5 Gene Contributes to Bortezomib Resistance in T-Lymphoblastic Lymphoma/Leukemia Cells Derived from Jurkat Line. Exp Hematol. 2008 Oct;36(10):1278–84. doi: 10.1016/j.exphem.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Lu S, Chen Z, Yang J, Chen L, Zhou H, Xu X, Li J, Han F, Wang J. The Effects of Proteasome Inhibitor Bortezomib on a P-Gp Positive Leukemia Cell Line K562/A02. Int J Lab Hematol. 2010 Feb;32(1 Pt 1):e123–31. doi: 10.1111/j.1751-553X.2009.01145.x. [DOI] [PubMed] [Google Scholar]
- Lu S, Yang J, Chen Z, Gong S, Zhou H, Xu X, Wang J. Different Mutants of Psmb5 Confer Varying Bortezomib Resistance in T Lymphoblastic Lymphoma/Leukemia Cells Derived from the Jurkat Cell Line. Exp Hematol. 2009 Jul;37(7):831–7. doi: 10.1016/j.exphem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- McAndrew PE, Parsons DW, Simard LR, Rochette C, Ray PN, Mendell JR, Prior TW, Burghes AH. Identification of Proximal Spinal Muscular Atrophy Carriers and Patients by Analysis of Smnt and Smnc Gene Copy Number. Am J Hum Genet. 1997 Jun;60(6):1411–22. doi: 10.1086/515465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tanaka K, Matsunobu T, Okada T, Nakatani F, Sakimura R, Hanada M, Iwamoto Y. The Mechanism of Cross-Resistance to Proteasome Inhibitor Bortezomib and Overcoming Resistance in Ewing’s Family Tumor Cells. Int J Oncol. 2007 Oct;31(4):803–11. [PubMed] [Google Scholar]
- O’Connor R, Ooi MG, Meiller J, Jakubikova J, Klippel S, Delmore J, Richardson P, et al. The Interaction of Bortezomib with Multidrug Transporters: Implications for Therapeutic Applications in Advanced Multiple Myeloma and Other Neoplasias. Cancer Chemother Pharmacol. 2013 May;71(5):1357–68. doi: 10.1007/s00280-013-2136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Drug Transport across the Blood-Brain Barrier. J Cereb Blood Flow Metab. 2012 Nov;32(11):1959–72. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D. Choroid Plexus Epithelial Expression of Mdr1 P Glycoprotein and Multidrug Resistance-Associated Protein Contribute to the Blood-Cerebrospinal-Fluid Drug-Permeability Barrier. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3900–5. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, Lin B, Qiu J, Chan LL, Bates SE. Rapid Detection of Abc Transporter Interaction: Potential Utility in Pharmacology. J Pharmacol Toxicol Methods. 2011 May-Jun;63(3):217–22. doi: 10.1016/j.vascn.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE. Overexpression of the Atp-Binding Cassette Half-Transporter, Abcg2 (Mxr/Bcrp/Abcp1), in Flavopiridol-Resistant Human Breast Cancer Cells. Clin Cancer Res. 2001 Jan;7(1):145–52. [PubMed] [Google Scholar]
- Rumpold H, Salvador C, Wolf AM, Tilg H, Gastl G, Wolf D. Knockdown of Pgp Resensitizes Leukemic Cells to Proteasome Inhibitors. Biochem Biophys Res Commun. 2007 Sep 21;361(2):549–54. doi: 10.1016/j.bbrc.2007.07.049. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Liddelow SA, Dziegielewska KM. Barrier Mechanisms in the Developing Brain. Front Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, et al. Disruption of the Mouse Mdr1a P-Glycoprotein Gene Leads to a Deficiency in the Blood-Brain Barrier and to Increased Sensitivity to Drugs. Cell. 1994 May 20;77(4):491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-Glycoprotein in the Blood-Brain Barrier of Mice Influences the Brain Penetration and Pharmacological Activity of Many Drugs. J Clin Invest. 1996 Jun 1;97(11):2517–24. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E, Horton JK, Yang CH, Nakagawa M, Cowan KH. Multidrug Resistance-Associated Protein Gene Overexpression and Reduced Drug Sensitivity of Topoisomerase Ii in a Human Breast Carcinoma Mcf7 Cell Line Selected for Etoposide Resistance. Cancer Res. 1994 Jan 1;54(1):152–8. [PubMed] [Google Scholar]
- Wiberg K, Carlson K, Aleskog A, Larsson R, Nygren P, Lindhagen E. In Vitro Activity of Bortezomib in Cultures of Patient Tumour Cells--Potential Utility in Haematological Malignancies. Med Oncol. 2009;26(2):193–201. doi: 10.1007/s12032-008-9107-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Increasing amounts of HEK293 cell lysate were incubated with the Suc-LLVY-AMC fluorogenic compound. Fluorescence (excitation: 360 nm, emission: 460 nm) increased over time with increased concentration of protein lysate. Based on these data we chose to use 70 μg of protein lysate for all subsequent experiments (b) No lysate and no substrate controls have minimal background florescence. Purified proteasome released fluorogenic cleavage products leading to readings that were far in excess of that obtained using 70 μg of protein lysate after 30 minutes, illustrating that our experimental conditions were well within the dynamic range of the assay. We verified that the cleavage activity measured was due to the proteasome using the chymotryptic activity specific proteasome inhibitor MG132.
Bortezomib is transported by P-gp in cultured cells. Cells stably expressing either P-gp or an empty vector were treated with tariquidar (1 μM) or DMSO (1 μl per 1 ml) for 15 min then with bortezomib (0.5 μM) for 30 min. The chymotrypsin-like activity of the proteasome was assessed by examining the cleavage of the fluorogenic peptide Suc-LLVY-AMC (a and b) when added to the cell lysate. Values represent chymotrypsin-like activity relative to vehicle control ± SEM,*P<0.05, n=6.
Tariquidar treatment does not alter proteasome activity. Cell transfected with an empty vector were treated with tariquidar (1 μM) or DMSO (1 μL per 1 ml) for 15 min. The chymotrypsin-like activity of the proteasome in cell lines was analyzed by assessing the cleavage of the fluorogenic peptide Suc-LLVY-AMC (c) when added to cell lysate. Values represent chymotrypsin-like activity relative to vehicle control ± SEM, p>0.05, n=6.
Single dose treatment with bortezomib does not alter proteasome protein expression. Mice at P12 were injected with bortezomib (0.15 mg/kg) and sacrificed 1 h later. PSMB5 protein levels are consistent in the spinal cords between wildtype and (pgp1a/b−/−:bcrp−/−) triple knockout mice. A densitometry analysis was performed on the respective western blots to ascertain relative SMN protein levels. Values represent mean relative to tariquidar-treated control ± SEM. p>0.05, n=6,
Tariquidar plus bortezomib treatment of unaffected animals do not alter the weight gain seen during the first two weeks of life compared to tariquidar only treated mice. Mice at P4 were treated with tariquidar (13 mg/kg) or vehicle (DMSO) 15 min before bortezomib (0.15 mg/kg) or vehicle (Ringer’s solution) injection every other day.
There is a trend to increased bortezomib penetration of the BSCB when mice are pre-treated with tariquidar. Mice at P4 were treated with tariquidar (13 mg/kg) or vehicle (DMSO) 15 min before bortezomib (0.15 mg/kg) or vehicle (Ringer’s solution) i.p. injection every other day and sacrificed at P12. The spinal cords were analyzed for bortezomib using LCMS/MS. p>0.05, n=6.
Bortezomib requires prior treatment with tariquidar to increase SMN protein levels in the CNS. Mice at P4 were treated with tariquidar (13 mg/kg) or vehicle (DMSO, 1 μl/g) 15 min before bortezomib (0.15 mg/kg – 0.075 mg/kg) or vehicle (Ringer’s solution, 1 μ/g) injection every other day and sacrificed at P12. The 13 mg/kg tariquidar pre-treatment result was not significantly different from bortezomib alone. A densitometry analysis was performed on the resulting western blots to ascertain relative SMN protein levels. Values represent mean relative to tariquidar-treated control ± SEM. p>0.05, n=6.