Abstract
Paracetamol overdose prior to the introduction of acetylcysteine was associated with significant morbidity. Acetylcysteine is now the mainstay of treatment for paracetamol poisoning and has effectively reduced rates of hepatotoxicity and death. The current three‐bag intravenous regimen with an initial high loading dose was empirically derived four decades ago and has not changed since. This regimen is associated with a high rate of adverse effects due mainly to the high initial peak acetylcysteine concentration. Furthermore, there are concerns that the acetylcysteine concentration is not adequate for ‘massive’ overdoses and that the dose and duration may need to be altered. Various novel regimens have been proposed, looking to address these issues. Many of these modified regimens aim to decrease the rate of adverse reactions by slowing the loading dose and thereby decrease the peak concentration. We used a published population pharmacokinetic model of acetylcysteine to simulate these modified regimens. We determined mean peak and 20 h acetylcysteine concentrations and area under the under the plasma concentration–time curve to compare these regimens. Those regimens that resulted in a lower peak acetylcysteine concentration have been shown in studies to have a lower rate of adverse events. However, these studies were too small to show whether they are as effective as the traditional regimen. Further research is still needed to determine the optimum dose and duration of acetylcysteine that results in the fewest side‐effects and treatment failures. Indeed, a more patient‐tailored approach might be required, whereby the dose and duration are altered depending on the paracetamol dose ingested or paracetamol concentrations.
Keywords: acetylcysteine, antidote, overdose, paracetamol
Background
Paracetamol is one of the most common medications leading to hospital presentations and admissions following deliberate self‐poisoning and accidental overdose worldwide. Furthermore, it is the commonest cause of acute liver failure in North America, Europe and Australia 1, 2. Since the 1970s, the mainstay of treatment has been acetylcysteine, which dramatically decreased mortality and rates of hepatotoxicity secondary to paracetamol poisoning. Neither the oral nor intravenous regimen, when proposed, was subject to a randomized controlled trial, nor was an optimum regimen established by dose‐ranging studies. As a result, acetylcysteine treatment is usually given using the dose calculated by Prescott et al., with a single standard dosage based on weight, which has been essentially unchanged since the 1970s 3. This regimen has been successful but has several issues; adverse effects are common and the infusion schedule is complex and can result in errors 4. It is based on the assumption that ‘one size fits all,’ and questions still exist as to whether those who take large overdoses require higher doses of acetylcysteine. If it is accepted that the dose should be adjusted, this then raises further questions. For example, what dose of acetylcysteine is optimum? What are the indications for altering the dose? Can we better tailor the duration of the acetylcysteine infusion? Should we shorten or extend the infusion time, depending on the paracetamol can be serum or plasma concentration, liver functions tests or other clinical parameters? The present article will review the history of acetylcysteine and the new regimens that have been studied, and simulate their expected plasma acetylcysteine concentrations.
Paracetamol pharmacokinetics
The pharmacokinetics of paracetamol has been well established in volunteer studies 5. At therapeutic doses, paracetamol has a half‐life of 1.5–2.5 h 6. It is extensively metabolized, with only a small proportion excreted unchanged 5. At therapeutic doses in adults, the major metabolites are sulphate and glucuronide conjugates, which account for 30% and 55% of paracetamol metabolites, respectively.
A highly reactive toxic metabolite, N‐acetyl‐p‐benzoquinone imine (NAPQI), is also formed by cytochrome P450 2E1. NAPQI is responsible for the hepatocellular injury that occurs with paracetamol toxicity. The small amounts of NAPQI produced after therapeutic doses of paracetamol are detoxified by glutathione‐dependent reactions. NAPQI is detoxified via irreversible glutathione conjugation to two nontoxic metabolites, mercapturic acid and cysteine conjugates. At therapeutic doses, these two metabolites are excreted at 4% (as a fraction of the parent dose) each, with over 80% excreted in the urine in the first 12 h following ingestion 5, 6.
Similar rates of mercapturic acid and cysteine conjugate excretion were seen in a study of patients with paracetamol poisoning without liver damage, with the proportion of mercapturate plus cysteine excreted ranging from 6.4% to 10.6%. In the same study, those patients with severe liver damage had an increased proportion of excreted mercapturate plus cysteine (13.8–16.5%), regardless of treatment. Excretion was also slower and delayed by up to 30 h 6. This might indicate that a higher proportion of the paracetamol dose ingested is converted to NAPQI in those who develop severe hepatotoxicity 3.
In overdose, the formation of NAPQI depletes glutathione. Once glutathione is depleted, NAPQI covalently binds to critical cellular proteins 7. It is hypothesized that this results in the loss of activity and function of critical proteins and eventually results in hepatic cell death. In animals, hepatic necrosis is observed once glutathione is depleted by approximately 70% 3.
How the acetylcysteine regimen was evaluated and optimized
Several antidotes that replenish glutathione and detoxify NAPQI were evaluated in the 1970s, including methionine, cysteine, cysteamine and dimercaprol 8. Cysteamine and methionine have been shown in small randomized controlled trials to decrease the risk of developing hepatotoxicity 9, 10. Cysteine was associated with severe headache, as well as nausea and vomiting in nearly all patients. While methionine was only available as an oral preparation. Hence, the use of these antidotes was limited 9, 10. In an observational study from Edinburgh, intravenous acetylcysteine as a first‐line treatment was found to be equally effective as cysteamine and methionine, and noticeably free of adverse effects 11. Ever since that finding, acetylcysteine has been accepted as an antidote for paracetamol overdose and has become the standard treatment, as either a 20–21‐h intravenous regimen or a longer oral regimen 12, 13. Acetylcysteine is effective in paracetamol toxicity as it is hydrolysed intracellularly to cysteine, which replenishes glutathione 14. Glutathione can then covalently bind to NAPQI in a 1:1 ratio. Acetylcysteine also supplies thiol groups, which can directly bind to NAPQI in hepatocytes 15.
Two different acetylcysteine regimens were initially developed. A 20.25‐h intravenous regimen (Table 1: Traditional three‐bag protocol) was used in the UK. In the US, a 3‐day oral regimen was used owing to a lack of licensing for intravenous acetylcysteine 3, 16. The dosage regimen for intravenous acetylcysteine was developed in Edinburgh; it involved giving a large loading dose as patients were thought to be glutathione depleted on presentation 17. The intravenous regimen was 20.25 h, based on five times the theoretical 4‐h half‐life of paracetamol 3, 17. In the US, they were concerned about the prolonged paracetamol half‐life seen in overdose in some patients. Therefore, based on a 12‐h half‐life that can occur in overdose, a 72‐h oral regimen was developed 3. In 2004, intravenous acetylcysteine was approved for use in the US, with the same three‐bag 20.25 h regimen (Table 1: Traditional three‐bag protocol) as used in the UK. Initially, the recommended duration of the loading dose infusion was 15 min; however, this was subsequently increased to 60 min, with the aim of reducing the number of infusion‐related adverse reactions 18.
The pharmacokinetics of intravenous acetylcysteine was studied by Prescott et al . 19 in 18 presentations of paracetamol overdose requiring acetylcysteine. These patients received the traditional three‐bag 20.25‐h intravenous protocol. The mean maximum plasma concentration was 554 mg l–1 (range: 304–875 mg l–1), with the first concentration measured at 15 min. Concentrations then fell rapidly; steady‐state concentrations occurred at 12 h and were maintained with a mean concentration of 35 mg l–1. However, there was considerable individual variation in clearance, and steady‐state concentrations ranged from 11 mg l–1 to 90 mg l–1. When the infusion was discontinued, acetylcysteine disappeared with a mean half‐life of 5.7 h 19.
In this same study, adverse reactions tended to occur early in the infusion, when acetylcysteine concentrations were highest. Therefore, these reactions are likely to be concentration related 19. Furthermore, it is unknown whether the initial very high concentrations of acetylcysteine are necessary to protect against hepatotoxicity. In this small study, liver damage was prevented just as effectively, regardless of the maximum concentration achieved. The authors concluded that the traditional regimen (developed by the same centre) was effective but that it was suboptimal in terms of adverse effects, the initial concentration, time profile and even the duration of treatment 11. However, for four decades the intravenous on‐label dose regimen for acetylcysteine regimen has remained essentially unchanged.
Acetylcysteine adverse effects: rates and risk factors
Since the introduction of acetylcysteine, there have been reports of adverse reactions, ranging from mild to severe. These include rash, nausea and vomiting, angioedema, flushing, tachycardia, bronchospasm, hypotension and death 20, 21, 22, with the most common reactions to intravenous acetylcysteine being nausea, vomiting and cutaneous systemic hypersensitivity reactions 21. Nausea and vomiting can also be secondary to the paracetamol overdose itself, with a rate of vomiting of approximately 12% before antidote treatment reported in some observational studies 23.
The main mechanism for adverse reactions is a non‐immunoglobulin (Ig) E‐mediated systemic hypersensitivity (anaphylactic) reaction. This is consistent with patients suffering moderate‐to‐severe adverse reactions having a 2.5‐fold increase in histamine concentrations without elevated tryptase concentrations 24. By contrast, IgE‐mediated reactions cause mast cell degranulation and elevations in both histamine and tryptase.
Adverse reactions appear to be concentration dependent, occurring almost exclusively within the first hour of treatment and corresponding to peak acetylcysteine concentrations 21. Various risk factors have been identified for adverse reactions, with higher rates in females, and those with a family history of allergy or a past history of asthma 21, 24, 25, 26. A lower paracetamol concentration has been associated with both an increased and a decreased risk of adverse reactions in various studies 20, 27.
The rates of adverse reactions varies depending on whether they are measured prospectively or retrospectively and which adverse effects are measured (e.g. total vs. gastrointestinal vs. systemic hypersensitivity reactions). Figure 1 (Table supplementary material) reviews a number of prospective and larger retrospective studies for the rate of adverse reactions to acetylcysteine. The reported rate varies widely between studies, from 8.5% to 77% 20, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40.
Figure 1.
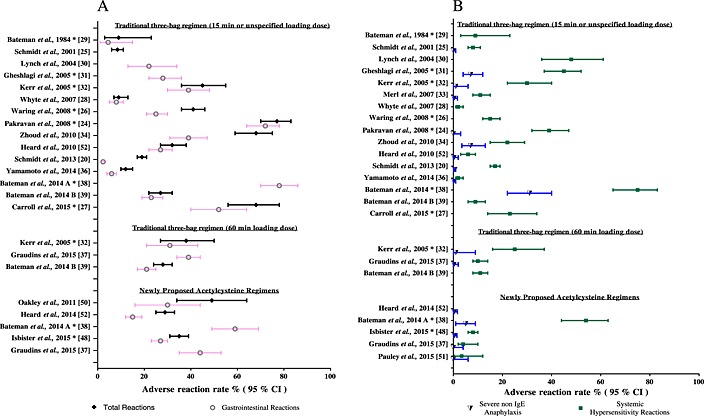
Percentage [95% confidence interval (CI)] of patients with adverse reaction, as reported in various retrospective and prospective studies. *Prospective study. All other studies are retrospective
Acetylcysteine treatment ‘failures’: rates and risk factors
Treatment with acetylcysteine within 8 h of paracetamol ingestion ensures a nonfatal outcome in nearly all patients; however, it does not guarantee that the patient will not develop hepatotoxicity [defined as an alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) level of > 1000 IU l–1). There are numerous case reports of treatment ‘failures’, where patients have developed hepatotoxicity despite receiving acetylcysteine treatment within 8 h 41, 42. Some observational studies of both intravenous and oral acetylcysteine treatment report rates of hepatotoxicity in those treated within 8 h as high as 3–7% 16, 43.
Risk factors for those developing hepatotoxicity in spite of early treatment include high initial paracetamol concentrations and persistently elevated paracetamol concentrations at the completion of the 21‐h intravenous acetylcysteine regimen 43. Various reasons have been proposed for these treatment ‘failures’, including: incorrect timing of ingestion, acetylcysteine protocol error, inadequate duration of treatment, an insufficient dose of acetylcysteine (particularly in large overdoses) or a combination of factors.
In a study in advanced cancer patients receiving up to 1 g kg–1 of paracetamol, a significant number of patients had delayed and prolonged absorption. The time to peak concentration occurred within 4 h in only 46% of the treatment courses, with 49% having a time to maximum concentration between 4 h and 8 h 44. Four percent had two distinct peaks of paracetamol concentration but a significant number of patients were on opioid analgesics, which can delay gastric emptying, which can delay gastric emptying 44. This phenomenon of two peaks of paracetamol has also been reported following large overdoses. Second peaks occurred at >30 h postingestion in some cases, and these patients developed hepatotoxicity despite early acetylcysteine treatment 41, 45. The probable cause of this double peak is a pharmacological bezoar and/or co‐ingestion of gastrointestinal tract‐slowing medications 41. A concern in these cases is that the decrease in the dose of acetylcysteine after 4–5 h and/or the cessation of acetylcysteine at the finish of the standard 21 h protocol occurs too early and is likely to contribute to increased rates of hepatotoxicity 41, 45.
The current acetylcysteine regimen is adequate for the majority of overdoses but many clinical toxicologists feel that simply following the standard three‐bag intravenous protocol might not be adequate in all paracetamol overdoses 3. It is common practice that acetylcysteine is continued beyond the 20–21‐h regimen if patients develop abnormal liver function tests or continue to have high paracetamol concentrations 46. It has been suggested in large overdoses that the dose of acetylcysteine is inadequate and should be increased 43. Theoretically, with increasing paracetamol dose and hepatic damage, there is an increase in the paracetamol half‐life and in the total and proportion of NAPQI produced. Furthermore, a prolonged paracetamol half‐life of greater than 4 h is known to correlate with the degree of subsequent liver damage 47. Increasing the dose and duration of acetylcysteine is logical. However, there have been no studies to determine the ingested dose or concentration of paracetamol at which the acetylcysteine dose should be increased, and furthermore what that dose increase should be. Another issue is that, when the paracetamol concentration is still elevated at the completion of the standard regimen, it is unclear what dose of acetylcysteine should be continued beyond the third bag.
Studied modified intravenous acetylcysteine protocols
Various different intravenous acetylcysteine regimens have been studied. Some of these protocols have been outlined in Figure 2 and Table 1. They were selected as they represent a divergent group from the protocols studied. Most of these regimens aim to decrease the rate of adverse events 32, 37, 38, 48 and administrative errors 49, 50, 51, and also the treatment time 38, while some deliver higher doses of acetylcysteine 49, 51, 52. Two were subject to a randomized controlled trial 32, 38 against the traditional regimen and the remainder have been evaluated in observational studies. Figure 2 shows the rates of infusion and cumulative acetylcysteine dose administered in a 70 kg patient, for some of these modified acetylcysteine regimens. The adverse event rates have been published for some of these regimens and are shown in Figure 1 and in the Supporting Information.
Figure 2.
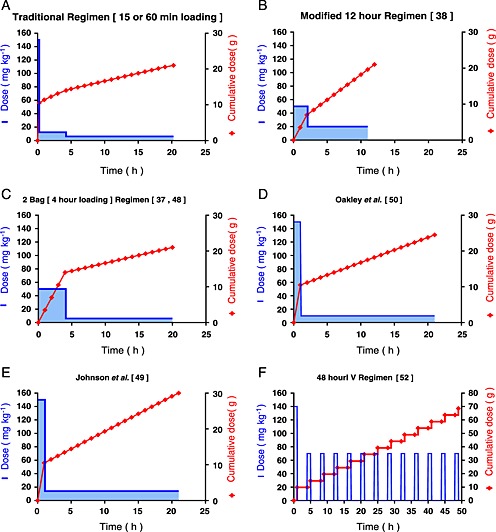
Acetylcysteine infusion (mg kg–1) and cumulative acetylcysteine dose (g) in a 70 kg patient vs. time, for the traditional and newly proposed regimens. (A) Traditional regimen (15 min or 60 min loading). (B) Modified 12‐h regimen 38. (C) Two‐bag (4‐h loading) regimen 37, 48. (D) Oakley et al. (1 h loading followed by 10mg/kg/h for 20 h) 50. (E) Johnson et al. (1 h loading followed by 14mg/kg/h for 20 h) 49. (F) 48‐hour intravenous regimen 52. Note: The 48‐h intravenous regimen has a different scale for the acetylcysteine cumulative dose and time.
Table 1.
Traditional and modified acetylcysteine regimens, dose, duration and simulated acetylcysteine concentrations
| Regimen | Dose | Duration (h) | Total acetylcysteine received (mg kg–1) over entire infusion | Simulated concentrations | |||
|---|---|---|---|---|---|---|---|
| Cmax (mg l–1) (mean: 5th, 95th centiles) | Tmax (h) | Concentration at 20 h post‐infusion starting (mg l–1) (mean: 5th, 95th centiles) | AUC (0–24 h) mg l–1.h | ||||
| Traditional three‐bag protocol | 1/ 100mg/kg over 15 mins2/ 50mg/kg over 4h3/100mg/kg over 16h | 20.25 | 300 mg kg–1 | 1216 (980, 1460) | 0.2 (12 min) | 39 [33, 47] | 1645 |
| Three‐bag protocol with 60 min loading | 1/100 mg kg–1 over 1 h2/50 mg kg–1 over 4 h 3/100 mg kg–1 over 16 h | 21 | 300 mg kg–1 | 566 (495, 642) | 1 | 39 [33, 47] | 1728 |
| 12‐h modified protocol (Bateman et al.) 38 | 1/100 mg kg–1 over 2 h2/200 mg kg–1 over 10 h | 12 | 300 mg kg–1 | 224 (193 254) | 2 | 18 [11, 28] | 1821 |
| Two‐bag protocol (Graudins et al. 37, Isbister et al.) 48 | 1/200 mg kg–1 over 4 h 2/100 mg kg–1 over 16 h | 20 | 300 mg/kg–1 | 260 (223, 299) | 4 | 41 [33, 49] | 1752 |
| Johnson et al. 49 | 1/150 mg kg – 1over 1 h 2/14 mg kg–1 h–1 for 20 h | 21 | 430 mg kg–1 | 564 (488, 653) | 1 | 86 (72, 101) | 2408 |
| Oakley et al. 50 | 1/ 150mg/kg over 1 h2/ 10mg/kg/hr for 20h | 21 | 350 mg kg–1 | 565 (494, 654) | 1 | 61 (51, 73) | 1968 |
| 48‐h intravenous protocol 52 | 1/ 140mg/kg over 1 h2/ 70mg/kg over1h every 4h for 12 doses | 48 | 980 mg/kg–1 | 526 (464, 600) | 1 | 44 (30, 62) | 2859.4 |
TheNote: Pauley et al. (2015) acetylcysteine regimen is similar to that of Johnson et al., with a two‐stage infusion; second infusion 15 mg kg–1 h–1 vs. 14 mg kg–1 h–1, respectively. The Pauley et al. acetylcysteine regimen duration is not fixed; hence, only the Johnson et al. regimen is simulated. AUC, area under the plasma concentration–time curve; Cmax, peak plasma concentration; Tmax, time to reach peak concentration.
Various protocols have lengthened the duration of administration of the loading dose to 1 h (Table 1) and this is the current recommended infusion rate in many parts of the world 39. However, in a randomized controlled trial of 180 patients, Kerr et al. found only a small and nonsignificant reduction in adverse outcomes with the 60‐min loading dose vs. the 15‐min (traditional) loading dose 32.
Oakley et al. 50 and Johnson et al. 49 also used a 150 mg kg–1 60‐min loading but with a two stage acetylcysteine regimen of 10 mg kg–1 h–1 and 14 mg kg–1 h–1 of acetylcysteine, respectively, over 20 h (Table 1). The aims of these regimens were to simplify acetylcysteine administration and reduce administration errors. Oakley et al. performed a retrospective observational study of his two‐bag regimen in a paediatric population (3 months to 17 years) and found that 49% had adverse events from acetylcysteine 50. Johnson used a single‐bag method, to simplify the administration, after the loading dose was administered all that was required was a decrease in infusion rate. When Johnson et al. looked retrospectively at rates of medications errors with his two‐stage, one bag regimen, they still found high rates of medications errors 49.
Pauley et al. 51 studied a similar regimen to Oakley et al. and Johnson et al. but with a patient‐tailored approach for the duration of the infusion of the second bag. The second bag was administered at a dose of 15 mg kg–1 h–1 and acetylcysteine was continued until the paracetamol concentration was lower than 10 mg l–1 and the liver enzymes remained normal or were falling 51. This regimen was reviewed retrospectively in a paediatric population (2 months to 18years). The median duration of the acetylcysteine infusion for acute overdoses was 26.3 h (range: 4.25–89 h, n = 56). The mean time for the paracetamol concentration to be measured as less than 10 mg l–1 was 31.5 ± 9.7 h. Only two patients (3.4%) developed hepatotoxicity 51. The authors did not specify in their protocol when investigations were repeated and therefore infrequent sampling might have accounted for the prolonged acetylcysteine infusions in the majority of patients.
One regimen of particular interest is the 12‐h modified protocol. This modified the loading dose to 100 mg kg–1 over 2 h and also shortened the duration of acetylcysteine treatment by 8 h, giving a second infusion of 200 mg kg–1 over 10 h. This regimen was derived using a Monte Carlo simulation of acetylcysteine concentrations based on a one‐compartment model 53. It was assumed that adverse reactions were concentration related and occurred at a peak concentration one standard deviation above the mean maximum concentration (650 mg l–1) found by Prescott et al. 19 [53]. The simulated data found that this modified regimen would result in peak acetylcysteine concentrations greater than 150 mg l–1 but less than 650 mg l–1 in 99% of patients, with a mean concentration of acetylcysteine of 30 mg l–1 at 20 h. The authors proposed that this regimen would administer the same total dose of acetylcysteine but with lower peak concentrations, and 20‐h plasma concentrations comparable to those in the conventional regimen 53. This modified 12‐h protocol was subject to a randomized controlled trial vs. the traditional protocol and was found to greatly reduce the incidence of vomiting or retching, or the need for antiemetics at 2 h (36% vs. 65%, respectively). There was also a reduced rate of severe anaphylaxis: 5% in the 12‐h modified arm vs. 31% in those receiving the traditional protocol (Figure 1). Furthermore, only six (6%, n = 108) in the modified 12‐h regimen required a prolonged acetylcysteine infusion 38.
More recently, other two‐bag regimens, with much slower initial infusions, to decrease the rate of adverse reactions, have been studied. These included a 4‐h loading dose of 200 mg kg–1 followed by 100 mg kg–1 over 16 h 37, and a two‐phase infusion protocol 48, 54 using a loading dose of 200 mg kg–1 over 11 h minus the time since ingestion or over 4 h (whichever is greater). This regimen commences acetylcysteine on arrival to hospital if >4 g paracetamol has been ingested. Observational studies of these two regimens reported fewer systemic hypersensitivity (4–8%) and severe non‐IgE anaphylactic (0–0.5%) reactions but rates of gastrointestinal reactions remained high (27–39%) 37, 48.
All modified regimens that have a much slower loading dose infusion have greatly reduced rates of adverse reactions 37, 38, 48. Although the effectiveness of these modified regimens appears to be the same as that of the conventional regimen, none of these studies was powered to determine efficacy 37, 38, 48. Early treatment is associated with very low rates of hepatotoxicity, so a trial with large numbers of patients would be required to prove non‐inferiority of one regimen over another in these circumstances.
Questions have been raised as to whether the dose of acetylcysteine is adequate in those who take large paracetamol overdoses. Acetylcysteine regimens have been proposed that increase the dose in those who ingest large paracetamol overdoses or who have high paracetamol concentrations. However, there are very few recommendations or studies concerning when to increase the acetylcysteine dose and by how much, and, in addition, it has not been established that this reduces risk. Proposed regimens commonly suggest doubling the dose of acetylcysteine in the third infusion in those with high paracetamol concentrations. A recent survey of international clinical toxicologists and poison centres found that 61% of the 164 respondents would increase the dose of acetylcysteine in the third infusion in patients with a high paracetamol concentration. However, the paracetamol concentration at which the dose should be increased varied widely between respondents 55.
Larger intravenous doses of acetylcysteine have been used safely. Heard et al. 52 (initial data published by Smilkstein et al. 56) reported the result of a single‐armed, multicentre trial from the US using the much higher total dose of intravenous acetylcysteine of 980 mg kg–1 over 48 h. In this trial, the intravenous dose mirrored the oral regimen, with a loading dose of 140 mg kg–1 over 1 h followed by 70 mg kg–1 over 1 h every 4 h for 12 doses in total (Table 1, Figure 2). The total adverse event rate was 28.9% (Figure 1). The overall rate of hepatotoxicity (ALT or AST >1000 U L–1) was 18.1% from 309 patients studied, with a rate of 3.4% in those treated within 10 h of ingestion. The authors concluded that this higher dose regimen, with its few adverse events and low rate of hepatotoxicity, might be useful in selected very large overdoses. However, the lack of a control arm in the study makes it difficult to draw conclusions on efficacy. The majority of patients do not require such a large or prolonged dose of acetylcysteine.
Rumack and Bateman hypothesized a modified acetylcysteine regimen based on ingested dose, and that the duration of treatment could be guided by the calculated paracetamol half‐life 3. Indeed, for large paracetamol overdoses, a more patient‐tailored approach might be postulated to be beneficial – for example, increasing the acetylcysteine dose if the patient has a high initial paracetamol concentration or a prolonged half‐life, and also taking into account liver function tests and coagulation profiles. In the future, novel biomarkers might also aid this decision 57. Further studies are required, to investigate which patients require an increase in the acetylcysteine dose, and the size and duration of this increase.
Simulation
To compare these various regimens further, acetylcysteine concentration–time profiles were simulated. The simulation was performed in MATLAB (version R2014a, MathWorks, Natick, MA, USA), and based on a published population pharmacokinetic model by Brown et al. 58, and was a three‐compartment model. The pharmacokinetic parameters of clearance, intercompartmental clearances and volumes of distribution were as outlined in a previous simulation of acetylcysteine by Shen et al. 54, and 500 patients were simulated from the model.
Table 1 and Figure 3 show the results of these simulations. Figure 3 shows the mean simulated acetylcysteine concentrations vs. time, and the 5th and 95th percentiles. For the purpose of the simulation, the two‐phase acetylcysteine infusion protocol of Shen et al. 54 was taken from 7 h post ingestion and hence became the same protocol as the two‐bag 200 mg kg–1 over 4 h loading protocol.
Figure 3.
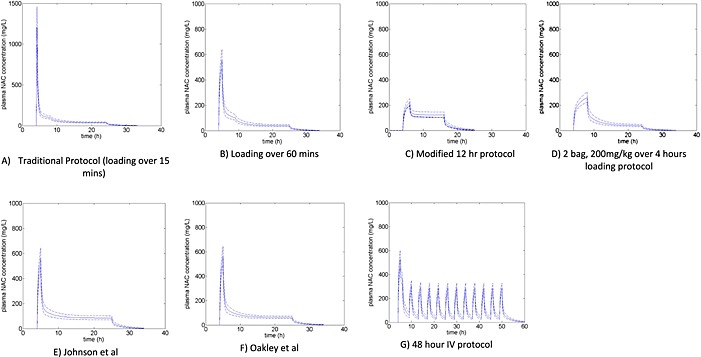
Simulated acetylcysteine concentrations vs. time for the traditional and proposed acetylcysteine regimens. (A) Traditional protocol (loading over 15 min); the scale of the y‐axis is larger in (A) than in (B) to (G). (B) Loading over 60 min. (C) Modified 12‐h protocol 38. (D) Two‐bag loading protocol, 200 mg kg–1 over 4 h 37, 48. (E) Johnson et al. (1 h loading followed by 14mg/kg/hr for 20 h). 50. F) Oakley et al. (1 h loading followed by 10mg/kg/hr for 20 h). 49. (G) 48‐h intravenous protocol 52; graph simulated to 60 h as a longer infusion of acetylcysteine. Note: for (A) to (G), the time of infusion commences at 4 h post‐ingestion.
There were notable differences in peak acetylcysteine concentrations between the conventional regimens and those with a longer‐duration or smaller loading dose, with mean peak acetylcysteine concentrations of: 1200 mg l–1 with a 150 mg kg–1 loading dose over15 min; 560 mg l–1 with 150 mg kg–1 over 60 min; 260 mg l–1 with 200 mg kg–1 over 4 h; and 225 mg l–1 with 100 mg kg–1 over 2 h–1. Importantly, those regimens with slower loading and lower reaction rates had consistently lower peak acetylcysteine concentrations in the modelling simulations.
The mean acetylcysteine concentrations at 20 h were greater than 40 mg l–1 in all regimens except the 12‐h modified regimen. The study protocol for the 12‐h modified regimen reported a simulated mean concentration of acetylcysteine of 30 mg l–1 at 20 h with a one‐compartment model, but when simulated with the more rigorous three‐compartment model it was only 18 mg l–1. Hence, this regimen does not appear to deliver the same acetylcysteine concentration at 20 h as initially proposed. However, it is not known what the minimum concentration of acetylcysteine should be at 20 h and, indeed, the majority of patients may not require such a high concentration. The main concern would be that this 20‐h concentration may be inadequate for larger and modified‐release paracetamol overdoses. In the 12‐h modified regimen, all patients underwent liver function tests and had the paracetamol concentration and international normalized ratio (INR) measured at completion, to determine the need for further acetylcysteine. In a subsequent commentary, the authors reported that all patients with no ALT rise also had no detectable paracetamol at the completion of the infusion 59. Lower 20‐h acetylcysteine concentrations are unlikely be an issue if paracetamol is no longer detected. Those with abnormal liver function tests, an INR >1.3 or detectable paracetamol had continued acetylcysteine treatment 46. This high‐risk group might indeed have benefited from continuation of a higher‐than‐usual dose of acetylcysteine (200 mg kg–1 over 10 h vs. 100 mg kg–1 over 16 h).
These simulations are useful in enabling the different regimens to be compared in terms of their peak concentration, area under the under the plasma concentration–time curve and steady‐state concentrations. By simulating different regimens, this could help researchers to better determine which regimens should be studied. However, the simulations cannot determine the most efficacious acetylcysteine concentration, the peak value that is required or which regimen will have the best balance between adverse effects and efficacy.
Conclusion
Acetylcysteine is a highly effective antidote for paracetamol poisoning and has decreased both morbidity and mortality following this very common drug overdose. Various modified regimens have been proposed, most aiming to decrease the number and severity by increasing the duration of the initial infusion and decreasing the peak acetylcysteine concentration. From the one randomized controlled trial and a few observational studies that have been carried out in this area, this approach appears to be successful. Whether these regimens are still as effective as traditional ones has yet to be determined. Further research is required to better determine the optimum acetylcysteine regimen, in terms of dose and duration of treatment. In the future, patient‐tailored regimens should aim to shorten treatment duration in low‐risk patients and decrease rates of treatment failures in high‐risk patients by altering the dose and/or duration of treatment.
Competing Interest
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years AND no other relationships or activities that could appear to have influenced the submitted work.
Supporting information
Table S1
Proportion of patients with adverse reactions, for the whole group and sub‐groups, following acetylcysteine infusion in patients receiving the traditional and newly proposed regimens
Supporting Info Item
Chiew, A. L. , Isbister, G. K. , Duffull, S. B. , and Buckley, N. A. (2016) Evidence for the changing regimens of acetylcysteine. Br J Clin Pharmacol, 81: 471–481. doi: 10.1111/bcp.12789.
References
- 1. Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM, Acute Liver Failure Study Group . Acetaminophen‐induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42: 1364–72. [DOI] [PubMed] [Google Scholar]
- 2. Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol 2015; 89: 193–9. [DOI] [PubMed] [Google Scholar]
- 3. Rumack BH, Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present and future. Clin Toxicol 2012; 50: 91–8. [DOI] [PubMed] [Google Scholar]
- 4. Hayes BD, Klein‐Schwartz W, Doyon S. Frequency of medication errors with intravenous acetylcysteine for acetaminophen overdose. Ann Pharmacother 2008; 42: 766–70. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H. Acetaminophen‐induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther 1974; 16: 676–84. [DOI] [PubMed] [Google Scholar]
- 6. Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol 1980; 10: 291S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen‐induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 1973; 187: 211–7. [PubMed] [Google Scholar]
- 8. Prescott LF, Sutherland GR, Park J, Smith IJ, Proudfoot AT. Cysteamine, methionine, and penicillamine in the treatment of paracetamol poisoning. Lancet 1976; 2: 109–13. [DOI] [PubMed] [Google Scholar]
- 9. Douglas AP, Hamlyn AN, James O. Controlled trial of cysteamine in treatment of acute paracetamol (acetaminophen) poisoning. Lancet 1976; 1: 111–5. [DOI] [PubMed] [Google Scholar]
- 10. Hamlyn AN, Lesna M, Record CO, Smith PA, Watson AJ, Meredith T, Volans GN, Crome P. Methionine and cysteamine in paracetamol (acetaminophen) overdose, prospective controlled trial of early therapy. J Int Med Res 1981; 9: 226–31. [DOI] [PubMed] [Google Scholar]
- 11. Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N‐acetylcystine: the treatment of choice for paracetamol poisoning. BMJ 1979; 2: 1097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williamson K, Wahl MS, Mycyk MB. Direct comparison of 20‐h IV, 36‐h oral, and 72‐h oral acetylcysteine for treatment of acute acetaminophen poisoning. Am J Ther 2013; 20: 37–40. [DOI] [PubMed] [Google Scholar]
- 13. Woo OF, Mueller PD, Olson KR, Anderson IB, Kim SY. Shorter duration of oral N‐acetylcysteine therapy for acute acetaminophen overdose. Ann Emerg Med 2000; 35: 363–8. [PubMed] [Google Scholar]
- 14. Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N‐acetylcysteine. Eur J Clin Pharmacol 1988; 34: 77–82. [DOI] [PubMed] [Google Scholar]
- 15. Jones AL. Mechanism of action and value of N‐acetylcysteine in the treatment of early and late acetaminophen poisoning: a critical review. J Toxicol Clin Toxicol 1998; 36: 277–85. [DOI] [PubMed] [Google Scholar]
- 16. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N‐acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988; 319: 1557–62. [DOI] [PubMed] [Google Scholar]
- 17. Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N‐acetylcysteine. Lancet 1977; 2: 432–4. [DOI] [PubMed] [Google Scholar]
- 18. Green JL, Heard KJ, Reynolds KM, Albert D. Oral and intravenous acetylcysteine for treatment of acetaminophen toxicity: a systematic review and meta‐analysis. West J Emerg Med 2013; 14: 218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prescott LF, Donovan JW, Jarvie DR, Proudfoot AT. The disposition and kinetics of intravenous N‐acetylcysteine in patients with paracetamol overdosage. Eur J Clin Pharmacol 1989; 37: 501–6. [DOI] [PubMed] [Google Scholar]
- 20. Schmidt LE. Identification of patients at risk of anaphylactoid reactions to N‐acetylcysteine in the treatment of paracetamol overdose. Clin Toxicol 2013; 51: 467–72. [DOI] [PubMed] [Google Scholar]
- 21. Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clin Toxicol 2009; 47: 81–8. [DOI] [PubMed] [Google Scholar]
- 22. Mant TG, Tempowski JH, Volans GN, Talbot JC. Adverse reactions to acetylcysteine and effects of overdose. BMJ 1984; 289: 217–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herren K, Clarke S. Best evidence topic reports: vomiting in paracetamol overdose. Emerg Med J 2002; 19: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pakravan N, Waring WS, Sharma S, Ludlam C, Megson I, Bateman DN. Risk factors and mechanisms of anaphylactoid reactions to acetylcysteine in acetaminophen overdose. Clin Toxicol 2008; 46: 697–702. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt LE, Dalhoff K. Risk factors in the development of adverse reactions to N‐acetylcysteine in patients with paracetamol poisoning. Br J Clin Pharmacol 2001; 51: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waring WS, Stephen AF, Robinson OD, Dow MA, Pettie JM. Lower incidence of anaphylactoid reactions to N‐acetylcysteine in patients with high acetaminophen concentrations after overdose. Clin Toxicol 2008; 46: 496–500. [DOI] [PubMed] [Google Scholar]
- 27. Carroll R, Benger J, Bramley K, Williams S, Griffin L, Potokar J, Gunnell D. Epidemiology, management and outcome of paracetamol poisoning in an inner city emergency department. Emerg Med J 2015; 32: 155–60. [DOI] [PubMed] [Google Scholar]
- 28. Whyte IM, Francis B, Dawson AH. Safety and efficacy of intravenous N‐acetylcysteine for acetaminophen overdose: analysis of the Hunter Area Toxicology Service (HATS) database. Curr Med Res Opin 2007; 23: 2359–68. [DOI] [PubMed] [Google Scholar]
- 29. Bateman DN, Woodhouse KW, Rawlins MD. Adverse reactions to N‐acetylcysteine. Hum Toxicol 1984; 3: 393–8. [DOI] [PubMed] [Google Scholar]
- 30. Lynch RM, Robertson R. Anaphylactoid reactions to intravenous N‐acetylcysteine: a prospective case controlled study. Accid Emerg Nurs 2004; 12: 10–5. [DOI] [PubMed] [Google Scholar]
- 31. Gheshlaghi F, Eizadi‐Mood N. Atopic diseases: risk factor in developing adverse reaction to intravenous N‐acetylcysteine. J Res Med Sci 2006; 11: 108–10. [Google Scholar]
- 32. Kerr F, Dawson A, Whyte IM, Buckley N, Murray L, Graudins A, Chan B, Trudinger B. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N‐acetylcysteine. Ann Emerg Med 2005; 45: 402–8. [DOI] [PubMed] [Google Scholar]
- 33. Merl W, Koutsogiannis Z, Kerr D, Kelly AM. How safe is intravenous N‐acetylcysteine for the treatment of paracetamol poisoning? Hong Kong J Emerg Med 2007; 14: 198–203. [Google Scholar]
- 34. Zyoud SH, Awang R, Sulaiman SA, Al‐Jabi SW. Effects of delay in infusion of N‐acetylcysteine on appearance of adverse drug reactions after acetaminophen overdose: a retrospective study. Pharmacoepidemiol Drug Saf 2010; 19: 1064–70. [DOI] [PubMed] [Google Scholar]
- 35. The Toxicology Investigator Network Authorship Group . A multicenter comparison of the safety of oral versus intravenous acetylcysteine for treatment of acetaminophen overdose. Clin Toxicol 2010; 48: 424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamamoto T, Spencer T, Dargan PI, Wood DM. Incidence and management of N‐acetylcysteine‐related anaphylactoid reactions during the management of acute paracetamol overdose. Eur J Emerg Med 2014; 21: 57–60. [DOI] [PubMed] [Google Scholar]
- 37. Graudins A, Harper A. Comparison of adverse drug reaction rates using a two‐bag to a standard three‐bag intravenous acetylcysteine regimen for paracetamol poisoning (Abstract: EAPCCT). Clin Toxicol 2015; 53: 249. [DOI] [PubMed] [Google Scholar]
- 38. Bateman DN, Dear JW, Thanacoody HK, Thomas SH, Eddleston M, Sandilands EA, Coyle J, Cooper JG, Rodriguez A, Butcher I, Lewis SC, Vliegenthart AD, Veiraiah A, Webb DJ, Gray A. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet 2014; 383: 697–704. [DOI] [PubMed] [Google Scholar]
- 39. Bateman DN, Carroll R, Pettie J, Yamamoto T, Elamin ME, Peart L, Dow M, Coyle J, Cranfield KR, Hook C, Sandilands EA, Veiraiah A, Webb D, Gray A, Dargan PI, Wood DM, Thomas SH, Dear JW, Eddleston M. Effect of the UK's revised paracetamol poisoning management guidelines on admissions, adverse reactions and costs of treatment. Br J Clin Pharmacol 2014; 78: 610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yarema MC, Johnson DW, Berlin RJ, Sivilotti ML, Nettel‐Aguirre A, Brant RF, Spyker DA, Bailey B, Chalut D, Lee JS, Plint AC, Purssell RA, Rutledge T, Seviour CA, Stiell IG, Thompson M, Tyberg J, Dart RC, Rumack BH. Comparison of the 20‐h intravenous and 72‐h oral acetylcysteine protocols for the treatment of acute acetaminophen poisoning. Ann Emerg Med 2009; 54: 606–14. [DOI] [PubMed] [Google Scholar]
- 41. Hendrickson RGMN, West PL, Burke CR. Bactrian (“double hump”) acetaminophen pharmacokinetics: a case series and review of the literature. J Med Toxicol 2010; 6: 337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang GS, Monte A, Bagdure D, Heard K. Hepatic failure despite early acetylcysteine following large acetaminophen–diphenhydramine overdose. Pediatrics 2011; 127: e1077–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Doyon S, Klein‐Schwartz W. Hepatotoxicity despite early administration of intravenous N‐acetylcysteine for acute acetaminophen overdose. Acad Emerg Med 2009; 16: 34–9. [DOI] [PubMed] [Google Scholar]
- 44. Kobrinsky NLHD, Horner H, Maksymiuk A, Minuk GY, White DF, Feldstein TJ. Treatment of advanced malgnancies with high‐dose acetaminophen and N‐acetylcysteine rescue. Cancer Invest 1996; 14: 202–10. [DOI] [PubMed] [Google Scholar]
- 45. Smith SW, Howland MA, Hoffman RS, Nelson LS. Acetaminophen overdose with altered acetaminophen pharmacokinetics and hepatotoxicity associated with premature cessation of intravenous N‐acetylcysteine therapy. Ann Pharmacother 2008; 42: 1333–9. [DOI] [PubMed] [Google Scholar]
- 46. Bateman DN. Paracetamol poisoning: beyond the nomogram. Br J Clin Pharmacol 2015; 80: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schiodt FV, Ott P, Christensen E, Bondesen S. The value of plasma acetaminophen half‐life in antidote‐treated acetaminophen overdosage. Clin Pharmacol Ther 2002; 71: 221–5. [DOI] [PubMed] [Google Scholar]
- 48. Isbister GK, Downes MA, Mcnamara K, Berling I, Whyte IM, Page CB. A novel infusion protocol for the administration of acetylcysteine (Abstract EAPCTT). Clin Toxicol 2015; 53: 249–50. [DOI] [PubMed] [Google Scholar]
- 49. Johnson MT, McCammon CA, Mullins ME, Halcomb SE. Evaluation of a simplified N‐acetylcysteine dosing regimen for the treatment of acetaminophen toxicity. Ann Pharmacother 2011; 45: 713–20. [DOI] [PubMed] [Google Scholar]
- 50. Oakley E, Robinson J, Deasy C. Using 0.45% saline solution and a modified dosing regimen for infusing N‐acetylcysteine in children with paracetamol poisoning. Emerg Med Australas 2011; 23: 63–7. [DOI] [PubMed] [Google Scholar]
- 51. Pauley KA, Sandritter TL, Lowry JA, Algren DA. Evaluation of an alternative intravenous N‐acetylcysteine regimen in pediatric patients. J Pediatr Pharmacol Ther 2015; 20: 178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heard K, Rumack BH, Green JL, Bucher‐Bartelson B, Heard S, Bronstein AC, Dart RC. A single‐arm clinical trial of a 48‐h intravenous N‐acetylcysteine protocol for treatment of acetaminophen poisoning. Clin Toxicol 2014; 52: 512–8. [DOI] [PubMed] [Google Scholar]
- 53. Thanacoody HK, Gray A, Dear JW, Coyle J, Sandilands EA, Webb DJ, Lewis S, Eddleston M, Thomas SH, Bateman DN. Scottish and Newcastle antiemetic pre‐treatment for paracetamol poisoning study (SNAP). BMC Pharmacol Toxicol 2013; 14: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shen F, Coulter CV, Isbister GK, Duffull SB. A dosing regimen for immediate N‐acetylcysteine treatment for acute paracetamol overdose. Clin Toxicol 2011; 49: 643–7. [DOI] [PubMed] [Google Scholar]
- 55. Juma SA, Villeneuve E, Elliot A, Palmer RB, Gosselin S. Doubling the third dose of intravenous N‐acetylcysteine survey: an international practice perspective (Abstract: EAPCCT). Clin Toxicol 2015; 53: 253–4. [Google Scholar]
- 56. Smilkstein MJ, Bronstein AC, Linden C, Augenstein WL, Kulig KW, Rumack BH. Acetaminophen overdose: a 48‐h intravenous N‐acetylcysteine treatment protocol. Ann Emerg Med 1991; 20: 1058–63. [DOI] [PubMed] [Google Scholar]
- 57. Vliegenthart AD, Antoine DJ, Dear JW. Target biomarker profile for the clinical management of paracetamol overdose. Br J Clin Pharmacol 2015; 80: 351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brown M, Bjorksten A, Medved I, McKenna M. Pharmacokinetics of intravenous N‐acetylcysteine in men at rest and during exercise. Eur J Clin Pharmacol 2004; 60: 717–23. [DOI] [PubMed] [Google Scholar]
- 59. Bateman DN, Dear JW, Thanacoody HK, Thomas SH, Eddleston M. Treatment of paracetamol overdose – authors' reply. Lancet 2014; 383: 1383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Proportion of patients with adverse reactions, for the whole group and sub‐groups, following acetylcysteine infusion in patients receiving the traditional and newly proposed regimens
Supporting Info Item


