Abstract
Seizures are a common complication of drug intoxication, and up to 9% of status epilepticus cases are caused by a drug or poison. While the specific drugs associated with drug‐induced seizures may vary by geography and change over time, common reported causes include antidepressants, stimulants and antihistamines. Seizures occur generally as a result of inadequate inhibitory influences (e.g., gamma aminobutyric acid, GABA) or excessive excitatory stimulation (e.g. glutamate) although many other neurotransmitters play a role. Most drug‐induced seizures are self‐limited. However, status epilepticus occurs in up to 10% of cases. Prolonged or recurrent seizures can lead to serious complications and require vigorous supportive care and anticonvulsant drugs. Benzodiazepines are generally accepted as the first line anticonvulsant therapy for drug‐induced seizures. If benzodiazepines fail to halt seizures promptly, second line drugs include barbiturates and propofol. If isoniazid poisoning is a possibility, pyridoxine is given. Continuous infusion of one or more anticonvulsants may be required in refractory status epilepticus. There is no role for phenytoin in the treatment of drug‐induced seizures. The potential role of ketamine and levetiracetam is promising but not established.
Keywords: anticonvulsants, poisoning, seizures
Introduction
Seizures are a common toxic complication of numerous drugs and poisons, as well as drug withdrawal syndromes. Studies have estimated that 6% of new‐onset seizures and up to 9% of status epilepticus cases are due to drug toxicity 1, 2. Several case series have identified a variety of drugs and other substances associated with seizures 3, 4, 5, 6, 7, 8. Antidepressants, diphenhydramine, stimulants (including cocaine and methamphetamine), tramadol and isoniazid account for the majority of cases. However, substances implicated in drug‐induced seizures have evolved over time as new drugs enter the market. For example, a California case series analyzing calls to the regional poison control centre found that over a 10 year interval, newer antidepressants replaced tricyclics as the most common cause of seizures and the frequency of cocaine and theophylline cases fell dramatically (with reports of theophylline decreasing to zero) 7. Causes of drug‐induced seizures also vary by geographic region. In two recent US studies bupropion was the leading drug 5, 7, whereas a Swiss study found mefenamic acid and citalopram were the most commonly reported seizure‐causing drugs 8. In Iran 9 and Austrialia 10 tramadol overdose is a common cause of seizures. In developing countries and agricultural regions herbicides and insecticides are an important consideration 11, 12, 13, 14, 15.
Most drug‐induced seizures are self‐limited and do not cause permanent sequelae. However, repeated or prolonged seizure activity may lead to irreversible neurological injury 16 as well as other life‐threatening complications such as hypoxia, hypotension, pulmonary aspiration, hyperthermia, rhabdomyolysis and metabolic acidosis. In retrospective studies of drug‐induced seizures reported to a regional poison control centre, status epilepticus (defined as continuous seizure activity lasting more than 30 min or two or more seizures without full recovery of consciousness between seizures) occurred in 3.6 % to 10% of cases 7, 17. Thus, prompt treatment including good supportive care and administration of effective anticonvulsant drugs are imperative. In this article, we review the mechanisms and treatment of drug‐induced seizures.
Pathophysiology
Exposure to certain drugs and chemical substances can result in the abrupt onset of altered mental status with or without localized or generalized motor activity (convulsions) combined with epileptic‐like brain activity (seizures) seen on the electroencephalogram (EEG). This altered EEG electrical activity is the result of abnormal neuronal discharges that start in cortical or subcortical brain regions and may persist for an extended amount of time. This is not epilepsy, which is a disease state associated with recurrent spontaneous paroxysmal epileptic brain activity on EEG with or without associated motor activity. While the clinical condition of patients with epilepsy certainly can be made worse (with more severe or frequent seizures) by exposure to many drugs or chemicals, this is usually not considered a primary drug‐induced seizure.
Drug‐induced seizures can occur as a direct result of altering neural pathways and specific excitatory or inhibitory transmitters and receptors within those pathways. Figure 1 offers a simplified representation of these processes. In Figure 1A, the normal balance is represented between excitatory and inhibitory neural pathways, transmitters and receptors. Gamma aminobutyric acid (GABA) mediated receptors and pathways are inhibitory, while those involving glutamate are excitatory. Decreasing excitatory pathway activity, neurotransmitters or receptor function, or increasing inhibitory actions, leads to reduced neural activity that can manifest itself as clinical sedation (Figure 1B). Reducing inhibitory pathways, neurotransmitters or receptor function by drugs or chemicals, or increasing excitatory activity, can result in over activation and seizures (Figure 1C). If the effect of a drug is to reduce GABA activity (e.g. isoniazid or a cephalosporin), seizures can result. Drugs such as barbiturates or benzodiazepines can increase the functional effect of GABA‐mediated inhibitory activity and as a result prevent or terminate drug‐induced or drug‐withdrawal seizures. Using this model, the sudden withdrawal of high doses of drugs applied chronically that have a strong inhibitory neuronal function (e.g. ethanol or barbiturates) can result in acute withdrawal seizures. This may be exacerbated by up‐regulation of the excitatory N‐methyl‐D‐aspartate (NMDA) subtype of the glutamate receptor/pathway 18, 19.
Figure 1.
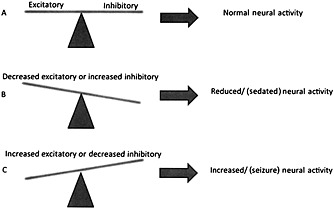
A model of drug‐ or chemical‐induced effects on neural activity. (A) The normal balance between excitatory and inhibitory neuronal activity, receptor function, transmitters and pathways. (B) Decreasing excitatory or increasing inhibitory neuronal activity can result in sedation, and is likely involved in terminating seizure activity. (C) When increased excitatory or decreased inhibitory neuronal activity results from drug or chemical exposure, seizures may occur. Although greatly simplified in this model, in actuality feedback loops, transmitter depletion and up‐and down‐regulation of receptor numbers and sensitivities are but a few of the complexities of this physiological process
Neuronal pathways are much more complex than represented in Figure 1 20. Besides GABA and glutamate, central nervous system (CNS) neurotransmitter systems known to be involved in seizure generation in animal models of epilepsy include norepinephrine, dopamine, serotonin, acetylcholine, histamine 21, 22 and adenosine 23, 24. Presynaptic and postsynaptic adenosine receptor stimulation generates an inhibitory effect on many excitatory pathways such as those that are glutamate mediated. Presynaptic adenosine receptor stimulation may reduce the release of the excitatory neurotransmitter glutamate and postsynaptic adenosine receptor stimulation may directly inhibit excitatory pathways. Adenosine type one (A1) receptor antagonists like theophylline and caffeine can reduce seizure thresholds and prolong seizures by interfering with mechanisms of seizure termination.
Because CNS neuronal interactions are complex, with both direct and indirect drug and chemical effects, and variable pro‐ or anticonvulsant effects depending on concentration, no single mechanism exists for explaining all cases of drug‐induced seizures. For example, in animal models both acetylcholine receptor antagonists 25 and acetylcholinesterase inhibitors 12, 26 can cause seizures. In animal models of nerve agent poisoning there is an early phase in which anticholinergic agents are effective in terminating seizures, followed by a phase in which these drugs are less effective and prolonged epileptiform activity appears to be mediated by stimulation of NMDA receptors by excitatory amino acids 26.
Many drugs and toxins can also cause seizures as a result of indirect effects on brain perfusion, oxygenation or metabolic disturbances. Toxic exposures can reduce brain blood flow by depressing cardiac contractility, vasomotor tone, heart rate or inducing cardiac arrhythmias. Pneumonia due to pulmonary aspiration of gastric contents or direct chemical injury to lung parenchyma can cause hypoxaemia. Other poisons, such as carbon monoxide and cyanide, can interfere with oxygen delivery or cellular oxygen utilization, simulating cellular hypoxia. Electrolyte and metabolic disturbances such as hyponatraemia, hypomagnesaemia and hypoglycaemia can also be an indirect cause of drug‐induced seizures 4.
Some poisons can induce convulsive activity that resembles seizures but does not originate in the cerebral cortex. Glycine is the major inhibiotry neurotransmitter of motor neurons in the spinal cord and brain stem and contributes to the suppression of reflex arcs 27. Strychnine competitively inhibits the action of glycine on postsynaptic receptors 28. Strychnine poisoning results in so‐called ‘spinal seizures’ (involuntary muscle contraction, myoclonus, hyperreflexia and opisthotonus) without loss of consciousness until the victim becomes hypoxic due to impaired ventilation 29. Inhibition of presynaptic glycine release by tetanus toxin produces an identical syndrome 30.
Management of drug‐induced seizures
Initial stabilization and investigation
Most drug‐induced seizures manifest as generalized tonic‐clonic motor activity (grand mal). Convulsive muscle activity, especially if prolonged, can lead to hypoxia, hypercarbia, pulmonary aspiration of gastric contents, lactic acidosis, hyperthermia and rhabdomyolysis. Initial treatment should include airway management with adequate oxygenation and ventilation, stabilization of the blood pressure and heart rate and rapid bedside testing of serum glucose concentration and core body temperature. Hyperthermia is a critical complication of status epilepticus and needs to be treated promptly to prevent death or serious end‐organ damage 17. If initial anticonvulsant therapy does not stop excessive muscle activity and the temperature remains above 40ºC, neuromuscular paralysis should be employed along with external cooling measures. Note that after neuromuscular paralysis, central neuronal seizure activity may persist without peripheral convulsions, and EEG monitoring is recommended to monitor response to additional anticonvulsants. It is critical to monitor and correct abnormalities in serum glucose and electrolytes. Gastric decontamination and enhanced elimination or antidote administration may be appropriate in some patients, and consultation with a medical toxicologist is recommended.
Computed tomography (CT) is usually not necessary in patients with self‐limited and uncomplicated drug‐induced seizures. A prospective observation study showed that patients presenting to the emergency department with altered mental status or headache caused by poisoning or drug overdose had a low likelihood of abnormal findings on head CT scan 31. However, another study found that 6.2% of patients presenting to ED with their first alcohol withdrawal seizure had an intracranial lesion on CT scan 32. It is reasonable to consider a CT scan in patients with evidence of head trauma, focal or repeated seizures, focal neurological deficits or prolonged alteration of consciousness.
Figure 2 provides a stepwise approach to treatment.
Figure 2.
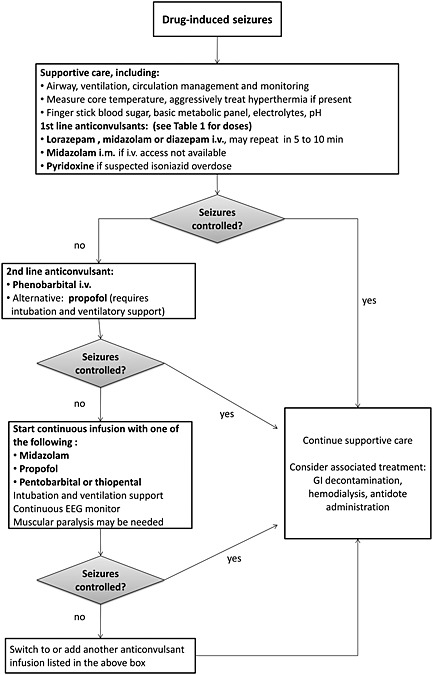
Recommended treatment approach for drug‐induced seizures
Initial anticonvulsant therapy
Benzodiazepines
Benzodiazepines are commonly recommended as the first line anticonvulsant therapy in drug‐induced seizures. Benzodiazepines enhance GABAA activity by increasing the frequency of chloride channel opening, leading to neuronal hyperpolarization 33, 34. If available, intravenous lorazepam is the preferred initial benzodiazepine, although intravenous midazolam is also widely used. We were unable to find any randomized controlled trial or prospective study regarding the effectiveness of benzodiazepines specifically for drug‐induced seizures. However, a Cochrane review and a large randomized controlled trial for status epilepticus of any cause found that intravenous lorazepam was better than intravenous diazepam or intravenous phenytoin alone for cessation of status epilepticus 35, 36. If intravenous access is not readily available, the more water‐soluble midazolam can be given intramuscularly as it is readily absorbed by this route and is at least as safe and effective as intravenous lorazepam for prehospital seizure cessation 37. Benzodiazepines can also be given via the intra‐osseous route. Detailed dosage information for commonly used anticonvulsants is shown in Table 1. Large doses of benzodiazepines can contribute to respiratory depression. In addition, some benzodiazepines are formulated with the diluent propylene glycol, which is metabolized to lactic acid and can cause hyperosmolality and acidosis in patients receiving prolonged high dose infusion 38.
Table 1.
| Drug | Initial/Loading dose | Continuous infusion |
|---|---|---|
| Diazepam | 5–10 mg i.v. (children: 0.2–0.5 mg kg–1) over 2–5 min (max 10 mg/dose); may repeat every 5–20 min. | Note: contains propylene glycol. |
| Lorazepam | 2–4 mg i.v. (children: 0.05–0.1 mg kg–1, max 4 mg/dose); may repeat every 5–10 min (max rate: 2 mg min–1). | Note: contains propylene glycol. |
| Midazolam * | i.v.: 0.05–0.2 mg kg–1 (children: 0.1–0.3 mg kg–1) over 20–30 s (max 10 mg). i.m.*: 0.1–0.2 mg kg–1 (max 10 mg). | 0.05–2 mg kg–1 h–1 titrated to EEG. |
| Pentobarbital † | 5–15 mg kg–1 i.v. (children: 3–15 mg kg–1) no faster than 1 mg kg–1 min–1. | 0.5–5 mg kg–1 h–1, titrated to EEG. |
| Phenobarbital † | 15–20 mg kg–1 i.v. no faster than 1 mg kg–1 min–1. An additional 5–10 mg kg–1 dose may be given 10 min after initial dose. | Note: contains propylene glycol. |
| Propofol †, ‡ | 1–2 mg kg–1 i.v. | 1.5–10 mg kg–1 h–1 titrated to EEG. Note: doses >5 mg kg–1 h–1 over prolonged periods may increase risk of propofol infusion syndrome. |
| Thiopental † | 2–7 mg kg–1 i.v. no faster than 1 mg kg–1 min–1. | 0.5–5 mg kg–1 h–1 titrated to EEG. |
Consider intramuscular route when there is no i.v. access.
May cause deep sedation requiring endotracheal intubation.
Propofol is not recommended for infants and young children. 78
Second line anticonvulsants
Barbiturates
Barbiturates are recommended as the next treatment if benzodiazepines are ineffective 33, 34. Barbiturates also bind to the GABAA complex, prolonging the duration of chloride channel opening and chloride ion influx. In addition, at high concentrations some barbiturates directly open the chloride channel 34. Among various available barbiturates, phenobarbital is usually the drug of choice. Dosages for phenobarbital, pentobarbital and thiopental are shown in Table 1. Phenobarbital is as effective as lorazepam for patients with status epilepticus, although it requires a longer infusion time 36. Barbiturates have been reported effective in treating citutoxin and fluvoxamine‐induced seizures which were not responsive to benzodiazepines and phenytoin 39, 40. Experimental evidence supports that phenobarbital is superior to phenytoin in prevention of theophylline‐induced seizure and death 41, 42. However, no prospective controlled studies have evaluated the effectiveness of barbiturates as a second line anticonvulsant for drug‐induced seizures.
Propofol
Propofol is an intravenous anaesthetic. Its anticonvulsant mechanism of action is not totally understood. It enhances GABA binding to its receptor on the chloride channel and it may also open the chloride channel directly at high concentrations 43, 44. It may have an additive or synergistic effect when used with benzodiazepines or barbiturates 45, 46. Propofol also antagonizes the NMDA receptor, theoretically an advantage where seizures may be secondary to increased NMDA activity 47.
Propofol is usually reserved for patients with refractory status epilepticus 48. It was as effective as thiopental in treatment of refractory status seizures (unselected causes) 49, 50. Refractory convulsive activity caused by amoxapine 51 and star fruit (Averrhoa carambola) 52 was successfully suppressed by propofol infusion (although EEG confirmation was not reported). The dose required for status epilepticus is generally greater than that for sedation and may approach the dose required for induction of general anaesthesia (2–5 mg kg–1) 53. Patients will nearly always require endotracheal intubation and ventilatory support. Propofol has been suggested as a second line anticonvulsant for drug‐induced seizures 53, 54. However, it should be noted that there are no randomized controlled trials or large case series regarding the use of propofol for drug‐induced seizures.
Disadvantages of propofol are its relatively high cost, the potential to cause hypertriglyceridaemia, propofol infusion syndrome and neuroexcitatory events such as opisthotonos, muscle rigidity and choreoathetoid movements 45. Propofol infusion syndrome is a rare but fatal complication, mostly reported in children and adolescents after prolonged high dose propofol infusion. Features include bradycardia, hypotension, rhabdomyolysis and metabolic acidosis. A dose of more than 5 mg kg–1 h–1 over a prolonged period should be avoided in paediatric patients. 55
Pyridoxine (vitamin B6)
Pyridoxine is the drug of choice for seizures due to suspected isoniazid (INH) toxicity, and may also be useful in poisoning by certain hydrazine‐containing Gyromitra mushrooms 56. These toxins interfere with the enzyme that converts glutamate into GABA, reducing GABA levels. Pyridoxine is an essential cofactor in GABA synthesis 57, and it effectively restores GABA synthesis and suppresses seizures within minutes of administration 58. Empiric dosing (5 g i.v. in an adult and 70 mg kg–1 i.v. in a child) should be administered if the INH dose is not known. If the amount ingested is known, then a pyridoxine dose equivalent to the amount of INH ingested (gram for gram) should be given. Synergistic effects between diazepam and pyridoxine have been reported in dogs and rats 59, 60.
Not recommended: phenytoin
Phenytoin binds to and inhibits voltage‐dependent sodium channels, increasing the membrane threshold for depolarization, which inhibits the propagation of seizure activity 61. Although it may be effective in preventing the spread of abnormal electrical activity from an epileptic focus, its role in drug‐induced seizures is questionable. It would not be expected to suppress the characteristically diffuse lowering of seizure threshold or oppose the increase of neuronal excitability induced by drugs or toxins 34. Numerous experimental studies and human case reports have shown that phenytoin does not effectively terminate seizures produced by a variety of substances 62. Moreover, based on animal studies, phenytoin may be harmful when used to treat seizures induced by lidocaine, theophylline or tricyclic antidepressants 41, 63, 64. Phenytoin was also ineffective in preventing recurrent alcohol withdrawal seizures in several prospective, randomized, double‐blind studies 65, 66, 67. We do not recommend its use for drug‐induced seizures.
Other drugs
There are only a few reports of other possible anticonvulsants for drug‐induced seizures. Valproic acid was reported to increase the threshold for theophylline‐induced seizures in an animal study 68 and it has been recommended for the prophylaxis of clozapine‐induced seizures 69. Further studies are needed to verify these findings. During status epilepticus, GABAA receptors become less responsive while NMDA receptors become more responsive 20, 70 and there may be a role for NMDA/glutamate antagonists 71. Ketamine proved useful in at least one case report of refractory status epilepticus 72 and in two cases of tetramine poisoning in which seizures were refractory to benzodiazepines and thiopental 14. Levetiracetam is a novel anticonvulsant with several potential mechanisms of activity. It has been reported effective in patients with status epilepticus 73 and in animal models of nerve agent and pilocarpine neurotoxicity 74, 75. Other potentially effective therapies still in development include adenosine analogues 76 and cannabinoid receptor agonists 77.
Conclusion
While many drug‐induced seizures are brief and uncomplicated, prolonged or recurrent seizure activity may cause serious complications. Benzodiazepines are the first‐line treatment for drug‐induced seizures, with addition of pyridoxine if isoniazid or other hydrazine toxicity is suspected. If benzodiazepines fail to terminate seizures, second‐line agents include barbiturates and propofol. There is no role for phenytoin in the management of drug‐induced seizures. The role of valproic acid, levetiracetam, ketamine, adenosine agonists and other drugs is not established.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Chen, H.‐Y. , Albertson, T. E. , and Olson, K. R. (2016) Treatment of drug‐induced seizures. Br J Clin Pharmacol, 81: 412–419. doi: 10.1111/bcp.12720.
References
- 1. Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology 1993; 43: 483–8. [DOI] [PubMed] [Google Scholar]
- 2. Pesola GR, Avasarala J. Bupropion seizure proportion among new‐onset generalized seizures and drug related seizures presenting to an emergency department. J Emerg Med 2002; 22: 235–9. [DOI] [PubMed] [Google Scholar]
- 3. Alldredge BK, Lowenstein DH, Simon RP. Seizures associated with recreational drug abuse. Neurology 1989; 39: 1037–9. [DOI] [PubMed] [Google Scholar]
- 4. Delanty N, Vaughan CJ, French JA. Medical causes of seizures. Lancet 1998; 352: 383–90. [DOI] [PubMed] [Google Scholar]
- 5. Finkelstein Y, Hutson JR, Freedman SB, Wax P, Brent J, Toxicology Investigators Consortium Case Registry . Drug‐induced seizures in children and adolescents presenting for emergency care: current and emerging trends. Clin Toxicol 2013; 51: 761–6. [DOI] [PubMed] [Google Scholar]
- 6. Olson KR, Kearney TE, Dyer JE, Benowitz NL, Blanc PD. Seizures associated with poisoning and drug overdose. Amer J Emerg Med 1993; 11: 565–8. [DOI] [PubMed] [Google Scholar]
- 7. Thundiyil JG, Kearney TE, Olson KR. Evolving epidemiology of drug‐induced seizures reported to a Poison Control Center System. J Med Toxicol 2007; 3: 15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reichert C, Reichert P, Monnet‐Tschudi F, Kupferschmidt H, Ceschi A, Rauber-Luthy C. Seizures after single‐agent overdose with pharmaceutical drugs: Analysis of cases reported to a poison center. Clin Toxicol 2014; 52: 629–34. [DOI] [PubMed] [Google Scholar]
- 9. Shadnia S, Brent J, Mousavi‐Fatemi K, Hafezi P, Soltaninejad K. Recurrent seizures in tramadol intoxication: implications for therapy based on 100 patients. Basic Clin Pharmacol Toxicol 2012; 111: 133–6. [DOI] [PubMed] [Google Scholar]
- 10. Ryan MN, Isbister GK. Tramadol overdose causes seizures and respiratory depression but serotonin toxicity appears unlikely. Clin Toxicol 2015; 53: 545–50. [DOI] [PubMed] [Google Scholar]
- 11. Mohamed F, Senarathna L, Percy A, Abeyewardene M, Eaglesham G, Cheng R, Azher S, Hittarage A, Dissanayake W, Rezvi Sheriff MH, Davies W, Buckley N, Eddleston M. Human self‐poisoning with the N‐phenylpyrazole insecticide fipronil – a GABAA‐gated chloride channel blocker. J Toxicol Clin Toxicol 2004; 42: 955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waheed S, Sabeen A, Ullah KN. New onset refractory status epilepticus as an unusual presentation of a suspected organophosphate poisoning. Case Reports in Emerg Med 2014; 2014: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts DM, Dissanayake W, Rezvi Sheriff MH, Eddleston M. Refractory status epilepticus following self‐poisoning with the organochlorine pesticide endosulfan. J Clin Neurosci 2004; 11: 760–2. [DOI] [PubMed] [Google Scholar]
- 14. Chau CM, Leung AKH, Tan IKS. Tetramine poisoning. Hong Kong Med J 2005; 11: 511–4. [PubMed] [Google Scholar]
- 15. Moon JM, Chun BJ. Acute endosulfan poisoning: a retrospective study. Hum Exp Toxicol 2009; 28: 309–16. [DOI] [PubMed] [Google Scholar]
- 16. Legriel S, Azoulay E, Resche‐Rigon M, Lemiale V, Mourvillier B, Kouatchet A, Troche G, Wolf M, Galliot R, Dessertaine G, Combaux D, Jacobs F, Beuret P, Megarbane B, Carli P, Lambert Y, Bruneel F, Bedos JP. Functional outcome after convulsive status epilepticus. Crit Care Med 2010; 38: 2295–303. [DOI] [PubMed] [Google Scholar]
- 17. Thundiyil JG, Rowley F, Papa L, Olson KR, Kearney TE. Risk factors for complications of drug‐induced seizures. J Med Toxicol 2011; 7: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buck KJ, Hahner L, Sikela J, Harris RA. Chronic ethanol treatment alters brain levels of gamma‐aminobutyric acid A receptor subunit mRNAs: relationship to genetic differences in ethanol withdrawal seizure severity. J Neurochem 1991; 57: 1452–5. [DOI] [PubMed] [Google Scholar]
- 19. Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma‐aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther 2004; 101: 211–26. [DOI] [PubMed] [Google Scholar]
- 20. Werner FM, Covenas R. Review: Classical neurotransmitters and neuropeptides involved in generalized epilepsy in a multi‐neurotransmitter system: How to improve the antiepileptic effect? Epilepsy Behav 2015; Mar 26 [Epub ahead of print] [PMID: 25819950]. [DOI] [PubMed] [Google Scholar]
- 21. Cerminara C, El‐Malhany N, Roberto D, Lo Castro A, Curatolo P. Seizures induced by desloratadine, a second‐generation antihistamine: clinical observations. Neuropediatrics 2013; 44: 222–4. [DOI] [PubMed] [Google Scholar]
- 22. Rejdak K, Nieoczym D, Czuczwar M, Kis J, Wlaz P, Turski WA. Orphenadrine‐induced convulsive status epilepticus in rats responds to the NMDA antagonist dizocilpine. Pharmacol Rep 2014; 66: 399–403. [DOI] [PubMed] [Google Scholar]
- 23. Avsar E, Empson RM. Adenosine acting via A1 receptors, controls the transition to status epilepticus‐like behaviour in an in vitro model of epilepsy. Neuropharmacol 2004; 47: 427–37. [DOI] [PubMed] [Google Scholar]
- 24. Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist 2005; 11: 25–36. [DOI] [PubMed] [Google Scholar]
- 25. Wygnanski‐Jaffe T, Nucci P, Goldchmidt M, Mezer E. Epilpetic seizures induced by cycloplegic eye drops. Cutan OculToxicol 2014; 33: 103–8. [DOI] [PubMed] [Google Scholar]
- 26. McDonough JH Jr, Shih TM. Neuropharmacological mechanisms of nerve agent‐induced seizure and neuropathology. Neurosci Biobehav Rev 1997; 21: 559–79. [DOI] [PubMed] [Google Scholar]
- 27. Curtis DR, Hosli L, Johnston GAR. A pharmacological study of the depression of spinal neurons by glycine and related amino acids. Exp Brain Res 1968; 6: 1–18. [DOI] [PubMed] [Google Scholar]
- 28. Probst A, Cortes R, Palacois JM. The distribution of glycine receptors in the human brain. A light microscopic autoradiograhic study using [3H] strychnine. Neuroscience 1986; 17: 11–35. [DOI] [PubMed] [Google Scholar]
- 29. Makarovsky I, Markel G, Hoffman A, Schein O, Brosh‐Nissimov T, Tashma Z, Dushnitsky T, Eisenkraft A. Strychnine ‐ a killer from the past. Isr Med Assoc J 2008; 10: 142–5. [PubMed] [Google Scholar]
- 30. Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR. Montecucco C. Tetanus and botulinum‐B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 1992; 359: 832. [DOI] [PubMed] [Google Scholar]
- 31. Patel MM, Tsutaoka BT, Banerji S, Blanc PD, Olson KR. ED utilization of computed tomography in a poisoned population. Am J Emerg Med 2002; 20: 212–7. [DOI] [PubMed] [Google Scholar]
- 32. Earnest MP, Feldman H, Marx JA, Harris JA, Biletch M, Sullivan LP. Intracranial lesions shown by CT scans in 259 cases of first alcohol‐related seizures. Neurology 1988; 38: 1561–5. [DOI] [PubMed] [Google Scholar]
- 33. Rao RB. Neurologic Principles In: Goldfrank's Toxicologic Emergencies, eds Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. New York: McGraw‐Hill, 2014. [Google Scholar]
- 34. Wallace KL. Toxin‐induced seizures In: Critical Care Toxicology: Diagnosis and Management of the Critically Poisoned Patient, eds Brent J, Wallace K, Burkhart K. Philadelphia, Pennsylvania: Elsevier, 2005. [Google Scholar]
- 35. Prasad M, Krishnan PR, Sequeira R, Al‐Roomi K. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev 2014; 9: CD003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, Handforth A, Faught E, Calabrese VP, Uthman BM, Ramsay RE, Mamdani MB. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. New Engl J Med 1998; 339: 792–8. [DOI] [PubMed] [Google Scholar]
- 37. Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, Barsan W, NETT Investigators . Intramuscular versus intravenous therapy for prehospital status epilepticus. New Engl J Med 2012; 366: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cawley MJ. Short‐term lorazepam infusion and concern for propylene glycol toxicity: case report and review. Pharmacother 2001; 21: 1140–4. [DOI] [PubMed] [Google Scholar]
- 39. Starreveld E, Hope E. Cicutoxin poisoning (water hemlock). Neurology 1975; 25: 730–4. [DOI] [PubMed] [Google Scholar]
- 40. Wood DM, Rajalingam Y, Greene SL, Morgan PE, Gerrie D, Jones AL, Dargan PI. Status epilepticus following intentional overdose of fluvoxamine: a case report with serum fluvoxamine concentration. Clin Toxicol 2007; 45: 791. [DOI] [PubMed] [Google Scholar]
- 41. Blake KV, Massey KL, Hendeles L, Nickerson D, Neims A. Relative efficacy of phenytoin and phenobarbital for the prevention of theophylline‐induced seizures in mice. Ann Emerg Med 1988; 17: 1024–8. [DOI] [PubMed] [Google Scholar]
- 42. Goldberg MJ, Spector R, Miller G. Phenobarbital improves survival in theophylline‐intoxicated rabbits. J Toxicol Clin Toxicol 1986; 24: 203–11. [DOI] [PubMed] [Google Scholar]
- 43. Bali M, Akabas MH. Defining the propofol binding site location on the GABA‐A receptor. Molecular Pharmacol 2004; 65: 68–76. [DOI] [PubMed] [Google Scholar]
- 44. Curry SC, O'Connor AD, Graeme KA, Mills KC, Sholnik AB. Neurotransmitters and Neuromodulators In: Goldfrank's Toxicologic Emergencies, eds Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. New York: McGraw‐Hill, 2014. [Google Scholar]
- 45. Brown LA, Levin GM. Role of propofol in refractory status epilepticus. Ann Pharmacother 1998; 32: 1053–9. [DOI] [PubMed] [Google Scholar]
- 46. Trapani G, Latrofa A, Franco M, Altomare C, Sanna E, Usala M, Biggio G, Liso G. Propofol analogues. Synthesis, relationships between structure and affinity at GABAA receptor in rat brain, and differential electrophysiological profile at recombinant human GABAA receptors. J Medicinal Chem 1998; 41: 1846–54. [DOI] [PubMed] [Google Scholar]
- 47. Orser BA, Bertlik M, Wang LY, MacDonald JF. Inhibition by propofol (2,6 di‐isopropylphenol) of the N‐methyl‐D‐aspartate subtype of glutamate receptor in cultured hippocampal neurones. Br J Pharmacol 1995; 116: 1761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, Laroche SM, Riviello JJ Jr, Shutter L, Sperling MR, Treiman DM, Vespa PM, Neurocritical Care Society Status Epilepticus Guideline Writing Committee . Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012; 17: 3–23. [DOI] [PubMed] [Google Scholar]
- 49. Prabhakar H, Bindra A, Singh GP, Kalaivani M. Propofol versus thiopental sodium for the treatment of refractory status epilepticus (Review). Evid Based Child Health 2013; 8: 1488–508. [DOI] [PubMed] [Google Scholar]
- 50. Rossetti AO, Reichhart MD, Schaller MD, Despland PA, Bogousslavsky J. Propofol treatment of refractory status epilepticus: a study of 31 episodes. Epilepsia 2004; 45: 757–63. [DOI] [PubMed] [Google Scholar]
- 51. Merigian KS, Browning RG, Leeper KV. Successful treatment of amoxapine‐induced refractory status epilepticus with propofol (diprivan). Acad Emerg Med 1995; 2: 128–33. [DOI] [PubMed] [Google Scholar]
- 52. Wang YC, Liu BM, Supernaw RB, Lu YH, Lee PY. Management of star fruit‐induced neurotoxicity and seizures in a patient with chronic renal failure. Pharmacother 2006; 26: 143–6. [DOI] [PubMed] [Google Scholar]
- 53. Sharma AN, Hoffman RJ. Toxin‐related seizures. Emerg Med Clin North Am 2011; 29: 125–39. [DOI] [PubMed] [Google Scholar]
- 54. Seizures. In POISINDEX(R) Managements. Greenwood Village, CO: Thomson Micromedex. Updated periodically. Accessed in 2015.
- 55. Fudickar A, Bein B. Propofol infusion syndrome: update of clinical manifestation and pathophysiology. Minerva Anestesiol 2009; 75: 339–44. [PubMed] [Google Scholar]
- 56. Berger KJ, Guss DA. Mycotoxins revisitied: Part II. J Emerg Med 2005; 28: 175–83. [DOI] [PubMed] [Google Scholar]
- 57. Lheureux P, Penaloza A, Gris M. Pyridoxine in clinical toxicology: a review. Eur J Emerg Med 2005; 12: 78–85. [DOI] [PubMed] [Google Scholar]
- 58. Brent J, Vo N, Kulig K, Rumack BH. Reversal of prolonged isoniazid‐induced coma by pyridoxine. Arch Internal Med 1990; 150: 1751–3. [PubMed] [Google Scholar]
- 59. Chin L, Sievers ML, Herrier RN, Picchioni AL. Potentiation of pyridoxine by depressants and anticonvulsants in the treatment of acute isoniazid intoxication in dogs. Toxicol Appl Pharmacol 1981; 58: 504–9. [DOI] [PubMed] [Google Scholar]
- 60. Chin L, Sievers ML, Laird HE, Herrier RN, Picchioni AL. Evaluation of diazepam and pyridoxine as antidotes to isoniazid intoxication in rats and dogs. Toxicol Appl Pharmacol 1978; 45: 713–22. [DOI] [PubMed] [Google Scholar]
- 61. Tunnicliff G. Basis of the antiseizure action of phenytoin. Gen Pharmacol 1996; 27: 1091–7. [DOI] [PubMed] [Google Scholar]
- 62. Shah AS, Eddleston M. Should phenytoin or barbiturates be used as second‐line anticonvulsant therapy for toxicological seizures? Clin Toxicol 2010; 48: 800–5. [DOI] [PubMed] [Google Scholar]
- 63. Callaham M, Schumaker H, Pentel P. Phenytoin prophylaxis of cardiotoxicity in experimental amitriptyline poisoning. J Pharmacol Exp Ther 1988; 245: 216–20. [PubMed] [Google Scholar]
- 64. Sawaki K, Ohno K, Miyamoto K, Hirai S, Yazaki K, Kawaguchi M. Effects of anticonvulsants on local anaesthetic‐induced neurotoxicity in rats. Pharmacol Toxicol 2000; 86: 59–62. [DOI] [PubMed] [Google Scholar]
- 65. Alldredge BK, Lowenstein DH, Simon RP. Placebo‐controlled trial of intravenous diphenylhydantoin for short‐term treatment of alcohol withdrawal seizures. Am J Med 1989; 87: 645–8. [DOI] [PubMed] [Google Scholar]
- 66. Chance JF. Emergency department treatment of alcohol withdrawal seizures with phenytoin. Ann Emerg Med 1991; 20: 520–2. [DOI] [PubMed] [Google Scholar]
- 67. Rathlev NK, D'Onofrio G, Fish SS, Harrison PM, Bernstein E, Hossack RW, Pickens L. The lack of efficacy of phenytoin in the prevention of recurrent alcohol‐related seizures. Ann Emerg Med 1994; 23: 513–8. [DOI] [PubMed] [Google Scholar]
- 68. Hoffman A, Pinto E, Gilhar D. Effect of pretreatment with anticonvulsants on theophylline‐induced seizures in the rat. J Crit Care 1993; 8: 198–202. [DOI] [PubMed] [Google Scholar]
- 69. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs 2015; 29: 101–11. [DOI] [PubMed] [Google Scholar]
- 70. Zeiler FA. Early Use of the NMDA receptor antagonist ketamine in refractory and superrefractory status epilepticus. Crit Care Res Pract 2015; Jan 12 2015: 831260. [PMID: 25649724]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shakarjian MP, Ali MS, Veliskova J, Stanton PK, Heck DE, Velisek L. Combined diazepam and MK‐801 therapy provides synergistic protection from tetramethylenedisulfotetramine‐induced tonic‐clonic seizures and lethality in mice. Neurotoxicology 2015; March 14 [Epub ahead of print] [PMID: 25783504]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kramer AH. Early ketamine to treat refractory status epilepticus. Neurocrit Care 2012; 16: 299–305. [DOI] [PubMed] [Google Scholar]
- 73. Deshpande LS, DeLorenzo RJ. Mechanisms of levetiracetam in the control of status epilpeticus and epilepsy. Front Neurol 2014; 5: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Oliveira AA, Nogueira CRA, Nascimento VS, Aguiar LMV, Freitas RM, Sousa FCF, Viana GSB, Fonteles MMF. Evaluation of levetiracetam effects on pilocarpine‐induced seizures: Cholinergic muscarinic system involvement. Neurosci Lett 2005; 385: 184–8. [DOI] [PubMed] [Google Scholar]
- 75. Myhrer T, Enger S, Jonassen M, Aas P. Enhanced efficacy of anticonvulsants when combined with levetiracetam in soman‐exposed rats. Neurotoxicol 2011; 32: 923–30. [DOI] [PubMed] [Google Scholar]
- 76. Li M, Kang R, Shi J, Liu G, Zhang J. Anticonvulsant activity of B2, an adenosine analog, on chemical convulsant‐induced seizures. PLoS One 2013; 8: e67060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Andres‐Mach M, Zolkowska D, Barcicka‐Klosowska B, Haratym‐Maj A, Florek‐Luszczki M, Luszczki JJ. Effect of ACEA ‐ a selective cannabinoid CB1 receptor agonist on the protective action of different antiepileptic drugs in the mouse pentylenetetrazole‐induced seizure model. Prog Neuropsychopharmacol Biol Psychiatry 2012; 39: 301–9. [DOI] [PubMed] [Google Scholar]
- 78. Loddenkemper T, Goodkin HP. Treatment of pediatric status epilepticus. Curr Treat Options Neurol 2011; 13: 560–73. [DOI] [PubMed] [Google Scholar]
- 79. Goralka JM. Propofol In: Poisoning & Drug Overdose, 6th edn, ed Olson KR. New York: McGraw‐Hill, 2012. [Google Scholar]


