Abstract
Background:
Platelets and P-selectin (CD62P) play an unequivocal role in the pathology of hepatic ischemia/reperfusion (I/R) injury. Inhibition or knock-out of P-selectin or immunodepletion of platelets results in amelioration of post-ischemic inflammation, reduced hepatocellular damage, and improved survival. However, P-selectin expression on platelets and endothelial cells, which concurs with platelet activation, has never been clearly demonstrated in I/R-subjected livers.
Aims:
To determine whether platelets become activated and degranulate in the acute phase of liver I/R and whether the platelets interact with neutrophils.
Methods:
Hepatic I/R was induced in male C57BL/6J mice (N = 12) using 37.5-min ischemia time. Platelets, endothelial cells, and neutrophils were fluorescently labeled by systemic administration of non-blocking antibodies. Cell kinetics were monitored by intravital spinning disk confocal microscopy during 90 min of reperfusion. Image analysis and quantification was performed with dedicated software.
Results:
Platelets adhered to sinusoids more extensively in post-ischemic livers compared to livers not subjected to I/R and formed aggregates, which occurred directly after ischemia. Platelets and endothelial cells did not express P-selectin in post-ischemic livers. There was no interaction between platelets and neutrophils.
Conclusions:
Platelets aggregate but do not become activated and do not degranulate in post-ischemic livers. There is no platelet-neutrophil interplay during the early reperfusion phase in a moderate model of hepatic I/R injury. The mechanisms underlying the biological effects of platelets and P-selectin in this setting warrant further investigation.
Relevance for patients:
I/R in surgical liver patients may compromise outcome due to post-ischemic oxidative stress and sterile inflammation. Both processes are mediated in part by platelets. Understanding platelet function during I/R is key to developing effective interventions for I/R injury and improving clinical outcomes.
Keywords: hepatic ischemia/reperfusion injury, sterile inflammation, platelet-neutrophil interactions, CD62P or P-selectin
1. Introduction
In the last decade the paradigm of platelet function has expanded from primary hemostasis to also include intravascular redox signaling and sterile inflammation. Inasmuch as both oxidative stress and a sterile immune response are prominent hallmarks of hepatic ischemia/reperfusion (I/R) injury [1,2], the role of platelets has been studied in the context of I/R damage and post-ischemic liver repair. The main findings of these studies are summarized in Table 1.
Table 1. Summary of in vivo studies on the role of P-selectin (CD62P), platelets, and leukocytes in warm hepatic ischemia/reperfusion injury. Data reported versus controls.
| Ref. Species | Major findings |
|---|---|
| [3] RAT |
|
| [4] RAT |
|
| [5] MOUSE |
|
| [6] MOUSE |
|
| [7] MOUSE |
---------------------------------------------------------------------------------- ---------------------------------------- ---------------------------------------- NOTE: platelet activation status was not assessed |
| [8] MOUSE |
|
| [9] MOUSE |
|
| [10] MOUSE |
|
| [11] MOUSE |
---------------------------------------------------------------------------------- ---------------------------------------- ---------------------------------------- NOTE: platelet activation status was not assessed NOTE: platelets were fluorescently labeled ex vivo (rhodamine 6G) and reinfused |
| [12] MOUSE |
---------------------------------------------------------------------------------- ---------------------------------------- ---------------------------------------- NOTE: platelet activation status was not assessed NOTE: platelets were fluorescently labeled ex vivo (rhodamine 6G) and reinfused |
| [13] MOUSE |
NOTE: platelet activation status was not assessed NOTE: platelets were fluorescently labeled ex vivo (rhodamine 6G) and reinfused |
| [14] MOUSE |
---------------------------------------------------------------------------------- ---------------------------------------- ---------------------------------------- NOTE: platelet activation status was not assessed |
| [15] RAT |
|
| [16] RAT |
------------------------------------------------------------------------------------------------------------------------------------------------------------------ * No data were shown NOTE: platelets were fluorescently labeled ex vivo (rhodamine 6G) and reinfused |
| [17] RAT |
---------------------------------------------------------------------------------- ---------------------------------------- ---------------------------------------- NOTE: platelet activation status was not assessed NOTE: platelets were fluorescently labeled ex vivo (rhodamine 6G) and reinfused |
| [18] RAT |
------------------------------------------------------------------------------------------------------------------------------------------------------------------------ NOTE: platelet activation status was not assessed NOTE: platelets were fluorescently labeled ex vivo (rhodamine 6G) and reinfused |
| [19] RAT |
---------------------------------------------------------------------------------- ---------------------------------------- ---------------------------------------- NOTE: platelet activation status was not assessed NOTE: platelets were fluorescently labeled ex vivo (rhodamine 6G) and reinfused |
| [20] MOUSE |
------------------------------------------------------------------------------- ---------------------------------------- ---------------------------------------- NOTE: platelet activation status was not assessed NOTE: platelets were fluorescently labeled ex vivo (rhodamine 6G) and reinfused |
Abbreviations (alphabetically): ALT, alanine aminotransferase; AST, aspartate aminotransferase; CD31, platelet endothelial cell adhesion molecule (PECAM-1); CD41, integrin alpha-IIb; CD49b, integrin, alpha 2 (alpha 2 subunit of VLA-2 receptor); CD62P, P-selectin; Cl2MDP, dichloromethylene diphosphonate; CoPP, cobalt protoporphyrin; FITC, fluorescein isothiocyanate; HO-1, heme oxygenase 1; ICAM-1, intercellular adhesion molecule-1 (CD54); IFM, intravital fluorescence microscopy; IL, interleukin; I/R, ischemia/reperfusion; LDH, lactate dehydrogenase; MIP, macrophage inflammatory protein; MPO, myeloperoxidase; mRNA, messenger ribonucleic acid; PAR-4, protease-activated receptor 4; PCNA, proliferating cell nuclear antigen; PE, phycoerythrin; PMN, polymorphonuclear; PSGL-1, P-selectin glycoprotein ligand-1 (CD162); RT-PCR, reverse transcription polymerase chain reaction; TBARS, thiobarbituric acid-reactive substances; TNF-α, tumor necrosis factor α; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling deoxynucleotidyl transferase dUTP nick end labeling; WT, wild-type.
In the early reperfusion phase, warm hepatic I/R is associated with exacerbated platelet rolling and adhesion in the hepatic microcirculation [B11, B12] and perturbed blood flow[B13]. This interaction is facilitated by intercellular adhesion molecule-1 (ICAM-1) [B11], fibrinogen [B11], and protease-activated receptor-4 (PAR-4) [B20], which is involved in the initiation of secondary hemostasis (coagulation) [B21]. Inhibition or knock-out of P-selectin (CD62P) or immunodepletion of platelets results in amelioration of post-ischemic inflammation [B14], reduced hepatocellular damage [B11,B12], and improved survival [B3], altogether attesting to a role of platelets and P-selectin in post-ischemic hepatopathology.
As such, Khandoga et al. [B11] postulated (or concluded) that, since “activated platelets are able to generate reactive oxygen radicals and nitric oxide and to release pro-inflammatory mediators, …activated platelets have the potential to induce I/R injury by both direct impact and aggravation of microcirculatory derangements.” Despite the apparent involvement of platelets in I/R injury, Woolbright and Jaeschke [B22] recently questioned whether the data available to date unequivocally corroborate a mediatory role of platelets in post- ischemic injury or platelet activation. On the basis of Table 1 it can be concluded that post-ischemic platelet activation and corollary degranulation (surface exposure of P-selectin), i.e., the trigger for the inflammatory processes, repair mechanisms, and the initiation of secondary hemostasis, have indeed never been closely investigated.
Accordingly, we have conducted several focused hepatic I/R experiments in mice using intravital spinning disk confocal microscopy and platelet labeling [B23] to elucidate the platelet activation status in the acute reperfusion phase (0-90 min) [B1]. The experiments yielded some unexpected and contradictory findings. Platelet aggregation occurred in the hepatic microcirculation during the acute reperfusion phase but was not associated with degranulation, which is necessary for the biological effects reported in literature (Table 1). Evidently, these findings have important implications on the regulatory role of platelets in hepatic I/R.
2. Materials and Methods
All supplementary material is indicated with a prefix ‘S.’
2.1. Animal model and surgery
The study was approved by the animal ethics committee of the University of Calgary (protocol#AC12-0162) and all animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, rev. 2011). Male C57BL/6J mice (N = 12, Charles River, Montreal, Quebec, Canada) weighing between 22-25 g were housed under standard laboratory conditions with ad libitum access to regular chow and water. The animals were acclimated for at least 2 d before entering the experiment.
Mice received analgesia by subcutaneous administration of buprenorphine (0.06 mg/kg, Temgesic, Schering-Plough, Kenilworth, NJ) following induction anesthesia with isoflurane (2.5% isoflurane in O2, 1 L/min, Forene, Abbott Laboratories, Queensborough, UK). Anesthesia was subsequently maintained with isoflurane (1.5% in O2, 0.5 L/min) during the experimental procedure. Body temperature was measured with a rectal temperature probe and was maintained at 37 °C with a heating pad (Fig. S1A, orange pad) connected to a self-regulating TR-200 homeothermic temperature controller (Fine Science Tools, Heidelberg, Germany). The unit automatically adjusted the temperature of the heating pad on the basis of the signal received from the rectal temperature probe. The animals were fixed dorsally onto the heating pad, which in turn was secured to a mobile microscope stage (Fig. S1A) placed on a Vibraplane optical table (Kinetic Systems, Boston, MA) for surgery and intravital microscopy.
Following a midline laparotomy, the left medial-, right medial-, and left lateral lobes were exteriorized, gently retracted cranially, and secured with a PBS-drenched gauze as described in [B24]. The liver hilus was mobilized and 70% ischemia was induced by clamping the portal and arterial blood supply with a 4 × 1-mm microvessel clip (MEHDORN, Aesculaep, Center Valley, PA) [B24]. Following 37.5-min ischemia, which is associated with moderate liver injury [B24], the clip was removed and a customized metal transabdominal stage (Home Depot, Calgary, Alberta, Canada) was placed over the animal’s abdomen (Fig. S1A) as described in [B25]. The transabdominal segment of the stage was convexly shaped and wrapped in gauze to ensure proper fixation of the liver lobe, elimination of breathing artifacts, and an optimal focal plane during intravital microscopy. The stage-wrapped gauze was wetted with 0.9% NaCl solution and the left lateral lobe was gently flipped onto the stage and fixed with acryl-based tissue glue (Vetbond tissue adhesive, 3M Animal Care Products, St. Paul, MN) at the distal and lateral ends of the lobe (relative to the head). Following a flush with 0.9% NaCl solution, the liver lobe was covered with saran wrap to prevent desiccation [B25]. The saran wrap was secured to the stage with a thin strip of tape (not over the liver) and the liver lobe was imaged by intravital microscopy (Fig. S1B).
2.2. Systemic cell labeling for intravital microscopy
Antibodies were added to sterile 0.9% NaCl solution (B. Braun Melsungen, Melsungen, Germany) to a final infusion volume of 100 μL. The used antibodies and antibody concentrations were: sinusoidal endothelial cells: rat anti-mouse CD31-PE, 10 μL of 200 μg/mL (cat.#12-0311-83, clone 390, eBioscience, San Diego, CA) or rat anti-mouse CD31-Alexa Fluor 647, 5 μL of 1000 μg/mL (cat.#16-0311-85, clone 390, eBioscience, labeled with Alexa Fluor 647 protein labeling kit, cat.#A-20173, Life Technologies, Carlsbad, CA); resting platelets: hamster anti-mouse CD49b-Alexa Fluor 647, 7 μL of 500 μg/mL (cat.#103511, clone HMα2, Biolegend, San Diego, CA); activated platelets, rat anti-mouse CD62P-FITC, 10 μL of 500 μg/mL (cat.#553744, clone RB40.34, BD Pharmingen, Franklin Lakes, NJ); neutrophils: rat anti-mouse Ly-6G (Gr-1)-FITC, 10 μL of 500 μg/mL (cat.#108406, clone R86-8C5, BioLegend). The mixture was infused into the penile vein directly before surgery using a 1 mL insulin syringe, after which the puncture wound was sealed with an electro-surgical cauterizer. Before the liver I/R experiments, in vivo thrombus staining by the CD62P-FITC antibodies was verified in a puncture-induced thrombosis model in the murine saphenous artery (N = 2, Fig. S2).
2.3. Intravital microscopy
Intravital microscopy was performed with a Quorum Wave FX-X1 spinning disk confocal system that consisted of an upright Olympus IX51 microscope (Olympus Corporation, Tokyo, Japan) equipped with a Yokogawa CSU-X1 scan head (Yokogawa Electric, Tokyo, Japan), a back-thinned Hamamatsu EMCCD camera (model C9100-13, Hamamatsu Photonics, Hamamatsu City, Japan), and 491-, 561-, and 640-nm excitation lasers. The following emission filters were used for the antibody-conjugated fluorophores: 536 ± 40 nm (CD62P-FITC and Gr-1-FITC), 593 ± 40 nm (CD31-PE), and 692 ± 40 nm (CD31-Alexa Fluor 647 and CD49b-Alexa Fluor 647), respectively. The emission filters were under the control of a MAC 6000 Modular Automation Controller (Ludl Electronic Products, Hawthorne, NY). Imaging was performed with an Olympus UPlanFL-N, 10×, NA = 0.2 objective. The hardware settings were kept constant during all experiments (FITC channel: laser power 60, exposure time 80 ms, camera gain 1, camera sensitivity 209; PE channel: laser power 71, exposure time 120 ms, camera gain 1, camera sensitivity 224; Alexa Fluor 647 channel: laser power 85, exposure time 120 ms, camera gain 1, camera sensitivity 171). Image acquisition was performed under Volocity software control (Version 6.3.1, Perkin Elmer, Waltham, MA). Image acquisition was performed for 1 min at a frame rate of 11 Hz (3 fluorophores) or 13 Hz (2 fluorophores) after removal of the microvascular clip (the time interval between clip removal and image acquisition was on average 4 min) and at t = 4 + 15, 30, 45, 60, 75, and 90 min reperfusion. The abdominal cavity was hydrated via the base of the liver lobe after each image acquisition sequence with a syringe containing 0.9% NaCl solution (37 °C).
2.4. Image analysis
Image analysis was performed using ImageJ/FIJI (NIH, Bethesda, MD). Volocity files were imported into Fiji as colorized hyperstacks using the Bio-Formats Importer. After splitting the fluorescence channels, the total pixel intensity per frame was measured for each channel using FIJI’s automated analysis module (“analyze→measure”). The fluorescence intensity from platelets (i.e., CD62P or CD49b) was first normalized per frame to the fluorescence intensity from endothelium (i.e., CD31) to correct for loss-of-focus within the ROI during image acquisition. Subsequently, the normalized pixel intensity per frame was averaged per time point (i.e., the mean normalized pixel intensity of 11 or 13 frames was calculated for each 1-min imaging sequence). Data are presented as the mean platelet fluorescence/endothelial fluorescence ratio per time point.
3. Results
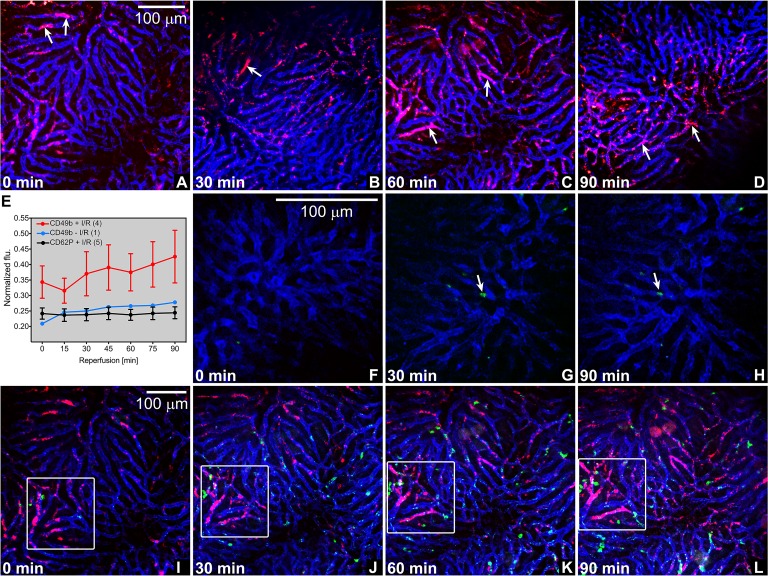
First, systemically labeled platelets (CD49b-Alexa Fluor 647, pan-platelet staining) adhered to sinusoids more extensively in post-ischemic livers (Fig. 1A-E) compared to livers not subjected to I/R (Fig. S3) and formed aggregates, which occurred from the very onset of reperfusion. It is therefore likely that platelet-vascular wall interactions transpired mainly during ischemia, particularly since the platelet aggregates did not notably increase during 90-min reperfusion and were not abundantly present in sham-operated animals (Fig. 1E). Second, P-selectin staining of activated platelets and endothelial cells was entirely absent in post-ischemic livers (Fig. 1F-H), indicating that neither α-granule nor Weibel-Palade body secretion from platelets and endothelial cells, respectively, occurs during ischemia and early reperfusion. Third, to investigate the interplay between platelet aggregates and neutrophils (demonstrated in [B26]), systemic staining was performed for endothelium (CD31-PE), platelets (CD49b-Alexa Fluor 647), and neutrophils (Gr-1-FITC) before the induction of I/R. Of note, platelet-neutrophil interactions are facilitated by glycoprotein Ibα (CD42b) [B26], which were not blocked by the mentioned fluorescently labeled antibodies. As shown in Fig. 1I-H, there was no notable heterotypic aggregate formation that involved platelets and neutrophils. Whereas the extent of platelet aggregate formation remained relatively stable over the reperfusion time (Fig. 1E), the influx and adhesion of neutrophils increased with reperfusion time (manuscript in preparation).
Figure 1. Intravital imaging of platelet aggregation and platelet activation status following hepatic I/R in mice. (A-D) Platelet aggregates (red (CD49b), arrows) in hepatic microcirculation (blue, CD31) as a function of reperfusion time (left bottom, all imaging panels). Representative panels are shown per time point, taken from the video footage of 3 animals. Scale bar applies to all panels. (E) The mean pixel intensity per fluorescence channel (y-axis) was quantitated for each time point (x-axis) for every experimental group (resting platelets following I/R (CD49b + I/R), resting platelets in sham-operated animals (CD49b – I/R), and activated platelets following I/R (CD62P + I/R)) using FiJi/ImageJ software. Platelet fluorescence (flu) was normalized to endothelial fluorescence (mean ± SEM, sample size is given in parentheses in the legend). (F-H) Absence of P-selectin staining (green, CD62P) in post-ischemic liver microcirculation (blue, CD31). Incidental P-selectin-positive foci are indicated with arrows, corresponding to the same location at different reperfusion times. (I-L) Absence of platelet (red, CD49b) and neutrophil (green, Gr1) colocalization in post-ischemic hepatic microcirculation (blue, CD31). The quadrant corresponds to the same location at different reperfusion times, whereas the time lapse series in I-L correspond to panel C. Note the gradual increase in platelet aggregation in the demarcated region in this animal.
4. Discussion
Taken together, these data demonstrate that [a] platelets aggregate but do not become activated in post-ischemic livers and [b] there is no platelet-neutrophil interplay during the early reperfusion phase. Platelet aggregation following liver I/R in mice was visualized with spinning-disk intravital confocal microscopy using fluorescent anti-CD49b antibodies as an in vivo pan-platelet label. Although the use of anti-CD49b antibodies deters a potential interaction of platelets with certain substrates (e.g., collagen [B27]), this staining method has a high labeling efficiency, does not affect platelet phenotype [B23], and obviates the need for intricate ex vivo platelet staining procedures that may affect platelet phenotype or function [B28]. The finding that platelets adhere extensively to sinusoidal endothelium during the first 90 min of reperfusion is in line with previous intravital imaging observations in murine liver I/R models [11-13;20;29]. However, the biological significance of this phenomenon is less clear. As mentioned in the introduction, platelet aggregation has been causally linked to, e.g., microvascular perfusion defects, apoptotic cell death, vascular oxidative stress, and an inflamed endothelium [11-13;20], all alluding to a pathophysiological connection between platelets and surgery-induced hepatopathology. There are, however, important considerations that need to be kept in mind when interpreting the results from the cited studies.
The parameter that has been most extensively used to study platelet function in liver I/R is P-selectin, which is released from α-granules during platelet activation and is subsequently expressed on the platelet outer membrane leaflet to facilitate thrombosis via platelet-platelet and platelet-neutrophil interactions [B12]. Although P-selectin-deficient animals generally exhibit an attenuated liver I/R injury profile (Table 1), suggesting pathological platelet behavior, this genetic model does not differentiate between platelet and endothelial P-selectin. This distinction is crucial insofar as endothelial P-selectin enables leukocyte adhesion in postsinusoidal venules under inflammatory conditions [30-32], which could explain the hepatoprotective efficacy of a generalized P-selectin deficiency. In addition, it has been posited that anti-P-selectin therapies reduce hepatic I/R injury via protective effects on the intestinal microcirculation [B32] rather than through direct effects on the liver, which underscores that a more targeted approach is necessary to selectively explore platelet P-selectin function. In light of these considerations, it should be noted that endothelial P-selectin expression was not observed during the first 90 min of reperfusion in the current experiments (Fig. 1F-H), which might be due to the fact that leukocyte adhesion typically occurs at later stages of I/R injury (i.e., the chronic reperfusion phase [B1]) and/or the fact that sinusoidal endothelium mainly relies on ICAM-1 instead of P-selectin to immobilize chemoattracted leukocytes following hepatic I/R. The latter may also relate to the reported absence of P-selectin-containing Weibel-Palade bodies in sinusoidal endothelial cells, albeit contradictory findings on this subject have been published (discussed in [B33,B34]).
In order to properly assess platelet function under inflammatory conditions, it is imperative to also determine platelet activation status, which is a frequent omission in platelet-centered liver I/R studies ([B22], see also Table 1). Using a validated antibody-based in vivo P-selectin labeling method (Fig. S2), it was shown that liver I/R in mice triggers platelet aggregation without notable P-selectin expression (Fig. 1F-H), which deviates from the putative platelet activation paradigm. Several factors could explain this observation. First, platelets can form reversible aggregates based on integrin-fibrinogen or integrin-endothelial interactions only, which can occur independently of α-granule (i.e., P-selectin) release and does not require soluble platelet agonists such as ADP or thrombin [35-37]. Based on Fig. 1I-L, however, the reversible nature of such aggregates is entirely absent given the fact that all aggregates in the demarcated region are expanding in time over a 90-min time span. Alternatively, and more plausibly, autocrine and/or paracrine signals may terminate the platelet activation cascade before α-granule secretion takes place [B38]. Such signals can be either derived from platelets (e.g., release of tissue factor pathway inhibitor and/or protein S) or mediated by activated endothelium (e.g., through production of prostacyclin I2 and/or nitric oxide) [B38]. Tissue factor pathway inhibitor and protein S are localized in intracellular storage granules of platelets and endothelial cells and hence require activation-stimulated degranulation and release [39-42] to become biologically available. The lack of degranulation in aggregated platelets and endothelial cells, as evidenced by the absence of P-selectin-positive staining, therefore precludes that this mechanism was mediated by intracellular tissue factor pathway inhibitor and protein S. However, tissue factor pathway inhibitor and protein S are abundant in plasma [B43,B44] and could therefore facilitate the activation of the cascade-terminating process.
Notwithstanding the lack of P-selectin expression, Fig. 1A-D unequivocally confirm that platelets aggregate extensively during early reperfusion, leading to complete obstruction of the vascular lumen in some sinusoids. It is unclear whether this extent of platelet aggregation can occur independently of P- selectin release and corollary coagulation activation (i.e., thrombus formation). With respect to the latter, thrombosis following I/R has only been documented by one research group in rats [B29], albeit a disproportionally severe I/R injury model was employed. On the other hand, our group has shown that the coagulation cascade is activated following I/R in rats (30 min partial liver ischemia), which resulted in thrombin and fibrin formation in the acute reperfusion phase (30 min) [B45]. Thrombin and to a lesser extent fibrin [B46] induce platelet activation and aggregation, which begs the question why no P-selectin exposure was observed.
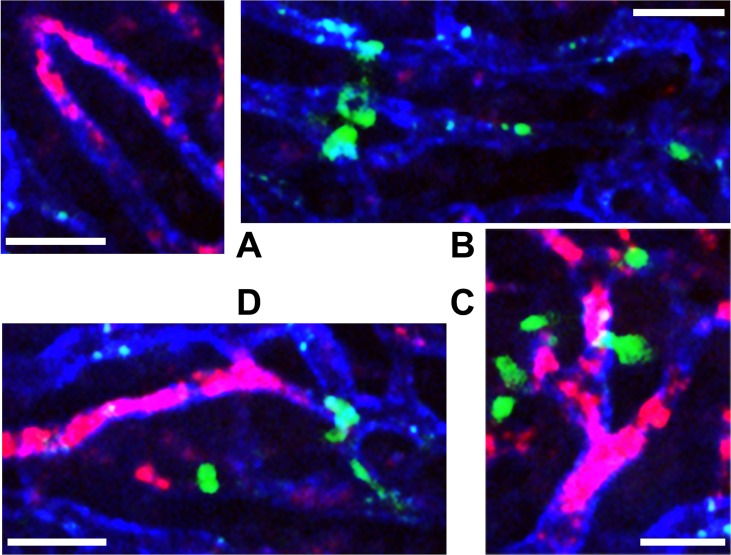
Consequently, the findings in this study render the thrombosis paradigm in liver I/R injury equivocal and elusive for two reasons. First, the most important biological processes that lead to intravascular thrombosis, which include platelet aggregation, endothelial damage, and innate immune activity, are well-established liver I/R injury hallmarks. It was therefore expected that thrombosis, during which P-selectin is translocated to the outer membrane such that systemically administered antibodies can bind, would be detected. Second, local perfusion deficits have been routinely described in murine liver I/R models [B47]. However, (micro)vascular perfusion failure has been attributed to vasoconstriction, edema [B45], and consequent leukocyte plugging rather than platelet aggregation. The data presented here present an inverse scenario, in which platelet aggregates actually occlude the vascular lumen first, with an apparent minor role for neutrophils or neutrophil-platelet interactions (Fig. 2) in the acute reperfusion phase. This finding is in agreement with the paradigm that neutrophils are slow to accumulate during the first hours of reperfusion and in this stage do not (yet) contribute significantly to oxidative stress [B48]. Any effect of platelets on neutrophils, if there is one, therefore only has very limited impact on the pathophysiology of liver I/R injury.
Figure 2. Absence of platelet-neutrophil interactions after hepatic I/R in mice. Systemic triple staining and intravital imaging of platelets (red), endothelium (blue), and neutrophils (green) was performed as described in sections 2.2 and 2.3, and [B23]. Panels A and B show close-ups of I/R-induced platelet plugs and sinusoidal neutrophil adhesion, respectively. In contrast to recent venous thrombosis literature [B26], neutrophils did not mediate the formation of platelet aggregates after I/R, as evidenced by the lack of neutrophil-platelet co-location (C, D). Scale bar = 30 μm.
Collectively, these findings place the biological significance of platelets in post-ischemic liver pathology in a different light. Owing to their established involvement in thrombotic and inflammatory processes, platelets are often deemed harmful by default. Recent reports, however, challenge this claim. First, it has been shown that immunodepletion of platelets using CD41 antibodies does not protect mice from hepatic I/R injury, but does delay functional liver recovery in the long run [B14]. Corroboratively, it has been postulated that platelets relay the protective signals of remote ischemic preconditioning [B49] and are essential for the liver to regenerate properly following a partial liver resection [B50,B51]. The same trend is seen in immunological platelet studies, which increasingly recognize that platelets are not merely cytokine factories, but also coordinate antimi-crobial responses in close collaboration with Kupffer cells [B52]. Insofar as an association between platelets and KCs has been repeatedly shown in murine liver I/R studies [B18,B19], these findings indicate that platelets might actually play a beneficial role in liver I/R injury.
In summary, it is concluded that platelet aggregation in post-ischemic livers does not abide by the putative thrombosis-inflammation mechanisms [B26]. How platelets aggregate without becoming activated and how they mediate the biological and immunological processes alluded to previously and in Table 1 without degranulating should be subjected to experimental scrutiny.
Acknowledgements
This project was enabled by a Young Talent Fund award to RFvG. RFvG was supported by an AMC Scholarship. HJ is supported by grants from the National Institutes of Health (R01 DK070195 and R01 AA12916) and the National Institute of General Medical Sciences (8P20GM103549-07). MH received research grants from the Stichting Nationaal Fonds Tegen Kanker in Amsterdam and Stichting Technologische Wetenschap. We are grateful to Lori Zbytnuik, Justin Deniset, and Michelle Willson from the Kubes Lab for technical and infrastructural support.
Footnotes
The authors declare that there are no conflicts of interest present.
References
- [1].van Golen RF, van Gulik TM, Heger M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic Biol Med. 2012;52:1382–1402. doi: 10.1016/j.freeradbiomed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- [2].van Golen RF, van Gulik TM, Heger M. The sterile immune response during hepatic ischemia/reperfusion. Cytokine Growth Factor Rev. 2012;23:69–84. doi: 10.1016/j.cytogfr.2012.04.006. [DOI] [PubMed] [Google Scholar]
- [3].Garcia-Criado FJ, Toledo-Pereyra LH, Lopez-Neblina F, Phillips ML, Paez-Rollys A, Misawa K. Role of P-selectin in total hepatic ischemia and reperfusion. J Am Coll Surg. 1995;181:327–334. [PubMed] [Google Scholar]
- [4].Dulkanchainun TS, Goss JA, Imagawa DK, Shaw GD, Anselmo DM, Kaldas F, Wang T, Zhao D, Busuttil AA, Kato H, Murray NG, Kupiec-Weglinski JW, Busuttil RW. Reduction of hepatic ischemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann Surg. 1998;227:832–840. doi: 10.1097/00000658-199806000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zibari GB, Brown MF, Burney DL, Granger N, McDonald JC. Role of P-selectin in the recruitment of leukocytes in mouse liver exposed to ischemia and reperfusion. Transplant Proc. 1998;30:2327–2330. doi: 10.1016/s0041-1345(98)00641-1. [DOI] [PubMed] [Google Scholar]
- [6].Singh I, Zibari GB, Brown MF, Granger DN, Eppihimer M, Zizzi H, Cruz L, Meyer K, Gonzales E, McDonald JC. Role of P-selectin expression in hepatic ischemia and reperfusion injury. Clin Transplant. 1999;13:76–82. doi: 10.1034/j.1399-0012.1999.130103.x. [DOI] [PubMed] [Google Scholar]
- [7].Yadav SS, Howell DN, Steeber DA, Harland RC, Tedder TF, Clavien PA. P-selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology. 1999;29:1494–1502. doi: 10.1002/hep.510290505. [DOI] [PubMed] [Google Scholar]
- [8].Sawaya DE, Zibari GB, Minardi A, Bilton B, Burney D, Granger DN, McDonald JC, Brown M. P-selectin contributes to the initial recruitment of rolling and adherent leukocytes in hepatic venules after ischemia/reperfusion. Shock. 1999;12:227–232. doi: 10.1097/00024382-199909000-00010. [DOI] [PubMed] [Google Scholar]
- [9].Martinez-Mier G, Toledo-Pereyra LH, McDuffie JE, Warner RL, Ward PA. P-selectin and chemokine response after liver ischemia and reperfusion. J Am Coll Surg. 2000;191:395–402. doi: 10.1016/s1072-7515(00)00360-4. [DOI] [PubMed] [Google Scholar]
- [10].Young CS, Palma JM, Mosher BD, Harkema J, Naylor DF, Dean RE, Crockett E. Hepatic ischemia/reperfusion injury in P-selectin and intercellular adhesion molecule-1 double-mutant mice. Am Surg. 2001;67:737–744. [PubMed] [Google Scholar]
- [11].Khandoga A, Biberthaler P, Enders G, Axmann S, Hutter J, Messmer K, Krombach F. Platelet adhesion mediated by fibrinogen-intercelllular adhesion molecule-1 binding induces tissue injury in the postischemic liver in vivo. Transplantation. 2002;74:681–688. doi: 10.1097/00007890-200209150-00016. [DOI] [PubMed] [Google Scholar]
- [12].Khandoga A, Biberthaler P, Enders G, Teupser D, Axmann S, Luchting B, Hutter J, Messmer K, Krombach F. P-selectin mediates platelet-endothelial cell interactions and reperfusion injury in the mouse liver in vivo. Shock. 2002;18:529–535. doi: 10.1097/00024382-200212000-00008. [DOI] [PubMed] [Google Scholar]
- [13].Khandoga A, Biberthaler P, Messmer K, Krombach F. Platelet-endothelial cell interactions during hepatic ischemia-reperfusion in vivo: a systematic analysis. Microvasc Res. 2003;65:71–77. doi: 10.1016/s0026-2862(02)00018-3. [DOI] [PubMed] [Google Scholar]
- [14].Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, Clavien PA. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369–376. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- [15].Xue F, Wang G, Pang Z, Liu C, Liang T. Protective effect of glutathione against liver warm ischemia-reperfusion injury in rats is associated with regulation of P-selectin and neutrophil infiltration. Anat Rec. 2008;291:1016–1022. doi: 10.1002/ar.20725. [DOI] [PubMed] [Google Scholar]
- [16].Nakano Y, Kondo T, Matsuo R, Hashimoto I, Kawasaki T, Kohno K, Myronovych A, Tadano S, Hisakura K, Ikeda O, Watanabe M, Murata S, Fukunaga K, Ohkohchi N. Platelet dynamics in the early phase of postischemic liver in vivo. J Surg Res. 2008;149:192–198. doi: 10.1016/j.jss.2007.09.016. [DOI] [PubMed] [Google Scholar]
- [17].Pak S, Kondo T, Nakano Y, Murata S, Fukunaga K, Oda T, Sasaki R, Ohkohchi N. Platelet adhesion in the sinusoid caused hepatic injury by neutrophils after hepatic ischemia reperfusion. Platelets. 2010;21:282–288. doi: 10.3109/09537101003637265. [DOI] [PubMed] [Google Scholar]
- [18].Tamura T, Kondo T, Pak S, Nakano Y, Murata S, Fukunaga K, Ohkohchi N. Interaction between Kupffer cells and platelets in the early period of hepatic ischemia-reperfusion injury − an in vivo study. J Surg Res. 2012;178:443–451. doi: 10.1016/j.jss.2011.12.010. [DOI] [PubMed] [Google Scholar]
- [19].Tamura T, Kondo T, Ogawa K, Fukunaga K, Ohkohchi N. Protective effect of heme oxygenase-1 on hepatic ischemia-reperfusion injury through inhibition of platelet adhesion to the sinusoids. J Gastroenterol Hepatol. 2013;28:700–706. doi: 10.1111/jgh.12075. [DOI] [PubMed] [Google Scholar]
- [20].Mende K, Reifart J, Rosentreter D, Manukyan D, Mayr D, Krombach F, Rentsch M, Khandoga A. Targeting platelet migration in the postischemic liver by blocking protease-activated receptor 4. Transplantation. 2014;97:154–160. doi: 10.1097/01.TP.0000437430.89485.a0. [DOI] [PubMed] [Google Scholar]
- [21].Vretenbrant K, Ramstrom S, Bjerke M, Lindahl TL. Platelet activation via PAR4 is involved in the initiation of thrombin generation and in clot elasticity development. Thromb Haemost. 2007;97:417–424. [PubMed] [Google Scholar]
- [22].Woolbright BL, Jaeschke H. Heme oxygenase-1 and platelets in hepatic ischemia reperfusion injury. J Gastroenterol Hepatol. 2013;28:756–757. doi: 10.1111/jgh.12124. [DOI] [PubMed] [Google Scholar]
- [23].Jenne CN, Wong CH, Petri B, Kubes P. The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation. PLoS One. 2011;6:e25109. doi: 10.1371/journal.pone.0025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van Golen RF, Reiniers MJ, Heger M, Verheij J. Solutions to the discrepancies in the extent of liver damage following ischemia/reperfusion in standard mouse models. J Hepatol. 2015;62:975–977. doi: 10.1016/j.jhep.2014.12.014. [DOI] [PubMed] [Google Scholar]
- [25].Kloek JJ, Marechal X, Roelofsen J, Houtkooper RH, van Kuilenburg AB, Kulik W, Bezemer R, Neviere R, van Gulik TM, Heger M. Cholestasis is associated with hepatic microvascular dysfunction and aberrant energy metabolism before and during ischemia-reperfusion. Antioxid Redox Signal. 2012;17:1109–1123. doi: 10.1089/ars.2011.4291. [DOI] [PubMed] [Google Scholar]
- [26].von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Monocytes Massberg S. neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kamata T, Takada Y. Direct binding of collagen to the I domain of integrin alpha 2 beta 1 (VLA-2, CD49b/CD29) in a divalent cation-independent manner. J Biol Chem. 1994;269:26006–26010. [PubMed] [Google Scholar]
- [28].Berger G, Hartwell DW, Wagner DD. P-Selectin and platelet clearance. Blood. 1998;92:4446–4452. [PubMed] [Google Scholar]
- [29].Okajima K, Harada N, Kushimoto S, Uchiba M. Role of microthrombus formation in the development of ischemia/reperfusioninduced liver injury in rats. Thromb Haemost. 2002;88:473–480. [PubMed] [Google Scholar]
- [30].Essani NA, Fisher MA, Simmons CA, Hoover JL, Farhood A, Jaeschke H. Increased P-selectin gene expression in the liver vasculature and its role in the pathophysiology of neutrophilinduced liver injury in murine endotoxin shock. J Leukoc Biol. 1998;63:288–296. doi: 10.1002/jlb.63.3.288. [DOI] [PubMed] [Google Scholar]
- [31].Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, Bliss GA, Zimmerman GA, McEver RP. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343:757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- [32].Kubes P, Payne D, Woodman RC. Molecular mechanisms of leukocyte recruitment in postischemic liver microcirculation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G139–G147. doi: 10.1152/ajpgi.00058.2002. [DOI] [PubMed] [Google Scholar]
- [33].Smedsrod B, De Bleser PJ, Braet F, Lovisetti P, Vanderkerken K, Wisse E, Geerts A. Cell biology of liver endothelial and Kupffer cells. Gut. 1994;35:1509–1516. doi: 10.1136/gut.35.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lalor PF, Lai WK, Curbishley SM, Shetty S, Adams DH. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J Gastroenterol. 2006;12:5429–5439. doi: 10.3748/wjg.v12.i34.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- [36].Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25:155–167. doi: 10.1016/j.blre.2011.03.002. [DOI] [PubMed] [Google Scholar]
- [37].Broos K, De Meyer SF, Feys HB, Vanhoorelbeke K, Deckmyn H. Blood platelet biochemistry. Thromb Res. 2012;129:245–249. doi: 10.1016/j.thromres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [38].Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93:327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- [39].Wood JP, Ellery PE, Maroney SA, Mast AE. Biology of tissue factor pathway inhibitor. Blood. 2014;123:2934–2943. doi: 10.1182/blood-2013-11-512764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maroney SA, Haberichter SL, Friese P, Collins ML, Ferrel JP, Dale GL, Mast AE. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109:1931–1937. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schwarz HP, Heeb MJ, Wencel-Drake JD, Griffin JH. Identification and quantitation of protein S in human platelets. Blood. 1985;66:1452–1455. [PubMed] [Google Scholar]
- [42].Novotny WF, Girard TJ, Miletich JP, Broze GJ. Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein associated coagulation inhibitor. Blood. 1988;72:2020–2025. [PubMed] [Google Scholar]
- [43].Walker FJ. Protein S and the regulation of activated protein C. Semin Thromb Hemost. 1984;10:131–138. doi: 10.1055/s-2007-1004415. [DOI] [PubMed] [Google Scholar]
- [44].Lindahl AK, Sandset PM, Abildgaard U. The present status of tissue factor pathway inhibitor. Blood Coagul Fibrinolysis. 1992;3:439–449. [PubMed] [Google Scholar]
- [45].Kloek JJ, Levi M, Heger M, van der Loos CM, Gouma DJ, van Gulik TM. Cholestasis enhances liver ischemia/reperfusion- induced coagulation activation in rats. Hepatol Res. 2010;40:204–215. doi: 10.1111/j.1872-034X.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- [46].Niewiarowski S, Regoeczi E, Stewart GJ, Senyl AF, Mustard JF. Platelet interaction with polymerizing fibrin. J Clin Invest. 1972;51:685–699. doi: 10.1172/JCI106857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- [48].Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- [49].Oberkofler CE, Limani P, Jang JH, Rickenbacher A, Lehmann K, Raptis DA, Ungethuem U, Tian Y, Grabliauskaite K, Humar R, Graf R, Humar B, Clavien PA. Systemic protection through remote ischemic preconditioning is spread by platelet-dependent signaling in mice. Hepatology. 2014;60:1409–1417. doi: 10.1002/hep.27089. [DOI] [PubMed] [Google Scholar]
- [50].Starlinger P, Assinger A, Haegele S, Wanek D, Zikeli S, Schauer D, Birner P, Fleischmann E, Gruenberger B, Brostjan C, Gruenberger T. Evidence for serotonin as a relevant inducer of liver regeneration after liver resection in humans. Hepatology. 2014;60:257–266. doi: 10.1002/hep.26950. [DOI] [PubMed] [Google Scholar]
- [51].Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- [52].Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol. 2013;14:785–792. doi: 10.1038/ni.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]