Abstract
Torsades de pointes (TdP) is a characteristic polymorphic ventricular arrhythmia associated with delayed ventricular repolarization as evidenced on the surface electrocardiogram by QT interval prolongation. It typically occurs in self‐limiting bursts, causing dizziness and syncope, but may occasionally progress to ventricular fibrillation and sudden death. Acquired long QT syndromes are mainly caused by cardiac disease, electrolyte abnormalities or exposure to drugs that block rectifying potassium channels, especially IKr. Management of TdP or marked QT prolongation includes removal or correction of precipitants, including discontinuation of culprit drugs and institution of cardiac monitoring. Electrolyte abnormalities and hypoxia should be corrected, with potassium concentrations maintained in the high normal range. Immediate treatment of TdP is by intravenous administration of magnesium sulphate, terminating prolonged episodes using electrical cardioversion. In refractory cases of recurrent TdP, the arrhythmia can be suppressed by increasing the underlying heart rate using isoproterenol (isoprenaline) or transvenous pacing. Other interventions are rarely needed, but there are case reports of successful use of lidocaine or phenytoin. Anti‐arrhythmic drugs that prolong ventricular repolarization should be avoided. Some episodes of TdP could be avoided by careful prescribing of QT prolonging drugs, including an individualized assessment of risks and benefits before use, performing baseline and periodic electrocardiograms and measurement of electrolytes, especially during acute illnesses, using the lowest effective dose for the shortest possible time and avoiding potential drug interactions. These steps are particularly important in those with underlying repolarization abnormalities and those who have previously experienced drug‐induced TdP.
Keywords: magnesium sulphate, management, QT interval, torsades de pointes
Introduction
It is now half a century since the French cardiologist François Dessertenne published his original report of a characteristic polymorphic ventricular tachycardia and coined the phrase ‘torsades de pointes’ (TdP) or ‘twisting of the points’ to describe its ECG appearance 1. This uncommon arrhythmia characteristically occurs in self‐terminating bursts, causing dizziness or syncope and occasionally convulsions, but can occasionally degenerate into ventricular fibrillation, resulting in sudden cardiac death.
TdP occurs when there is delayed ventricular repolarization with associated triggered activity due to early afterdepolarizations (EADs). Drug‐induced delayed repolarization characteristically occurs because of blockade of rectifying potassium channels and is reflected on the 12 lead ECG by prolongation of the QT, which almost invariably precedes TdP. EADs occur because of continuing late calcium entry resulting from delayed inactivation of voltage‐gated calcium channels as a consequence of prolonged ventricular depolarization.
The QT interval
The QT interval extends from the onset of the QRS complex to the end of the T wave and reflects the duration of ventricular depolarization and repolarization. Factors that affect depolarization, such as sodium channel blockade, may prolong the QT interval as a result of increases in QRS duration. The majority of the QT interval, however, from the J point to the end of the T wave (the JT interval), reflects ventricular repolarization and the QT interval is more sensitive to factors that influence this component.
Prolongation of the QT interval can be congenital (genetic) or acquired. Congenital long QT syndromes (cLQTS), not considered in detail in this article, are caused by genetic loss‐of‐function mutations that affect the rectifying potassium channels primarily responsible for cardiac repolarization, such as IKr, IKs or IK1, or gain‐of‐function mutations affecting sodium or rarely L‐type calcium channels 2. Acquired LQTS (aLQTS) is caused by heart disease, electrolyte abnormalities and/or exposure to precipitating (‘culprit’) medications, of which a large number have been implicated (Table 1). These drugs usually delay cardiac repolarization by blocking relevant potassium channels, especially IKr, which is encoded by the gene previously referred to as HERG (the human ether a go go‐related gene) but now termed KCNH2. The risk of TdP with individual drugs is often not well defined, but is generally higher for most anti‐arrhythmic agents (e.g. quinidine, sotalol) than non‐cardiovascular drugs. Amiodarone is thought to be an exception, carrying a low risk of TdP. Anti‐arrhythmics are also used in patients likely to have other risk factors.
Table 1.
Examples of currently marketed drugs that have been associated with torsade de pointes
| Anti‐arrhythmics | |
| Class Ia | Quinidine |
| Disopyramide | |
| Class Ic | Flecainide |
| Class III | Amiodarone |
| Dofetilide | |
| Dronedarone | |
| Ibutilide | |
| Sotalol | |
| Anaesthetics | Sevoflurane |
| Propofol | |
| Antimalarials | Chloroquine |
| Halofantrine | |
| Antimicrobials | |
| Macrolides | Azithromycin |
| Clarithromycin | |
| Erythromycin | |
| Quinolones | Levofloxacin |
| Moxifloxacin | |
| Antipsychotics | Haloperidol |
| Pimozide | |
| Thioridazine | |
| Chlorpromazine | |
| Amisulpride | |
| Antidepressants | Citalopram |
| Escitalopram | |
| Amitriptyline | |
| Anticancer | Arsenic trioxide |
| Vandetanib | |
| Sunitinib | |
| Others | Pentamidine |
| Fluconazole | |
| Ketoconazole | |
| HIV | |
| Cocaine | |
| Methadone | |
| Ondansetron | |
| Domperidone | |
| Anagrelide | |
| Donepezil | |
| Cilostazol |
Assessing risk
The extent of QT prolongation is one of several important risk factors for the development of TdP. Clinical use of the QT interval as a biomarker for risk of TdP is, however, hampered by practical difficulties in measurement in a clinical context. While many modern ECG machines provide automated measurements of QT interval upon which many clinicians rely 3, these are not always accurate, so manual confirmation is essential, especially when the ECG is abnormal. Manual measurements are, however, often problematic due to uncertainty over the end of the T wave, especially in patients with repolarization abnormalities and/or prominent U waves. Indeed variability of measurement amongst cardiologists and even cardiac electrophysiologists can be worryingly high 4.
It is also necessary to take account of the heart rate, because the QT interval shortens as heart rate increases. Although TdP due to aLQTS is uncommon in patients with tachycardia, accurate assessment of delayed repolarization is still important because of the risk of TdP in the event of subsequent bradycardia or pauses. Various formulae are available to provide a QT interval corrected to a heart rate of 60 min−1 (QTc). The original and still most commonly used formula is that of Bazett (QTc = QT/[RR0.5]) 5, but a major limitation is that this over‐corrects the QT interval in patients with tachycardia resulting in spurious apparent QTc prolongation, with the reverse occurring in those with bradycardia. These effects are less marked with more sophisticated correction formulae such as the Fridericia (QTc = QT/[RR0.33]) or Framingham [QTc = QT + 0.154(1 – RR)] methods 6. All these formulae, however, require calculation based on the RR interval and even if the measurement of QT interval is accurate, this is challenging in a clinical environment. Also, because of individual variation in the relationship between heart rate and QT interval 7, no universal formula will provide an ideal heart rate correction for a specific patient.
Women have, on average, longer QT intervals than men, a difference that emerges around puberty, and are at higher risk of TdP. Using the Bazett formula, a QTc greater than 450 ms in a male and 470 ms in a female is considered abnormal in adults 6, 8. More recently it has been suggested that the 99th centiles for otherwise healthy adults are used as limits of normality, 470 ms for males and 480 ms for females 9. TdP is unusual if the Bazett QTc is less than 500 ms, although it can occur, especially in those with bradycardia. Heart rate correction is therefore probably unnecessary in those with heart rates below 60 beats min−1. In one study the average QT and Bazett QTc intervals preceding drug‐induced TdP were 580 and 590 ms respectively 10.
More recently a heart rate–QT interval nomogram has been described for risk stratification (Figure 1). This method is attractive because it avoids the need for correction formulae and is based on heart rate rather than RR interval. Because the relationship between QT interval, heart rate and risk is uncertain in those with more rapid heart rates, an interrupted nomogram line is used for heart rates above 104 beats min−1. As well as being simple to use, the heart rate–QT nomogram separates patients who develop TdP from those who do not with improved sensitivity and specificity compared with the various heart rate correction formulae described above 11.
Figure 1.
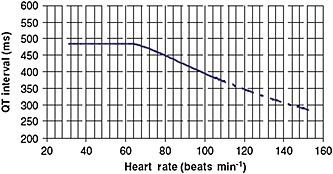
QT interval nomogram for determining ‘at risk’ QT‐heart rate pairs from a single 12‐lead ECG. Reproduced from reference 11, with permission
The QT interval is just one important determinant of the risk of TdP and other risk factors should also be taken into account when planning patient management. In aLQTS the arrhythmia is more likely to occur in those with bradycardias or pauses because impairment of IKr produces a prolonged and often dispersed or variable repolarization. Ventricular premature contractions may increase risk because they are associated with compensatory pauses that predispose to TdP. They may result from EADs occurring above a certain threshold required to cause triggered activity that can induce arrhythmia. The arrhythmia is more likely to develop if there is instability of repolarization as reflected on the ECG by T/U wave variability, such as T wave alternans or short term variations in the QT interval 9, 12. TdP is more common in those with structural heart diseases, including heart failure, myocardial infarction and left ventricular hypertrophy, as well as those with cLQTS, which may be concealed. Other risk factors include advanced age, female gender, alcoholic liver disease, recent conversion from atrial fibrillation, hypokalaemia, hypomagnesaemia, hypocalcaemia and digoxin or diuretic therapy. High concentrations or rapid intravenous infusion rates of a QT‐prolonging drug increase risk, such as following overdose or when there is reduced drug elimination due to liver or renal impairment 9, 13, 14.
Typically, development of severe QT prolongation or TdP requires several precipitating factors to occur in combination, to overcome a ‘repolarization reserve’ generated by the presence of multiple rectifying potassium channels 9. Precipitants of aLQTS exacerbate repolarization delay in those with cLQTS, while genetic mutations affecting relevant cardiac channels have been identified in a proportion of patients with aLQTS 15, 16. Common genetic variation may also predispose to aLQTS but investigation has so far proven either limited or disappointing 17. Patients receiving culprit drug therapies may remain well and have a normal ECG until other factors supervene such as electrolyte abnormalities, bradycardias or pauses, when QT prolongation and TdP may develop (‘late pro‐arrhythmia’).
Prevention of TdP
Over the past three decades drug regulatory authorities have taken steps to reduce the risk of licensed medicines causing TdP and this has resulted in several drugs being removed from the market or their use severely restricted. Drug development now requires detailed assessment of the electrophysiological effects of new drugs and, unless justification can be provided, human studies assessing effects on the QT interval 6. This makes it less likely that unexpected TdP will occur after drugs are marketed, such as occurred previously for several drugs such as probucol, terfenadine, astemizole, grepafloxacin, thioridazine and cisapride. It does however increase the cost of drug development and the chance of late failure of a novel drug to proceed to market.
Many episodes of TdP might be avoided by more skilful prescribing and monitoring of QT prolonging therapy. The benefit–risk balance of drug use should be considered on an individualized basis, taking into account the patient's medical history, baseline ECG and blood test results. This is challenging for prescribers as the risks associated with such patient factors are not clearly defined. When a QT prolonging drug is needed, the lowest effective dose should be used and the drug should be discontinued when no longer required. It may be difficult for prescribers to find information about the propensity of drugs to prolong the QT interval or cause TdP, but frequently updated information is available, for example from the CredibleMeds website 18.
Drug interactions are important precipitants of TdP and avoiding these is essential. These may be pharmacokinetic, for example via inhibition of metabolism (Table 2), or pharmacodynamic, where for example two QT prolonging drugs are used together. There are numerous clinical examples of TdP occurring after interactions of both main categories 19.
Table 2.
Examples of potential pharmacokinetic interactions affecting drugs that prolong the QT interval, inhibit cytochrome P450 (CYP) inhibitors, or both
| CYP isoform affected | |||
|---|---|---|---|
| CYP1A2 | CYP2D6 | CYP3A4 | |
| QT prolonging drugs | Haloperidol | Amitriptyline | Erythromycin |
| Amitriptyline | Haloperidol | SSRIs† | |
| Imipramine | Imipramine | Amiodarone | |
| Quinidine | Cisapride | ||
| Haloperidol | |||
| Quinidine | |||
| Pimozide | |||
| Methadone | |||
| CYP inhibitors | Fluoroquinolones* | Ritonavir | Protease inhibitors |
| Cimetidine | SSRIs* | Imidazole fungicides* | |
| Amiodarone* | Amiodarone* | Diltiazem | |
| Grapefruit juice | Quinidine* | SSRIs†, * | |
| Methadone* | Erythromycin* | ||
| Grapefruit juice | |||
May also prolong QT interval.
Selective serotonin re‐uptake inhibitors.
It is important to have access to an ECG in all patients receiving drugs that may prolong the QT interval. Depending upon the clinical and individual circumstances and the risk associated with the drug being used, it may be appropriate to perform baseline ECGs and blood tests before prescribing and repeating these after dose increases, with co‐prescription of drugs that may interact or during times of intercurrent illness. Useful advice on risks and the need for ECG monitoring may be found in the drug's summary of product characteristics. Monitoring potassium, magnesium and calcium concentrations is particularly important if there are clinical reasons for concern, for example with gastrointestinal disturbances or institution of diuretic therapy.
Management
The American College of Cardiology (ACC), American Heart Association (AHA) and European Society for Cardiology ESC) published guidelines for management of ventricular arrhythmias, including drug‐induced TdP, in 2006 20 and the key recommendations have been endorsed in a more recent ACC/AHA statement 9.
QT prolongation without torsade
There remains considerable variation in practice in the management of patients with clinically important QT prolongation (e.g. above the nomogram at risk line) in the absence of observed episodes of TdP. If feasible, the predicating drug should be discontinued and other modifiable risk factors addressed, including correction of oxygen saturation and plasma potassium, calcium and magnesium concentrations as necessary. Potassium administration to achieve a concentration between 4.7 and 5.2 mmol l−1 has been shown to shorten QT interval and reduce QT dispersion in human volunteers administered quinidine 21.
Patients with clinically important QT prolongation should undergo continuous ECG monitoring. The QT interval on the 12 lead ECG should be re‐evaluated periodically, with the frequency depending on the clinical circumstances and the extent of QT prolongation.
Some clinicians administer intravenous magnesium sulphate to patients with QT prolongation in the absence of TdP 3, but this is probably unnecessary unless multiple risk factors are present and especially if ECG features suggest instability, such as frequent premature contractions or unstable T/U intervals. Although risks from magnesium administration are small, the risk of harm from TdP is also low in appropriately monitored hospitalized patients with isolated aLQTS and there is currently no evidence of benefit. Because magnesium does not affect the QT interval, it is not possible to measure response.
Torsades de pointes
Prolonged episodes of continuous TdP associated with severe hypotension or cardiac arrest should be terminated by electrical cardioversion 9. It is more typical, however, for TdP to occur in recurrent self‐terminating bursts. Under these circumstances treatment is directed at stabilizing the myocardium using magnesium sulphate and by shortening repolarization by increasing heart rate using chronotropic drugs such as isoproterenol (isoprenaline) or cardiac pacing 20. Benefit may also be obtained using anti‐arrhythmic drugs, although some of these, especially class 1A and class 3 agents, may worsen the arrhythmia.
As for isolated QT prolongation, responsible agent(s) should be discontinued and modifiable risk factors such as hypokalaemia, hypomagnesaemia and hypoxia should be addressed. Several sources recommend that serum potassium is maintained in the high normal range (4.5–5.0 mmol l−1), although evidence is limited 9, 20.
Magnesium sulphate
Administration of magnesium sulphate is currently recommended as immediate first line treatment for TdP 20. The mechanism for benefit is uncertain 9, but magnesium may reduce the amplitude of EADs by inhibiting the late calcium influx via L‐type calcium channels that are associated with delayed ventricular repolarization. As a result, EADs are less likely to reach threshold potential and provoke or sustain TdP 22. As a co‐factor for the sodium potassium ATPase, magnesium may stabilize the membrane potential by facilitating potassium influx, correcting dispersed repolarization without shortening the action potential duration. Efficacy has not been demonstrated in a randomized controlled trial, but in a case series of 12 adult patients with TdP (in nine induced by anti‐arrhythmic drugs) a single 2 g (8 mmol) dose of magnesium sulphate administered intravenously over 1–2 min caused resolution in nine patients, with a second dose being effective in the remaining three, without reducing the QT interval 23. Similar results were reported from a French study, where TdP was completely abolished after administration of intravenous magnesium 1–3 g in four of six patients. In the remaining two patients, TdP improved but recurred in one and was suppressed only partially in the other 24. An initial intravenous bolus of 2.3–12 mg kg−1 magnesium sulphate gave a complete response in five of six children with cLQTS or aLQTS, including both of the children with aLQTS. The serum magnesium concentration was 3.9 ± 1.0 mg dl−1 (1.60 ± 0.4 mmol l−1) with a range of 2.9–5.4 mg dl−1 (1.2–2.2 mmol l−1) immediately after bolus injection 25.
Magnesium therapy is simple and relatively safe to administer. Recommended doses in the UK are shown in Table 3. The most prominent adverse effect is flushing, but nausea and vomiting, hypotension and drowsiness can occur, especially with higher doses. Substantial hypermagnesaemia may cause confusion, slurred speech, double vision, neuromuscular blockade, respiratory depression, hypophosphatemia, hyperosmolar dehydration, cardiac arrhythmias, coma and cardiac arrest. Severe toxicity is usually encountered with concentrations >3.5 mmol l−1.
Table 3.
Drugs used for treating TdP
| Dose (adult) | Notes | |
|---|---|---|
| Magnesium sulphate | Adults 2 g* (8 mmol) Children 25–50 mg kg−1 * (0.1–0.2 mmol kg−1) to maximum of 2 g. | Give as i.v. infusion over 10–15 min |
| Measure plasma magnesium after administration | ||
| Repeated doses may be needed | ||
| Isoproterenol | Adults 0.5–5 µg min−1 | Give as continuous i.v.infusion |
| Children 0.1–1.0 µg kg−1 min−1 | An initial bolus of 20–60 µg may be used in adults (0.3–1 µg kg−1 in children) | |
| Titrate infusion rate to heart rate of 90–110 beats min−1. Higher heart rates may be used if TdP recurs. |
Magnesium sulphate heptahydrate.
Isoproterenol
Isoproterenol increases the heart rate due to non‐selective β1/β2‐adrenoceptor agonist actions. This shortens the QT interval and effective refractory period. In a dog model, isoproterenol prevented quinine‐induced TdP 26, 27. There are no randomized controlled trials of isoproterenol use for TdP in humans, but occasional case reports suggest benefit 28. It is probably particularly useful as a bridge to temporary pacing in patients unresponsive to magnesium sulphate. Dose recommendations are shown in Table 3. Isoproterenol may, however, be contraindicated in cLQTS as it may prolong the QT interval and induce EADs 29 and may also enhance dispersion of repolarization in some subtypes 30. It may also worsen ventricular tachycardia if this is not TdP. Other adverse effects include palpitations, flushing, hypertension or hypotension and worsening of cardiac ischaemia.
Atropine is an alternative pharmacological method for increasing heart rate. It not only increases heart rate but also supresses QT prolongation and TdP induced by intracoronary acetylcholine in patients with cLQTS 31. Although atropine has been used to for treatment of TdP 32, it is not usually recommended as it can induce paradoxical bradycardia with consequent worsening of the arrhythmia 33.
Pacing
Transvenous pacing increases the heart rate, prevents pauses and can suppress or abolish episodes of TdP. Animal models indicate efficacy for pacing in dofetilide‐induced torsade 34, although in a dog model atrial pacing was relatively ineffective at preventing quinidine‐induced torsade 27. Case reports and case series in humans have suggested benefits from pacing for treating TdP induced by quinidine, disopyramide 35, sotalol 36 or amitriptyline 37, including in patients unresponsive to magnesium sulphate 38. Evidence suggests that pacing rates of at least 70 beats min−1 are required 39. Typically rates of 100 to 110 beats min−1 are used, while occasionally rates of up to 140 beats min−1 may be needed 38, 40. Pacing is generally considered as an option after magnesium sulphate. Its efficacy in comparison with isoproterenol is uncertain, but it may be a better option when the risk of torsade may persist over longer periods, for example with longer acting precipitant drugs.
Anti‐arrhythmic drugs
Few patients experiencing TdP fail to respond to the steps described above, and use of conventional anti‐arrhythmic drugs is rarely required. Those that prolong repolarization, the class 1A or III drugs, may in theory worsen TdP and should be avoided. There is, however, limited evidence of benefit from use of class 1B agents. Lidocaine was effective in dog 27 and rabbit 41 models of TdP and there are case reports of benefit for treating TdP in humans 42, 43. Phenytoin has also been reported to be successful in case reports 28, 44.
Novel approaches
Several drugs are in development to enhance delayed rectifier conductance and these may have future value for treatment of aLQTS. One of these, NS1643, supressed dofetilide‐induced QT prolongation and TdP in a rabbit model 45. Activators of cardiac ATP‐sensitive potassium channels (KATP) such as pinacidil and cromakalim have also had beneficial effects for aLQTS associated with some but not all culprit drugs 46.
α2‐adrenoceptor agonists attenuate L‐type calcium channels and supress EADs. Promising results were obtained for clonidine and dexmedetomidine, with both drugs shortening the QT interval and reducing the incidence of TdP in a rabbit model of aLQTS 47.
The anti‐ischaemic agent ranolazine blocks the late sodium current which is responsible for prolonging action potential duration and which also makes a contribution to EADs and has been shown to prevent or terminate clofilium‐induced TdP in rabbits. It may, however, prolong action potential duration and QT interval at higher concentrations due to IKr blockade 41.
EADs are facilitated by protein kinases and there is limited evidence that kinase inhibition may be a viable anti‐arrhythmic strategy, reducing inducibility of TdP 48.
Aftercare
In patients with aLQTS, the risk of further episodes of TdP is substantially reduced once precipitants and/or the culprit agent have been removed and this should result in normalization of the QTc interval. If it does not, further evaluation to exclude cLQTS should be considered, including obtaining a family history focusing on syncope, epilepsy and/or sudden death. Cardiological and genetic evaluation will then be required for patients to confirm evidence for the condition and for the family members of those in whom cLQTS is confirmed 49.
In view of the widespread use of QT prolonging drugs, it is important that these are not prescribed inadvertently to patients who have previously experienced TdP, as the risk of further episodes would be high 50. Patients and their primary clinicians should be alerted to this risk and to available sources of information about these drugs 18.
Competing Interests
Both authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Thomas, S. H. L. , and Behr, E. R. (2016) Pharmacological treatment of acquired QT prolongation and torsades de pointes. Br J Clin Pharmacol, 81: 420–427. doi: 10.1111/bcp.12726.
Footnotes
The correct orthography is disputed, with several variations used in the literature (including by French authors). These include torsades des pointes, torsade de pointes, etc. This article uses Dessertenne's original form ‐ torsades de pointes.
References
- 1. Dessertenne F. Ventricular tachycardia with 2 variable opposing foci. Arch Mal Coeur Vaiss 1966; 59: 263–72. [PubMed] [Google Scholar]
- 2. Wong L, Behr E. Acquired long QT syndrome: as risky as congenital long QT syndrome? Europace 2012; 14: 310–1. [DOI] [PubMed] [Google Scholar]
- 3. Othong R, Devlin JJ, Kazzi ZN. Medical toxicologists' practice patterns regarding drug‐induced QT prolongation in overdose patients: a survey in the United States of America, Europe, and Asia Pacific region. Clin Toxicol (Phila) 2015; 53: 204–9. [DOI] [PubMed] [Google Scholar]
- 4. Viskin S, Rosovski U, Sands AJ, Chen E, Kistler PM, Kalman JM, Rodriguez Chavez L, Iturralde Torres P, Cruz FFE, Centurión OA, Fujiki A, Maury P, Chen X, Krahn AD, Roithinger F, Zhang L, Vincent GM, Zeltser D. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm 2005; 2: 569–74. [DOI] [PubMed] [Google Scholar]
- 5. Bazett H. An analysis of the time‐relations of the electrocardiogram. Heart 1920; 7: 353–62. [Google Scholar]
- 6. European Medicines Agency . ICH topic E 14. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs. 2005. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002879.pdf (last accessed 1 June 2015).
- 7. Batchvarov VN, Ghuran A, Smetana P, Hnatkova K, Harries M, Dilaveris P, Camm AJ, Malik M. QT‐RR relationship in healthy subjects exhibits substantial intersubject variability and high intrasubject stability. Am J Physiol Heart Circ Physiol 2002; 282: H2356–63. [DOI] [PubMed] [Google Scholar]
- 8. Gupta A, Lawrence AT, Krishnan K, Kavinsky CJ, Trohman RG. Current concepts in the mechanisms and management of drug‐induced QT prolongation and torsade de pointes. Am Heart J 2007; 153: 891–9. [DOI] [PubMed] [Google Scholar]
- 9. Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010; 121: 1047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stratmann HG, Kennedy HL. Torsades de pointes associated with drugs and toxins: recognition and management. Am Heart J 1987; 113: 1470–82. [DOI] [PubMed] [Google Scholar]
- 11. Chan A, Isbister GK, Kirkpatrick CM, Dufful SB. Drug‐induced QT prolongation and torsades de pointes: evaluation of a QT nomogram. QJM 2007; 100: 609–15. [DOI] [PubMed] [Google Scholar]
- 12. Sauer AJ, Newton‐Cheh C. Clinical and genetic determinants of torsade de pointes risk. Circulation 2012; 125: 1684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE. Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review. Scientific World Journal 2012; 2012: 212178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roden DM. Drug‐induced prolongation of the QT interval. N Engl J Med 2004; 350: 1013–22. [DOI] [PubMed] [Google Scholar]
- 15. Newton‐Cheh C, Eijgelsheim M, Rice KM, de Bakker PI, Yin X, Estrada K, Bis JC, Marciante K, Rivadeneira F, Noseworthy PA, Sotoodehnia N, Smith NL, Rotter JI, Kors JA, Witteman JC, Hofman A, Heckbert SR, O'Donnell CJ, Uitterlinden AG, Psaty BM, Lumley T, Larson MG, Stricker BH. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat Genet 2009; 41: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Behr ER, Roden D. Drug‐induced arrhythmia: pharmacogenomic prescribing? Eur Heart J 2013; 34: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Behr ER, Ritchie MD, Tanaka T, Kaab S, Crawford DC, Nicoletti P, Floratos A, Sinner MF, Kannankeril PJ, Wilde AA, Bezzina CR, Schulze‐Bahr E, Zumhagen S, Guicheney P, Bishopric NH, Marshall V, Shakir S, Dalageorgou C, Bevan S, Jamshidi Y, Bastiaenen R, Myerburg RJ, Schott JJ, Camm AJ, Steinbeck G, Norris K, Altman RB, Tatonetti NP, Jeffery S, Kubo M, Nakamura Y, Shen Y, George AL Jr, Roden DM. Genome wide analysis of drug‐induced torsades de pointes: lack of common variants with large effect sizes. PLoS One 2013; 8: e78511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. CredibleMeds . Resources for healthcare professionals . Available at https://www.crediblemeds.org/healthcare‐providers/ (last accessed 1 June 2015).
- 19. Bauman JL. The role of pharmacokinetics, drug interactions and pharmacogenetics in the aquired long QT syndrome. Eur Heart J 2001; 3: K93–100. [Google Scholar]
- 20. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death‐‐executive summary: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur Heart J 2006; 27: 2099–140. [DOI] [PubMed] [Google Scholar]
- 21. Choy AM, Lang CC, Chomsky DM, Rayos GH, Wilson JR, Roden DM. Normalization of acquired QT prolongation in humans by intravenous potassium. Circulation 1997; 96: 2149–54. [DOI] [PubMed] [Google Scholar]
- 22. Kaye P, O'Sullivan I. The role of magnesium in the emergency department. Emerg Med J 2002; 19: 288–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tzivoni D, Banai S, Schuger C, Benhorin J, Keren A, Gottlieb S, Stern S. Treatment of torsade de pointes with magnesium sulfate. Circulation 1988; 77: 392–7. [DOI] [PubMed] [Google Scholar]
- 24. Etienne Y, Blanc JJ, Songy B, Boschat J, Guiserix J, Etienne E, Egreteau JP, Penther P. Antiarrhythmic effects of intravenous magnesium sulfate in torsade de pointes. Apropos of 6 cases. Arch Mal Coeur Vaiss 1986; 79: 362–7. [PubMed] [Google Scholar]
- 25. Hoshino K, Ogawa K, Hishitani T, Isobe T, Eto Y. Optimal administration dosage of magnesium sulfate for torsades de pointes in children with long QT syndrome. J Am Coll Nutr 2004; 23: 497S–500. [DOI] [PubMed] [Google Scholar]
- 26. Yamamoto H, Bando S, Nishikado A, Shinohara A, Hamai K, Yamamoto K, Ikefuji H, Ito S. The efficacy of isoproterenol on quinidine induced torsade de pointes. Tokushima J Exp Med 1991; 38: 1–4. [PubMed] [Google Scholar]
- 27. Inoue H, Matsuo H, Mashima S, Murao S. Effects of atrial pacing, isoprenaline and lignocaine on experimental polymorphous ventricular tachycardia. Cardiovasc Res 1984; 18: 538–47. [DOI] [PubMed] [Google Scholar]
- 28. Omar HR, Sprenker C, Karlnoski R, Mangar D, Camporesi EM. The use of isoproterenol and phenytoin to reverse torsade de pointes. Am J Emerg Med 2014; 32: 683 e5–7. [DOI] [PubMed] [Google Scholar]
- 29. Shimizu W, Ohe T, Kurita T, Takaki H, Aihara N, Kamakura S, Matsuhisa M, Shimomura K. Early afterdepolarizations induced by isoproterenol in patients with congenital long QT syndrome. Circulation 1991; 84: 1915–23. [DOI] [PubMed] [Google Scholar]
- 30. Khan IA, Gowda RM. Novel therapeutics for treatment of long‐QT syndrome and torsade de pointes. Int J Cardiol 2004; 95: 1–6. [DOI] [PubMed] [Google Scholar]
- 31. Furushima H, Niwano S, Chinushi M, Yamaura M, Taneda K, Washizuka T, Aizawa Y. Effect of atropine on QT prolongation and torsade de pointes induced by intracoronary acetylcholine in the long QT syndrome. Am J Cardiol 1999; 83: 714–8. [DOI] [PubMed] [Google Scholar]
- 32. Tan HL, Wilde AA, Peters RJ. Suppression of torsades de pointes by atropine. Heart 1998; 79: 99–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banai S, Tzivoni D. Drug therapy for torsade de pointes. J Cardiovasc Electrophysiol 1993; 4: 206–10. [DOI] [PubMed] [Google Scholar]
- 34. Oosterhoff P, Thomsen MB, Maas JN, Atteveld NJ, Beekman JD, Van Rijen HV, Van Der Heyden MA, Vos MA. High‐rate pacing reduces variability of repolarization and prevents repolarization‐dependent arrhythmias in dogs with chronic AV block. J Cardiovasc Electrophysiol 2010; 21: 1384–91. [DOI] [PubMed] [Google Scholar]
- 35. Keren A, Tzivoni D, Golhman JM, Corcos P, Benhorin J, Stern S. Ventricular pacing in atypical ventricular tachycardia. J Electrocardiol 1981; 14: 201–5. [DOI] [PubMed] [Google Scholar]
- 36. Totterman KJ, Turto H, Pellinen T. Overdrive pacing as treatment of sotalol‐induced ventricular tachyarrhythmias (torsade de pointes). Acta Med Scand Suppl 1982; 668: 28–33. [DOI] [PubMed] [Google Scholar]
- 37. Davison ET. Amitriptyline‐induced torsade de pointes. Successful therapy with atrial pacing. J Electrocardiol 1985; 18: 299–301. [DOI] [PubMed] [Google Scholar]
- 38. Charlton NP, Lawrence DT, Brady WJ, Kirk MA, Holstege CP. Termination of drug‐induced torsades de pointes with overdrive pacing. Am J Emerg Med 2010; 28: 95–102. [DOI] [PubMed] [Google Scholar]
- 39. Pinski SL, Eguia LE, Trohman RG. What is the minimal pacing rate that prevents torsades de pointes? Insights from patients with permanent pacemakers. Pace 2002; 25: 1612–5. [DOI] [PubMed] [Google Scholar]
- 40. Faber TS, Zehender M, Just H. Drug‐induced torsade‐de‐pointes ‐ incidence, management and prevention. Drug Saf 1994; 11: 463–76. [DOI] [PubMed] [Google Scholar]
- 41. Wang WQ, Robertson C, Dhalla AK, Belardinelli L. Antitorsadogenic effects of ({+/−})‐N‐(2,6‐dimethyl‐phenyl)‐(4[2‐hydroxy‐3‐(2‐methoxyphenoxy)propyl]‐1‐pipera zine (ranolazine) in anesthetized rabbits. J Pharmacol Exp Ther 2008; 325: 875–81. [DOI] [PubMed] [Google Scholar]
- 42. Assimes TL, Malcolm I. Torsade de pointes with sotalol overdose treated successfully with lidocaine. Can J Cardiol 1998; 14: 753–6. [PubMed] [Google Scholar]
- 43. Takahashi N, Ito M, Inoue T, Koumatsu K, Takeshita Y, Tsumabuki S, Tamura M, Inoue K, Maeda T, Saikawa T. Torsades de pointes associated with acquired long QT syndrome: observation of 7 cases. J Cardiol 1993; 23: 99–106. [PubMed] [Google Scholar]
- 44. Vukmir RB, Stein KL. Torsades de pointes therapy with phenytoin. Ann Emerg Med 1991; 20: 198–200. [DOI] [PubMed] [Google Scholar]
- 45. Diness T, Yeh YH, Qi X, Chartier D, Tsuji Y, Hansen RS, Olesen SP, Grunnet M, Nattel S. Antiarrhythmic properties of a rapid delayed‐rectifier current activator in rabbit models of acquired long QT syndrome. Cardiovasc Res 2008; 79: 61–9. [DOI] [PubMed] [Google Scholar]
- 46. Testai L, Cecchetti V, Sabatini S, Martelli A, Breschi MC, Calderone V. Effects of K openers on the QT prolongation induced by HERG‐blocking drugs in guinea‐pigs. J Pharm Pharmacol 2010; 62: 924–30. [DOI] [PubMed] [Google Scholar]
- 47. Tsutsui K, Hayami N, Kunishima T, Sugiura A, Mikamo T, Kanamori K, Yamagishi N, Yamagishi S, Watanabe H, Ajiki K, Murakawa Y. Dexmedetomidine and clonidine inhibit ventricular tachyarrhythmias in a rabbit model of acquired long QT syndrome. Circ J 2012; 76: 2343–7. [DOI] [PubMed] [Google Scholar]
- 48. Mazur A, Roden DM, Anderson ME. Systemic administration of calmodulin antagonist W‐7 or protein kinase A inhibitor H‐8 prevents torsade de pointes in rabbits. Circulation 1999; 100: 2437–42. [DOI] [PubMed] [Google Scholar]
- 49. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 2013; 15: 1389–406. [DOI] [PubMed] [Google Scholar]
- 50. Barra S, Agarwal S, Begley D, Providencia R. Post‐acute management of the acquired long QT syndrome. Postgrad Med J 2014; 90: 348–58. [DOI] [PubMed] [Google Scholar]


