Abstract
How commensal-specific T cells are controlled in the periphery is poorly understood. In a recent issue of Science, Hepworth et al. (2015) show that ILC3s induce apoptosis of microbiota-specific CD4 T cells in a form of extrathymic negative selection.
Discriminating between self and non-self is one of the most important and challenging functions of the immune system. This includes the ability to generate responses against non-self, i.e., immunity, while avoiding overt responses to self, i.e., autoimmunity. During T cell development in the thymus, potentially dangerous T cell clones recognizing host self-antigens are either eliminated or re-directed to alternative fates, such as the T regulatory (Treg) cell lineage. The deletion of autoreactive T cells, or negative selection, is directed by ectopic expression of tissue-restricted self-antigens presented by MHCII on medullary thymic epithelial cells (mTEC).
The mammalian gut contains trillions of resident commensal bacteria that are physically and functionally integrated with the host. In order to avoid self-reactivity in the host-microbe superorganism, the immune system must ensure unresponsiveness to the commensal “self.” The mechanisms that constrain activation of commensal-specific T cells are poorly understood. As bacterial antigens are not encoded in the host genome, commensal-specific CD4 T cells escape deletion in the thymus. Nevertheless, commensal-specific effector CD4 T cells are not expanded or engaged in the intestine at steady state. This is mainly achieved by limiting the exposure of the immune system to most commensal bacteria and their antigens (Hooper and Macpherson, 2010). However, even though they do not normally gain access across the epithelial barrier, commensal bacteria and their products are sampled by the host. This seems to be a well-regulated process mediated by transport of small amounts of antigens to mesenteric lymph nodes (MLNs) by lamina propria (LP) dendritic cells (DCs) (Hapfelmeier et al., 2010; Macpherson and Uhr, 2004). At steady state, this directed sampling by mostly tolerogenic LP DCs restricts commensal CD4 T cell reactivities by diverting them into the Treg cell lineage (Grainger et al., 2014).
In a recent study in Science, the Sonnenberg group reports another mechanism of preventing commensal-specific CD4 T cell expansion (Hepworth et al., 2015). They find that MHCII-expressing group 3 innate lymphoid cells (ILC3s) can curtail commensal-specific CD4 T cells in a process akin to negative selection in the thymus, termed “intestinal selection.” ILC3s regulate MHCII expression similarly to mTECs and are capable of inducing apoptosis of CD4 T cells in an MHCII-and antigen-dependent manner.
Previous work demonstrated a role for ILC3s in controlling CD4 T cell responses in the gut via expression of MHCII (Goto et al., 2014; Hepworth et al., 2013). Conditional ablation of MHCII expression on ILC3s (in MHCIIΔILC3 mice) leads to uncontrolled intestinal inflammation with significant expansion of activated CD4 T cells that produce pro-inflammatory cytokines (Hepworth et al., 2013). MHCIIΔILC3 mice in a different colony show expansion of T helper 17 (Th17) cells in the small intestine in the absence of inflammation, suggesting that the defect in CD4 T cell homeostasis is the initiating factor and that specific microbiota might drive inflammation (Goto et al., 2014). Indeed, antibiotic treatment abolishes both CD4 T cell activation and intestinal inflammation in this model, proving that inflammation is microbiota-driven and suggesting that the expanded CD4 T cells recognize commensal antigens (Hepworth et al., 2013). How exactly MHCII+ ILC3s control mucosal CD4 T cells remained unclear.
In order to address this question, Hepworth et al. characterized the regulation of MHCII expression on ILC3s. This expression is restricted to a subset of ILC3s known as lymphoid-tissue inducer (Lti) cells that express the chemokine receptor CCR6 and are enriched in the MLN and intestinal LP. In contrast to non-professional antigen-presenting cells (APCs) such as epithelial cells, MHCII expression on ILC3s is constant and independent of cytokine signals, presence of microbiota, and microbial-derived stimuli. In contrast to peripheral APCs such as B cells and DCs, MHCII expression on ILC3s is under the control of the pIV promoter of the master MHCII transcriptional regulator CIITA. The only other APCs with similar MHCII regulation are mTECs, which direct negative selection in the thymus. This led the authors to explore the possibility that ILC3s mediate extrathymic negative selection of commensal CD4 T cells. Indeed, ILC3-specific deletion of MHCII in MHCIIΔILC3 mice leads to loss of tolerance to the microbiota and expansion of activated CD4 T cells that recognize commensal bacteria in the LP.
Do ILC3s restrain all types of CD4 T cells? To address this question, Hepworth et al. examined the effects of ILC3s on TCR transgenic (Tg) T cells specific for either food antigens (chicken ovalbumin, OTII) or commensal bacteria (commensal flagelin, CBir1). Using a combination of genetic approaches, the authors demonstrate that ILC3s restrict expansion of CD4 T cells only in the presence of cognate antigen. As ILC3s in the MLN present mostly commensal antigens, they preferentially control CD4 T cells recognizing such antigens. Indeed, in the absence of exogenous antigen, expansion of CD4 T cells and intestinal inflammation occur only in MHCIIΔILC3 mice crossed to CBir1-Tg, but not to OTII-Tg mice, and only in intestinal tissues. In contrast, preferential CD4 T cell expansion is not detected in the thymus or spleen. Moreover, transfer of activated CD4 T cells together with cognate antigen into congenic animals in which only ILC3s express MHCII results in decreased recovery of CBir1 Tg T cells in the MLN and LP compared to MHCII-negative hosts. Interestingly, the decreased recovery is not a result of decreased trafficking or proliferation of transferred T cells, nor does it seem to re-direct cells to the Treg cell fate. This led the authors to examine whether ILC3s induce MHCII-mediated apoptosis of commensal T cells. To better study this question, they developed an in vitro co-culture system, where they first show that intestinal ILC3s can restrict expansion of activated CD4 T cells in an MHCII-dependent and antigen-dependent manner, underscoring the specificity of this effect and recapitulating the in vivo phenomenon. ILC3s induce apoptosis of target CD4 T cells in vitro in an antigen-dependent manner. Similarly to mTEC-mediated central negative selection, ILC3-induced apoptosis is accompanied by induction of Nur77 and the proapoptotic factor Bim in CD4 T cells, and ILC3s do not induce death in Bim-deficient CD4 T cells. Because Bim-dependent apoptotic cell death can be induced by cytokine starvation and ILC3s constitutively express common gamma chain cytokine receptors, the authors examined whether addition of several key cytokines can rescue apoptotic CD4 T cells. Indeed, addition of recombinant interleukin-2 (IL-2) rescues CD4 T cells from ILC3-induced apoptosis and mutant T cells with constitutively active IL-2 signaling can escape deletion by ILC3s both in vitro and following adoptive transfer in vivo.
Altogether, the data in this study establish a model in which presentation of commensal antigens by ILC3s leads to apoptosis of cognate CD4 T cells in the MLN, mediated by sequestration of IL-2 by ILCs (Figure 1). This results in a significant decrease in commensal-specific activated CD4 T cells in the gut mucosa, which is crucial for preserving host-commensal mutualism. Further expanding the impact of their study, the authors report that MHCII expression by ILC3s is also present in human intestinal tissues and is dysregulated during inflammatory bowel disease (IBD). Crohn's disease patients have significantly decreased expression of MHCII on mucosal ILC3s, which inversely correlates with frequencies of pro-inflammatory colonic Th17 cells and the amount of circulating, commensal bacteria-specific IgG. Thus, ILC3-mediated “intestinal selection” may represent a genuine therapeutic target in IBD.
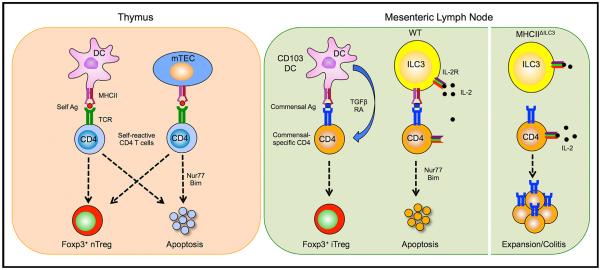
Figure 1. Selection of Self-Reactive and Commensal-Specific CD4 T Cells.
(Left) In the thymus, bone marrow-derived APCs, such as DCs, and mTECs eliminate self-reactive CD4 T cells either through the induction of apoptosis during negative selection or re-direction to the Treg cell lineage.
(Right) In the MLN, activated commensal-specific CD4 T cells are either directed to the Treg cell lineage by CD103+ DCs or deleted through induction of apoptosis by MHCII+ ILC3s in a process of “intestinal selection.” ILC3s induce apoptosis by IL-2 withdrawal, which activates Nur77 and the proapoptotic factor Bim. Conditional deletion of MHCII on ILC3 (MHCIIΔILC3 mice) prevents deletion and leads to expansion of commensal-specific CD4 T cells, which results in intestinal inflammation in the presence of appropriate microbiota.
Several questions remain to be addressed in future studies, central of which is the contribution of this new pathway of intestinal selection to steady state commensal homeostasis in vivo. In the LP, ILC3s are key participant in the crosstalk between the microbiota and innate and adaptive immune cells (Mortha et al., 2014). It will be important to examine whether the molecular mechanisms of induced apoptosis outlined by the elegant in vitro studies are the only mechanisms mediating MHCII+ ILC3 effects in vivo. It is important that ILC3s do not completely purge commensal-reactive CD4 T cells from the repertoire, because this would be detrimental. Indeed, Hepworth et al. show that only activated, and not naive, CD4 T cells could be controlled by ILC3s. This ensures preservation of commensal-reactive TCRs in the naive CD4 T cell pool that can deal with commensal dissemination in the event of epithelial barrier dysfunction. The mechanisms underlying this difference in specificity remain to be elucidated. The nature, location, and mode of acquisition of commensal antigens by ILC3s will also be important to ascertain. Very few commensal bacteria gain access to the MLN (Hapfelmeier et al., 2010) and at steady state most are delivered by tolerogenic DCs that induce Treg cells. MLN DCs from wild-type mice fail to stimulate CBir1 T cells in vitro, suggesting that they lack endogenous CBir1 antigens at steady state (Cong et al., 2009). This agrees with the results in the current study where adoptively transferred CBir1 T cells require administration of exogenous antigen for control by ILC3s. How do ILC3s then acquire commensal antigens and what are these antigens? One possibility is that this pathway is initiated under conditions of increased commensal antigen burden. Another possibility is that ILC3s sample antigens and restrict T cell activation against a subset of commensal bacteria. ILC3s might also receive antigens from DCs in either the MLN or LP. Although ILC3s co-localize with T cells in the MLN, they can also traffic from the LP to the MLN (Mackley et al., 2015) and might acquire antigens or exert intestinal selection in the mucosa itself.
Understanding how the immune system preserves mutualism with commensal microbiota is central to our ability to sustain healthy immune homeostasis. The study by Hepworth et al. describes not only a new function for ILCs, but also a new mechanism for maintaining intestinal homeostasis that introduces exciting opportunities for unconventional therapeutic approaches to chronic inflammatory disorders.
REFERENCES
- Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. Proc. Natl. Acad. Sci. USA. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger JR, Askenase MH, Guimont-Desrochers F, da Fonseca DM, Belkaid Y. Immunol. Rev. 2014;259:75–87. doi: 10.1111/imr.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, et al. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, Withers DR, Hugues S, Farrar MA, Reith W, et al. Science. 2015;348:1031–1035. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Macpherson AJ. Nat. Rev. Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- Mackley EC, Houston S, Marriott CL, Halford EE, Lucas B, Cerovic V, Filbey KJ, Maizels RM, Hepworth MR, Sonnenberg GF, et al. Nat. Commun. 2015;6:5862. doi: 10.1038/ncomms6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]