Abstract
Objective
To determine whether the commencement and length of puberty influences dual x-ray absorptiometry (DXA) values of bone mineral content (BMC) and bone mineral density (BMD) in the axial and appendicular skeleton at skeletal maturity.
Study design
From the Bone Mineral Density in Childhood Study, we identified children who began puberty and completed sexual and skeletal development and examined whether the timing and length of puberty influence DXA values of BMC and BMD at skeletal maturity.
Results
A total of 78 girls and 85 boys began puberty and completed skeletal maturity; 4.4 ± 0.8 and 4.5 ± 0.8 years later, respectively. Multiple linear regression analyses indicated that the age of onset of puberty was a strong negative predictor of DXA bone measurements at skeletal maturity, independent of bone values at the beginning of puberty, and the length of puberty. This negative relation was observed for all BMC and BMD measurements at all skeletal sites, in both boys and girls (all P < .0001). In contrast, length of puberty had no relation to any measures of bone.
Conclusions
In healthy adolescent males and females, bone mass and bone density at skeletal maturity are inversely related to the timing of puberty.
Peak bone mass (PBM), a major determinant of the future risk of fractures in the elderly, is largely achieved by the end of sexual and skeletal maturity.1,2 The greatest accretion of bone occurs during puberty, and low PBM may result from clinical states associated with abnormal pubertal development.1–3 Idiopathic delayed puberty in females is a cause for reduced PBM,4 and amenorrheic teenage girls have lower bone density than girls with normal menses.5 Likewise, delayed puberty and constitutional delay in male teenagers results in decreased bone mineralization and lower PBM.6–8 Genetic males with complete androgen insensitivity (testicular feminization) experience increased pubertal growth but achieve a bone mass less than expected of androgen-replete men.9–11 Estrogens are also needed for the achievement of adequate PBM in males, and aromatase deficiency or estrogen receptor defects in men result in tall stature and severe osteoporosis.12–15
Although the amount of bone gained during adolescence is the main contributor to PBM, a greater understanding of the influence that normal variations in sexual development have on bone acquisition during growth would facilitate the planning of strategies to enhance PBM. Previously, we have shown that, in healthy girls and boys, bone mass in the axial and appendicular skeletons at early puberty is the strongest predictor of values at sexual maturity.16 However, both age and duration of puberty vary greatly; whether the marked variability in pubertal development, even within the normal range, also influences PBM has yet to be defined. The purpose of this prospective longitudinal multicenter study was to determine whether the timing of commencement and length of puberty in contemporary children in the United States influence dual x-ray absorptiometry (DXA) values of bone mineral content (BMC) and bone mineral density (BMD) in the axial and appendicular skeletons at skeletal maturity.
Methods
The Bone Mineral Density in Childhood Study is an ongoing multicenter longitudinal study examining bone accretion in 1554 healthy children and teenagers of both sexes and different ethnic groups in the United States. From this unique subject pool, we identified children who began puberty and completed sexual and skeletal development during the duration of the study. Participants were recruited from July 2002 to November 2003 at 5 medical centers: Children’s Hospital Los Angeles (Los Angeles, California), Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio), Creighton University (Omaha, Nebraska), Children’s Hospital of Philadelphia (Philadelphia, Pennsylvania), and Columbia University (New York, New York). Participants were seen annually for measurements. The Institutional Review Board at each clinical center approved the protocol, and consent was obtained from each participant’s parent or guardian, and assent was obtained from the study participants.
From this cohort, we chose all 78 girls and 85 boys that started puberty and achieved sexual and skeletal maturity during the duration of the study. For the purpose of this study, baseline measures were obtained at Tanner II stage of sexual development, and follow-up examinations were taken when subjects reached sexual (Tanner V) and skeletal maturity; for this study skeletal maturation was defined as epiphyseal closure of the phalanges and metacarpals, corresponding to bone ages of 16 for females and 17 for males.
Detailed information about the study participants, inclusion/exclusion criteria, and study procedures have been published previously.17 Briefly, the sample was selected to reflect healthy, normally developing children in the United States. The following inclusion criteria were used: residence in United States for at least 3 years, school placement within 1 year of that expected for age, full-term birth (≥37 weeks gestation), birth weight > 2.3 kg, and no evidence of precocious or delayed puberty. For this study normal puberty was defined as breast development beginning between 8 and 13 years for girls, and testes size of at least 4 mL between 9 and 14 years for boys.
Exclusion criteria were height, weight, or body mass index (BMI; kg/m2) less than third or more than ninety-seventh percentile; current or previous medical condition known to affect growth, maturation, physical activity, or nutritional status, and medications known to affect growth, maturation, or bone mineral accrual such as steroids. Subjects with indwelling hardware; abnormality of the skeleton or spine such as scoliosis 20 degrees or more, kyphosis, or skeletal dysplasia by history; current or previous pregnancy; same-sex sibling enrolled in the Bone Mineral Density in Childhood Study; and participation in a diet or exercise intervention study in the previous year were also excluded from participation.
Height and weight measurements were obtained with participants dressed in examination gowns or lightweight clothing, without shoes. Body mass index (BMI) percentile was calculated with the Centers for Disease Control 2000 growth charts.18 All subjects underwent a physical examination. The maturational stage of breast development in girls and testicular volume by orchidometer in boys was evaluated on the basis of standard endocrine practice and the criteria of Tanner.19 Skeletal maturity was assessed on the basis of roentgenograms of the left hand and wrist obtained according to the method of Greulich and Pyle.20
Bone Densitometry
DXA scans were performed with Hologic, Inc. (Bedford, Massachusetts) bone densitometers (QDR4500A, QDR4500W, and Delphi A models). Scans were performed on a single densitometer at each center. The software versions used for acquisition varied from version 11.1 to 12.3. The following scans were performed according to manufacturer guidelines for subject positioning: whole body, posteroanterior lumbar spine (L1–L4, fast array), nondominant forearm, and left proximal femur (fast array). At study baseline and in year 3, the calibration of scanners was assessed by having all centers scan a single set of traveling phantoms that included the European Spine and Forearm Phantoms (QRM Inc, Mohrendorf, Germany) and the Hologic block, hip, and whole-body phantoms. The long-term calibration stability was monitored at each clinical site with two site-specific phantoms (Hologic anthropomorphic spine and whole-body phantoms) that were scanned weekly. All scans were analyzed centrally by the DXA Core Laboratory (University of California, San Francisco). The precision error for BMD and BMC were less than 1% for the spine phantom, and less than 2.5% for the whole-body phantom.
Statistical Analysis
Statistical analyses were conducted with Statview (version 5.0.1; SAS Institute Inc, Cary, North Carolina). Pearson correlations were used to examine associations between variables, and multiple regression analyses were used to determine the influence of the baseline bone measure, baseline chronological/bone age, and pubertal duration on values at PBM, corresponding to the time that both Tanner V and skeletal maturity were achieved.
To exclude the possibility of multicollinearity on the multiple regression models, postestimation procedures were used to calculate a condition number for the regression models and comparing the condition number with the suggested cutoff value of 15.21 Models with condition numbers less than 15 were judged to not have any substantial collinearity problems that would affect the results or the conclusions. The goodness of fit for the regression models was evaluated with the postestimation procedures of STATA (StataCorp, College Station, Texas). All models presented passed the following goodness of fit criteria: residuals appeared random and no strong influence or leverage points were present, on the basis of both a graphical and distribution evaluation.
Results
The age, anthropometric characteristics, and DXA bone measures at baseline and follow-up for boys and girls are described in Table I. As expected, the height, weight, and DXA measurements of BMC at all sites were significantly higher in males than in females at baseline and follow-up (all P < .001). Values for BMD for total body and the appendicular skeleton were also higher in males than in females (all P < .001), but there were no sex-related differences in BMD values for the axial skeleton (P = .45 and .57).
Table I.
Age, anthropometric characteristics, and DXA measures for 78 females and 85 males at baseline (Tanner 2) and follow-up (sexual and skeletal maturity)
| Females
|
Males
|
|||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| Age (y) | 10.7 ± 1.0 | 15.1 ± 1.0 | 11.7 ± 1.0 | 16.2 ± 0.9 |
| Bone age (y) | 11.2 ± 1.0 | 16.1 ± 0.4 | 11.3 ± 1.6 | 17.2 ± 0.4 |
| Height (cm) | 144.0 ± 7.3 | 163.5 ± 6.1 | 148.3 ± 6.8 | 174.6 ± 5.8 |
| Weight (kg) | 39.2 ± 8.1 | 59.3 ± 8.8 | 41.5 ± 7.5 | 67.3 ± 9.6 |
| BMI (kg/m2) | 18.8 ± 2.9 | 22.1 ± 2.9 | 18.8 ± 2.6 | 22.1 ± 3.1 |
| BMI percentile (%) | 59.9 ± 28.4 | 65.5 ± 23.1 | 58.1 ± 28.2 | 58.1 ± 26.8 |
| Whole body BMC (g) | 938 ± 193 | 1572 ± 245 | 1015 ± 172 | 1973 ± 243 |
| Whole body BMD (g/cm2) | 0.75 ± 0.07 | 0.94 ± 0.80 | 0.79 ± 0.06 | 1.03 ± 0.08 |
| Spine BMC (g) | 28.5 ± 6.4 | 54.6 ± 9.8 | 30.3 ± 5.6 | 62.8 ± 8.2 |
| Spine BMD (g/cm2) | 0.67 ± 0.10 | 0.98 ± 0.12 | 0.65 ± 0.08 | 0.97 ± 0.10 |
| Upper extremities BMC (g) | 74.4 ± 15.0 | 133.6 ± 21.6 | 82.2 ± 16.8 | 178.3 ± 25.5 |
| Upper extremities BMD (g/cm2) | 0.57 ± 0.05 | 0.70 ± 0.04 | 0.63 ± 0.05 | 0.80 ± 0.05 |
| Lower extremities BMC (g) | 246.5 ± 52.0 | 378.7 ± 62.3 | 266.4 ± 49.7 | 483.6 ± 63.9 |
| Lower extremities BMD (g/cm2) | 0.90 ± 0.09 | 1.12 ± 0.10 | 0.94 ± 0.08 | 1.24 ± 0.11 |
| Tanner 2 to sexual maturity (y) | - | 3.1 ± 1.0 | - | 2.8 ± 1.0 |
| Tanner 2 to skeletal maturity (y) | - | 4.4 ± 0.8 | - | 4.5 ± 0.8 |
All measures Mean ± SD
All subjects achieved sexual maturity earlier than skeletal maturity; on average 1.3 ± 1.0 years earlier for girls and 1.7 ± 1.2 years earlier for boys. Boys overall commenced puberty 1 year later and achieved both sexual and skeletal maturity approximately 1 year later than girls. When divided by ethnic group, 33 were African American (19 male and 14 female), 10 subjects were Asian (6 male and 4 female), 90 subjects were Caucasian (46 male and 44 female), and 30 were Hispanic (14 male and 16 female). Regardless of sex, there were no differences in the duration of puberty between African Americans, Asians, Caucasians, and Hispanics. Whereas there was no ethnic or racial difference in the age at the onset of puberty (Tanner 2) or completion (Tanner 5) in males, African American and Hispanic females started and completed puberty approximately 6 months earlier than Caucasians.
Values for the simple correlations between DXA measurements and age and anthropometrics at baseline (Tanner II) and follow-up (skeletal maturity) are described in Table II (available at www.jpeds.com). These correlations were stronger at baseline than at follow-up, and tended to be stronger for height and weight than for age and bone age in females and males. There were also moderate correlations between baseline and follow-up BMC and BMD values at all skeletal sites (r values between 0.53 and 0.77, all P values < .001). Regardless of sex, significant correlations were present between the age of pubertal commencement (Tanner 2) and all baseline DXA measurements (r values between 0.29 and 0.55, all P values < .02). In contrast, pubertal length did not correlate with baseline DXA measurements in boys or girls (r values between 0.02 and −0.15, all P values > .05). Additionally, pubertal length and age of pubertal commencement (Tanner 2) did not correlate significantly (r = −0.17 and P = .14 for females and r = −0.18 and P = .10 for males).
Table II.
Correlations between anthropometric measures and DXA bone values at baseline and follow-up
| Females
|
Males
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Bone age | Height | Weight | BMI | Age | Bone age | Height | Weight | BMI | |
| Baseline | ||||||||||
| Whole body BMC | 0.55 | 0.50 | 0.79 | 0.74 | 0.44 | 0.52 | 0.68 | 0.67 | 0.63 | 0.38 |
| Whole body BMD | 0.47 | 0.49 | 0.69 | 0.66 | 0.41 | 0.35 | 0.48 | 0.49 | 0.42 | 0.23 |
| Spine BMC | 0.48 | 0.43 | 0.72 | 0.65 | 0.37 | 0.43 | 0.61 | 0.55 | 0.44 | 0.22 |
| Spine BMD | 0.33 | 0.36 | 0.59 | 0.63 | 0.43 | 0.27 | 0.36 | 0.32 | 0.28 | 0.16 |
| Upper extremities BMC | 0.55 | 0.43 | 0.72 | 0.72 | 0.47 | 0.47 | 0.65 | 0.63 | 0.67 | 0.46 |
| Upper extremities BMD | 0.40 | 0.50 | 0.59 | 0.58 | 0.39 | 0.29 | 0.38 | 0.48 | 0.40 | 0.23 |
| Lower extremities BMC | 0.54 | 0.53 | 0.81 | 0.73 | 0.42 | 0.50 | 0.68 | 0.67 | 0.63 | 0.38 |
| Lower extremities BMD | 0.48 | 0.53 | 0.68 | 0.69 | 0.46 | 0.38 | 0.53 | 0.50 | 0.46 | 0.28 |
| Follow-up | ||||||||||
| Whole body BMC | 0.20 | 0.07 | 0.76 | 0.63 | 0.28 | 0.10 | −0.06 | 0.49 | 0.52 | 0.32 |
| Whole body BMD | 0.05 | 0.02 | 0.43 | 0.35 | 0.16 | −0.02 | −0.09 | 0.20 | 0.25 | 0.17 |
| Spine BMC | 0.16 | 0.12 | 0.64 | 0.42 | 0.11 | 0.05 | 0.02 | 0.33 | 0.36 | 0.22 |
| Spine BMD | 0.01 | 0.09 | 0.44 | 0.41 | 0.21 | 0.04 | −0.03 | 0.08 | 0.37 | 0.35 |
| Upper extremities BMC | 0.26 | 0.11 | 0.69 | 0.69 | 0.39 | 0.10 | 0.00 | 0.40 | 0.58 | 0.43 |
| Upper extremities BMD | 0.12 | 0.03 | 0.35 | 0.30 | 0.16 | −0.01 | −0.04 | 0.23 | 0.36 | 0.28 |
| Lower extremities BMC | 0.17 | 0.04 | 0.75 | 0.59 | 0.25 | 0.08 | −0.08 | 0.49 | 0.44 | 0.24 |
| Lower extremities BMD | 0.02 | 0.01 | 0.40 | 0.33 | 0.17 | −0.03 | −0.08 | 0.16 | 0.16 | 0.09 |
Multiple linear regression analyses indicated that both baseline bone values and the age of the onset of puberty independently predicted DXA measurements at skeletal maturity. This was true for BMC and BMD measurements at all skeletal sites and for boys and girls, regardless of whether chronological age or bone age was used in the model (Tables III and IV). However, although the baseline bone value had a positive predictive value with all DXA phenotypes, a negative effect was observed between the age of pubertal onset and all of these measures. The independent reciprocal relations between the timing of puberty commencement and all DXA measurements persisted even after adjusting for the possible confounding effect of height at all sites in both sexes (Tables V and VI, available at www.jpeds.com).
Table III.
Multiple regression models of DXA measures for 78 females with length of puberty, Tanner 2 chronological age, and Tanner 2 bone measure as independent variables
| B | σ | β | P | |
|---|---|---|---|---|
| Whole body peak BMC (R2 = 0.56) | ||||
| Tanner 2 chronological age | −78.234 | 23.473 | −0.311 | .001 |
| Tanner 2 whole body BMC | 1.093 | 0.117 | 0.860 | .000 |
| Length of puberty | 35.906 | 19.690 | 0.142 | .072 |
| Whole body peak BMD (R2 = 0.60) | ||||
| Tanner 2 chronological age | −0.029 | 0.007 | −0.359 | .000 |
| Tanner 2 whole body BMD | 0.963 | 0.093 | 0.868 | .000 |
| Length of puberty | 0.009 | 0.006 | 0.116 | .126 |
| Spine peak BMC (R2 = 0.48) | ||||
| Tanner 2 chronological age | −2.565 | 0.974 | −0.254 | .010 |
| Tanner 2 spine BMC | 1.160 | 0.146 | 0.758 | .000 |
| Length of puberty | 1.473 | 0.857 | 0.146 | .090 |
| Spine peak BMD (R2 = 0.69) | ||||
| Tanner 2 chronological age | −0.038 | 0.008 | −0.316 | .000 |
| Tanner 2 spine BMD | 1.020 | 0.080 | 0.880 | .000 |
| Length of puberty | 0.008 | 0.008 | 0.067 | .309 |
| Upper extremities peak BMC (R2 = 0.52) | ||||
| Tanner 2 chronological age | −6.671 | 2.152 | −0.300 | .003 |
| Tanner 2 upper extremities BMC | 1.209 | 0.138 | 0.838 | .000 |
| Length of puberty | 2.234 | 1.808 | 0.101 | .220 |
| Upper extremities peak BMD (R2 = 0.58) | ||||
| Tanner 2 chronological age | −0.011 | 0.004 | −0.245 | .018 |
| Tanner 2 upper extremities BMD | 0.533 | 0.082 | 0.652 | .000 |
| Length of puberty | 0.007 | 0.004 | 0.170 | .075 |
| Lower extremities peak BMC (R2 = 0.59) | ||||
| Tanner 2 chronological age | −19.264 | 5.721 | −0.300 | .001 |
| Tanner 2 lower extremities BMC | 1.055 | 0.106 | 0.879 | .000 |
| Length of puberty | 9.445 | 4.829 | 0.147 | .054 |
| Lower Extremities Peak BMD (R2 = 0.58) | ||||
| Tanner 2 chronological age | −0.036 | 0.009 | −0.357 | .000 |
| Tanner 2 lower extremities BMD | 0.949 | 0.095 | 0.866 | .000 |
| Length of puberty | 0.007 | 0.008 | 0.070 | .363 |
B, Unstandardized coefficients; σ, standard error of B; β, standardized coefficients.
Table IV.
Multiple regression models of DXA measures for 85 males with length of puberty, baseline chronological age, and baseline bone measure as independent variables
| B | σ | β | P | |
|---|---|---|---|---|
| Whole body peak BMC (R2 = 0.31) | ||||
| Tanner 2 age | −64.625 | 26.130 | −0.270 | .016 |
| Tanner 2 whole body BMC | 0.910 | 0.152 | 0.646 | .000 |
| Length of puberty | 0.086 | 22.717 | 0.000 | .997 |
| Whole body peak BMD (R2 = 0.57) | ||||
| Tanner 2 age | −0.025 | 0.006 | −0.313 | .000 |
| Tanner 2 whole body BMD | 1.015 | 0.099 | 0.804 | .000 |
| Length of puberty | 0.005 | 0.006 | 0.057 | .446 |
| Spine peak BMC (R2 = 0.40) | ||||
| Tanner 2 age | −1.591 | 0.782 | −0.197 | .045 |
| Tanner 2 spine BMC | 1.011 | 0.141 | 0.688 | .000 |
| Length of puberty | −0.132 | 0.717 | −0.016 | .854 |
| Spine peak BMD (R2 = 0.57) | ||||
| Tanner 2 age | −0.022 | 0.007 | −0.232 | .003 |
| Tanner 2 spine BMD | 0.900 | 0.088 | 0.776 | .000 |
| Length of puberty | −0.004 | 0.007 | −0.040 | .590 |
| Upper extremities peak BMC (R2 = 0.34) | ||||
| Tanner 2 age | −7.000 | 2.649 | −0.278 | .010 |
| Tanner 2 upper extremities BMC | 0.996 | 0.157 | 0.657 | .000 |
| Length of puberty | 0.018 | 2.357 | 0.001 | .994 |
| Upper extremities peak BMD (R2 = 0.45) | ||||
| Tanner 2 age | −0.015 | 0.004 | −0.302 | .001 |
| Tanner 2 upper extremities BMD | 0.728 | 0.090 | 0.695 | .000 |
| Length of puberty | 0.000 | 0.004 | 0.007 | .937 |
| Lower extremities peak BMC (R2 = 0.36) | ||||
| Tanner 2 age | −17.763 | 6.565 | −0.282 | .008 |
| Tanner 2 lower extremities BMD | 0.882 | 0.132 | 0.686 | .000 |
| Length of puberty | 1.721 | 5.769 | 0.027 | .766 |
| Lower extremities peak BMD (R2 = 0.56) | ||||
| Tanner 2 age | −0.038 | 0.008 | −0.363 | .000 |
| Tanner 2 lower extremities BMD | 1.015 | 0.100 | 0.809 | .000 |
| Length of puberty | 0.003 | 0.008 | 0.032 | .673 |
B, Unstandardized coefficients; σ, standard error of B; β, standardized coefficients.
Table V.
Multiple regression models of DXA measures for 78 females with length of puberty, Tanner 2 chronological age, Tanner 2 height, and Tanner 2 bone measure as independent variables
| B | σ | β | P | |
|---|---|---|---|---|
| Whole body peak BMC (R2 = 0.61) | ||||
| Tanner 2 chronological age | −113.554 | 25.370 | −0.451 | .000 |
| Tanner 2 whole body BMC | 0.782 | 0.154 | 0.615 | .000 |
| Tanner 2 height | 13.704 | 4.661 | 0.407 | .004 |
| Length of puberty | 40.790 | 18.819 | 0.162 | .034 |
| Whole body peak BMD (R2 = 0.60) | ||||
| Tanner 2 chronological age | −0.029 | 0.008 | −0.357 | .001 |
| Tanner 2 whole body BMD | 0.966 | 0.114 | 0.870 | .000 |
| Tanner 2 height | 0.000 | 0.001 | −0.004 | .973 |
| Length of puberty | 0.009 | 0.006 | 0.116 | .129 |
| Spine peak BMC (R2 = 0.52) | ||||
| Tanner 2 chronological age | −4.031 | 1.122 | −0.400 | .001 |
| Tanner 2 spine BMC | 0.889 | 0.180 | 0.581 | .000 |
| Tanner 2 height | 0.461 | 0.191 | 0.342 | .018 |
| Length of puberty | 1.682 | 0.834 | 0.167 | .048 |
| Spine peak BMD (R2 = 0.69) | ||||
| Tanner 2 chronological age | −0.042 | 0.011 | −0.347 | .000 |
| Tanner 2 spine BMD | 0.993 | 0.094 | 0.856 | .000 |
| Tanner 2 height | 0.001 | 0.002 | 0.058 | .579 |
| Length of puberty | 0.008 | 0.008 | 0.069 | .302 |
| Upper extremities peak BMC (R2 = 0.55) | ||||
| Tanner 2 chronological age | −8.879 | 2.421 | −0.400 | .001 |
| Tanner 2 upper extremities BMC | 1.032 | 0.166 | 0.715 | .000 |
| Tanner 2 height | 0.733 | 0.391 | 0.247 | .064 |
| Length of puberty | 2.466 | 1.782 | 0.111 | .171 |
| Upper extremities peak BMD (R2 = 0.37) | ||||
| Tanner 2 chronological age | −0.009 | 0.006 | −0.213 | .099 |
| Tanner 2 upper extremities BMD | 0.551 | 0.094 | 0.675 | .000 |
| Tanner 2 height | 0.000 | 0.001 | −0.060 | .680 |
| Length of puberty | 0.007 | 0.004 | 0.170 | .077 |
| Lower extremities peak BMC (R2 = 0.63) | ||||
| Tanner 2 chronological age | −27.279 | 6.288 | −0.425 | .000 |
| Tanner 2 lower extremities BMC | 0.783 | 0.145 | 0.652 | .000 |
| Tanner 2 height | 3.145 | 1.193 | 0.367 | .010 |
| Length of puberty | 10.411 | 4.660 | 0.162 | .029 |
| Lower extremities peak BMD (R2 = 0.58) | ||||
| Tanner 2 chronological age | −0.038 | 0.011 | −0.378 | .001 |
| Tanner 2 lower extremities BMD | 0.926 | 0.114 | 0.846 | .000 |
| Tanner 2 height | 0.001 | 0.002 | 0.045 | .722 |
| Length of puberty | 0.007 | 0.008 | 0.071 | .362 |
B, Unstandardized coefficients; σ, standard error of B; β, standardized coefficients.
Table VI.
Multiple regression models of DXA measures for 85 males with length of puberty, Tanner 2 chronological age, Tanner 2 height, and Tanner 2 bone measure as independent variables
| B | σ | B | P | |
|---|---|---|---|---|
| Whole body peak BMC (R2 = 0.37) | ||||
| Tanner 2 chronological age | −111.352 | 30.129 | −0.465 | .000 |
| Tanner 2 whole body BMC | 0.670 | 0.169 | 0.476 | .000 |
| Tanner 2 height | 14.393 | 5.138 | 0.406 | .006 |
| Length of puberty | 4.493 | 21.870 | 0.019 | .838 |
| Whole body peak BMD (R2 = 0.57) | ||||
| Tanner 2 chronological age | −0.024 | 0.008 | −0.307 | .004 |
| Tanner 2 whole body BMD | 1.018 | 0.107 | 0.807 | .000 |
| Tanner 2 height | 0.000 | 0.001 | −0.009 | .936 |
| Length of puberty | 0.005 | 0.006 | 0.057 | .451 |
| Spine peak BMC (R2 = 0.41) | ||||
| Tanner 2 chronological age | −2.308 | 0.987 | −0.285 | .022 |
| Tanner 2 spine BMC | 0.943 | 0.151 | 0.642 | .000 |
| Tanner 2 height | 0.186 | 0.157 | 0.156 | .239 |
| Length of puberty | −0.086 | 0.716 | −0.010 | .905 |
| Spine peak BMD (R2 = 0.57) | ||||
| Tanner 2 chronological age | −0.020 | 0.010 | −0.211 | .046 |
| Tanner 2 spine BMD | 0.905 | 0.090 | 0.781 | .000 |
| Tanner 2 height | 0.000 | 0.002 | −0.032 | .764 |
| Length of puberty | −0.004 | 0.007 | −0.041 | .584 |
| Upper extremities peak BMC (R2 = 0.37) | ||||
| Tanner 2 chronological age | −10.932 | 3.157 | −0.435 | .001 |
| Tanner 2 upper extremities BMC | 0.815 | 0.175 | 0.538 | .000 |
| Tanner 2 height | 1.150 | 0.528 | 0.309 | .032 |
| Length of puberty | 0.690 | 2.325 | 0.027 | .767 |
| Upper extremities peak BMD (R2 = 0.46) | ||||
| Tanner 2 chronological age | −0.018 | 0.006 | −0.345 | .004 |
| Tanner 2 upper extremities BMD | 0.706 | 0.098 | 0.674 | .000 |
| Tanner 2 height | 0.001 | 0.001 | 0.070 | .582 |
| Length of puberty | 0.001 | 0.004 | 0.010 | .904 |
| Lower extremities peak BMC (R2 = 0.42) | ||||
| Tanner 2 chronological age | −30.204 | 7.612 | −0.479 | .000 |
| Tanner 2 lower extremities BMC | 0.662 | 0.147 | 0.515 | .000 |
| Tanner 2 height | 3.786 | 1.307 | 0.406 | .005 |
| Length of puberty | 3.015 | 5.541 | 0.047 | .588 |
| Lower extremities peak BMD (R2 = 0.56) | ||||
| Tanner 2 chronological age | −0.034 | 0.011 | −0.322 | .003 |
| Tanner 2 lower extremities BMD | 1.038 | 0.107 | 0.828 | .000 |
| Tanner 2 height | −0.001 | 0.002 | −0.069 | .536 |
| Length of puberty | 0.003 | 0.008 | 0.030 | .688 |
B, Unstandardized coefficients; σ, standard error of B; β, standardized coefficients.
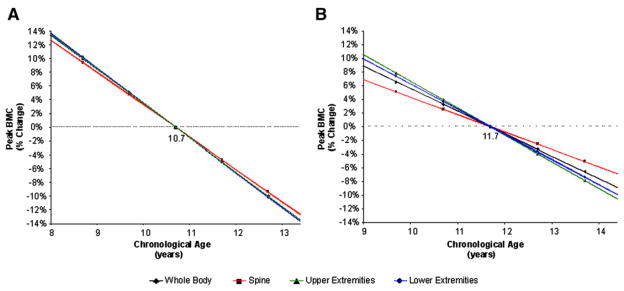
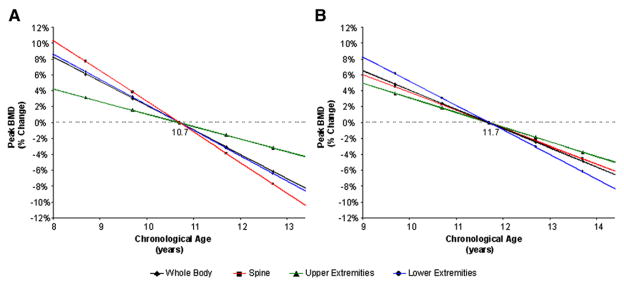
On the basis of the equations obtained from the multiple regression models, percent change in peak BMC and BMD values were calculated. Variations in predicted peak BMC and BMD measures for total body, spine, and upper and lower extremities for girls, 8 to 13 years of age, in relation to the mean pubertal age (10.7 ± 1.0 years) are shown in Figures 1, A, and 2, B (available at www.jpeds.com). On average, for every year, peak BMC values change 4.7% to 5.1%, and peak BMD values change 1.6% to 3.9% depending on skeletal site. Likewise, Figures 1, B, and 2, B, show the percent changes in BMC and BMD for boys starting puberty between ages 9 and 14 in reference to the mean age of pubertal commencement (11.7 ± 1.0 years) (Figure 2, B). On average, for every year, peak BMC values change between 2.5% to 3.9%, and peak BMD values change 1.9% to 3.1%. Similar findings for all bone phenotypes were observed when percent change in peak BMC and BMD in relation to the timing of pubertal commencement were calculated by use of the skeletal age (data not shown).
Figure 1.
Predicted percent change in peak BMC over the normal range of pubertal commencement for A, girls and B, boys as compared with the mean peak BMC at mean age of pubertal commencement (10.7 for girls and 11.7 for boys). Data points represent one and two standard deviations from the mean age of pubertal onset.
Figure 2.
Predicted percent change in peak BMD over the normal range of pubertal commencement for A, girls and B, boys as compared with the mean peak BMD at mean age of pubertal commencement (10.7 for girls and 11.7 for boys). Data points represent one and two standard deviations from the mean age of pubertal onset.
Discussion
The amount of bone gained during puberty is the main contributor to PBM, which, in turn, is a major determinant of osteoporosis and fracture risk in the elderly. The results of this longitudinal study provide strong evidence that the timing of puberty is a negative independent predictor of PBM. We found a strong reciprocal relation between all DXA values of bone mass and bone density at skeletal maturity and variations in the timing of puberty within the normal range. This negative relation was observed in both healthy young males and females, was present in the axial and appendicular skeleton, and was independent of the major known determinant of PBM: the bone value at the beginning of puberty. On average, healthy girls starting puberty a year earlier had approximately 5% greater BMC measures and 2.5% greater BMD values at skeletal maturity, but those starting a year later had 5% and 2.5% less. Similar findings of a slightly smaller magnitude were observed in healthy boys. Our findings that changes in the tempo of puberty within the normal range in males and females negatively effects PBM at all skeletal sites were also demonstrated when bone age rather than chronological age was used for the analyses.
Although the earlier the beginning of puberty, the higher the PBM at skeletal maturity, variations in pubertal length did not significantly influence bone accretion because both slow and fast sexually maturing male and female teenagers achieve similar PBM. This lack of association was observed for both BMC and BMD measurements at all skeletal sites.
Pubertal activation of sexual development accelerates skeletal growth and bone accretion, leading to epiphyseal fusion. In comparison with previous reports, our study is strengthened by highly detailed and standardized assessments of these physiological changes associated with pubertal development. The degrees of sexual and skeletal development were assessed yearly by pediatric endocrinologists and pediatric radiologists, respectively, and all DXA bone measurements were analyzed at a central core facility following rigorous acquisition and analyses protocols. Hence, although the number of subjects examined in this study is relatively small, they represent a well-characterized longitudinally analyzed cohort of healthy, normally developing adolescents in the United States.
This study has some notable limitations. Although we did not account for known determinants of bone accretion during growth, such as dietary intake and physical activity, it is unlikely that this omission would affect our findings because it pertained to all subjects. Another limitation of this study is that the evaluation was restricted to adolescents with normally timed puberty, and replication of our findings in the extremes of the normal population is needed to further strengthen the claim that the age of pubertal commencement is a strong determinant of PBM. Nonetheless, even greater impairments in PBM are likely to occur in subjects with constitutional delay of puberty, a common clinical condition potentially affecting up to 3% of otherwise normal adolescents. If left untreated, these children will attain a full sexual maturity spontaneously, albeit at later chronological age and, as available data would suggest, with lower PBM than their peers.4,7,8,11,14,15,22,23
The effect of pubertal timing on PBM has become the center of considerable attention because of reports of adverse effects of treatments aimed at augmenting the height of adolescents with short stature. It was recently shown that prolonging the growth period of short children with normally timed puberty, by delaying sex hormone-induce growth-plate senescence, may increase final height but substantially decreases PBM.24 Our findings indicate that minor delays in pubertal growth and maturation, even within the normal range, result in a deficit in PBM. This concurs with these foregoing observations, thus stressing the need for caution in the use of treatments aimed at prolonging the growth period, as they might result in reduced adult bone mass.
The care of patients with osteoporosis is difficult, and most interventions increase bone density by modest amounts despite long periods of treatment. In contrast, large increases in bone density occur over a relatively brief period during puberty.25,26 Because the rate of decline in bone mass in adulthood is approximately 1% to 2% per year, a 10% to 20% difference in bone density because of the normal variations in the timing of puberty corresponds to an additional 10 to 20 years of protection against the normal age-related decline in skeletal mass.27,28 The 2000 National Institutes of Health Consensus Development Conference on Osteoporosis Prevention, Diagnosis, and Therapy identified bone mineral accretion during adolescence as a critical determinant of osteoporosis risk later in life.29 The results of this study provide further evidence of the importance of the timing of pubertal commencement as a strong independent predictor of bone mass and bone density in healthy young adults. They underscore the need for additional studies to establish whether the potential deficiency in PBM in adolescents with delays in pubertal commencement, even within the normal range, can be prevented as a result of simple nutritional, mechanical, or pharmacologic intervention.
Acknowledgments
Funded by the National Institute of Child Health and Human Development (NICHD) contracts NO1-HD-1-3228, -3329, -3330, -3331, -3332, and -3333.
Special thanks is given to Margaret Frederick, PhD, and Xiangke Huang, MD, for their work at the Data Coordinating Center, Thomas Hangartner, PhD, for his work as DXA Quality Control Officer, and Frank Biro, MD, Jean-Claude DesMangles, MD, Deborah Elder, MD, Lynda Fisher, MD, Mitchell Geffner, MD, Debra Jeandron, MD, Andrea Kelly, MD, David Langdon, MD, Steven Mittelman, MD, Pisit Pitukcheewanont, MD, Sue Rose, MD, and Peggy Stenger, MD for their help in assessing the Tanner stages of sexual development.
Glossary
- BMC
Bone mineral content
- BMD
Bone mineral density
- BMI
Body mass index
- DXA
Dual x-ray absorptiometry
- PBM
Peak bone mass
Footnotes
The authors declare no conflicts of interest.
References
- 1.Gilsanz V, Gibbens DT, Carlson M, Boechat MI, Cann CE, Schulz EE. Peak trabecular vertebral density: a comparison of adolescent and adult females. Calcif Tissue Int. 1988;43:260–2. doi: 10.1007/BF02555144. [DOI] [PubMed] [Google Scholar]
- 2.Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. J Clin Invest. 1994;93:799–808. doi: 10.1172/JCI117034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabatier JP, Guaydier-Souquieres G, Laroche D, Benmalek A, Fournier L, Guillon-Metz F, et al. Bone mineral acquisition during adolescence and early adulthood: a study in 574 healthy females 10–24 years of age. Osteoporos Int. 1996;6:141–8. doi: 10.1007/BF01623938. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein JS, Klibanski A, Neer RM. A longitudinal evaluation of bone mineral density in adult men with histories of delayed puberty. J Clin Endocrinol Metab. 1996;81:1152–5. doi: 10.1210/jcem.81.3.8772591. [DOI] [PubMed] [Google Scholar]
- 5.Hergenroeder AC. Bone mineralization, hypothalamic amenorrhea, and sex steroid therapy in female adolescents and young adults. J Pediatr. 1995;126:683–9. doi: 10.1016/s0022-3476(95)70393-4. [DOI] [PubMed] [Google Scholar]
- 6.Moreira-Andres MN, Canizo FJ, de la Cruz FJ, Gomez-de la Camara A, Hawkins FG. Evaluation of radial bone mineral content in prepubertal children with constitutional delay of growth. J Pediatr Endocrinol Metab. 2000;13:591–7. doi: 10.1515/jpem.2000.13.6.591. [DOI] [PubMed] [Google Scholar]
- 7.Yap F, Hogler W, Briody J, Moore B, Howman-Giles R, Cowell CT. The skeletal phenotype of men with previous constitutional delay of puberty. J Clin Endocrinol Metab. 2004;89:4306–11. doi: 10.1210/jc.2004-0046. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein JS, Neer RM, Biller BMK, Crawford JD, Klibanski A. Osteopenia in men with a history of delayed puberty. N Engl J Med. 1992;326:600–4. doi: 10.1056/NEJM199202273260904. [DOI] [PubMed] [Google Scholar]
- 9.Danilovic DL, Correa PH, Costa EM, Melo KF, Mendonca BB, Arnhold IJ. Height and bone mineral density in androgen insensitivity syndrome with mutations in the androgen receptor gene. Osteoporos Int. 2007;18:369–74. doi: 10.1007/s00198-006-0243-6. [DOI] [PubMed] [Google Scholar]
- 10.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. The influence of pubertal timing on bone mass acquisition: a predetermined trajectory detectable five years before menarche. J Clin Endocrinol Metab. 2009;94:3424–31. doi: 10.1210/jc.2009-0241. [DOI] [PubMed] [Google Scholar]
- 11.Marcus R, Leary D, Schneider DL, Shane E, Favus M, Quigley CA. The contribution of testosterone to skeletal development and maintenance: lessons from the androgen insensitivity syndrome. J Clin Endocrinol Metab. 2000;85:1032–7. doi: 10.1210/jcem.85.3.6428. [DOI] [PubMed] [Google Scholar]
- 12.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. New Engl J Med. 1997;337:91–5. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 13.Morishima A, Gumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80:3689–98. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 14.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 15.Dunkel L, Wickman S. Novel treatment of short stature with aromatase inhibitors. J Steroid Biochem Mol Biol. 2003;86:345–56. doi: 10.1016/s0960-0760(03)00344-3. [DOI] [PubMed] [Google Scholar]
- 16.Loro ML, Sayre J, Roe TF, Goran MI, Kaufman FR, Gilsanz V. Early identification of children predisposed to low peak bone mass and osteoporosis later in life. J Clin Endocrinol Metab. 2000;85:3908–18. doi: 10.1210/jcem.85.10.6887. [DOI] [PubMed] [Google Scholar]
- 17.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–99. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 18.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 19.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 20.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2. Stanford: Stanford University Press; 1959. [Google Scholar]
- 21.Belsley DA, Kuh E, Welsch RE. Regression diagnostics. New York: Wiley; 1980. [Google Scholar]
- 22.Bertelloni S, Baroncelli GI, Federico G, Cappa M, Lala R, Saggese G. Altered bone mineral density in patients with complete androgen insensitivity syndrome. Horm Res. 1998;50:309–14. doi: 10.1159/000023296. [DOI] [PubMed] [Google Scholar]
- 23.Kindblom JM, Lorentzon M, Norjavaara E, Hellqvist A, Nilsson S, Mellstrom D, et al. Pubertal timing predicts previous fractures and BMD in young adult men: the GOOD study. J Bone Miner Res. 2006;21:790–5. doi: 10.1359/jbmr.020602. [DOI] [PubMed] [Google Scholar]
- 24.Yanovski JA, Rose SR, Municchi G, Pescovitz OH, Hill SC, Cassorla FG, et al. Treatment with a luteinizing hormone-releasing hormone agonist in adolescents with short stature. N Engl J Med. 2003;348:908–17. doi: 10.1056/NEJMoa013555. [DOI] [PubMed] [Google Scholar]
- 25.Mora S, Gilsanz V. Establishment of peak bone mass. Endocrinol Metab Clin North Am. 2003;32:39–63. doi: 10.1016/s0889-8529(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 26.Root AW. Bone strength and the adolescent. Adolesc Med. 2002;13:53–72. vi. [PubMed] [Google Scholar]
- 27.Magaziner J, Wehren L, Hawkes WG, Orwig D, Hebel JR, Fredman L, et al. Women with hip fracture have a greater rate of decline in bone mineral density than expected: another significant consequence of a common geriatric problem. Osteoporos Int. 2006;17:971–7. doi: 10.1007/s00198-006-0092-3. [DOI] [PubMed] [Google Scholar]
- 28.Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in rate of decline in bone mass in older men: the Baltimore men’s osteoporosis study. J Bone Miner Res. 2005;20:1228–34. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 29.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7–29, 2000: highlights of the conference. South Med J. 2001;94:569–73. [PubMed] [Google Scholar]