Abstract
Sometimes mistakenly characterized as a ‘universal antidote,’ activated charcoal (AC) is the most frequently employed method of gastrointestinal decontamination in the developed world. Typically administered as a single dose (SDAC), its tremendous surface area permits the binding of many drugs and toxins in the gastrointestinal lumen, reducing their systemic absorption. Like other decontamination procedures, the utility of SDAC attenuates with time, and, although generally safe, it is not free of risk. A large body of evidence demonstrates that SDAC can reduce the absorption of drugs and xenobiotics but most such studies involve volunteers and have little generalizability to clinical practice. Few rigorous clinical trials of SDAC have been conducted, and none validate or refute its utility in those patients who are intuitively most likely to benefit. Over the past decade, a growing body of observational data have demonstrated that SDAC can elicit substantial reductions in drug absorption in acutely poisoned patients. The challenge for clinicians rests in differentiating those patients most likely to benefit from SDAC from those in whom meaningful improvement is doubtful. This is often a difficult determination not well suited to an algorithmic approach. The present narrative review summarizes the data supporting the benefits and harms of SDAC, and offers pragmatic suggestions for clinical practice.
Keywords: activated charcoal, gastrointestinal decontamination, overdose, poisoning
Background
Charcoal for medicinal use is created by the controlled pyrolytic decomposition of carbon‐based compounds, such as coconut shells or peat 1. Thereafter, ‘activation’ with gases at high temperature removes previously adsorbed substances and further reduces particle size, resulting in an exceptionally porous final product 2. Indeed, some ‘superactivated’ charcoal preparations have a surface area of up to 3500 m2 g–1, or about 175 000 m2 per 50 g bottle 1. (For perspective, the area of a large football pitch is about 10 000 m2.) This allows the adsorption of drugs and toxins through weak intermolecular forces, with non‐ionized, organic compounds binding more avidly than dissociated, inorganic ones 1.
The first reported use of charcoal as an antidote occurred in 1811, when the French chemist Michel Bertrand reportedly ingested charcoal with 5 g of arsenic trioxide 1, 3. In 1852, Touéry showed no ill effects after consuming a large dose of strychnine with charcoal before sceptical colleagues of the French Academy of Medicine 4. Dramatic anecdotes aside, SDAC was infrequently used in the management of acute poisoning until 1963, when a review article in the Journal of Pediatrics concluded that: ‘This agent, presently somewhat neglected, has a wide spectrum of activity and when properly used is probably the most valuable single agent we possess’ 5. In the 1970s and 1980s, SDAC was a common element of gastrointestinal (GI) decontamination after acute poisoning, as were gastric emptying manoeuvres such as lavage and ipecac‐induced emesis.
Unlike gastric lavage and ipecac, SDAC remains a common element of therapy for acutely poisoned patients, although its use has decreased substantially in recent years. For example, in 1999, poison centres in the United States recommended SDAC more than 136 000 times, compared to only about 50 000 times in 2013. The declining use of SDAC reflects an appreciation of its limitations and potential risks, along with waning enthusiasm for GI decontamination more generally.
It bears mention that decontamination with SDAC is conceptually different from the use of multiple‐dose activated charcoal (MDAC), a less commonly deployed intervention involving the administration of multiple (typically, two to six) smaller doses of AC, with the goal of enhancing the total body clearance of a limited number of compounds such as dapsone 6, carbamazepine 7, 8, phenobarbital 9, 10 and methylxanthines 11. As such, the goal of MDAC is enhanced toxin elimination rather than reduced absorption per se. The balance of the present review focuses on the use of SDAC following acute overdose.
Effectiveness
Several lines of research demonstrate that SDAC effectively binds to a diverse range of toxins.
In vitro and animal studies
Dozens of in vitro simulations and animal studies convincingly show that SDAC binds to a wide range of drugs to varying degrees 1, 12. Importantly, some compounds do not bind to SDAC, even under ideal conditions. Metals (notably including salts of iron and lithium), hydrocarbons and caustics are common exposures not suited to SDAC on this basis. Although toxic alcohols and cyanide are sometimes also listed as substances not adsorbed to AC, this is incorrect. Decker and colleagues 13 demonstrated that SDAC does indeed bind to both methanol and ethylene glycol; this is of little clinical utility, however, because the amounts of these typically ingested yield an unfavourable stoichiometric ratio of charcoal:toxin (discussed below). Also, although AC adsorbs cyanide less avidly than many drugs, the maximum binding ratio (35 mg of cyanide per gram of AC) 3 might have clinical utility if SDAC is given shortly after ingestion, as a standard 50 g dose of SDAC could theoretically bind more than a gram of cyanide. Indeed, in an animal model of massive cyanide poisoning, 14 of 26 rats given AC with potassium cyanide (35–40 mg kg–1) displayed no signs of cyanide toxicity, whereas all rats not given AC died 14.
Many animal studies generally corroborate the findings of in vitro studies but they cannot be freely generalized to humans because of interspecies differences in GI morphology, function and other aspects involving pharmacokinetics 12.
Studies in human volunteers
The most recent iteration of the American Academy of Clinical Toxicology (AACT)/European Association of Poison Centres and Clinical Toxicologists (EAPCCT) joint position paper on SDAC observed that 46 drugs have been the subject of 122 evaluations of the effect of SDAC in healthy volunteers 12. Most of these are small crossover studies examining the extent to which SDAC influences the area under the curve (AUC) of drug concentration vs. time.
These studies employed varying doses of SDAC (0.5–100 g) at intervals of up to 6 h following ingestion of paracetamol 15, 16, 17, acetylsalicylic acid and other anti‐inflammatory agents 18, 19, 20, 21, valproate 22 and calcium channel blockers 23, 24, 25, 26, among others. Of 122 total comparisons, 84 (69%) involved the administration of SDAC within 5 min of drug ingestion, demonstrating a mean reduction in absorption of 74%. Among studies in which the dose of AC was at least 50 g, the average reduction in systemic drug absorption was 47.3% at 30 min, 40.1% at 60 min and 16.5% at 120 min 12.
In addition to recruiting medically well subjects, an important limitation of volunteer studies is that they involve subtoxic drug exposures. Although ethically inescapable, this seriously undermines their applicability to acutely poisoned patients who ingest much larger doses, often of multiple drugs, and who present with complications that might unfavourably influence the safety of charcoal, including altered mental status and airway compromise.
Studies in poisoned patients
Only two randomized trials have directly examined the efficacy of AC in acutely poisoned patients, demonstrating no clear benefit from the intervention. In a single‐centre study, Cooper et al. randomized 327 acutely poisoned patients to receive either 50 g of SDAC or no decontamination within 12 h of ingestion 27. In the primary analysis, they found no difference in the length of hospital stay between the SDAC and control arms (6.8 h vs. 5.5 h, respectively; P = 0.11), even in the subset of patients treated within 2 h of ingestion. However, the ability of this study to detect a benefit of SDAC might have been limited by the enrolment of patients destined to do well without AC (benzodiazepines and paracetamol represented more than half of all poisonings), and by the exclusion of a small number of patients presenting within 1 h of a potentially life‐threatening ingestion. The latter group represents those most likely to benefit from the intervention.
In the largest study to date, Eddleston and colleagues randomized 4632 patients at three Sri Lankan hospitals to one of three treatment arms: SDAC (50 g) once; every 4 h for six doses; or no charcoal 28. In the primary analysis, no mortality difference was evident among groups, and secondary analyses revealed no differences in the need for intubation or the risk of seizures. Limitations of this study include a median delay from ingestion to treatment of more than 4 h in all groups and uncertain generalizability to prescription drugs, as fully half of study subjects ingested pesticides, and more than a third ingested yellow oleander.
Although a major advance over previous decontamination trials, the studies of Cooper et al. and Eddleston et al. should not be overinterpreted. In spite of their negative findings, it must be emphasized that no randomized controlled trial has evaluated the efficacy of SDAC when given promptly (within 1–2 h) following ingestions likely to result in serious toxicity or death. Such a trial is impracticable, however, given the lack of equipoise in such subjects and the associated ethical barriers to a control group. Put differently, we should not anticipate that randomized trials will ever definitively address the utility of SDAC in those poisoned patients most likely to benefit from it.
The effect of SDAC following overdose has recently been the subject of several population pharmacokinetic and pharmacodynamic modelling studies. This is a promising avenue of research because it leverages detailed, prospectively collected data derived from ‘real‐world’ overdose patients. Moreover, it accounts for important uncertainties (amount ingested, co‐ingestants, timing, etc.) frequently encountered in clinical practice. Friberg and colleagues 29 evaluated 63 instances of citalopram overdose in 53 patients [median dose 270 (range 20–1700) mg], including 16 instances in which SDAC (50 g) was administered within 4 h of ingestion. The authors estimated that SDAC reduced citalopram bioavailability by 22% and increased total body clearance by 72%. Comparable studies estimate that early administration of SDAC following overdose reduces the absorption of quetiapine by 35% 30, sertraline by 27% 31, escitalopram by 31% 32 and venlafaxine by 29% 33. It is not difficult to envision scenarios in which effects of this magnitude could favourably influence clinically important outcomes, perhaps even mortality.
Indeed, thematically similar studies suggest that SDAC can be associated not only with altered pharmacokinetics, but also with improvements in clinical outcomes. For example, in patients who have taken large overdoses of citalopram, SDAC reduced the chance of high‐risk QT‐RR combinations by approximately 60% 34, and, when given within 2 h of promethazine overdose, reduced the risk of delirium by more than half 35. In another study involving paracetamol, SDAC reduced the likelihood of a concentration above the N‐acetylcysteine treatment line on the Rumack–Matthew nomogram 36. By design, these studies yield insights into the utility of SDAC in real‐world practice not obtainable by other means. Despite their observational nature, they provide what is arguably the most clinically relevant evidence supporting the use of SDAC after acute overdose.
Harms
Although generally safe, SDAC is not free of risk 37. The most widely cited concern associated with SDAC is pulmonary aspiration, although the risk of this complication is low. Indeed, in a cohort study of more than 4500 overdose patients, 71 (1.6%) developed aspiration pneumonitis. Emesis, seizure and altered mental status were among the independent predictors of aspiration but the administration of AC was not 38.
Nevertheless, aspiration following SDAC is well documented in isolated case reports, some of them dramatic 37, 39, 40, 41. In one case, a young child died following aspiration of SDAC given to treat the ingestion of an unknown liquid from a chemistry set 37. In another, a 34‐year‐old woman was given SDAC by nasogastric tube after a mixed drug overdose while intubated. The immediate appearance of AC in the endotracheal tube was followed by the development of acute respiratory distress syndrome and persistent parenchymal disease 41. This case reflects suboptimal airway management but also illustrates the rare potential for harm resulting from aspiration of AC. Chronic lung disease 39, obstructive laryngitis with glottic oedema 42, 43, granulomatous lung mass 43, charcoal empyema 40 and bronchiolitis obliterans 44 have also been reported as pulmonary complications of SDAC.
GI complications represent another potential risk of SDAC administration. Published reports describe bowel obstruction 45, 46, bezoars 47, 48 and stercoliths 49 after SDAC use. Patients with pre‐existing motility disorders, those receiving opioids or antimuscarinic drugs, and those treated with MDAC might be at greater risk but, on balance, the likelihood of GI complications following SDAC therapy is low.
Other considerations
Tolerability
Most patients tolerate SDAC well, in spite of its gritty, unpalatable taste. This can be a particular problem for children, for whom palatability can be improved by administration with cola or chocolate milk 50, 51. In a substudy of the largest randomized trial to date, adults allocated to treatment with AC consumed 83% of their first dose, on average, and 27% subsequently vomited a portion of the dose 52. This might partly reflect the emetogenic nature of the ingestions in that study as earlier studies suggested that the incidence of charcoal‐associated emesis is considerably lower, on the order of 6–7% 53, 54.
Timing of SDAC
As with all decontamination methods, the balance of benefit vs. risk becomes less favourable with time. Figure 1 shows the reduction in AUC observed when SDAC was given at various lag times after ingestion in volunteer studies. The most recent AACT/EAPCCT position paper 12 states that: ‘Although volunteer studies demonstrate that the reduction of drug absorption decreases to values of questionable clinical importance when charcoal is administered at times greater than 1 h, the potential for benefit after 1 h cannot be excluded’. This statement is problematic because volunteer studies permit no inferences regarding clinical importance, and the casual reader might conclude that SDAC has little utility more than 1 h after ingestion. Common sense and the population studies cited earlier suggest otherwise. Although most toxicologists recommend SDAC at 1 h following a significant ingestion 55, 56, in practice most acknowledge the potential benefits of later administration in certain settings.
Figure 1.
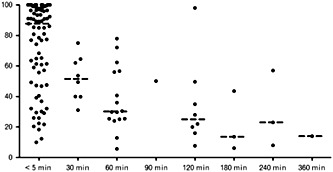
Summary of human volunteer studies of activated charcoal. Figure shows reduction in area under the curve (AUC) vs. time to single‐dose activated charcoal. Included studies are those cited in the 2005 American Academy of Clinical Toxicology/European Association of Poison Centres and Clinical Toxicologists Position Statement. Reproduced from Isbister et al. 58 with permission
It is unsurprising that delays to administration render SDAC less effective, but factors other than timing must be taken into account. Chief among these are the expected toxicity of the ingestion and the availability of other specific therapies. For example, a large overdose of colchicine or cyclic antidepressant presents a very high risk of morbidity and mortality, with limited adjunctive therapies to offer. In such cases, there is little to be lost in the administration of SDAC up to 4 h following ingestion. By contrast, for a patient with a large opioid overdose, naloxone and ventilatory support make late administration of SDAC at 4 h less easy to justify.
Other factors to consider include the co‐ingestion of drugs that slow gastric emptying, including antimuscarinics or opioids as well as the presence of food. A case can be made for administration of SDAC within 2 h (and perhaps longer) following ingestion, when the clinician has reason to believe that a clinically important amount of drug remains in the GI tract and that its adsorption to charcoal might favourably influence the patient's clinical course. Although it is not possible to specify with certainty when SDAC represents an appropriate intervention, Table 1 lists factors that cumulatively increase its appropriateness.
Table 1.
Factors that cumulatively increase the appropriateness of single‐dose activated charcoal
| – Serious toxicity anticipated |
| – Recent ingestion |
| – Alert, cooperative patient |
| – Intact airway |
| – Lack of a specific antidote |
| – Favourable stoichiometry (mass of charcoal:mass of drug >40:1) |
| – Ingestion of a modified‐release product |
| – Substance known to adsorb to activated charcoal |
| – Absence of ileus or intestinal obstruction |
Stoichiometry
Because SDAC adsorbs drugs and other xenobiotics, it follows that a stoichiometric relationship must exist under which a higher ratio of charcoal:drug will more effectively inhibit systemic absorption. Put differently, one might expect that 50 g of AC would more effectively prevent the absorption of sixty 50 mg tablets of amitriptyline (3 g of drug) than of sixty 500 mg tablets of valproic acid (30 g of drug), all other factors being equal. Previous studies bear this out 57. It is commonly taught that an AC:drug ratio of 10:1 is ideal but Olson suggests that a ratio of perhaps 40:1 might be superior 1. Clearly, neither is possible in patients with ‘high‐mass ingestions’ – i.e, those involving many tablets of valproic acid, acetylsalicylic acid, verapamil or metformin, for example. Common sense dictates that administering more than 50 g of AC might be worthwhile in such cases but this is speculative and often impractical.
Modified‐release products
Many drugs exist as modified‐release formulations in which the release of medication is retarded by, for example, an enteric coating, an osmotic pump delivery system or incorporation into a matrix. Examples frequently encountered in acute poisoning include acetylsalicylic acid, calcium channel blockers, methylxanthines, long‐acting opioids, paracetamol and valproic acid. In such instances, clinical toxicity can be both delayed and sustained. It follows that these cases might be more amenable to treatment with SDAC, and also that administration of SDAC several hours after ingestion might be advisable when significant toxicity is anticipated. The potential benefit of delayed SDAC in a modified‐release overdose was illustrated, for example, in a volunteer study in which SDAC given 4 h after ingestion of 2.9 g of enteric‐coated acetylsalicylic acid reduced absorption by 57% 18.
Summary
In patients with acute overdose, SDAC remains an important therapeutic option, and recent studies involving real‐world patients have reinforced the long‐held belief that it can significantly reduce systemic drug absorption when given shortly after overdose. Although generally well tolerated, SDAC is rarely associated with significant complications, most notably pulmonary aspiration. The decision to use it is not well suited to an algorithmic approach but rather requires clinical judgement involving a global assessment of several patient‐specific factors. As with all medical interventions, SDAC should be given only to patients in whom its use can reasonably be expected to reduce morbidity (or perhaps even mortality) in a manner that exceeds its risks.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Juurlink, D. N. (2016) Activated charcoal for acute overdose: a reappraisal. Br J Clin Pharmacol, 81: 482–487. doi: 10.1111/bcp.12793.
References
- 1. Olson KR. Activated charcoal for acute poisoning: one toxicologist's journey. J Med Toxicol 2010; 6: 190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seger D. Single‐dose activated charcoal‐backup and reassess. J Toxicol Clin Toxicol 2004; 42: 101–10. [DOI] [PubMed] [Google Scholar]
- 3. Cooney DO. Activated Charcoal in Medical Applications (2nd edn). New York, NY: Marcel Dekker, 1995. [Google Scholar]
- 4. Ferner RE. Our poisoned patients. QJM 2001; 94: 117–20. [DOI] [PubMed] [Google Scholar]
- 5. Holt LE Jr, Holz PH. The black bottle. A consideration of the role of charcoal in the treatment of poisoning in children. J Pediatr 1963; 63: 306–14. [DOI] [PubMed] [Google Scholar]
- 6. Neuvonen PJ, Elonen E, Mattila MJ. Oral activated charcoal and dapsone elimination. Clin Pharmacol Ther 1980; 27: 823–7. [DOI] [PubMed] [Google Scholar]
- 7. Wason S, Baker RC, Carolan P, Seigel R, Druckenbrod RW. Carbamazepine overdose – the effects of multiple dose activated charcoal. J Toxicol Clin Toxicol 1992; 30: 39–48. [DOI] [PubMed] [Google Scholar]
- 8. Brahmi N, Kouraichi N, Thabet H, Amamou M. Influence of activated charcoal on the pharmacokinetics and the clinical features of carbamazepine poisoning. Am J Emerg Med 2006; 24: 440–3. [DOI] [PubMed] [Google Scholar]
- 9. Berg MJ, Rose JQ, Wurster DE, Rahman S, Fincham RW, Schottelius DD. Effect of charcoal and sorbitol–charcoal suspension on the elimination of intravenous phenobarbital. Ther Drug Monit 1987; 9: 41–7. [DOI] [PubMed] [Google Scholar]
- 10. Frenia ML, Schauben JL, Wears RL, Karlix JL, Tucker CA, Kunisaki TA. Multiple‐dose activated charcoal compared to urinary alkalinization for the enhancement of phenobarbital elimination. J Toxicol Clin Toxicol 1996; 34: 169–75. [DOI] [PubMed] [Google Scholar]
- 11. Position statement and practice guidelines on the use of multi‐dose activated charcoal in the treatment of acute poisoning. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol 1999; 37: 731–51. [DOI] [PubMed] [Google Scholar]
- 12. Chyka PA, Seger D, Krenzelok EP, Vale JA. Position paper: Single‐dose activated charcoal. Clin Toxicol 2005; 43: 61–87. [DOI] [PubMed] [Google Scholar]
- 13. Decker WJ, Corby DG, Hilburn RE, Lynch RE. Adsorption of solvents by activated charcoal, polymers, and mineral sorbents. Vet Hum Toxicol 1981; 23 (Suppl. 1): 44–6. [PubMed] [Google Scholar]
- 14. Lambert RJ, Kindler BL, Schaeffer DJ. The efficacy of superactivated charcoal in treating rats exposed to a lethal oral dose of potassium cyanide. Ann Emerg Med 1988; 17: 595–8. [DOI] [PubMed] [Google Scholar]
- 15. Christophersen AB, Levin D, Hoegberg LC, Angelo HR, Kampmann JP. Activated charcoal alone or after gastric lavage: a simulated large paracetamol intoxication. Br J Clin Pharmacol 2002; 53: 312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Green R, Grierson R, Sitar DS, Tenenbein M. How long after drug ingestion is activated charcoal still effective? J Toxicol Clin Toxicol 2001; 39: 601–5. [DOI] [PubMed] [Google Scholar]
- 17. Green R, Sitar DS, Tenenbein M. Effect of anticholinergic drugs on the efficacy of activated charcoal. J Toxicol Clin Toxicol 2004; 42: 267–72. [DOI] [PubMed] [Google Scholar]
- 18. Kirshenbaum LA, Mathews SC, Sitar DS, Tenenbein M. Whole‐bowel irrigation versus activated charcoal in sorbitol for the ingestion of modified‐release pharmaceuticals. Clin Pharmacol Ther 1989; 46: 264–71. [DOI] [PubMed] [Google Scholar]
- 19. Dawling S, Chand S, Braithwaite RA, Crome P. In vitro and in vivo evaluation of two preparations of activated charcoal as adsorbents of aspirin. Hum Toxicol 1983; 2: 211–6. [DOI] [PubMed] [Google Scholar]
- 20. De NR. Antidotal efficacy of activated charcoal in presence of jam, starch and milk. Am J Hosp Pharm 1976; 33: 965–6. [PubMed] [Google Scholar]
- 21. Decker WJ, Shpall RA, Corby DG, Combs HF, Payne CE. Inhibition of aspirin absorption by activated charcoal and apomorphine. Clin Pharmacol Ther 1969; 10: 710–3. [DOI] [PubMed] [Google Scholar]
- 22. al Shareef A, Buss DC, Shetty HG, Ali N, Routledge PA. The effect of repeated‐dose activated charcoal on the pharmacokinetics of sodium valproate in healthy volunteers. Br J Clin Pharmacol 1997; 43: 109–11. [DOI] [PubMed] [Google Scholar]
- 23. Tanaka C, Ohtani H, Tsujimoto M, Ohdo S, Taniguchi M, Mizooku Y, Saitoh Y, Kimura M, Uchimaru H, Irie S, Sawada Y. Effects of dosing interval on the pharmacokinetic interaction between oral small spherical activated charcoal and amlodipine in humans. J Clin Pharmacol 2007; 47: 904–8. [DOI] [PubMed] [Google Scholar]
- 24. Laine K, Kivisto KT, Neuvonen PJ. Effect of delayed administration of activated charcoal on the absorption of conventional and slow‐release verapamil. J Toxicol Clin Toxicol 1997; 35: 263–8. [DOI] [PubMed] [Google Scholar]
- 25. Lapatto‐Reiniluoto O, Kivisto KT, Neuvonen PJ. Efficacy of activated charcoal versus gastric lavage half an hour after ingestion of moclobemide, temazepam, and verapamil. Eur J Clin Pharmacol 2000; 56: 285–8. [DOI] [PubMed] [Google Scholar]
- 26. Lapatto‐Reiniluoto O, Kivisto KT, Neuvonen PJ. Gastric decontamination performed 5 min after the ingestion of temazepam, verapamil and moclobemide: charcoal is superior to lavage. Br J Clin Pharmacol 2000; 49: 274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cooper GM, Le Couteur DG, Richardson D, Buckley NA. A randomized clinical trial of activated charcoal for the routine management of oral drug overdose. QJM 2005; 98: 655–60. [DOI] [PubMed] [Google Scholar]
- 28. Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Dissanayake W, Hittarage A, Azher S, Jeganathan K, Jayamanne S, Sheriff MR, Warrell DA; Ox‐Col Poisoning Study collaborators . Multiple‐dose activated charcoal in acute self‐poisoning: a randomised controlled trial. Lancet 2008; 371: 579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friberg LE, Isbister GK, Hackett LP, Duffull SB. The population pharmacokinetics of citalopram after deliberate self‐poisoning: a Bayesian approach. J Pharmacokinet Pharmacodyn 2005; 32: 571–605. [DOI] [PubMed] [Google Scholar]
- 30. Isbister GK, Friberg LE, Hackett LP, Duffull SB. Pharmacokinetics of quetiapine in overdose and the effect of activated charcoal. Clin Pharmacol Ther 2007; 81: 821–7. [DOI] [PubMed] [Google Scholar]
- 31. Cooper JM, Duffull SB, Saiao AS, Isbister GK. The pharmacokinetics of sertraline in overdose and the effect of activated charcoal. Br J Clin Pharmacol 2015; 79: 307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Gorp F, Duffull S, Hackett LP, Isbister GK. Population pharmacokinetics and pharmacodynamics of escitalopram in overdose and the effect of activated charcoal. Br J Clin Pharmacol 2012; 73: 402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar VV, Oscarsson S, Friberg LE, Isbister GK, Hackett LP, Duffull SB. The effect of decontamination procedures on the pharmacokinetics of venlafaxine in overdose. Clin Pharmacol Ther 2009; 86: 403–10. [DOI] [PubMed] [Google Scholar]
- 34. Friberg LE, Isbister GK, Duffull SB. Pharmacokinetic–pharmacodynamic modelling of QT interval prolongation following citalopram overdoses. Br J Clin Pharmacol 2006; 61: 177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Page CB, Duffull SB, Whyte IM, Isbister GK. Promethazine overdose: clinical effects, predicting delirium and the effect of charcoal. QJM 2009; 102: 123–31. [DOI] [PubMed] [Google Scholar]
- 36. Duffull SB, Isbister GK. Predicting the requirement for N‐acetylcysteine in paracetamol poisoning from reported dose. Clin Toxicol 2013; 51: 772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tomaszewski C Activated charcoal–treatment or toxin? J Toxicol Clin Toxicol 1999; 37: 17–8. [DOI] [PubMed] [Google Scholar]
- 38. Isbister GK, Downes F, Sibbritt D, Dawson AH, Whyte IM. Aspiration pneumonitis in an overdose population: frequency, predictors, and outcomes. Crit Care Med 2004; 32: 88–93. [DOI] [PubMed] [Google Scholar]
- 39. Graff GR, Stark J, Berkenbosch JW, Holcomb GW III, Garola RE. Chronic lung disease after activated charcoal aspiration. Pediatrics 2002; 109: 959–61. [DOI] [PubMed] [Google Scholar]
- 40. Justiniani FR, Hippalgaonkar R, Martinez LO. Charcoal‐containing empyema complicating treatment for overdose. Chest 1985; 87: 404–5. [DOI] [PubMed] [Google Scholar]
- 41. De WA, Snoeckx A, Germonpre P, Jorens PG. Rapid‐onset adult respiratory distress syndrome after activated charcoal aspiration. A pitch‐black tale of a potential to kill. Am J Respir Crit Care Med 2015; 191: 344–5. [DOI] [PubMed] [Google Scholar]
- 42. Donoso A, Linares M, León J, Rojas G, Valverde C, Ramírez M, Oberpaur M. Activated charcoal laryngitis in an intubated patient. Pediatr Emerg Care 2003; 19: 420–1. [DOI] [PubMed] [Google Scholar]
- 43. Seder DB, Christman RA, Quinn MO, Knauft ME. A 45‐year‐old man with a lung mass and history of charcoal aspiration. Respir Care 2006; 51: 1251–4. [PubMed] [Google Scholar]
- 44. Elliott CG, Colby TV, Kelly TM, Hicks HG. Charcoal lung. Bronchiolitis obliterans after aspiration of activated charcoal. Chest 1989; 96: 672–4. [DOI] [PubMed] [Google Scholar]
- 45. Francis RC, Schefold JC, Bercker S, Temmesfeld‐Wollbrück B, Weichert W, Spies CD, Weber‐Carstens S. Acute respiratory failure after aspiration of activated charcoal with recurrent deposition and release from an intrapulmonary cavern. Intensive Care Med 2009; 35: 360–3. [DOI] [PubMed] [Google Scholar]
- 46. Goulbourne KB, Cisek JE. Small‐bowel obstruction secondary to activated charcoal and adhesions. Ann Emerg Med 1994; 24: 108–10. [DOI] [PubMed] [Google Scholar]
- 47. Chan JC, Saranasuriya C, Waxman BP. Bezoar causing small bowel obstruction after repeated activated charcoal administration. Med J Aust 2005; 183: 537. [DOI] [PubMed] [Google Scholar]
- 48. Ray MJ, Radin DR, Condie JD, Halls JM. Charcoal bezoar. Small‐bowel obstruction secondary to amitriptyline overdose therapy. Dig Dis Sci 1988; 33: 106–7. [DOI] [PubMed] [Google Scholar]
- 49. Gomez HF, Brent JA, Munoz DC, Mimmack RF, Ritvo J, Phillips S, McKinney P. Charcoal stercolith with intestinal perforation in a patient treated for amitriptyline ingestion. J Emerg Med 1994; 12: 57–60. [DOI] [PubMed] [Google Scholar]
- 50. Dagnone D, Matsui D, Rieder MJ. Assessment of the palatability of vehicles for activated charcoal in pediatric volunteers. Pediatr Emerg Care 2002; 18: 19–21. [DOI] [PubMed] [Google Scholar]
- 51. Cheng A, Ratnapalan S. Improving the palatability of activated charcoal in pediatric patients. Pediatr Emerg Care 2007; 23: 384–6. [DOI] [PubMed] [Google Scholar]
- 52. Mohamed F, Sooriyarachchi MR, Senarathna L, Azhar S, Sheriff MH, Buckley NA, Eddleston M. Compliance for single and multiple dose regimens of superactivated charcoal: a prospective study of patients in a clinical trial. Clin Toxicol 2007; 45: 132–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boyd R, Hanson J. Prospective single blinded randomised controlled trial of two orally administered activated charcoal preparations. J Accid Emerg Med 1999; 16: 24–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fischer TF, Singer AJ. Comparison of the palatabilities of standard and superactivated charcoal in toxic ingestions: a randomized trial. Acad Emerg Med 1999; 6: 895–9. [DOI] [PubMed] [Google Scholar]
- 55. Juurlink DN, McGuigan MA. Gastrointestinal decontamination for enteric‐coated aspirin overdose: what to do depends on who you ask. J Toxicol Clin Toxicol 2000; 38: 465–70. [DOI] [PubMed] [Google Scholar]
- 56. Juurlink DN, Szalai JP, McGuigan MA. Discrepant advice from poison centres and their medical directors. Can J Clin Pharmacol 2002; 9: 101–5. [PubMed] [Google Scholar]
- 57. Jurgens G, Hoegberg LC, Graudal NA. The effect of activated charcoal on drug exposure in healthy volunteers: a meta‐analysis. Clin Pharmacol Ther 2009; 85: 501–5. [DOI] [PubMed] [Google Scholar]
- 58. Isbister GK, Kumar VV. Indications for single‐dose activated charcoal administration in acute overdose. Curr Opin Crit Care 2011; 17: 351–7. [DOI] [PubMed] [Google Scholar]


