Abstract
Aims
This study aimed to describe the real‐life incidence of bleeding, arterial thrombotic events and death during vitamin K antagonist (VKA) treatment in atrial fibrillation (AF).
Methods
This was a cohort study in Echantillon Généraliste de Bénéficiaires, the 1/97 sample of the French national healthcare claims and hospitalization database, of new VKA users with definite or probable AF and no other indication, and of patients without AF, from 2007 to 2011. Prespecified outcomes were all‐cause death, hospitalization for bleeding, arterial thrombotic event (ATE), or acute coronary syndrome (ACS) or any of the above (composite outcome).
Results
Of 8894 new VKA users, 3345 had probable or certain AF, 51.7% were male, mean age was 75.1 years, 87.1% had a CHA2DS2‐VASc score ≥ 2 and 11.6% a HAS‐BLED score > 3. Among AF patients, during VKA exposure the incidence rate of bleeding was 2.8 [95% confidence interval (CI) 2.2, 3.4] per 100 patient‐years, including 0.6 (95% CI 0.3, 0.8) cerebral, 1.0 (95% CI 0.7, 1.3) digestive and 1.4 (95% CI 1.0, 1.7) other bleeds. There were 1.6 (95% CI 1.2, 2.0) ACS, 1.5 (95% CI 1.1, 1.8) ATE and 3.8 (95% CI 3.2, 4.4) deaths per 100 patient‐years. The incidence rate of the composite outcome was 9.1 per 100 patient‐years (95% CI 8.2, 10.0). When patients stopped VKA, bleeding decreased (RR 0.67, 95% CI 0.43, 1.04)), but death or thrombosis increased (RR 3.06, 95% CI 2.46, 3.81 and 1.75, 95% CI 1.14, 2.70, respectively). During VKA exposure non‐AF patients had similar rates of bleeding, but fewer deaths, ACS and ischaemic events.
Conclusions
Real‐life rates for bleeding, arterial thrombotic events, ACS and deaths in AF patients treated with VKA were similar to those observed in clinical trials.
Keywords: anticoagulant, cohort study, death, atrial fibrillation, cardiovascular outcomes, population study
What is already known about this subject
Vitamin K antagonists (VKAs) are anticoagulants used for prevention of thromboembolism in venous thromboembolism and atrial fibrillation (AF).
Patients using VKAs have a high risk of severe bleeding or thrombosis because of the very nature of VKAs and because of the common interactions with other drugs and with food.
There is very little information on real‐life outcomes related to treatment with these drugs, which may be more common than in clinical trials.
What this study adds
Rates of severe bleeding and other complications are apparently not much greater in real‐life use than in clinical trials.
Event rates are different according to the indication (AF vs. non‐AF)
Event rates for death and thrombosis increase after stopping VKAs in AF patients, with a reduced risk of bleeding.
Introduction
Vitamin K antagonists (VKAs) are effective drugs for the prevention of thrombotic or embolic events during atrial fibrillation (AF). They are however subject to a large number of interactions with other drugs, with genetic factors and with alimentary or environmental factors, resulting in a risk of bleeding or inefficacy. They are among the drugs most commonly involved in hospital admissions for serious adverse reactions 1, 2. Because they are old drugs, there was little reliable data on adverse reaction rates until the recent development of direct oral anticoagulants (DOAC) provided data from clinical trials and from pharmacoepidemiological studies 3, 4, 5, 6, 7, 8, 9. There is still not much quantitative information on real‐life outcomes with VKAs and the influence of risk factors on outcomes 10, 11, 12.
To provide this information, we estimated incidence rates of bleeding, arterial thrombotic events (ATE), acute coronary syndrome (ACS) and death in VKA‐treated patients with AF prior to the marketing of DOAC, using data from a population database 13, 14.
Methods
Study design
The study was a population‐based cohort study in a national healthcare claims database.
Study setting
This was a historical cohort study performed in the Echantillon Généraliste de Bénéficiaires (EGB) healthcare claims and hospitalization database, which is a permanent representative anonymized 1/97 sample of the national healthcare insurance system database (SNIIRAM) linked to the national hospital discharge summary database (PMSI) and the national death registry 13, 14. SNIIRAM includes over 90% of the French population. The EGB sample represents 500 to 700 000 persons depending on the years, since 2004. These databases have been described elsewhere 13, 14, 15, 16.
They contain basic demographic information, reimbursed healthcare claims without indications and any of 30 long term diseases or disease families (LTD, with over 3500 ICD10 codes) that warrant full insurance coverage. This is linked to hospital discharge summaries with main, associated and secondary diagnoses (ICD‐10 codes) as well as procedures for all public and private hospitals 14, 15, 16. In addition the dataset includes date but not cause of death, from the national death registry.
Patients
All patients with a first dispensation of VKA (ATC code B01AA) between 1 January 2007 and 31 December 2011, with at least 2 years of data before and at least 1 year of follow‐up after first dispensation (except in the case of death) were extracted from the EGB database.
Exposures
VKA exposure started at the date of first VKA dispensation (index date) and ended at the date of VKA stop or at the end of follow‐up. Date of VKA stop was defined at the date of last dispensation plus 1 month (31 days). Last dispensation was defined as a dispensation without further dispensation for at least 90 days. This additional ‘grace period’ of 60 days was used to take into account use of fractional doses to adjust the INR, potential poor compliance and short treatment discontinuations. Deaths occurring during that grace period were considered as exposed to avoid immortal time bias.
VKA post‐exposure period was from date of VKA stop as defined above to date of a new VKA dispensation, or to 1 year after date of VKA stop.
Diagnosis of atrial fibrillation
The diagnosis of AF was considered definite if there was an LTD or hospital discharge summary with the ICD‐10 code I48 recorded before the index date, and no other indication for VKA. In the absence of definite AF, probable AF was defined using a disease score. The AF disease score was a logit function of patient characteristics, including specialty of VKA prescriber, specific drugs and investigations for AF or other VKA indications (see online Appendix S1), using as reference the definite AF population. Patients with an AF probability greater than 0.65, with no other apparent indication for VKA use were included in the probable AF population.
The total AF population is made of the patients with definite or probable AF.
Other probable indications of VKA dispensation were (i) prevention of venous thromboembolic events after orthopaedic surgery defined as a first VKA dispensation within 2 months after hospital discharge for orthopaedic surgery and (ii) treatment of venous thromboembolic events defined as a first VKA dispensation within 2 months after hospital discharge for a pulmonary embolism or deep vein thrombosis (I26, I80, I81, I82 ICD‐10 codes).
Outcomes
Outcomes considered were:
All‐cause death.
Major bleed, defined as hospitalization with primary diagnosis of bleeding events including haemorrhagic stroke (list of diagnostic codes in online Appendix S2).
Arterial thrombotic events (ATE) defined as hospitalization with a primary diagnosis of ischemic or undefined stroke or systemic arterial embolism (ICD‐10 codes I63, I64, I74, N28, D73.5 and K76.3), not including coronary heart events.
Acute coronary syndrome (ACS), defined as hospitalization with primary diagnosis of myocardial infarction or ACS (ICD‐10 codes I20 and I21).
A composite outcome of the occurrence of any of the above.
Risk factors
Risk factors for events were gender and age, CHA2DS2‐VASc and HAS‐BLED scores 17, 18, 19 derived from the data present in the database, congestive heart failure, hypertension, vascular disease history, bleeding history, medication use predisposing for bleeding, stroke history, diabetes, abnormal renal function, abnormal liver function and cancer in the two previous years (Online Appendix S3).
Statistical analysis
The cumulative incidence of outcomes was estimated using Kaplan–Meier estimate and 95% confidence interval (CI) for definite and probable AF populations. Risk factors were analyzed using multiple Cox proportional hazards models.
Event rates were also computed after VKA discontinuation in AF and non‐AF patients.
Ethics approval
The national data protection commission (CNIL) has authorized data extractions and analyses of the anonymized EGB database. No individual patient approval is required. The synopsis of the study was submitted to the National Institute for Medical Research (INSERM), which authorized the study. This study was also declared to the ENCEPP e‐registry (www.encepp.eu) at the European Medicines Agency (EMA, London, UK).
Results
Patient recruitment and disposition (Figure 1)
Figure 1.

Identification and selection of patients
Of the 11 906 patients who received a first dispensation of VKA between 1 January 2007 and 31 December 2011, 8894 had at least 2 years previous history and 1 year follow‐up after first dispensation. Of the 8000 patients with no other probable indication, 2197 (24.7%) had a diagnosis of AF (definite AF), 1148 (12.9%) had probable AF and 4655 (52.3%) did not have any identified AF. Respectively 78 (0.9%), 107 (1.2%) and 709 (8.0%) had other indications with a diagnosis of AF, probable AF or no AF.
The study populations were therefore 1) a specific population of 2197 persons with definite AF, 2) a sensitive population of 3345 AF patients combining the definite and the probable AF patients (all AF) with no other indication for the use of VKA and 3) 5364 non‐AF patients.
Patient characteristics at inclusion
These are indicated in Table 1. There was little difference between AF categories, whether AF was definite or probable and with or without valvular diseases (not shown). Gender distribution was balanced in all groups. Average age in the AF patients was 75 years compared with 64 years in the non‐AF group, 70 to 84% of AF patients had a chronic condition vs. 54.5% in non‐AF patients, 63 to 93% of AF patients had been hospitalized in the 2 years before index date vs. 62% of non‐AF patients and 98% of all AF patients had at least one drug dispensation in addition to a VKA and had seen a physician at least once in the previous 2 years. In over 95% this was a general practitioner (GP), and over 80% had also visited a specialist. Over 90% had had at least one laboratory test and in over 94% this was a haematology test.
Table 1.
Patient characteristics at study inclusion
| Definite AF n = 2197 | Probable AF n = 1148 | All* AF n = 3345 | Non AFn = 5364 | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 1110 (50.5) | 621 (54.1) | 1731 (51.7) | 2520 (47.0) |
| Female | 1087 (49.5) | 527 (45.9) | 1614 (48.3) | 2844 (53.0) |
| Age at index date (years), mean (± SD) | 74.5 (11.6) | 76.4 (9.5) | 75.1 (11.0) | 63.7 (16.9) |
| VKA treatment, n (%) | ||||
| Warfarin | 193 (8.8) | 83 (7.2) | 276 (8.3) | 459 (8.6) |
| Fluindione | 1871 (85.2) | 972 (84.7) | 2843 (85.0) | 4538 (84.6) |
| Acenocoumarol | 133 (6.1) | 93 (8.1) | 226 (6.8) | 367 (6.8) |
| At least one LTD, n (%) | 1842 (83.8) | 808 (70.4) | 2650 (79.2) | 2924 (54.5) |
| At least one hospital admission, n (%) | 2046 (93.1) | 717 (62.5) | 2763 (82.6) | 3341 (62.3) |
| Atrial fibrillation stroke risk factors, n (%) | ||||
| Age 65–74 years | 499 (22.7) | 275 (24.0) | 774 (23.1) | 1173 (21.9) |
| Age ≥ 75 years | 1272 (57.9) | 739 (64.4) | 2011 (60.1) | 1689 (31.5) |
| Congestive heart failure | 709 (32.3) | 179 (15.6) | 888 (26.5) | 259 (4.8) |
| Hypertension | 1221 (55.6) | 384 (33.5) | 1605 (48.0) | 1125 (21.0) |
| Diabetes mellitus | 598 (27.2) | 323 (28.1) | 921 (27.5) | 904 (16.9) |
| History of stroke or transient ischaemic attack | 340 (15.5) | 92 (8.0) | 432 (12.9) | 271 (5.1) |
| Vascular disease history | 451 (20.5) | 215 (18.7) | 666 (19.9) | 474 (8.8) |
| Women | 1087 (49.5) | 527 (45.9) | 1614 (48.3) | 2844 (53.0) |
| CHA 2 DS 2 ‐VASc score, n (%) | ||||
| 0 | 82 (3.7) | 41 (3.6) | 123 (3.7) | 881 (16.4) |
| 1 | 200 (9.1) | 110 (9.6) | 310 (9.3) | 1440 (26.8) |
| ≥ 2 | 1915 (87.2) | 997 (86.8) | 2912 (87.1) | 3043 (56.7) |
| Bleeding risk factors (score), n (%) | ||||
| Hypertension (+1) | 1221 (55.6) | 384 (33.5) | 1605 (48.0) | 1125 (21.0) |
| Abnormal renal function (+1) | 287 (13.1) | 94 (8.2) | 381 (11.4) | 236 (4.4) |
| Abnormal liver function (+1) | 55 (2.5) | 17 (1.5) | 72 (2.2) | 99 (1.8) |
| Stroke history (+1) | 278 (12.7) | 79 (6.9) | 357 (10.7) | 245 (4.6) |
| Bleeding history (+1) | 43 (2.0) | 16 (1.4) | 59 (1.8) | 88 (1.6) |
| Age > 65 years (+1) | 1737 (79.1) | 996 (86.8) | 2733 (81.7) | 2768 (51.6) |
| Medication usage predisposing to bleeding (+1) | 1611 (73.3) | 884 (77.0) | 2495 (74.6) | 3962 (73.9) |
| Modified HAS BLED score (in categories), n (%) | ||||
| 0 | 56 (2.5) | 24 (2.1) | 80 (2.4) | 461 (8.6) |
| 1 | 363 (16.5) | 241 (21.0) | 604 (18.1) | 2269 (42.3) |
| 2 | 787 (35.8) | 535 (46.6) | 1322 (39.5) | 1835 (34.2) |
| 3 | 704 (32.0) | 248 (21.6) | 952 (28.5) | 633 (11.8) |
| > 3 | 287 (13.1) | 100 (8.7) | 387 (11.6) | 166 (3.1) |
| At least one dispensation of drugs before index date, n (%) | 2125 (96.7) | 1135 (98.9) | 3260 (97.5) | 5201 (97.0) |
| At least one medical visit before index date, n (%) | 2142 (97.5) | 1136 (99.0) | 3278 (98.0) | 5217 (97.3) |
| Number of medical visits per patient over 2 years before index date, mean (± SD) | 22.9 (16.6) | 22.4 (15.2) | 22.7 (16.1) | 20.5 (15.8) |
| At least one general practitioner visit before index date, n (%) | 2102 (95.7) | 1114 (97.0) | 3216 (96.1) | 5093 (94.9) |
| Number of general practitioner visits per patient over 2 years before index date, mean (± SD) | 17.1 (12.4) | 16.8 (11.1) | 17.0 (11.9) | 15.3 (11.8) |
| At least one specialist visit before index date, n (%) | 1779 (81.0) | 995 (86.7) | 2774 (82.9) | 4345 (81.0) |
| Number of specialist visits per patient over 2 years before index date, mean (± SD) | 7.2 (10.0) | 6.6 (9.9) | 7.0 (9.9) | 6.5 (8.5) |
| At least one laboratory test before index date, n (%) | 2045 (93.1) | 1094 (95.3) | 3139 (93.8) | 4870 (90.8) |
LTD, registration for chronic long term disease resulting in full coverage of all expenses related to the disease.
All AF, definite or probable AF.
Follow‐up and exposure
Average duration of follow‐up was similar between groups, from 28 to 29 months. VKA maintenance after index date was shorter in non‐AF patients (median 125 days, quartiles 60–240) than in AF patients (median 201 days, quartiles 89–395) (see supplementary online Figure S1).
Outcomes
Incidence rates during VKA exposure in all AF (Table 2, Figure 2) were 2.8 [95% confidence interval (CI) 2.2, 3.4] per 100 patient‐years for major bleeding, including 0.6 (95% CI 0.3, 0.8) for cerebral bleeding, 1.0 (95% CI 0.7, 1.3) for digestive bleeding and 1.4 (95% CI 1.0, 1.7) for other bleeds. Event rates were 1.5 (95% CI 1.1, 1.8) per 100 patient‐years for ATE and 1.6 (95% CI 1.2, 2.0) for ACS. In total 3.8 (95% CI 3.2, 4.4) patients died per 100 patient‐years, resulting in a composite outcome rate of 9.1 (95% CI 8.2, 10.0) events per 100 patient‐years. Event rates were stable beyond the first 6 months of treatment (Figure 2).
Table 2.
Incidence rates of primary outcomes during the drug exposure, per 100 person‐years, with 95% confidence intervals (CI)
| Definite AF n = 2624 | All AF n = 3977 | Non AFn = 4233 | |
|---|---|---|---|
| Composite endpoint (death, hospitalization for major bleeding, arterial thrombotic events or MI): n, per 100 person‐years (95% CI) | 249, 9.5 (8.4, 10.6) | 362, 9.1 (8.2,10.0) | 296, 7.0 (6.2, 7.8) |
| Death: n, per 100 person‐years (95% CI) | 108, 4.1 (3.4, 4.9) | 152, 3.8 (3.2, 4.4) | 117, 2.8 (2.3, 3.3) |
| Hospitalization for major bleeding: n, per 100 person‐years (95% CI) | 73, 2.8 (2.2, 3.4) | 113, 2.8 (2.2, 3.4) | 97, 2.3 (1.8, 2.7) |
| Hospitalization for arterial thrombotic events:x n, per 100 person‐years (95% CI) | 41, 1.6 (1.1, 2.0) | 58, 1.5 (1.1, 1.8) | 58, 1.4 (1.0, 1.7) |
| Hospitalization for MI or acute coronary syndrome (ACS): n, per 100 person‐years (95% CI) | 44, 1.7 (1.2, 2.2) | 64, 1.6 (1.2, 2.0) | 37, 0.9 (0.9, 1.2) |
Figure 2.
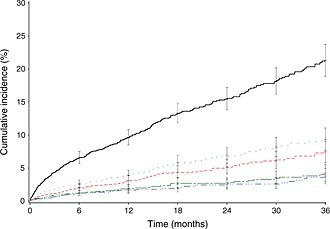
Cumulative incidence of primary outcomes during the VKA exposure period in patients with probable AF (Kaplan–Meier curve).  1 composite criterion,
1 composite criterion,  2 death,
2 death,  3 major bleeding,
3 major bleeding,  4 arterial thrombotic events,
4 arterial thrombotic events,  5 MI or acute coronary syndrome (ACS)
5 MI or acute coronary syndrome (ACS)
Event rates in the definite AF population were slightly higher but very similar in nature to those of the whole AF population or the probable AF population (Table 2).
Non‐AF patients had lower rates per 100 patient‐years of major bleeding (2.3, 95% CI 1.8, 2.7, ATE (1.4, 95% CI 1.0, 1.7), the composite outcome (7.0, 95% CI 6.2, 7.8), death (2.8, 95% CI 2.3, 3.3), and ACS (0.9, 95% CI 0.6, 1.2) (Table 2).
Occurrence of the outcomes during VKA exposure for AF increased non‐linearly with increasing CHAD2DS2‐VASc and HAS‐BLED score (see online supplemental Table S1). In multivariate analyses, factors associated with increased occurrence of the different outcomes are described in Table 3.
Table 3.
Multivariate analysis of factors associated with composite endpoint in the all AF population
| Composite endpoint RR (95% CI) | Death RR (95% CI) | Bleeding RR (95% CI) | Arterial thombosis RR (95% CI) | MI or ACS RR (95% CI) | |
|---|---|---|---|---|---|
| Gender Male | 1.23 (0.98,1.53) | 1.59 (1.12, 2.25) | 1.04 (0.70, 1.53) | 1.56 (0.89, 2.76) | 0.64 (0.38,1.08) |
| Age at index date (in categories) | |||||
| < 65 years | 1 | 1 | 1 | 1 | 1 |
| [65–74] years | 1.05 (0.74, 1.50) | 1.10 (0.63,1.92) | 1.25 (0.61,2.54) | 1.03 (0.45,2.37) | 0.85 (0.39,1.88) |
| [75–84] years | 1.14 (0.82,1.58) | 0.97 (0.58,1.64) | 1.69 (0.89,3.21) | 1.09 (0.51,2.35) | 1.00 (0.49,2.05) |
| > 84 years | 2.40 (1.68,3.43) | 3.17 (1.87,5.38) | 3.08 (1.53,6.23) | 1.89 (0.78,4.54) | 0.90 (0.36,2.28) |
| Congestive heart failure | 1.34 (1.07, 1.69) | 1.77 (1.26,2.48) | 1.07 (0.70,1.64) | 1.51 (0.87,2.65) | 1.04 (0.60,1.81) |
| Hypertension | 1.25 (1.00, 1.57) | 1.04 (0.74,1.48) | 1.52 (1.01,2.28) | 1.02 (0.58,1.78) | 1.81 (1.02,3.21) |
| Vascular disease history | 1.60 (91.26,2.04) | 1.56 (1.07,2.28) | 0.90 (0.56,1.46) | 2.38 (1.34,4.21) | 2.18 (1.28,3.72) |
| Bleeding history | 1.41 (0.72,2.76) | 0.72 (0.18,2.93) | 3.19 (1.37,7.46) | 0.96 (0.13,7.08) | 0.87 (0.12,6.41) |
| Medication use predisposing to bleeding | 1.06 (0.85,1.31) | 0.58 (0.41,0.82) | 1.38 (0.93,2.02) | 1.08 (0.63,1.87) | 2.85 (1.56,5.19) |
| Stroke history | 1.39 (1.03,1.87) | 1.67 (1.08,2.59] | 1.42 (0.85,2.38) | 1.90 (0.95,3.79) | 0.70 (0.28,1.75) |
| Diabetes mellitus | 1.16 (0.92,1.46) | 0.98 (0.68,1.41) | 0.98 (0.64,1.48) | 1.39 (0.80,2.42) | 1.66 (1.00,2.78) |
| Abnormal renal function | 1.70 (1.29,2.24) | 2.32 (1.56,3.44) | 1.31 (0.76,2.24) | 1.32 (0.64,2.70) | 1.40 (0.72,2.71) |
| Abnormal liver function | 1.08 (0.55,2.12) | 1.81 (0.78,4.22) | 0.48 (0.07,3.50) | 1.40 (0.33,5.96) | 0.53 (0.07,3.91) |
| Cancer in the two previous years | 2.10 (1.55,2.85) | 2.09 (1.32,3.29) | 2.97 (1.80,4.88) | 1.15 (0.45,2.92) | 1.44 (0.61,3.37) |
Reference groups are female gender, or the absence of the relevant condition;
RR (95% CI), relative risk and 95% confidence interval.
Among AF patients who stopped VKA, 29% had restarted VKA at 3 months, 50% at 6 months and 56% at 12 months, compared with 13, 22 and 26% after 3, 6 and 12 months, respectively, in patients without AF.
Incidence rates for composite outcome, deaths and ATE increased after VKA withdrawal in AF (Table 4) whereas bleeding tended to decrease. There was no significant difference for ACS.
Table 4.
Event rates for all AF and non‐AF populations during VKA exposure and after VKA withdrawal per 100 patient‐years
| All AF During exposure | All AF After exposure | RR (95% CI) After exposure/during exposure | No AF During exposure | Non‐AF After exposure | RR (95% CI) After exposure/during exposure | |
|---|---|---|---|---|---|---|
| Patient‐years | 3977 | 1214 | 4233 | 3431 | ||
| Composite outcome (n, %) | 362 (9.1) | 206 (17.0) | 1.86 (1.59,2.18) | 296 (7.0) | 235 (6.9) | 0.98 (0.83, 1.16) |
| Death n (%) | 152 (3.8) | 142 (11.7) | 3.06 (2.46,3.81) | 117 (2.8) | 145 (4.2) | 1.53 (1.21, 1.93) |
| Major bleeding n (%) | 113 (2.8) | 23 (1.9) | 0.67 (0.43, 1.04) | 97 (2.3) | 43 (1.3) | 0.55 (0.38, 0.78) |
| Thrombotic event n (%) | 58 (1.5) | 31 (2.6) | 1.75 (1.14, 2.70) | 58 (1.4) | 46 (1.3) | 0.98 (0.67, 1.43) |
| Acute coronary event n (%) | 64 (1.6) | 23 (1.9) | 1.18 (0.73, 1.89) | 37 (0.9) | 21 (0.6) | 0.70 (0.41, 1.19) |
AF, atrial fibrillation.
In non‐AF patients, there was a decrease in bleeds after stopping the VKA, but an increase in all‐cause deaths. There was no significant change for the composite outcome or in thrombotic or coronary events.
Discussion
This study was designed to describe bleeding or thrombotic events occurring during the real‐life use of VKAs, at a time when DOAC were not yet on the market for the indication of stroke prevention in atrial fibrillation 3, 4, 5, 7, 9, 20, 21, 22.
To this end, we used the EGB, a 1/97 permanent representative sample of the national French population healthcare claims database 13, 14. Over 85% of anticoagulant used was fluindione, the rest being equally divided between warfarin and acenocoumarol. Fluindione, an indane‐dione, has similar effects and interactions as coumadin drugs 23, 24, 25.
We found 2.8 major bleeding per 100 patient‐years exposed and 3.8 deaths per 100 patient‐years in AF patients. When VKAs were stopped, overall event rates went up, and especially ATE and death, while the bleeding rates went down.
Bleeding and thrombotic event rates can be compared with results from other population‐based studies and from clinical trials. In the Danish population, the rate of major bleeds was 3.7 per 100 patient years for users of warfarin 26. In the more recent population studies in an elderly Medicare population by Graham et al. 5 and Hernandez et al. 7, all cause major bleeding incidence rates were 4.4% and 5.9%, respectively. Death rate with warfarin was 3.8% for Graham et al. 5. Hernandez et al. did not provide death rates 7.
In the warfarin arms of Re‐Ly 27, Rocket‐AF 8, Aristotle, 6 and Engage‐af TIMI 4 the main clinical trials of the DOAC vs. warfarin, there were, respectively, 3.36, 3.40, 3.09 and 3.43 cases of major bleeding per 100 patient‐years and all cause death rates were 4.1, 2.2, 9.4, and 4.4 per 100 patient‐years exposed, respectively.
As Roskell et al., we found similar bleeding and death rates in real‐life and in the clinical trials 28, despite a presumption that because of better monitoring patients in clinical trials would have lower bleeding rates than patients in real‐life.
Our patients had a similar age and gender distribution to those in the clinical trials, consistent with the epidemiology of atrial fibrillation. The risk factors we identified for the occurrence of bleeding corresponded to the usual risk scales 29, 30, 31, 32. We found non‐linear increases in risk for increasing CHA2DS2‐VASc and HAS‐BLED scores, which could explain the differences in bleeding rates between Graham et al. 5 who used matched patients analyses and Hernandez et al. who used adjusted analyses 7.
Finally we found that when VKAs were stopped in the AF population, thrombotic events and death rates went up, whereas bleeding rates went down. This might be related to a healthy continuer effect. However the nature and direction of the changes are very much in favour of a direct relationship with stopping VKA in a population that remains at high risk of peripheral arterial embolism from AF. Other studies also found a similar risk when stopping anticoagulation in AF patients and the potential benefits of resuming anticoagulants in patients with AF who stopped them 33, 34.
Non‐AF patients were quite different from AF patients. They were younger, with fewer concomitant diseases and treatments, and lower event rates for all outcomes except non‐myocardial thrombotic events 35. When VKAs were stopped, presumably because of treatment guidelines recommending short term anticoagulation, the risk of bleeding decreased, but the overall death rate increased significantly. This may be due to increased venous or pulmonary embolic complications, which we did not study. The best duration of anticoagulant treatment in non‐AF indications might certainly be further explored.
The EGB uses existing data that are not impacted by the study. It contains exhaustive information about treatments and use of healthcare resources. Since the EGB is representative of the French population and all covered drugs dispensed by pharmacies are recorded, there is no selection or attrition bias 13, 14.
VKAs are oral drugs that are covered by the health care insurance system. All dispensations of reimbursed drugs are captured in the national database at the time of dispensation, so it is very unlikely that any patient on VKAs would not be included.
Among VKA users, the diagnosis of AF was considered definite when it was recorded as a chronic disease or was mentioned in hospital discharge summaries. The FDA Sentinel programme found that the ICD‐9 code 427.31 (ICD10 code I48) had satisfactory positive predictive value (77–94%) to identify AF in administrative databases 36. However some patients may have chronic AF without hospital admissions or formal registration. These probable AF patients were identified by excluding other indications for VKAs and applying a disease risk score based on the definite AF patients. There was no difference between the definite and probable AF patients whereas non‐AF patients had very different profiles, reinforcing our belief that our AF patients indeed had AF. We did not identify or study patients with AF or undiagnosed AF not treated with VKAs.
Outcomes were defined using ICD‐10 discharge diagnoses and diagnosis‐related groups codes in the database. Miscoding cannot be excluded. However, the hospital coding was fully independent from the study, excluding an information bias. Differential misclassification was equally unlikely. This approach was used in the same database in another recent study of bleeding and arterial thrombosis with DOAC 37.
Since deaths are recorded in the database using the national death registry, there is no information bias for this endpoint.
In conclusion, real‐life event rates for bleeding, death and arterial thrombosis in a real‐life population using VKAs for AF were similar to those reported in clinical trials. It may be anticipated that the benefits or risks of DOAC identified in clinical trials will also apply to their actual use 5. The withdrawal of anticoagulation was accompanied by an increased risk of ATE and death in AF patients, not counterbalanced by the decreased rates of bleeding. This has apparently not been studied with DOAC, and would certainly warrant further exploration 38.
Disclosures
NM is a tenured professor of the University of Bordeaux. He has received honoraria from various pharmaceutical companies, none which were involved in the present study.
Bordeaux Pharmacoepi is a research platform of University of Bordeaux and INSERM. It does publicly and industry‐funded studies, usually at the request of regulatory bodies, such as post‐authorization efficacy or safety studies (PAES, PASS) or within its own research programmes. In addition to the specific funding of this study, Bordeaux Pharmacoepi has received funding from many pharmaceutical companies including those that market anticoagulant or antiplatelet drugs, for studies not involving the drugs concerned by this study.
Though the salaries of the persons working on the platform are covered by the funding of these projects, there is no direct link between any of the individual funding sources and individual salaries.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, other than the unrestricted grant by Boehringer Ingelheim to University of Bordeaux mentioned above and no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years. Bordeaux Pharmacoepi is presently preparing studies of the comparative effectiveness of direct‐acting anticoagulants, at the request of the regulatory authorities, with funding by Boehringer Ingelheim and Bayer AG. The present study was undertaken as background information for these future studies. NM and PB have frequent contacts with colleagues from pharmaceutical industry and regulatory bodies, on related or unrelated topics, that have no financial substrate per se but might lead to future contracts and research grants for our research team.
NM and PB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
NM and PB had the initial idea. The protocol was developed by PB with RL, CDuP, AA, CDrP and NM. Data analysis was performed by RL and AA. All authors contributed to the interpretation of the results. NM wrote the first draft and all authors contributed to improving the paper. They all approved its final version.
Funding
This study was supported by an unrestricted grant from Boehringer‐Ingelheim. The sponsor had no part in the design or conduct of the study, collection, management, analysis, and interpretation of the data and preparation, review or approval of the manuscript.
This study is registered in the ENCEPP e‐Register of studies at EMA (www.encepp.eu), as ENGEL (‘rEal‐life aNticoaGulants bEnefit‐risk in atrial fibrilLation in France’).
Supporting information
Appendix S1
Appendix S1.1 Diagnostic elements of probable atrial fibrillation
Appendix S1.2 Factors associated with definite AF vs. no indication: multiple logistic regression analyses
Appendix S1.3 Probability pi of an individual i being a patient with definite AF (LTD for AF or hospitalization with an AF diagnosis without other probable indication criteria) among patients with at least one VKA dispensation
Appendix S2 List of ICD‐10 codes for major bleeding
Appendix S3 Confounders considered in the analysis
Table S1 Risk scores and occurrence of the study endpoint in the total AF population
Table S2 Incidence rate (in % person‐years) of primary outcomes after VKA withdrawal (during 365 days after drug withdrawal date) according to the VKA populations
Figure S1 VKA maintenance during the follow‐up period according to the VKA populations (Kaplan–Meier estimate)
Figure S2 Cumulative incidence of primary outcomes after VKA withdrawal (during 365 days after drug withdrawal date) in patients with AF (Kaplan–Meier curve).
Supporting info item
Blin, P. , Dureau‐Pournin, C. , Lassalle, R. , Abouelfath, A. , Droz‐Perroteau, C. , and Moore, N. (2016) A population database study of outcomes associated with vitamin K antagonists in atrial fibrillation before DOAC. Br J Clin Pharmacol, 81: 569–578. doi: 10.1111/bcp.12807.
References
- 1. Pouyanne P, Haramburu F, Imbs JL, Begaud B. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. French Pharmacovigilance Centres. BMJ 2000; 320: 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004; 329: 15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Connolly SJ, Wallentin L, Ezekowitz MD, Eikelboom J, Oldgren J, Reilly PA, Brueckmann M, Pogue J, Alings M, Amerena JV, Avezum A, Baumgartner I, Budaj AJ, Chen JH, Dans AL, Darius H, Di Pasquale G, Ferreira J, Flaker GC, Flather MD, Franzosi MG, Golitsyn SP, Halon DA, Heidbuchel H, Hohnloser SH, Huber K, Jansky P, Kamensky G, Keltai M, Kim SS, Lau CP, Le Heuzey JY, Lewis BS, Liu L, Nanas J, Omar R, Pais P, Pedersen KE, Piegas LS, Raev D, Smith PJ, Talajic M, Tan RS, Tanomsup S, Toivonen L, Vinereanu D, Xavier D, Zhu J, Wang SQ, Duffy CO, Themeles E, Yusuf S. The Long‐Term Multicenter Observational Study of Dabigatran Treatment in Patients With Atrial Fibrillation (RELY‐ABLE) Study. Circulation 2013; 128: 237–43. [DOI] [PubMed] [Google Scholar]
- 4. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, Engage Af‐Timi Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–104. [DOI] [PubMed] [Google Scholar]
- 5. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015; 131: 157–64. [DOI] [PubMed] [Google Scholar]
- 6. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, Aristotle Committees , Investigators . Apixaban versus warfarin in patients with atrial fibrillation. New Engl J Med 2011; 365: 981–92. [DOI] [PubMed] [Google Scholar]
- 7. Hernandez I, Baik SH, Pinera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med 2015; 175: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM, Investigators RA. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–91. [DOI] [PubMed] [Google Scholar]
- 9. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014; 383: 955–62. [DOI] [PubMed] [Google Scholar]
- 10. Gasse C, Hollowell J, Meier CR, Haefeli WE. Drug interactions and risk of acute bleeding leading to hospitalisation or death in patients with chronic atrial fibrillation treated with warfarin. Thromb Haemost 2005; 94: 537–43. [DOI] [PubMed] [Google Scholar]
- 11. Khan F, Datta YH. Risk of bleeding during long‐term anticoagulation with warfarin: a tertiary care center experience. Blood Coagul fibrinolysis 2015; 26: 110–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med 1989; 87: 144–52. [DOI] [PubMed] [Google Scholar]
- 13. Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merliere Y. French national health insurance information system and the permanent beneficiaries sample. Revue d'epidemiologie et de sante publique 2010; 58: 286–90. [DOI] [PubMed] [Google Scholar]
- 14. Moulis G, Lapeyre‐Mestre M, Palmaro A, Pugnet G, Montastruc JL, Sailler L. French health insurance databases: what interest for medical research? Rev Med Interne 2015; 36: 411–7. [DOI] [PubMed] [Google Scholar]
- 15. Blin P, Dureau‐Pournin C, Foubert‐Samier A, Grolleau A, Corbillon E, Jove J, Lassalle R, Robinson P, Poutignat N, Droz‐Perroteau C, Moore N. Parkinson's disease incidence and prevalence assessment in France using the national healthcare insurance database. Eur J Neurol 2015; 22: 464–71. [DOI] [PubMed] [Google Scholar]
- 16. Blin P, Lassalle R, Dureau‐Pournin C, Ambrosino B, Bernard MA, Abouelfath A, Gin H, Le Jeunne C, Pariente A, Droz C, Moore N. Insulin glargine and risk of cancer: a cohort study in the French national healthcare insurance database. Diabetologia 2012; 55: 644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Apostolakis S, Lane DA, Buller H, Lip GY. Comparison of the CHADS2, CHA2DS2‐VASc and HAS‐BLED scores for the prediction of clinically relevant bleeding in anticoagulated patients with atrial fibrillation: the AMADEUS trial. Thromb Haemost 2013; 110: 1074–9. [DOI] [PubMed] [Google Scholar]
- 18. Dzeshka MS, Lip GY. Specific risk scores for specific purposes: use CHA2DS2‐VASc for assessing stroke risk, and use HAS‐BLED for assessing bleeding risk in atrial fibrillation. Thromb Res 2014; 134: 217–8. [DOI] [PubMed] [Google Scholar]
- 19. Odum LE, Cochran KA, Aistrope DS, Snella KA. The CHADS(2) versus the new CHA2DS2‐VASc scoring systems for guiding antithrombotic treatment of patients with atrial fibrillation: review of the literature and recommendations for use. Pharmacotherapy 2012; 32: 285–96. [DOI] [PubMed] [Google Scholar]
- 20. Majeed A, Hwang HG, Connolly SJ, Eikelboom JW, Ezekowitz MD, Wallentin L, Brueckmann M, Fraessdorf M, Yusuf S, Schulman S. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation 2013; 128: 2325–32. [DOI] [PubMed] [Google Scholar]
- 21. Redberg RF. The importance of postapproval data for dabigatran. JAMA Intern Med 2015; 175: 25. [DOI] [PubMed] [Google Scholar]
- 22. Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013; 368: 1272–4. [DOI] [PubMed] [Google Scholar]
- 23. Bossavy JP, Sakariassen KS, Thalamas C, Boneu B, Cadroy Y. Antithrombotic efficacy of the vitamin K antagonist fluindione in a human ex vivo model of arterial thrombosis : effect of anticoagulation level and combination therapy with aspirin. Arterioscler Thromb Vasc Biol 1999; 19: 2269–75. [DOI] [PubMed] [Google Scholar]
- 24. Lacut K, Ayme‐Dietrich E, Gourhant L, Poulhazan E, Andro M, Becquemont L, Mottier D, Le Gal G, Verstuyft C. Impact of genetic factors (VKORC1, CYP2C9, CYP4F2 and EPHX1) on the anticoagulation response to fluindione. Br J Clin Pharmacol 2012; 73: 428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roncato M, Billaud‐Mesguich E, Fiessinger JN, Vitoux JF, Aiach M, Alexandre JM. Anticoagulant treatment with fluindione. Relation between the decrease of vitamin K‐dependent factors and blood fluindione. Therapie 1986; 41: 203–6. [PubMed] [Google Scholar]
- 26. Hansen ML, Sorensen R, Clausen MT, Fog‐Petersen ML, Raunso J, Gadsboll N, Gislason GH, Folke F, Andersen SS, Schramm TK, Abildstrom SZ, Poulsen HE, Kober L, Torp‐Pedersen C. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170: 1433–41. [DOI] [PubMed] [Google Scholar]
- 27. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, Re‐Ly Steering Committee , Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139‐51. [DOI] [PubMed] [Google Scholar]
- 28. Roskell NS, Samuel M, Noack H, Monz BU. Major bleeding in patients with atrial fibrillation receiving vitamin K antagonists: a systematic review of randomized and observational studies. Europace 2013; 15: 787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case‐fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med 2006; 166: 853–9. [DOI] [PubMed] [Google Scholar]
- 30. Donze J, Rodondi N, Waeber G, Monney P, Cornuz J, Aujesky D. Scores to predict major bleeding risk during oral anticoagulation therapy: a prospective validation study. Am J Med 2012; 125: 1095–102. [DOI] [PubMed] [Google Scholar]
- 31. Oldgren J, Alings M, Darius H, Diener HC, Eikelboom J, Ezekowitz MD, Kamensky G, Reilly PA, Yang S, Yusuf S, Wallentin L, Connolly SJ, Investigators R‐L. Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE‐LY trial. Ann Intern Med 2011; 155: 660–7, W204. [DOI] [PubMed] [Google Scholar]
- 32. Roldan V, Marin F, Fernandez H, Manzano‐Fernandez S, Gallego P, Valdes M, Vicente V, Lip GY. Predictive value of the HAS‐BLED and ATRIA bleeding scores for the risk of serious bleeding in a “real‐world” population with atrial fibrillation receiving anticoagulant therapy. Chest 2013; 143: 179–84. [DOI] [PubMed] [Google Scholar]
- 33. Witt DM, Delate T, Garcia DA, Clark NP, Hylek EM, Ageno W, Dentali F, Crowther MA. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172: 1484–91. [DOI] [PubMed] [Google Scholar]
- 34. Slomski A. Resuming warfarin after GI bleeding leads to better outcomes. JAMA 2014; 311: 349. [DOI] [PubMed] [Google Scholar]
- 35. Abraham NS, Singh S, Alexander GC, Heien H, Haas LR, Crown W, Shah ND. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ 2015; 350: h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012; 21 (Suppl 1): 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maura G, Blotiere PO, Bouillon K, Billionnet C, Ricordeau P, Alla F, Zureik M. Comparison of the short‐term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity‐matched cohort study. Circulation 2015; 132: 1252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chatterjee S, Sardar P, Giri JS, Ghosh J, Mukherjee D. Treatment discontinuations with new oral agents for long‐term anticoagulation: insights from a meta‐analysis of 18 randomized trials including 101,801 patients. Mayo Clin Proc 2014; 89: 896–907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S1.1 Diagnostic elements of probable atrial fibrillation
Appendix S1.2 Factors associated with definite AF vs. no indication: multiple logistic regression analyses
Appendix S1.3 Probability pi of an individual i being a patient with definite AF (LTD for AF or hospitalization with an AF diagnosis without other probable indication criteria) among patients with at least one VKA dispensation
Appendix S2 List of ICD‐10 codes for major bleeding
Appendix S3 Confounders considered in the analysis
Table S1 Risk scores and occurrence of the study endpoint in the total AF population
Table S2 Incidence rate (in % person‐years) of primary outcomes after VKA withdrawal (during 365 days after drug withdrawal date) according to the VKA populations
Figure S1 VKA maintenance during the follow‐up period according to the VKA populations (Kaplan–Meier estimate)
Figure S2 Cumulative incidence of primary outcomes after VKA withdrawal (during 365 days after drug withdrawal date) in patients with AF (Kaplan–Meier curve).
Supporting info item


