Abstract
Resting-state functional magnetic resonance imaging is a powerful technique to study the whole-brain neural connectivity that underlies cognitive systems. The present study aimed to define the changes in neural connectivity in their relation to language development. Longitudinal resting-state functional data were acquired from a cohort of preschool children at age 5 and one year later, and changes in functional connectivity were correlated with language performance in sentence comprehension. For this, degree centrality, a voxel-based network measure, was used to assess age-related differences in connectivity at the whole-brain level. Increases in connectivity with age were found selectively in a cluster within the left posterior superior temporal gyrus and sulcus (STG/STS). In order to further specify the connection changes, a secondary seed-based functional connectivity analysis on this very cluster was performed. The correlations between resting-state functional connectivity (RSFC) and language performance revealed developmental effects with age and, importantly, also dependent on the advancement in sentence comprehension ability over time. In children with greater advancement in language abilities, the behavioral improvement was positively correlated with RSFC increase between left posterior STG/STS and other regions of the language network, i.e., left and right inferior frontal cortex. The age-related changes observed in this study provide evidence for alterations in the language network as language develops and demonstrates the viability of this approach for the investigation of normal and aberrant language development.
Keywords: Preschool children, Language development, Resting-state fMRI, Intrinsic connectivity, Frontal-to-temporal connection
Introduction
Given the importance of language development during childhood, an increasing number of studies have investigated the neural basis of language acquisition. In recent years, functional magnetic resonance imaging (fMRI) has been widely used to detect the brain mechanisms underlying language processing in adults (Friederici, 2006, Friederici, 2011, Kinno et al., 2008, Makuuchi et al., 2009), as well as during childhood (Balsamo et al., 2006, Brauer and Friederici, 2007, Brauer et al., 2008, Knoll et al., 2012, Perani et al., 2011, Redcay et al., 2008, Szaflarski et al., 2006a, Szaflarski et al., 2006b).
Studies on language processing using fMRI in adults have consistently reported activation in the left posterior superior temporal gyrus and sulcus (STG/STS) and the left inferior frontal cortex (IFC) as crucial regions for language comprehension (for a review, see Friederici, 2011). Specifically, the left IFC has been constantly reported as being involved in processing syntactically complex sentence structures (Ben-Shachar et al., 2004, Bornkessel et al., 2005, Friederici et al., 2006b, Grewe et al., 2005, Obleser et al., 2011, Santi and Grodzinsky, 2010). Moreover, enhanced activation in the left posterior STG/STS has been reported for the processing of syntactic information in syntactically complex sentences in adults (Friederici et al., 2006a, Kinno et al., 2008, Röder et al., 2002). Developmental research has reported that the superior temporal cortex is required for rapid language acquisition during the second year of life (Redcay et al., 2008). A 10-year longitudinal study reported that bilateral superior temporal cortical activation played an increasing role in narrative comprehension from young children to adolescents (Szaflarski et al., 2012). In addition, the recruitment of left superior temporal cortex was shown for both semantic and syntactic processing in children aged 5 and 6 years (Brauer and Friederici, 2007) and for syntax–semantics interaction effects in 3–4- and 6–7-year-old children (Skeide et al., 2014).
It was furthermore shown that the maturation of structural connectivity correlates with the performance on processing complex sentences (Skeide et al., 2015), and that the structural connectivity is still not adult-like around the age of 7 years when children still have problems with processing such sentences (Brauer et al., 2011). Studies exploring the functional connectivity between the language-related areas so far have mostly been conducted with adults. They indicate a functional connectivity between the IFC and the STG/STS suggesting that these brain regions are functionally connected during sentence comprehension (den Ouden et al., 2012, Makuuchi and Friederici, 2013).
Spontaneous low frequency (< 0.1 Hz) fluctuations (LFFs) in the human brain at rest have been observed to be related to intrinsic brain activities (Biswal et al., 1995). During the past two decades, a large number of studies have used resting-state functional MRI data to map the brain organization underlying human cognition (e.g., Dosenbach et al., 2010, Fox et al., 2005, Fransson et al., 2011).
Functional connectivity of language regions was observed in LFFs factoring out task dependent activity when seeding in the respective brain regions (Friederici et al., 2011, Lohmann et al., 2010). Data from newborns using the same analysis method reveal that such a functional connectivity is not yet present early in life when infants start to acquire language (Perani et al., 2011). These findings suggest that the analysis of LFFs can serve the investigation of language development. And indeed a number of novel findings have expanded our knowledge on the development of functional and structural connectivity in infants and young children (de Bie et al., 2012, Fransson et al., 2011, Fransson et al., 2007, Gao et al., 2009, Lee et al., 2013, Power et al., 2010, van den Heuvel et al., 2014).
The development of the language network from childhood to adulthood was shown to be characterized by a development from inter- to intra-hemispheric connectivities (Friederici et al., 2011). So far, however, research using resting-state fMRI data to identify and explore the language related networks is still very limited (Antonenko et al., 2012, Muller and Meyer, 2014, Tomasi and Volkow, 2012, Turken and Dronkers, 2011, Xiang et al., 2010), and resting-state fMRI data to explore the development of language in children is even more sparse. Recent studies have shown that RSFC–behavior correlations are advantageous to reveal the neural basis of individual variation in cognitive performance (e.g., Hampson et al., 2006, Kelly et al., 2008, Koyama et al., 2011, Seeley et al., 2007, Stevens and Spreng, 2014, Wang et al., 2010, Zou et al., 2013). Given the interesting relation between task-dependent fMRI and seed based task-independent resting-state fMRI data on language processing in adults, we expect to elucidate the development of the functional connectivity networks related to language development by combining behavioral and resting-state fMRI data.
Here, we present an exploratory examination of developmental changes in intrinsic connectivity patterns of children from age 5 to age 6 by using a network measure, which allows an unbiased comparison at a voxel-wise level. The range was chosen since at this age the structural and functional development of the brain is in full progress (Gogtay et al., 2004, Knoll et al., 2012, Skeide et al., 2014) while at the same time performance in language functions increases steadily (Guasti, 2002, Sakai, 2005, Skeide et al., 2014). Combining resting-state functional connectivity (RSFC) with behavioral data on the development of sentence comprehension carries the potential to open new perspectives on the relation between brain maturation and the ontogeny of language in children. In order to explore the developmental changes in intrinsic connectivity patterns, longitudinal resting-state fMRI data were acquired from a cohort of typically developing children aged 5 years and one year later, and subjected to degree centrality analysis. As a measure of graph theory, degree centrality is among the most fundamental and most common centrality measures, and has been widely used to identify hubs in the human brain (e.g., Buckner et al., 2009, Cole et al., 2010, Tomasi and Volkow, 2011). Degree centrality has been found to be physiologically meaningful (Liang et al., 2013, Tomasi et al., 2015, Tomasi et al., 2013) and has been applied to investigate the changes in network connectivity associated with healthy aging (Hampson et al., 2012) and cognitive functions (van den Heuvel et al., 2009). Hubs, as highly connected central nodes in a network, are thought to play pivotal roles in the coordination of information flow (Sporns et al., 2007) and may also help to minimize wiring and metabolism costs by providing a limited number of long-distance connections that integrate local networks (Bassett and Bullmore, 2006). The approach used here is similar to that shown by Buckner et al. (2009) and Zuo et al. (2012). Binary network measure of degree centrality was computed in a voxel-wise manner and used in order to identify developmental changes in intrinsic connectivity over one year. Subsequently, the result of this analysis was used as a seed to further detect how the connections change with age and, moreover, to what extent the functional resting-state network is related to language abilities. We expected to find growing involvement of core regions of the language network with age, in particular the posterior STG/STS and IFC. The importance of these regions in functional networks supporting language functions should be reflected in a relation between the growing network architecture and language development.
Methods
Participants
Fifty-three typically developing preschool children at age 5 years (27 males; mean age 5.5 years, range 5.0 to 6.0 years) participated in the study, and longitudinal data were obtained in a one-year follow-up measurement (mean age 6.5 years, range 6.0 to 7.1 years). Prior to participation, children's parents gave written, informed consent, and children gave verbal assent for attendance. All participants were right-handed, monolingual German speakers with no history of neurological, medical, or psychological disorders. The study was approved by the Ethical Review Board of the University of Leipzig (Germany).
Behavioral testing
Sentence comprehension was assessed by the standardized German test of sentence-comprehension (Test zum Satzverstehen von Kindern (TSVK); Siegmüller et al., 2011). The test employs a picture matching task with three possible pictures in response to verbally presented sentences at varying syntactic difficulty. Participants were instructed to listen to stories and to select the picture that best fits the story. The number of correct responses was summed (in percent) and converted to standard scores (T values).
MRI scanning
All data were obtained at a 3T magnetic resonance scanner (Siemens Tim Trio, Germany) with a 12-channel head coil. During resting-state data acquisition, children were instructed to lie as still as possible, keep their eyes open and watch the visual presentation of a screensaver featuring a lava lamp. Resting-state fMRI whole-brain volumes were acquired with a T2*-weighted gradient-echo echo-planar imaging (EPI) sequence using the following parameters: TR 2000 ms, TE 30 ms, flip angle = 90°, slice thickness 3 mm, gap = 1 mm, FOV 19.2 cm, matrix = 64 × 64, 28 slices, 100 volumes. High-resolution 3-D structural images were acquired with a T1-weighted, magnetization prepared rapid gradient echo (MPRAGE) sequence using the following parameters: TR 1480 ms, TE 3.46 ms, flip angle = 10°; slice thickness 1.5 mm, gap 0 mm; matrix 250 × 250; spatial resolution 1 × 1 × 1.5 mm.
Preprocessing
Data preprocessing was carried out using the Data Processing Assistant for Resting-State fMRI (DPARSF) (Chao-Gan and Yu-Feng, 2010, http://www.restfmri.net) which is based on Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm) and Resting-State fMRI Data Analysis Toolkit (REST) (Song et al., 2011, http://www.restfmri.net). Before preprocessing, the first three EPI volumes were discarded to avoid possible effects of scanner instability and allow for signal equilibration. Preprocessing steps included: i) slice timing by shifting the signal measured in each slice relative to the acquisition of the slice at the mid-point of each TR; ii) 3D motion correction using a least squares approach and a 6 parameter (rigid body) spatial transformation; iii) reorienting both functional and MPRAGE images and then co-registering MPRAGE image to the mean functional image of each subject; iv) MPRAGE images were segmented into gray matter, white matter (WM), and cerebrospinal fluid (CSF) tissue based on the NIH pediatric atlas (NIHPD) (Fonov et al., 2011, http://www.bic.mni.mcgill.ca/ServicesAtlases/NIHPD-obj1), using the asymmetric T1 version of the NIHPD atlas, age range 4.5–8.5 years old (prepuberty), based on 82 subjects; v) spatial normalization by using the parameters from the segmentation procedure in each subject and resampling voxel size to 3 × 3 × 3 mm3; vi) spatial smoothing with a 6-mm full-width-at-half-maximum (FWHM) Gaussian kernel; vii) nuisance regression, including principal components (PCs) extracted from subject-specific WM and CSF mask (5 PC parameters) using a component based noise correction method (CompCor) (Behzadi et al., 2007), as well as Friston 24-parameter model (6 head motion parameters, 6 head motion parameters one time point before, and the 12 corresponding squared items) (Friston et al., 1996). The CompCor procedure comprised detrending, variance (i.e., WM and CSF) normalization and PC analysis according to Behzadi et al. (2007); viii) band-pass temporal filtering (0.01–0.1 Hz). For degree centrality calculation, spatial smoothing was not included in the preprocessing but performed after Z-normalization in order to prevent artefactual local correlations between voxels (Zuo et al., 2012).
CompCor was proposed to correct for physiological noise by regressing out PCs from noise regions of interest (ROIs) (Behzadi et al., 2007). Compared with mean signal regression, where average signal were extracted from WM and CSF mask, signals captured by PCs derived from these noise ROIs can better account for voxel-specific phase differences in physiological noise due to the potential of principle component analysis to identify temporal pattern of physiological noise (Thomas et al., 2002).
Given concerns regarding a possible confounding influence of micromovements in intrinsic functional connectivity analyses (Power et al., 2012, Power et al., 2014, Satterthwaite et al., 2012, Van Dijk et al., 2012), the framewise displacement (FD) of time series (Jenkinson et al., 2002) was calculated as it is preferable for its consideration of voxel-wise differences in its derivation (Yan et al., 2013a). Seven subjects with motion (mean FD) greater than mean + 2 ∗ SD (Yan et al., 2013b) were excluded, with threshold of 0.229 mm for children at age 5 and 0.221 mm at age 6, separately. For the remaining 46 data sets, the average of mean FD at age 5 was 0.101 mm (SD = 0.04 mm, range = 0.037–0.206 mm), and at age 6 was 0.092 mm (SD = 0.039 mm, range = 0.025–0.184 mm). Differences of mean FD were calculated by using paired t-test and showed no significant differences (t(45) = 1.349, p = .184). Nevertheless, the mean FD was controlled as a covariate of no interest in subsequent group-level statistical analyses to reduce any remaining potential effect of head motion.
Calculation of degree centrality maps
Degree centrality maps were computed by using the REST toolbox that employs an approach similar to that shown by Buckner et al. (2009) and Zuo et al. (2012). Specifically, for each voxel i the connectivity between the time course of this given voxel i and the time course of every other voxel within the mask of gray matter of the brain was computed. Then the correlation map of voxel i was converted to a binary map of connectivity thresholded at r = 0.25, setting all connections below the threshold to zero while setting all remaining connections to 1. The sum of all non-zero connections in this binary map was calculated to yield the degree centrality of the voxel i. This process was repeated for each voxel in the brain to produce a whole-brain map of the network degree.
The individual-level degree centrality maps were then standardized by converting to z-scores and maps were averaged across participants and compared (Buckner et al., 2009, Van Dijk et al., 2012, Zuo et al., 2012). The z-score transformation is achieved by subtracting mean degree and dividing standard deviation of degree across all voxels as described in previous studies (Buckner et al., 2009, Zuo et al., 2012). Group-level degree centrality map for each age group was obtained by implementing one-sample t-test. Multiple comparisons were corrected at the cluster-level using Gaussian random field theory (| Z | > 3.5, cluster-wise p < .001, GRF corrected).
The threshold used to compute degree centrality in this study was chosen to be consistent with previous studies (Buckner et al., 2009, Hampson et al., 2012, Van Dijk et al., 2012), and different threshold selections did not qualitatively change the results for the cortex (Buckner et al., 2009, Hampson et al., 2012). For an analysis with alternative thresholds, see Supplementary Fig. S1. Furthermore, the weighted version of degree centrality was also computed, assuring the robustness of the findings with nearly identical results as shown in Supplementary Fig. S2.
Developmental changes in degree centrality
The primary analysis of this study examined intrinsic connectivity differences in the longitudinal data identifying clusters that change their degree centrality with development. Paired t-test was performed to detect the developmental changes in voxel-wise connectivity maps from age 5 to age 6 years, controlled for head motion (mean FD).
Seed-based connectivity changes and relation to advances in language performance
However, the aforementioned primary analysis would not provide information about which connections are changing or the relation between the connections and language performance. To explore this, a secondary seed-based analysis was implemented. The resulting clusters from the primary analysis were subjected to a seed-based analysis on functional connectivity.
RSFC analyses were performed at both measurement time points using REST software. For RSFC calculation, the mean time series of the seed were first computed for each participant by averaging the time series of all the voxels in the seed (6-mm-radius sphere), and then an individual level RSFC correlation map (r-map) was produced within the whole brain. Next, r-maps were converted into z-maps with application of Fisher's r-to-z transformation to obtain approximately normally distributed values for further statistical analysis.
Average functional connectivity maps for both time points (age 5 and age 6) were computed based on z-transformed maps to illustrate the connectivity patterns of the cluster. In addition, the comparison of connectivity maps between the two time points was obtained by performing paired t-test, controlling for mean FD of each participant, and corrected at the cluster-level using Gaussian random field theory (Z > 2.3, cluster-wise p < .05, GRF corrected).
In order to model the relationship between changes in functional connectivity and changes in behavioral performance, the absolute changes of both connectivity strength and language comprehension (TSVK) performance were calculated for age 6 subtracting age 5, and results were then entered into a model of RSFC-behavior correlation. For further exploration of behavioral effects, the whole group data were divided into two subgroups by the median of changes in TSVK performance from age 5 to age 6. Participants with change value greater than median were considered to show greater advancement in language abilities (18 participants) whereas participants with change value smaller than median were considered to show less advancement in language abilities (20 participants). Subsequently, RSFC-behavioral correlation was obtained for each of the two subgroups. Finally, all statistical r-maps were transformed to z-maps and corrected at the cluster-level using Gaussian random field theory (Z > 2.3, cluster-wise p < .05, GRF corrected).
Results
Behavioral results
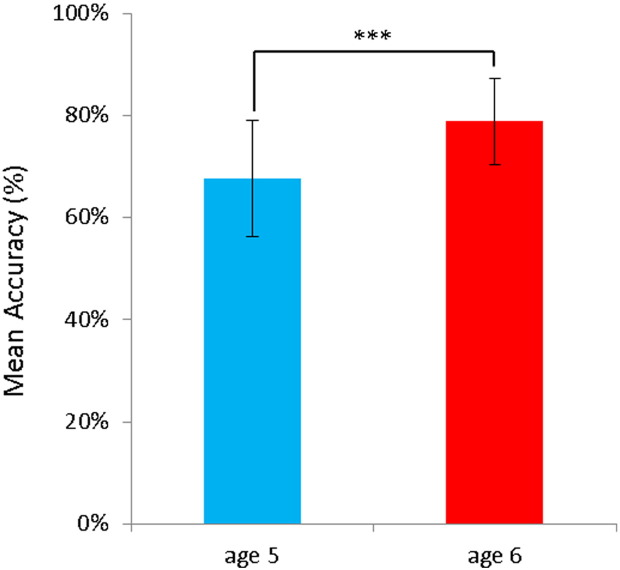
Mean accuracy for the sentence comprehension task was 67.7% (SD 11.38) at age 5 years and 78.8% (SD 8.37) at age 6 years. Performance was above chance at both time points (age 5: t(45) = 10.55, p < .001; age 6: t(45) = 23.34, p < .001), and there was a significant performance difference between the two measurement time points (t(45) = − 7.53, p < .001) (Fig. 1).
Fig. 1.
Mean accuracy of sentence comprehension performance (TSVK) at age 5 and 6 years. Error bars represent standard error of the mean (*** P < .001).
Group-level degree centrality and changes with age
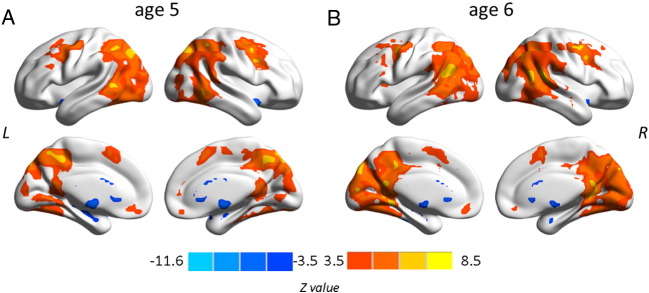
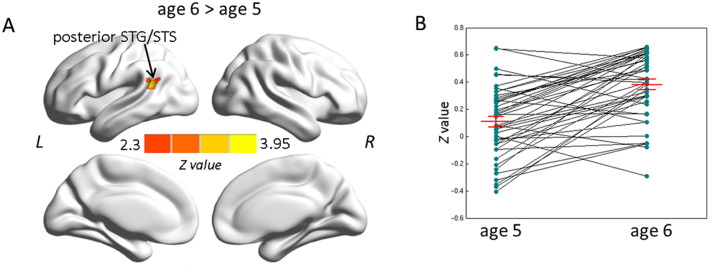
Degree centrality maps indicate that hubs at age 5 and age 6 years covered regions of the default mode network (DMN), including posterior cingulate cortex/precuneus (PCC), lateral temporal cortex, lateral parietal cortex, and medial/lateral prefrontal cortices (Fig. 2) as known from adult data (Buckner et al., 2009). Interestingly, the comparison between the two measurement time points yielded one cluster centered on the left posterior STG/STS (MNI coordinates: − 45, − 51, 21; peak z: 3.95; 170 voxels) with increased connectivity at age 6 compared to age 5 years (Fig. 3).
Fig. 2.
Voxel-wise degree centrality maps at age 5 (2A) and age 6 (2B). Red–yellow colors indicate positive connectivity, whereas blue colors indicate negative connectivity. Z value is the scale of degree centrality. Multiple comparisons were corrected at the cluster-level using Gaussian random field theory (| Z | > 3.5, cluster-wise p < .001, GRF corrected). L, left hemisphere; R, right hemisphere.
Fig. 3.
Comparison of degree centrality maps between age 5 and age 6 years (3A). Red–yellow colors indicates stronger degree centrality at age 6 compared to age 5 in the left posterior STG/STS. Multiple comparisons were corrected at the cluster level using Gaussian random field theory (Z > 2.3, cluster-wise p < .05, GRF corrected). Figure B illustrates individual variation in degree centrality of left posterior STG/STS and also includes the mean values of the cluster in posterior STG/STS at age 5 and age 6 years, as well as error bars representing standard error of the mean (3B). L, left hemisphere; R, right hemisphere. STG/STS, superior temporal gyrus and sulcus.
Seed-based connectivity changes and relation to advances in language performance
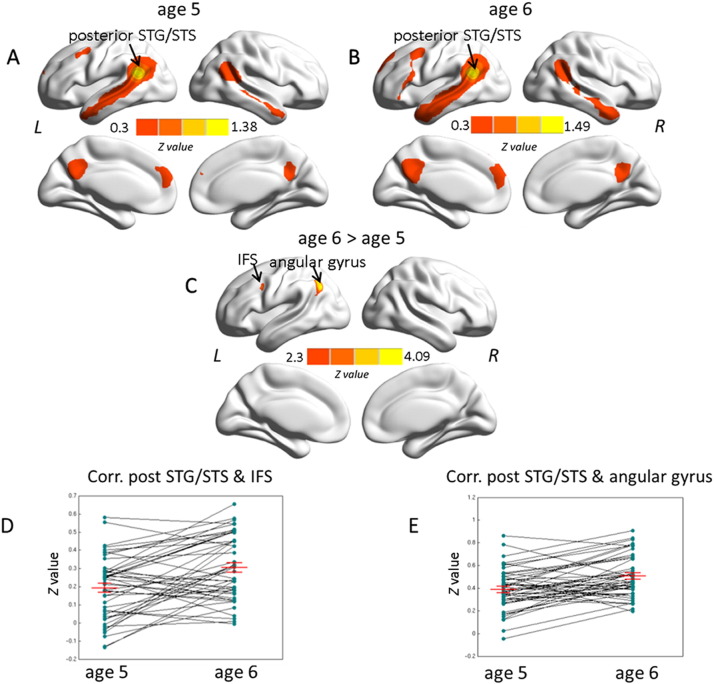
In a next step, the resulting cluster from the degree centrality analysis was used as a seed in order to examine functional connectivity of this cluster. This seeding in the left posterior STG/STS revealed a number of correlated regions at both ages, including middle frontal gyrus, bilateral PCC, dorsomedial prefrontal cortex, bilateral STG/STS and angular gyrus bilaterally (Figs. 4A and B). At age 6 years, the IFC was additionally involved (Fig. 4B). Direct comparison of functional connectivity between the two measurement time points showed developmental changes in the left inferior frontal sulcus (IFS) of the IFC and left angular gyrus from age 5 to age 6 years (Fig. 4C). Individual variations in correlations between left posterior STG/STS and left IFS as well as left angular gyrus are shown in Figs. 4D and E, respectively.
Fig. 4.
Average functional connectivity maps seeded in the left posterior STG/STS shown for children at age 5 (4A) and age 6 (4B). Significant correlations to left inferior frontal cortex are only found for age 6 (Z = 0.3 with minimal cluster size of 60 voxels). Fig. C depicts the direct contrast between the two time points (4C), with red–yellow colors indicating stronger connections at age 6 (Z > 2.3, cluster-wise p < .05, GRF corrected). In addition, the individual variation in correlations between left posterior STG/STS and left IFS (4D), as well as between posterior STG/STS and left angular gyrus (4E) are depicted including the mean correlation coefficients at age 5 and age 6. Error bars represent standard error of the mean. L, left hemisphere; R, right hemisphere. IFS, inferior frontal sulcus.
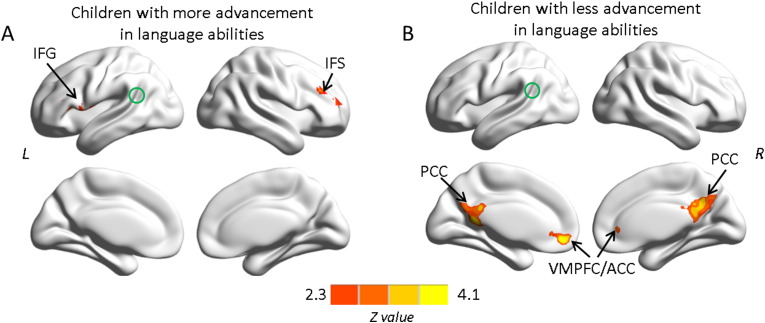
In order to further evaluate behavioral relevance of these functional networks, changes in RSFC were correlated with changes in language comprehension performance from age 5 to age 6 years. Participants were allocated to two subgroups with either greater or less change in language performance based on a median split. RSFC-behavior correlation for each subgroup showed distinct patterns. Specifically, correlations in the left and right IFC were observed in children with greater advancement in language abilities, whereas correlations in bilateral PCC, ventromedial prefrontal cortex and anterior cingulate cortex were observed in children with less advancement in language abilities (Figs. 5A and B; Table 1). All maps are displayed with the BrainNet Viewer (Xia et al., 2013, http://www.nitrc.org/projects/bnv/).
Fig. 5.
Correlations between changes in functional connectivity seeded in the left posterior STG/STS cluster (green circle) and changes in language comprehension performance from age 5 to age 6 in children with greater advancement in language abilities (5A) and children with less advancement in language abilities (5B). While for the former, significant correlations to the bilateral inferior frontal cortex were found, for the latter, no such correlations to other parts of the language network were observed and rather correlations to regions within the DMN exist. Multiple comparisons were corrected at the cluster level using Gaussian random field theory (Z > 2.3, cluster-wise p < .05, GRF corrected). L, left hemisphere; R, right hemisphere. IFG, inferior frontal gyrus; IFS, inferior frontal sulcus; PCC, posterior cingulate cortex/precuneus; VMPFC/ACC, ventromedial prefrontal cortex/anterior cingulate cortex.
Table 1.
Details of RSFC–behavior correlations in two subgroups of children with greater or less advancement in sentence comprehension over the one-year period from age 5 to 6 years.
| Subgroup | L/R | Region | BA | Peak MNI coordinates |
Voxels | Z value | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Children with greater advancement | L | IFG | 44 | − 42 | 24 | 9 | 60 | 3.31 |
| R | IFS | 46 | 51 | 39 | 30 | 100 | 3.38 | |
| Children with less advancement | L/R | PCC | 7 | 15 | − 30 | 9 | 705 | 4.10 |
| L/R | VMPFC, ACC | 32 | − 6 | 48 | − 9 | 148 | 4.01 | |
Note: L, left hemisphere; R, right hemisphere; BA, Brodmann's area. IFG, inferior frontal gyrus; IFS, inferior frontal sulcus; PCC, posterior cingulate cortex/precuneus; VMPFC, ventromedial prefrontal cortex; ACC, anterior cingulate cortex.
Discussion
The current study investigated the neural basis of language development in longitudinal resting-state functional MRI data in a cohort of typically developing children at age 5 and age 6 years. Using a data-driven approach to investigate degree centrality, we found at both ages a similar pattern of hubs covering regions of the DMN. A significant cluster of stronger intrinsic connectivity at age 6 compared to age 5 was observed in the left posterior STG/STS. The RSFC-behavior correlation revealed connections from this cluster to language-relevant regions in bilateral IFC for children with greater advancement in language abilities, whereas for children with less advancement in language abilities stronger connectivity of DMN regions was observed. These findings demonstrate the development of functional resting-state networks during a one-year period between age 5 and age 6 and its relation to concurrent development of language abilities.
Increased degree centrality in left posterior STG/STS with age
Importantly, we found increased connectivity between ages 5 to 6 years in the left posterior STG/STS. There was no other region that showed connectivity increase above threshold and there were no regions with concurrent decreased connectivity change. Accumulated evidence supports the role of the posterior STG/STS in language comprehension (for a review, see Friederici, 2011). Task-related activation in this region has been reported for processing syntactic information in word list (Humphries et al., 2005, Snijders et al., 2009), complex sentences (Cooke et al., 2002, Friederici et al., 2006a, Friederici et al., 2009, Kinno et al., 2008, Röder et al., 2002), and combined syntactic and semantic sentential information (Friederici et al., 2003, Friederici et al., 2010) as well as argument processing (Grewe et al., 2007, Grewe et al., 2006). Taken together, evidence suggests this region as a central component for the integration of linguistic information at different levels.
Note that the specific functional role of increased degree centrality in the left posterior STG/STS from age 5 to age 6 cannot be concluded directly from resting-state functional brain data alone. These changes can potentially be related to a variety of developmental changes in brain maturation and human development. However, based on the specific location of this increase in connectivity in the posterior STG/STS, we hypothesize that it is related to the central involvement of this region in the language network where changes in the functional network are manifested at that age when language abilities increase prominently (e.g., Guasti, 2002, Sakai, 2005). The posterior STG/STS had been shown a central part of the language network in studies with adults (Friederici, 2011, Hickok and Poeppel, 2007, Vigneau et al., 2006) and with children (Berl et al., 2010, Brauer et al., 2008, Knoll et al., 2012, Skeide et al., 2015). Therefore, a secondary analysis exploring changes in RSFC based on this region was performed to further examine which network connections terminating in this region are changing from age 5 to age 6, whether they are part of the language network, and whether there is a relation to behavioral changes in language abilities.
Frontal-to-temporal connections in children with greater advancement in language comprehension
Previous task-dependent fMRI experiments in adults and children have consistently reported enhanced activation in both left IFC and left posterior STG/STS when processing syntactically complex sentences (Kinno et al., 2008, Knoll et al., 2012, Obleser et al., 2011, Thompson et al., 2010). The left frontal-to-temporal network connection between language-relevant brain regions develops as the brain matures and is still structurally immature at the age of 5 to 6 years (Brauer et al., 2011). Furthermore, the common activity of left IFC and posterior STG/STS in the sense of a “default language network” has been observed in LFFs (Lohmann et al., 2010), which was, moreover, shown not yet fully developed at age 6 years (Friederici et al., 2011). Consistent with these findings, we found that RSFC between bilateral IFC (left inferior frontal gyrus and right IFS) and left posterior STG/STS was positively correlated with greater advancement in language comprehension, suggesting that this long-range connection is relevant for the progress in language abilities.
It has been widely acknowledged that the activation of left IFC is crucial for language comprehension (Friederici, 2011, Friederici et al., 2006a, Makuuchi et al., 2009, Santi and Grodzinsky, 2010). Other studies have shown increasing BOLD responses in the left IFC as task difficulty increases and have related this to increased working memory and phonological processing demands (Binder et al., 2005, Desai et al., 2006, Lehmann et al., 2006, Tregellas et al., 2006). A developmental study found that children with better syntactic processing skills showed more prominent activation in the left IFC compared to children with poorer syntactic processing skills (Nuñez et al., 2011). Particularly, mounting evidence from fMRI or behavioral studies has revealed that language performance is closely related with working memory (e.g., Baddeley, 2003, Gathercole and Susan, 2000, Määttä et al., 2014, Montgomery and Evans, 2009, van Daal et al., 2008). It was shown that for both, syntactic processes as well as working memory demands, the IFC is recruited (Makuuchi et al., 2009). The current findings of stronger functional connectivity between IFC and posterior STG/STS could be helpful for syntactic comprehension in a narrow sense but also in a more general sense for working memory related processes.
The involvement of DMN in children with less advancement in language comprehension
The DMN was originally defined as a set of brain areas that consistently show task-induced deactivation in functional imaging studies (Binder et al., 1999, Raichle et al., 2001, Shulman et al., 1997). Recent studies have found that the DMN was widely engaged in internal mentation (e.g., self-referential processing, mentalizing, affective cognition, theory of mind, episodic retrieval, autobiographical thought, mnemonic or prospective processes) (Andrews-Hanna et al., 2010, Andrews-Hanna et al., 2014a, Andrews-Hanna et al., 2014b, Buckner and Carroll, 2007, D'Argembeau et al., 2005, Gusnard et al., 2001, Gusnard and Raichle, 2001, Johnson, 2003, Northoff et al., 2006, Whitfield-Gabrieli et al., 2011).
Mounting evidence has confirmed the opposite relationship between behavioral performance and the suppression of the DMN (Anticevic et al., 2010, Daselaar et al., 2004, Shulman et al., 2007, White et al., 2013). For instance, successful performance on cognitive tasks has been related to a specific recruitment of task-relevant networks while deactivating resting-state networks such as the DMN (Anticevic et al., 2012, Anticevic et al., 2010, Hampson et al., 2010, Kelly et al., 2008). Similarly, less DMN suppression was associated with less efficient stimulus processing during attention lapses (Weissman et al., 2006). These findings support the view of a direct competition between exogenous and endogenous components for attentional and executive resources, and suggest that lower involvement of the DMN activity on a trial-by-trial basis is associated with better cognitive performance, indicating that the ability of DMN suppression is functionally important (for a review, see Anticevic et al., 2012). Therefore, it might be plausible to infer that the involvement of the DMN in functional connectivity for children with less advancement in language abilities is due to their insufficient suppression of the DMN.
Limitations
It is important to note that the interpretation of the current results should be limited to resting-state fMRI context, especially for the involvement of regions within the DMN, because the data presented here are not from a task-based fMRI experiment. Though, consistent activation of PCC was found in semantic processing, and it has been proposed that the involvement of PCC might have to do with the nature of episodic memory and PCC probably acts as an interface between the semantic retrieval and episodic encoding systems based on the fact of strong connections of PCC and hippocampus (Binder et al., 2009). Moreover, a model of involvement of regions within DMN was proposed when the task itself engages the semantic system (e.g., semantic tasks) (Binder et al., 2009), but it still requires more evidence with regarding to the role of the DMN in language processing. Therefore, in future studies, it would be necessary to identify to what extent the DMN is involved in language processing as well as the interactions between DMN and the language specific network by using language-related fMRI data. Another limitation of the present study is the relatively short acquisition time for the resting-state fMRI data. Considering the difficulties of data acquisition from typically developing young children during waking state, a total of 100 volumes resting-state fMRI data were collected, which is relatively short for intrinsic functional connectivity analysis. However, importantly, studies with comparably short acquisition of resting-state fMRI data observed stable correlation strengths with acquisition times as brief as 5 min (Van Dijk et al., 2010). Moreover, recent studies found good inter-session reliability for functional homogeneity analyses with scan durations as brief as 3 min (Zuo et al., 2013) and high reliability of resting-state fMRI measures available with data length of 3 min (Yan et al., 2013a). Hence, the present findings can be considered reliable and valid.
Conclusion
Exploring the development of intrinsic brain connectivity, increases in the left posterior STG/STS were identified as significant changes in the degree centrality during a one-year period in typically developing children between age 5 and age 6 years. The RSFC of left posterior STG/STS to language-relevant perisylvian regions is significantly associated with greater advancement in language abilities, whereas RSFC of left posterior STG/STS to regions within DMN is significantly correlated with less advancement in language abilities. These findings suggest that functional connectivity within the language network considerably develops from age 5 to age 6 and becomes behaviorally relevant. The present data provide evidence for alterations in functional networks with respect to language development during preschool age, and demonstrate the viability of these methods for characterizing the brain basis and ontogeny of language development in children.
Acknowledgments
We would like to thank Jeanine Auerswald, Kodjo Vissiennon, and Riccardo Cafiero for their contributions to data acquisition. We also thank Xiangyu Long and Seung-Goo Kim for their valuable comments on data analysis. This research was supported by an ERC advanced grant awarded to ADF (ERC-2010-AdG 269505, NEUROSYNTAX).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.12.008.
Appendix A. Supplementary data
Supplementary material.
References
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional–anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Saxe R., Yarkoni T. Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. NeuroImage. 2014;91:324–335. doi: 10.1016/j.neuroimage.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Repovs G., Shulman G.L., Barch D.M. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. NeuroImage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Cole M.W., Murray J.D., Corlett P.R., Wang X.J., Krystal J.H. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko D., Meinzer M., Lindenberg R., Witte A.V., Floel A. Grammar learning in older adults is linked to white matter microstructure and functional connectivity. NeuroImage. 2012;62:1667–1674. doi: 10.1016/j.neuroimage.2012.05.074. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory and language: an overview. J. Commun. Disord. 2003;36:189–208. doi: 10.1016/s0021-9924(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Balsamo L., Xu B., Gaillard W. Language lateralization and the role of the fusiform gyrus in semantic processing in young children. NeuroImage. 2006;31:1306–1314. doi: 10.1016/j.neuroimage.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar M., Palti D., Grodzinsky Y. Neural correlates of syntactic movement: converging evidence from two fMRI experiments. NeuroImage. 2004;21:1320–1336. doi: 10.1016/j.neuroimage.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Berl M.M., Duke E.S., Mayo J., Rosenberger L.R., Moore E.N., VanMeter J., Ratner N.B., Vaidya C.J., Gaillard W.D. Functional anatomy of listening and reading comprehension during development. Brain Lang. 2010;114:115–125. doi: 10.1016/j.bandl.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Frost J.A., Hammeke T.A., Bellgowan P., Rao S.M., Cox R.W. Conceptual processing during the conscious resting state: a functional MRI study. J. Cogn. Neurosci. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Medler D., Desai R., Conant L., Liebenthal E. Some neurophysiological constraints on models of word naming. NeuroImage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Zerrin Yetkin F., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bornkessel I., Zysset S., Friederici A.D., von Cramon D.Y., Schlesewsky M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. NeuroImage. 2005;26:221–233. doi: 10.1016/j.neuroimage.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Brauer J., Friederici A.D. Functional neural networks of semantic and syntactic processes in the developing brain. J. Cogn. Neurosci. 2007;19:1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Brauer J., Neumann J., Friederici A.D. Temporal dynamics of perisylvian activation during language processing in children and adults. NeuroImage. 2008;41:1484–1492. doi: 10.1016/j.neuroimage.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J., Anwander A., Friederici A.D. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb. Cortex. 2011;21:459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Carroll D.C. Self-projection and the brain. Trends Cogn. Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T., Andrews-Hanna J.R., Sperling R.A., Johnson K.A. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J. Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.W., Pathak S., Schneider W. Identifying the brain's most globally connected regions. NeuroImage. 2010;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Cooke A., Zurif E.B., DeVita C., Alsop D., Koenig P., Detre J., Gee J., Pinango M., Balogh J., Grossman M. Neural basis for sentence comprehension: grammatical and short-term memory components. Hum. Brain Mapp. 2002;15:80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A., Collette F., Van der Linden M., Laureys S., Del Fiore G., Degueldre C., Luxen A., Salmon E. Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Daselaar S., Prince S., Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. NeuroImage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- de Bie H.M., Boersma M., Adriaanse S., Veltman D.J., Wink A.M., Roosendaal S.D., Barkhof F., Stam C.J., Oostrom K.J., Delemarre-van de Waal H.A., Sanz-Arigita E.J. Resting-state networks in awake five- to eight-year old children. Hum. Brain Mapp. 2012;33:1189–1201. doi: 10.1002/hbm.21280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Ouden D.-B., Saur D., Mader W., Schelter B., Lukic S., Wali E., Timmer J., Thompson C.K. Network modulation during complex syntactic processing. NeuroImage. 2012;59:815–823. doi: 10.1016/j.neuroimage.2011.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R., Conant L.L., Waldron E., Binder J.R. FMRI of past tense processing: the effects of phonological complexity and task difficulty. J. Cogn. Neurosci. 2006;18:278–297. doi: 10.1162/089892906775783633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Nardos B., Cohen A.L., Fair D.A., Power J.D., Church J.A., Nelson S.M., Wig G.S., Vogel A.C., Lessov-Schlaggar C.N., Barnes K.A., Dubis J.W., Feczko E., Coalson R.S., Pruett J.R., Jr., Barch D.M., Petersen S.E., Schlaggar B.L. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Skiöld B., Horsch S., Nordell A., Blennow M., Lagercrantz H., Åden U. Resting-state networks in the infant brain. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Aden U., Blennow M., Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb. Cortex. 2011;21:145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The neural basis of language development and its impairment. Neuron. 2006;52:941–952. doi: 10.1016/j.neuron.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Rueschemeyer S.-A., Hahne A., Fiebach C.J. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb. Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Bahlmann J., Heim S., Schubotz R.I., Anwander A. The brain differentiates human and non-human grammars: functional localization and structural connectivity. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2458–2463. doi: 10.1073/pnas.0509389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D., Fiebach C.J., Schlesewsky M., Bornkessel I.D., Von Cramon D.Y. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb. Cortex. 2006;16:1709–1717. doi: 10.1093/cercor/bhj106. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Makuuchi M., Bahlmann J. The role of the posterior superior temporal cortex in sentence comprehension. Neuroreport. 2009;20:563–568. doi: 10.1097/WNR.0b013e3283297dee. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Kotz S.A., Scott S.K., Obleser J. Disentangling syntax and intelligibility in auditory language comprehension. Hum. Brain Mapp. 2010;31:448–457. doi: 10.1002/hbm.20878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D., Brauer J., Lohmann G. Maturation of the language network: from inter-to intrahemispheric connectivities. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., Smith J.K., Shen D., Gilmore J.H., Lin W. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole A.-M.A., Susan E. Limitations in working memory: implications for language development. Int. J. Lang. Commun. Disord. 2000;35:95–116. doi: 10.1080/136828200247278. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe T., Bornkessel I., Zysset S., Wiese R., von Cramon D.Y., Schlesewsky M. The emergence of the unmarked: a new perspective on the language-specific function of Broca's area. Hum. Brain Mapp. 2005;26:178–190. doi: 10.1002/hbm.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe T., Bornkessel I., Zysset S., Wiese R., von Cramon D.Y., Schlesewsky M. Linguistic prominence and Broca's area: the influence of animacy as a linearization principle. NeuroImage. 2006;32:1395–1402. doi: 10.1016/j.neuroimage.2006.04.213. [DOI] [PubMed] [Google Scholar]
- Grewe T., Bornkessel-Schlesewsky I., Zysset S., Wiese R., von Cramon D.Y., Schlesewsky M. The role of the posterior superior temporal sulcus in the processing of unmarked transitivity. NeuroImage. 2007;35:343–352. doi: 10.1016/j.neuroimage.2006.11.045. [DOI] [PubMed] [Google Scholar]
- Guasti M.T. MIT Press; Camrbidge, MA: 2002. Language acquisition: The growth of grammar. [Google Scholar]
- Gusnard D.A., Raichle M.E. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Driesen N.R., Skudlarski P., Gore J.C., Constable R.T. Brain connectivity related to working memory performance. J. Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Driesen N., Roth J.K., Gore J.C., Constable R.T. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn. Reson. Imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Tokoglu F., Shen X., Scheinost D., Papademetris X., Constable R.T. Intrinsic brain connectivity related to age in young and middle aged adults. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Humphries C., Love T., Swinney D., Hickok G. Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Hum. Brain Mapp. 2005;26:128–138. doi: 10.1002/hbm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson S.P. The nature of cognitive development. Trends Cogn. Sci. 2003;7:102–104. doi: 10.1016/s1364-6613(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Kelly A.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kinno R., Kawamura M., Shioda S., Sakai K.L. Neural correlates of noncanonical syntactic processing revealed by a picture-sentence matching task. Hum. Brain Mapp. 2008;29:1015–1027. doi: 10.1002/hbm.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll L.J., Obleser J., Schipke C.S., Friederici A.D., Brauer J. Left prefrontal cortex activation during sentence comprehension covaries with grammatical knowledge in children. NeuroImage. 2012;62:207–216. doi: 10.1016/j.neuroimage.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Koyama M.S., Di Martino A., Zuo X.-N., Kelly C., Mennes M., Jutagir D.R., Castellanos F.X., Milham M.P. Resting-state functional connectivity indexes reading competence in children and adults. J. Neurosci. 2011;31:8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Morgan B.R., Shroff M.M., Sled J.G., Taylor M.J. The development of regional functional connectivity in preterm infants into early childhood. Neuroradiology. 2013;55:105–111. doi: 10.1007/s00234-013-1232-z. [DOI] [PubMed] [Google Scholar]
- Lehmann C., Vannini P., Wahlund L.-O., Almkvist O., Dierks T. Increased sensitivity in mapping task demand in visuospatial processing using reaction-time-dependent hemodynamic response predictors in rapid event-related fMRI. NeuroImage. 2006;31:505–512. doi: 10.1016/j.neuroimage.2005.12.064. [DOI] [PubMed] [Google Scholar]
- Liang X., Zou Q., He Y., Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc. Natl. Acad. Sci. U. S. A. 2013;110:1929–1934. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G., Hoehl S., Brauer J., Danielmeier C., Bornkessel-Schlesewsky I., Bahlmann J., Turner R., Friederici A. Setting the frame: the human brain activates a basic low-frequency network for language processing. Cereb. Cortex. 2010;20:1286–1292. doi: 10.1093/cercor/bhp190. [DOI] [PubMed] [Google Scholar]
- Määttä S., Laakso M.-L., Tolvanen A., Ahonen T., Aro T. Children with differing developmental trajectories of prelinguistic communication skills: language and working memory at age 5. J. Speech Lang. Hear. Res. 2014;57:1026–1039. doi: 10.1044/2014_JSLHR-L-13-0012. [DOI] [PubMed] [Google Scholar]
- Makuuchi M., Friederici A.D. Hierarchical functional connectivity between the core language system and the working memory system. Cortex. 2013;49:2416–2423. doi: 10.1016/j.cortex.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Makuuchi M., Bahlmann J., Anwander A., Friederici A.D. Segregating the core computational faculty of human language from working memory. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8362–8367. doi: 10.1073/pnas.0810928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J.W., Evans J.L. Complex sentence comprehension and working memory in children with specific language impairment. J. Speech Lang. Hear. Res. 2009;52:269–288. doi: 10.1044/1092-4388(2008/07-0116). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A.M., Meyer M. Language in the brain at rest: new insights from resting state data and graph theoretical analysis. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nuñez S.C., Dapretto M., Katzir T., Starr A., Bramen J., Kan E., Bookheimer S., Sowell E.R. fMRI of syntactic processing in typically developing children: structural correlates in the inferior frontal gyrus. Dev. Cogn. Neurosci. 2011;1:313–323. doi: 10.1016/j.dcn.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J., Meyer L., Friederici A.D. Dynamic assignment of neural resources in auditory comprehension of complex sentences. NeuroImage. 2011;56:2310–2320. doi: 10.1016/j.neuroimage.2011.03.035. [DOI] [PubMed] [Google Scholar]
- Perani D., Saccuman M.C., Scifo P., Anwander A., Spada D., Baldoli C., Poloniato A., Lohmann G., Friederici A.D. Neural language networks at birth. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16056–16061. doi: 10.1073/pnas.1102991108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Fair D.A., Schlaggar B.L., Petersen S.E. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E., Haist F., Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev. Sci. 2008;11:237–252. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Röder B., Stock O., Neville H., Bien S., Rösler F. Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: a functional magnetic resonance imaging study. NeuroImage. 2002;15:1003–1014. doi: 10.1006/nimg.2001.1026. [DOI] [PubMed] [Google Scholar]
- Sakai K.L. Language acquisition and brain development. Science. 2005;310:815–819. doi: 10.1126/science.1113530. [DOI] [PubMed] [Google Scholar]
- Santi A., Grodzinsky Y. fMRI adaptation dissociates syntactic complexity dimensions. NeuroImage. 2010;51:1285–1293. doi: 10.1016/j.neuroimage.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H., Gur R.C., Gur R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G.L., Fiez J.A., Corbetta M., Buckner R.L., Miezin F.M., Raichle M.E., Petersen S.E. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Shulman G.L., Astafiev S.V., McAvoy M.P., d'Avossa G., Corbetta M. Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cereb. Cortex. 2007;17:2625–2633. doi: 10.1093/cercor/bhl170. [DOI] [PubMed] [Google Scholar]
- Siegmüller J., Kauschke C., van Minnen S., Bittner D. Elsevier GmbH; München: 2011. Test zum Satzverstehnen von Kindern — Eine profilorientierte Diagnostik der Syntax. [Google Scholar]
- Skeide M.A., Brauer J., Friederici A.D. Syntax gradually segregates from semantics in the developing brain. NeuroImage. 2014;100:106–111. doi: 10.1016/j.neuroimage.2014.05.080. [DOI] [PubMed] [Google Scholar]
- Skeide M.A., Brauer J., Friederici A.D. Brain functional and structural predictors of language performance. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv042. [DOI] [PubMed] [Google Scholar]
- Snijders T.M., Vosse T., Kempen G., Van Berkum J.J., Petersson K.M., Hagoort P. Retrieval and unification of syntactic structure in sentence comprehension: an fMRI study using word-category ambiguity. Cereb. Cortex. 2009;19:1493–1503. doi: 10.1093/cercor/bhn187. [DOI] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., Li S.F., Zuo X.N., Zhu C.Z., He Y., Yan C.G., Zang Y.F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O., Honey C.J., Kötter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2 doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens W.D., Spreng R.N. Resting-state functional connectivity MRI reveals active processes central to cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2014;5:233–245. doi: 10.1002/wcs.1275. [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P., Holland S.K., Schmithorst V.J., Byars A.W. fMRI study of language lateralization in children and adults. Hum. Brain Mapp. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J.P., Schmithorst V.J., Altaye M., Byars A.W., Ret J., Plante E., Holland S.K. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann. Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J.P., Altaye M., Rajagopal A., Eaton K., Meng X., Plante E., Holland S.K. A 10-year longitudinal fMRI study of narrative comprehension in children and adolescents. NeuroImage. 2012;63:1188–1195. doi: 10.1016/j.neuroimage.2012.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.G., Harshman R.A., Menon R.S. Noise reduction in BOLD-based fMRI using component analysis. NeuroImage. 2002;17:1521–1537. doi: 10.1006/nimg.2002.1200. [DOI] [PubMed] [Google Scholar]
- Thompson C.K., den Ouden D.-B., Bonakdarpour B., Garibaldi K., Parrish T.B. Neural plasticity and treatment-induced recovery of sentence processing in agrammatism. Neuropsychologia. 2010;48:3211–3227. doi: 10.1016/j.neuropsychologia.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Functional connectivity hubs in the human brain. NeuroImage. 2011;57:908–917. doi: 10.1016/j.neuroimage.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Resting functional connectivity of language networks: characterization and reproducibility. Mol. Psychiatry. 2012;17:841–854. doi: 10.1038/mp.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Wang G.-J., Volkow N.D. Energetic cost of brain functional connectivity. Proc. Natl. Acad. Sci. U. S. A. 2013;110:13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Shokri-Kojori E., Volkow N.D. High-Resolution Functional Connectivity Density: Hub Locations, Sensitivity, Specificity, Reproducibility, and Reliability. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas J.R., Davalos D.B., Rojas D.C. Effect of task difficulty on the functional anatomy of temporal processing. NeuroImage. 2006;32:307–315. doi: 10.1016/j.neuroimage.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Turken U., Dronkers N.F. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front. Syst. Neurosci. 2011;5 doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Daal J., Verhoeven L., van Leeuwe J., van Balkom H. Working memory limitations in children with severe language impairment. J. Commun. Disord. 2008;41:85–107. doi: 10.1016/j.jcomdis.2007.03.010. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Stam C.J., Kahn R.S., Pol H.E.H. Efficiency of functional brain networks and intellectual performance. J. Neurosci. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.P., Kersbergen K.J., de Reus M.A., Keunen K., Kahn R.S., Groenendaal F., de Vries L.S., Benders M.J. The neonatal connectome during preterm brain development. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Herve P.-Y., Duffau H., Crivello F., Houde O., Mazoyer B., Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wang L., Negreira A., LaViolette P., Bakkour A., Sperling R.A., Dickerson B.C. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010;20:345–351. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D., Roberts K., Visscher K., Woldorff M. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- White T.P., Jansen M., Doege K., Mullinger K.J., Park S.B., Liddle E.B., Gowland P.A., Francis S.T., Bowtell R., Liddle P. Theta power during encoding predicts subsequent-memory performance and default mode network deactivation. Hum. Brain Mapp. 2013;34:2929–2943. doi: 10.1002/hbm.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Moran J.M., Nieto-Castañón A., Triantafyllou C., Saxe R., Gabrieli J.D. Associations and dissociations between default and self-reference networks in the human brain. NeuroImage. 2011;55:225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H.-D., Fonteijn H.M., Norris D.G., Hagoort P. Topographical functional connectivity pattern in the perisylvian language networks. Cereb. Cortex. 2010;20:549–560. doi: 10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., Colcombe S., Craddock R.C., Di Martino A., Li Q., Zuo X.N., Castellanos F.X., Milham M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Craddock R.C., Zuo X.N., Zang Y.F., Milham M.P. Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. NeuroImage. 2013;80:246–262. doi: 10.1016/j.neuroimage.2013.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q., Ross T.J., Gu H., Geng X., Zuo X.N., Hong L.E., Gao J.H., Stein E.A., Zang Y.F., Yang Y. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Hum. Brain Mapp. 2013;34:3204–3215. doi: 10.1002/hbm.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Ehmke R., Mennes M., Imperati D., Castellanos F.X., Sporns O., Milham M.P. Network centrality in the human functional connectome. Cereb. Cortex. 2012;22:1862–1875. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]
- Zuo X.N., Xu T., Jiang L., Yang Z., Cao X.Y., He Y., Zang Y.F., Castellanos F.X., Milham M.P. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. NeuroImage. 2013;65:374–386. doi: 10.1016/j.neuroimage.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.