Abstract
Background
Immunotherapy is an ideal treatment modality to specifically target the diffusely infiltrative tumor cells of malignant gliomas while sparing the normal brain parenchyma. However, progress in the development of these therapies for glioblastoma has been slow due to the lack of immunogenic antigen targets that are expressed uniformly and selectively by gliomas.
Methods
We utilized human glioblastoma cell cultures to induce expression of New York–esophageal squamous cell carcinoma (NY-ESO-1) following in vitro treatment with the demethylating agent decitabine. We then investigated the phenotype of lymphocytes specific for NY-ESO-1 using flow cytometry analysis and cytotoxicity against cells treated with decitabine using the xCelligence real-time cytotoxicity assay. Finally, we examined the in vivo application of this immune therapy using an intracranially implanted xenograft model for in situ T cell trafficking, survival, and tissue studies.
Results
Our studies showed that treatment of intracranial glioma–bearing mice with decitabine reliably and consistently induced the expression of an immunogenic tumor-rejection antigen, NY-ESO-1, specifically in glioma cells and not in normal brain tissue. The upregulation of NY-ESO-1 by intracranial gliomas was associated with the migration of adoptively transferred NY-ESO-1–specific lymphocytes along white matter tracts to these tumors in the brain. Similarly, NY-ESO-1–specific adoptive T cell therapy demonstrated antitumor activity after decitabine treatment and conferred a highly significant survival benefit to mice bearing established intracranial human glioma xenografts. Transfer of NY-ESO-1–specific T cells systemically was superior to intracranial administration and resulted in significantly extended and long-term survival of animals.
Conclusion
These results reveal an innovative, clinically feasible strategy for the treatment of glioblastoma.
Keywords: cancer, decitabine, engineered T cells, glioblastoma, immunotherapy
T cell–based immunotherapy is a particularly appealing strategy for the treatment of malignant gliomas due to the inherent high specificity, lytic activity, and ability for T lymphocytes to traffic to distant tumor sites. These T cells could theoretically target infiltrating tumor cells that have permeated into the normal brain parenchyma beyond the reaches of standard surgical resection and are yet unrecognizable by conventional imaging methods.1,2 While there has been a long-standing interest in applying immunotherapy to primary malignant brain tumors, these efforts have shown little in the way of objective therapeutic efficacy. Part of the difficulty has been: (i) the lack of well-defined immunotherapeutically targetable antigens that are expressed consistently and uniformly by glioma cells, (ii) uncertainty about the ability of immune cells to traffic into the central nervous system, and (iii) systemic and local immune defects in glioblastoma patients that alter the ability of T cells to mediate injury to tumor cells. Here we demonstrate a strategy that can be used to overcome many of these critical barriers to effective brain tumor immunotherapy.
The therapeutic use of engineered lymphocytes to target tumor-associated antigens is gaining traction as a viable potential therapeutic modality for cancer. In this study, we combined the selective in vivo induction of New York–esophageal squamous cell carcinoma (NY-ESO-1), an immunogenic tumor-rejection antigen, in glioblastomas with the adoptive transfer of T cells retrovirally transduced to express the NY-ESO-1 T-cell receptor (TCR). We demonstrated that engineered TCR-transduced T cells migrate through the parenchyma of the brain, and elicited enhanced therapeutic benefit after systemic delivery in animals harboring well-established intracranial gliomas.
Materials and Methods
Cell Lines and Biological Samples
The human glioblastoma cell lines U-251MG and T98G were obtained from the American Type Culture Collection. The primary glioblastoma cell explant, 13-06-MG, was established from surgically resected tissue of a human leukocyte antigen (HLA)–A*0201+ individual.3 The human primary melanoma cell lines M407 and 624.38, which express endogenous NY-ESO-1, were supplied by Antoni Ribas. Further details are provided in the Supplemental Methods. Peripheral blood mononuclear cells (PBMCs) were obtained from healthy human volunteers at UCLA Medical Center after leukapheresis. Lymphocytes were isolated by density gradient centrifugation as previously described.4 Written informed consent and institutional review board approval were obtained for all studies involving human blood and tissues.
NY-ESO-1 TCR-Transduced Lymphocytes
The PG13-based stable retroviral packaging cell line encoding an HLA-A*0201–restricted NY-ESO157–165 specific TCR was generated as described5 and obtained from Dr Paul Robbins (Surgery Branch, NCI/NIH). Briefly, anti-CD3 (clone OKT-3; 50 ng/mL) was used to stimulate human PBMCs for 48 h prior to double transfection with supernatants from the PG13-based retroviral producer cell line, as described.5,6 The cells were expanded for 3 days in the presence of interleukin (IL)-2 and then rested for 2 days in the absence of IL-2.
In vitro Glioma Cell Treatment with Decitabine
Decitabine (DAC), supplied by Eisai Pharmaceuticals, was reconstituted in dimethyl sulfoxide as a 10-μM stock solution. After allowing tumor cells (106 cells/mL) to culture overnight, the cell culture medium was supplemented with 1 μM DAC. The cells were treated again the following day with fresh cell culture medium with or without DAC. On the third day of culture, fresh medium without DAC was placed onto the cells and cultured for additional time periods before assaying.
In vitro Cytotoxicity Assays
Cytotoxic killing of tumor cells was assessed using the xCELLigence Real-Time Cell Analyzer System (Acea Biotechnology). Target cells (U-251MG or 13-06-MG gliomas, DAC-treated [DAC+] or nontreated [DAC−] were plated at day 0 (105 cells/well) in 150 µL of medium. After overnight tumor cell adherence to the well bottom, effector cells (NY-ESO-1 TCR-transduced T cells) were added at effector:target (E:T) ratios of 10:1, 5:1, 1:1, and 1:10. Maximal cell release was obtained by adding 1% Triton X-100 to the wells. Cell index values (relative cell impedance) were collected over 30 h and normalized to the maximal cell index value immediately prior to effector cell plating. The percentage lysis was calculated as a proportion of the normalized cell index at a time point of interest versus the normalized cell index at the point of initial effector cell plating.7
In vivo Trafficking Studies
For evaluation of T cell trafficking, female NOD scid gamma-chain deficient (NSG) mice, 6–10 weeks of age, were implanted with U-251MG glioma cells (2.5 × 105 in 2 μL phosphate buffered saline [PBS]) in the right frontal lobe. Further details are provided in the Supplemental Methods. Mice bearing one-week established tumors then received i.p. DAC (10 mg/kg) or control PBS on days 3, 5, and 7 post tumor implant, for a total of 3 doses. Two days following the last DAC injection, intracranial administration of lymphocytes occurred using the same coordinates, but at the contralateral position from the tumor implant. Brains were collected 2 days after adoptive cell transfer (ACT) and processed for immunohistochemistry (IHC).
In vivo Survival Studies
For survival studies, mice were implanted with U-251MG or 13-06-MG glioma cells (2.5 × 105 in 2 μL PBS) as described before. Mice were then randomized into treatment groups (n = 6–8 mice/group) and injected i.p. with 10 mg/kg DAC or control as described. NY-ESO-1 TCR engineered T cells or nontransduced PBMCs (106/2 μL) were administered intratumorally (i.t.) at the same ipsilateral coordinates, or systemically by i.v. injection (107 cells/100 μL). Immediately after ACT, 105 IU of IL-2 in 100 μL or 100 μL of PBS 1× was administered i.p. and again at 24 and 48 h post cell transfer for T cell support. The mice were monitored over time for symptoms of disease progression and sacrificed according to institutional guidelines. The UCLA Animal Research Committee approved all experiments. Additional details are included in the Supplemental Methods section. Survival plots were generated using the Kaplan–Meier method and plotted using GraphPad Prism software.
In vivo Cellular Characterization Studies
For analysis requiring harvest of brain and splenic tissue, NSG mice were implanted with U-251MG glioma and received i.p. DAC one-week post-implant as described before. NY-ESO-1 TCR engineered T cells (106/2 μL) were administered intravenously or intratumorally as described previously. Animals were sacrificed 72 h after ACT and the tumor-bearing brain hemispheres and spleens were harvested. Infiltrating lymphocytes from brain were obtained using a Percoll gradient. Cells were stained with anti-human CD3 phycoerythrin (PE)-Cy7 (clone SK7; eBioscience), CD4 Alexa Fluor 700 (clone RPA-T4; BD Biosystems), CD8 Pacific Blue (clone SFCI21Thy2D3; BD Biosystems), and TCRvβ13.1-PE (clone IMMU 222; Beckman Coulter).
Quantitative Real-Time PCR
Total RNA was isolated with the RNeasy Mini kit (Qiagen) according to manufacturer's protocol. Human glioblastoma cancer cell lines (3 × 106) or tumor tissue specimens (25 mg) were used. Quantitative real-time (RT) PCR was performed with the LightCycler RT-PCR system (Roche), using the LightCycler SYBR green mastermix (Roche). Additional details are provided in the Supplemental Methods.
Western Blotting
Membrane-bound proteins were probed with monoclonal antibodies to NY-ESO-1 (Upstate Biotechnology) and β-actin (Sigma) and then visualized with a secondary antibody conjugated to horseradish peroxidase (1:2500, Jackson ImmunoResearch Laboratories) using a chemiluminescent substrate (Thermo-Scientific). X-ray films were scanned on a BioRad Gel Doc with Image Lab software.
Histology and Immunohistochemistry
Histology and IHC staining were performed as previously published.8 To evaluate the induction of NY-ESO-1 in orthotopic glioma xenografts, DAC-treated and untreated control mice (n = 8/group) were sacrificed on day 21 after implantation. Whole brains were rapidly harvested and placed in formalin overnight prior to being embedded in paraffin. Serial sections (5 μm) were deparaffinized, then either stained with hematoxylin and eosin or used for IHC with antibodies to NY-ESO-1 (Life Technologies), CD3, or CD8 (both Biocare Medical). Further details are provided in the Supplemental Methods.
Statistical Analysis
Continuous variables were compared using Student's t-test. Results comparing more than 2 groups were analyzed by ANOVA followed by Kruskal–Wallis statistics. Values were considered significant at P < .05. Median survival times were estimated using the Kaplan–Meier method. Within each glioma xenograft type, study comparisons between treatments were carried out using a log-rank test. A Cox proportional hazards model, stratifying on the glioma xenograft types, was also used to compare the effectiveness of treatments after combining the 2 different xenograft models. SAS 9.3 software was used for all statistical analyses.
Results
Decitabine Induces Durable Expression of NY-ESO-1 in Glioblastoma In vitro and In vivo
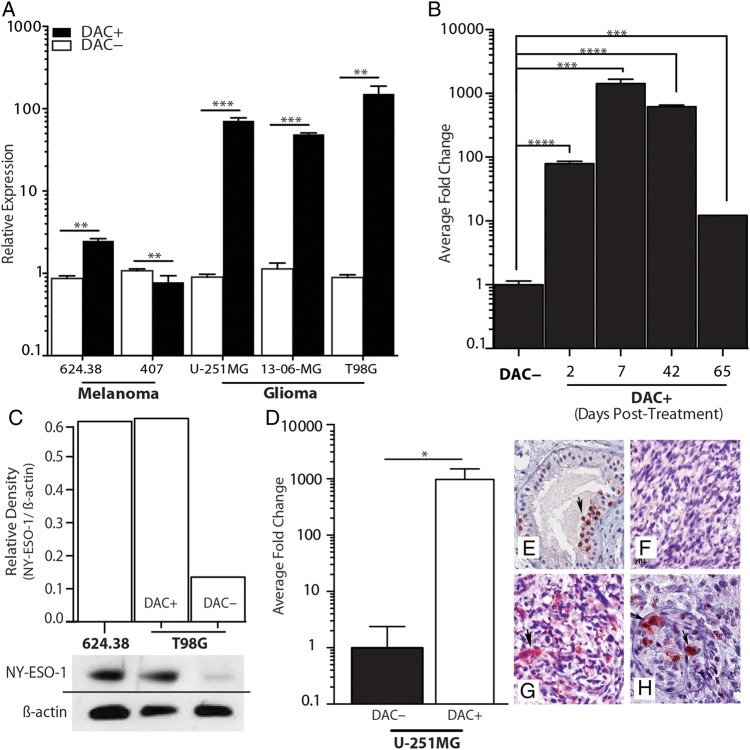
In our previous studies, we discovered that the cancer-testis antigen NY-ESO-1 was highly upregulated after a short, low-concentration in vitro exposure to the demethylating agent DAC.9 To translate these findings to a therapeutic model and assess the generalizability of NY-ESO-1 upregulation in glioblastoma, we examined the extent and durability of NY-ESO-1 upregulation in established glioma cell lines and on primary, surgically resected glioblastoma cells after a 48-h in vitro exposure to DAC. DAC induced rapid, robust expression of NY-ESO-1 in all glioma cells tested at both the mRNA and protein levels (Fig. 1A and C). This short exposure to DAC resulted in durable NY-ESO-1 upregulation that was observed up to day 65 in culture after a single course of DAC (Fig. 1B). NY-ESO-1 expression was also induced in situ in an orthotopic intracranial glioma model, when intracranial glioma–bearing mice (U-251MG) were treated with DAC (10 mg/kg/d, every other day for 3 injections). Quantitative PCR of NY-ESO-1 exhibited a 500-fold increase in NY-ESO-1 expression in tumor tissue isolated from animals treated with DAC compared with a placebo (Fig. 1D). Similarly, IHC staining for NY-ESO-1 protein also demonstrated tumor-restricted expression of NY-ESO-1 in glioma-bearing mice treated with DAC (Fig. 1E–H). Thus, short periods of DAC exposure resulted in rapid and extended upregulation of NY-ESO-1 in vitro and in vivo.
Fig. 1.
Upregulation of NY-ESO-1 by DAC treatment in vitro and in situ. (A) Relative expression of NY-ESO-1, with or without DAC (1 μM) treatment for 48 h (DAC+ and DAC−, respectively), was examined in 2 melanoma cell lines, 624.38 and 407, which endogenously express NY-ESO-1. Only slight upregulation was noted when treated with DAC. Glioma cell lines U-251MG, 13-06-MG, and T98G show dramatic upregulation of NY-ESO-1 after DAC treatment (**P < .01, ***P < .001, Student's t-test). (B) Time-course expression of NY-ESO-1 in U-251MG glioma cells after treatment with DAC. Expression measured by quantitative (q)PCR from cells harvested at the indicated time points (***P < 0.001, Student's t-test). (C) Verification of gene expression at the protein level in 624.38 and T98G glioma cells, with and without DAC treatment by western blot. Relative density (NY-ESO-1/β-actin) is plotted. (D) Mice with intracranial U-251MG tumors were treated with control (DAC−) or DAC (DAC+); tumors were then excised at day 19 post-implant and used either for qPCR or for IHC detection of NY-ESO-1 expression. The relative fold change in gene expression is graphed (*P < .05, Student's t-test). IHC staining on a positive testes control (E), as well as on brains from DAC− (F) and DAC+ (G and H) is displayed. Data shown are representative of one experiment repeated 3 times with similar findings.
Decitabine Treatment Immunosensitizes Gliomas to T Cell–Mediated Cytotoxicity Prior to Adoptive Cell Transfer
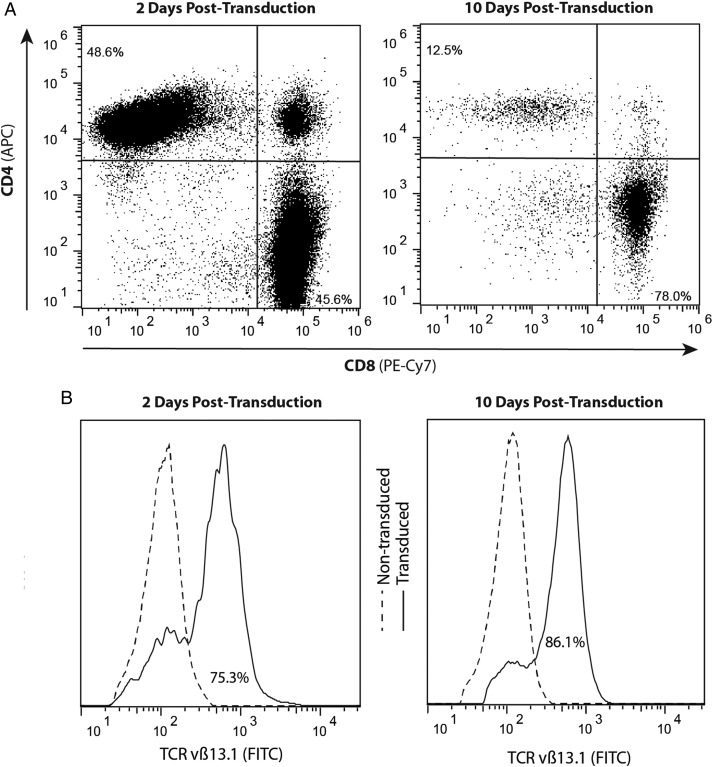
To evaluate whether DAC treatment sensitizes glioma cells to T cell recognition in vitro and in vivo, we transduced the NY-ESO-1–specific T cell receptor clone 1G4 into normal human peripheral blood lymphocytes utilizing a retroviral transduction system.5,6 Two days following transduction, flow cytometric analysis revealed equal distribution in CD4 and CD8 T cell populations. However, after 10 days of expansion culture containing IL-2, the population shifted predominantly to a CD8+ T cell phenotype (Fig. 2A). A representative analysis is shown in Fig. 2B, which reveals that ∼75% and 86% of the CD3+ T cells expressed the NY-ESO-1 TCR at days 2 and 10, respectively, posttransduction.
Fig. 2.
Retroviral transduction of the NY-ESO-1 TCR generates a high percentage of antigen-specific T cells, over a 10-day culture, prior to ACT. (A) Populations skew toward a CD3/CD8 cytotoxic T cell phenotype over 10 days of expansion. The percentages of CD8+ and CD4+ T cell populations were estimated by flow cytometry at 2 days (45.6% and 48.6%, respectively) and at 10 days (78.0% and 12.5%, respectively) following transduction and expansion in medium supplemented with 300 IU/mL of IL-2. APC, allophycocyanin. (B) Expression of TCRvβ13.1 by CD8+ T cells at 2 days (75.3%) and 10 days (86.1%) posttransduction compared with nontransduced T cells. Data shown are representative of one experiment repeated 4 times with similar findings. FITC, fluorescein isothiocyanate.
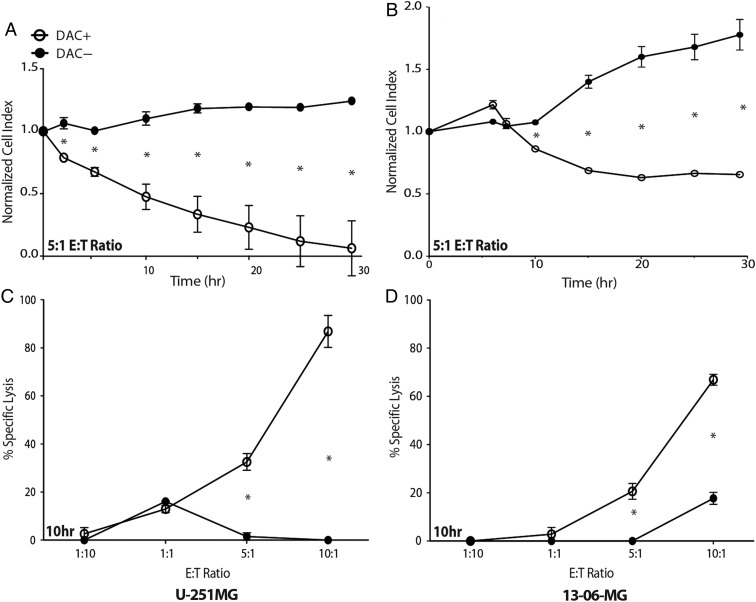
The antitumor cytotoxic effect of these engineered lymphocytes against DAC-treated and -untreated glioblastoma cells was evaluated using a real-time, impedance-based cytotoxicity assay (Fig. 3) and confirmed with a more conventional assay (Supplementary Fig. S1). NY-ESO-1 TCR-transduced T cells effectively and specifically lysed DAC-treated 13-06-MG and U-251MG targets (Fig. 3A–D). DAC-treated U-251MG and 13-06-MG cells were effectively killed at an E:T ratio as low as 5:1, with effects seen as early as 2 h (Fig. 3A and B) and in a dose-dependent manner (Fig. 3C and D). Little cytotoxicity to DAC-untreated cells was observed. Killing of U-251MG continued until almost complete lysis of target cells was achieved by 30 h (Fig. 3A).
Fig. 3.
Glioblastoma cells treated with DAC are susceptible to lysis by T cells engineered to express the NY-ESO-1 TCR. A real-time, impedance-based cytotoxicity assay (xCelligence) was used to evaluate the lysis of (A and C) U-251MG and (B and D) 13-06-MG glioma cells over a 30-h period when coincubated with NY-ESO-1 TCR-transduced effector T cells. (A and B) Representative data for glioma cell lysis at an E:T ratio of 5:1. DAC-treated (DAC+) cells are significantly more susceptible to lysis compared with untreated cells. (C and D) NY-ESO-1 TCR-transduced effector T cells were coincubated with (C) U-251MG and (D) 13-06-MG glioma cells at multiple E:T ratios (1:10, 1:1, 5:1, and 10:1). The percent specific lysis at 10 h is plotted (*P < .05, Student's t-test). Data shown are representative of one experiment repeated 7 times with similar findings.
NY-ESO-1–Specific T cells Administered Intracranially Effectively Traffic Through the Brain Parenchyma and Exhibit Antitumor Effects
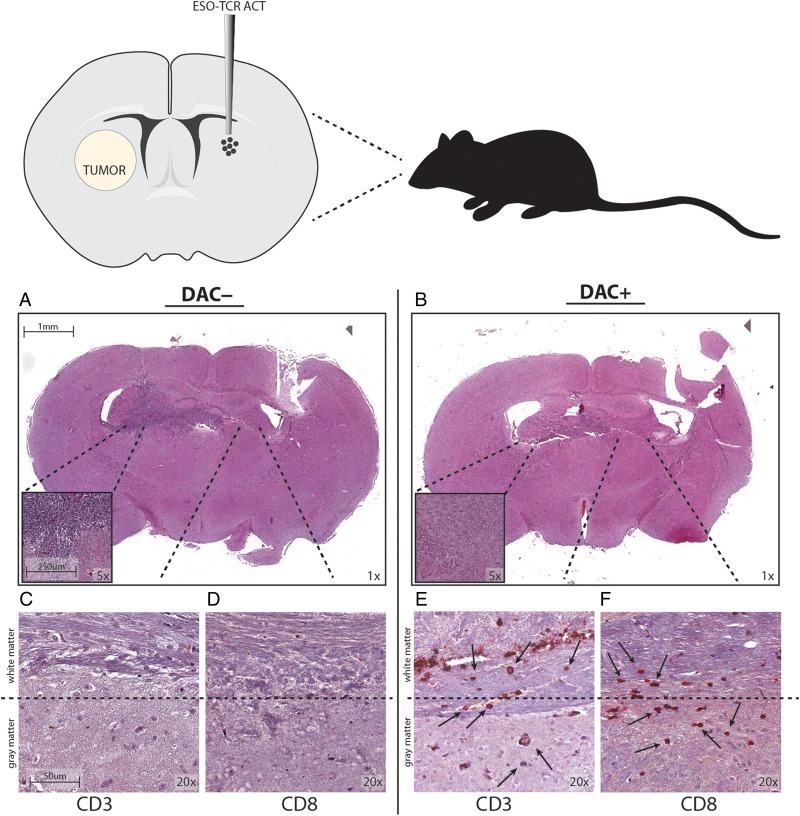
To evaluate the ability of NY-ESO-1–specific T cells to survive and traffic within the brain parenchyma, 2.5 × 105 U-251MG cells were stereotactically implanted into the right frontal lobe and allowed to establish for 7 days. DAC or control treatments were administered to animals systemically via i.p. injection. Subsequently, NY-ESO-1 engineered T cells (1 × 106) were injected into the contralateral hemisphere of the brain. Animals were sacrificed 48 hours after intracranial ACT and the brains were analyzed histologically for evidence of tumor and T cell trafficking. Tumor was readily detected in mice treated with NY-ESO-1 T cells in the absence of DAC (Fig. 4A), while no evidence of residual tumor was found in groups treated with DAC and NY-ESO-1 T cells (Fig. 4B). In mice treated with DAC, human CD3+ and CD8+ T lymphocytes were present at perivascular spaces within white matter (Fig. 4E and F). In particular, human T cells were noted within the white matter tracts of the corpus callosum, likely in transit from the contralateral hemisphere. Histologically evident tumor cell masses were found in all mice not treated with DAC. In these animals, T cells could be located only at their implantation site in the brain contralateral to the tumor (Fig. 4C and D). These studies indicate that DAC treatment induced changes that render tumor-specific T cells capable of migrating through white matter tracts toward tumor cells, even from the contralateral side of the brain.
Fig. 4.
DAC treatment of intracranial glioma-bearing mice permits trafficking of NY-ESO-1 TCR engineered T cells through the brain parenchyma. Mice with 1-week established intracranial U-251MG were treated with a vehicle control (DAC−) or with 10 mg/kg DAC i.p. × 3 days (DAC+). NY-ESO-1 TCR-transduced T cells were injected into the opposite hemisphere of the brain 2 days following the last DAC administration; 24 h later, the animals were sacrificed and the brains removed, sectioned, and immunolabeled for human CD3 and CD8. (A and B) Coronal sections (mag = 1×) stained with hematoxylin and eosin in mice given (A) control treatment or (B) DAC. (C–F) Injection of NY-ESO-1 TCR-transduced T cells into the hemisphere contralateral to the tumor implantation site at 2 days following the DAC treatment shows migration to contralateral human glioma xenografts. (C and D) DAC– group show lack of T cell infiltration into tumor, while (E and F) DAC+ group show trafficking through white matter (mag = 10×). Scale bar = 1 mm (A and B), 250 μm (A and B inset), and 50 μm (C–F). Data shown are representative of one experiment repeated twice with similar findings.
Prolonged Survival of Tumor-Bearing Mice Treated with Decitabine and Adoptive Cell Transfer of NY-ESO-1 TCR-Transduced Lymphocytes
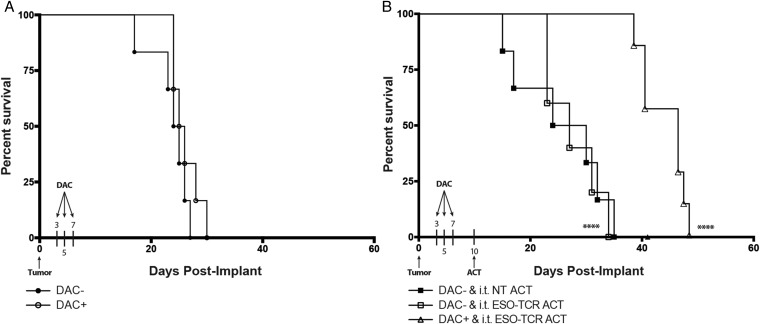
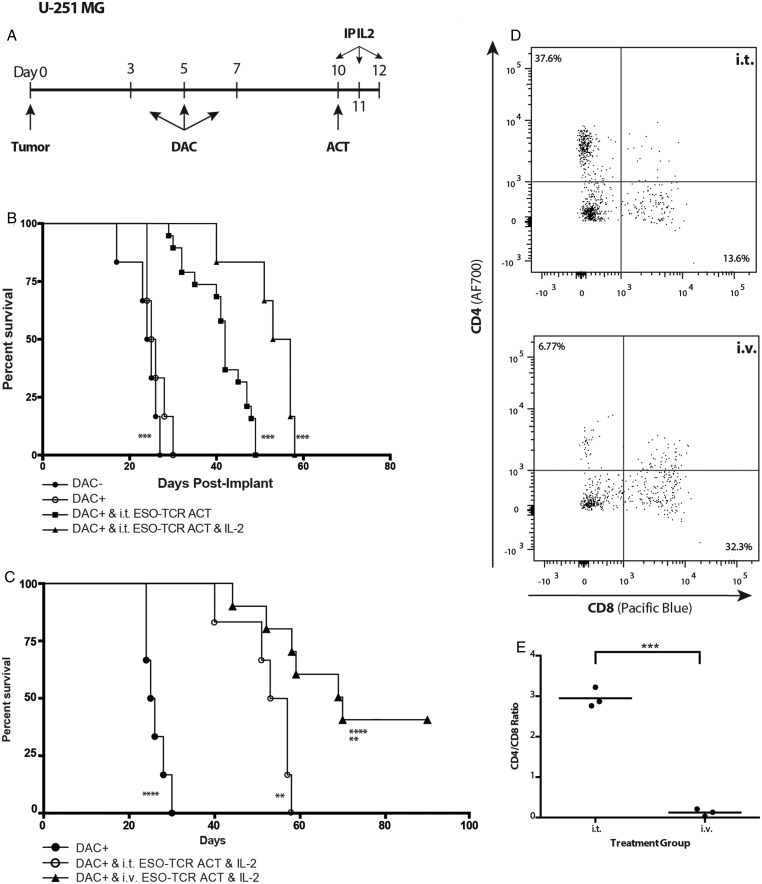
Finally, we evaluated whether a regimen of DAC chemotherapy prior to NY-ESO-1 targeted immunotherapy extended the overall survival of mice bearing intracranial glioma. Whereas DAC alone did not result in a significant survival differential between treated and untreated groups (Fig. 5A), intratumoral ACT of 106 NY-ESO-1–specific T cells following DAC treatment conferred a significant survival advantage for the combined treatment group compared with mice treated with nontransduced T lymphocytes or NY-ESO-1 TCR ACT in the absence of DAC chemotherapy (Fig. 5B). These findings were recapitulated when the same experimental paradigm was also performed with mice bearing a primary, patient-derived glioblastoma cell culture (13-06-MG; Supplementary Fig. S2A). Further sequential improvements in survival were observed with the addition of systemic IL-2 for T cell support (Fig. 6B).
Fig. 5.
Extended survival of glioma-bearing mice that received DAC treatment followed by i.t. ACT of NY-ESO-1–specific T cells. (A) Groups of NSG mice (n = 6/group) were implanted with 2.5 × 105 U-251MG glioma cells into the brain. Beginning at day 3 post tumor implant, the animals were treated with 10 mg/kg DAC i.p. every other day for a total of 3 doses (DAC+) or left untreated (DAC−). No significant difference in median survival was noted (DAC−, 24.5 d; DAC+, 25.5 d). (B) NSG mice (n = 6/group) were intracranially implanted with 2.5 × 105 U-251MG glioma cells. Two groups received i.t. ACT of NY-ESO-1 TCR engineered T cells (ESO-TCR ACT) or nontransduced T cells (NT ACT) (106 cells/2 μL) on day 10 in the absence of DAC treatment. One group received NY-ESO-1 TCR engineered T cells (ESO-TCR ACT) (106 cells/2 μL) on day 10 following DAC treatment regimen as described before. Significant differences were noted between DAC+ and i.t. ESO-TCR ACT and DAC− and i.t. NT ACT groups (****P < .0001, log-rank survival test). Data shown are representative of one experiment repeated 3 times with similar findings.
Fig. 6.
Systemic IL-2 support and i.v. route of delivery enhance the therapeutic benefit of NY-ESO-1–specific T cell adoptive transfer. (A) Treatment regimen. Mice were intracranially implanted with 2.5 × 105 U-251MG glioma cells. At day 3 post tumor implantation, the animals were treated with 10 mg/kg DAC (DAC+) i.p. every other day for a total of 3 doses (days 3, 5, 7 post tumor implant). Three days following the last injection (day 10 post tumor implant), mice received i.t. adoptive transfer of NY-ESO-1 TCR engineered T cells followed by IL-2 (500 000 IU in 200 μL) or PBS 1× (200 μL) on days 10, 11, and 12 post tumor implant. (B) The mice were then followed for survival. Significant differences were noted between DAC+ and i.t. ESO-TCR ACT and IL-2 and DAC−, as well as between DAC+ and i.t. ESO-TCR ACT and IL-2 and DAC+ and i.t. ESO-TCR ACT groups (***P < .001, log-rank survival test and Cox proportional hazards model). (C) Three days following the final DAC injection (day 10 post tumor implant), experimental mice received i.t. or i.v. ACT of NY-ESO-1 TCR engineered T cells (i.t., 106 cells/2 μL; i.v., 107 cells/100 μL). Treated mice received i.p. injection of IL-2 (500 000 IU in 200 μL) on days 10, 11, and 12 post tumor implant. The mice were then followed for survival. Significant survival differences were observed between DAC+ and DAC+ and i.v. ESO-TCR ACT and IL-2, between DAC+ and i.t. ESO-TCR ACT and IL-2, and between DAC+ and i.v. ESO-TCR ACT and IL-2 (**P < .01, ****P < .0001, log-rank survival test and Cox proportional hazards model). The survival curves are each representative of one experiment repeated 3 times with similar findings. (D) On day 13 post tumor implant, mice were sacrificed and brain tumor–infiltrating lymphocytes harvested and stained by fluorescence activated cell sorting. The histograms depict representative animals after either i.t. (top) or i.v. (bottom) delivery. (E) The tumor-infiltrating lymphocytic population shows a significantly decreased CD4/CD8 ratio in the i.v.-treated group compared with the i.t.-treated group (***P < .001, Student's t-test).
To evaluate whether the route of administration influenced our ability to treat mice bearing an established intracranial glioma, we treated animals with intravenous or intratumoral ACT of NY-ESO-1 TCR engineered T lymphocytes. Surprisingly, the systemic i.v. delivery of these lymphocytes conferred a significantly greater therapeutic benefit than i.t. delivery (Fig. 6C). More than 40% of the animals treated with systemic i.v. ACT completely rejected the intracranial tumor and survived long-term. Again, these findings were recapitulated with similar experiments performed in mice bearing 13-06-MG primary human patient–derived GBM intracranial xenografts (Supplementary Fig. S2B). Thus, the i.v. delivery of NY-ESO-1 TCR engineered T cell adoptive transfer was statistically more effective than i.t. delivery in conferring a survival benefit to mice bearing established intracranial glioma across multiple models (P < .05, log-rank test and stratified Cox regression; Supplementary Tables S1 and S2), suggesting that the blood–brain barrier may not be as limiting a factor to the efficacy of brain tumor immunotherapy as previously believed. These studies prove that a course of DAC renders human glioblastomas susceptible to NY-ESO-1–specific T cell adoptive transfer immunotherapy in an in vivo, orthotopic glioblastoma model.
Systemic Administration Route Promotes a CD8-Dominant Tumor-Infiltrating T Lymphocyte Population
In a parallel series of experiments, instead of monitoring animals for survival, animals were sacrificed 72 h following ACT; the tumor-bearing brain hemispheres and spleens were harvested and processed for flow cytometric analysis of T cell infiltration. NY-ESO-1 engineered T cell infiltration into tumor was observed in both i.t. and i.v. treatment models (Fig. 6D). However, mice that received T cells systemically had a significant increase in the TCRvβ13.1+CD8+ population and a complete reversal of the CD4/CD8 ratio observed (Fig. 6E). Similarly, i.v. delivery resulted in the trafficking of engineered T cells to the spleen. In mice that received i.t. delivery, very few transferred T cells could be found in any lymphoid organs (Supplementary Fig. S3). These findings suggest that i.v. delivery results in the expansion of T cells in vivo, preferential selection for a CD8+ population with a major histocompatibility complex class I–restricted TCR, and CNS homing toward antigen expression.
Discussion
Adoptive cell transfer and other immunotherapeutic modalities have been increasingly successful in the treatment of human cancers, such as malignant melanoma and other malignant solid tumors.6,10–12 Recent advances in techniques to generate the large numbers of antigen-specific T cells required for effective adoptive transfer have recently made the technique clinically feasible. In these studies, we utilized a retroviral system to engineer NY-ESO-1–specific T lymphocytes for the treatment of glioblastoma. Administration of the hypomethylating agent, DAC, selectively upregulated NY-ESO-1 on intracranial glioblastomas in vivo and allowed for effective ACT immunotherapy with NY-ESO-1 TCR engineered T lymphocytes. NY-ESO-1–specific T lymphocytes could traffic through the normal brain parenchyma and migrate along white matter tracts toward tumor cells in mice only pretreated with DAC. Interestingly, we found that the transfer of T cells by systemic infusion was superior to the local, intracranial delivery of NY-ESO-1–specific T cells in this orthotopic model. These important findings highlight the ability of a demethylating agent to upregulate a well-characterized, immunogenic cancer-testis antigen in the brain, which can be targeted by systemic intravenous delivery of T cells targeting the upregulated antigen.
This strategy overcomes several of the current shortcomings of immunotherapy for glioma. Our treatment regimen induced the expression of a highly immunogenic antigen target in all glioma cell lines tested thus far in vitro and in vivo. This demethylating agent, DAC, induced persistent and durable expression of NY-ESO-1 in situ. This drug is also known to cross the blood–brain barrier, making it amenable to produce the effects on intracranial tumors. Our dosing regimen also is safe and nontoxic enough to be administered repeatedly so that expression of NY-ESO-1 is maintained over time. This dose was calculated to be slightly less than the equivalent FDA-approved dose for patients with myelodysplastic syndrome.13 Engineered NY-ESO-1–specific T cells have potent antitumor functionality and can be generated relatively easily in large numbers in a short amount of time, which is critical when facing a rapidly progressing disease such as glioblastoma.
Initially, our adoptive transfer model focused on the intratumoral delivery of T cells, a strategy typically employed for intracranial glioma because of the perceived barriers of cellular delivery across the blood–brain barrier. Malignant gliomas rarely metastasize outside of the CNS, making local delivery a logical choice. In this manner, T cells can be delivered directly into tumor-containing areas and do not have to cross the blood–brain barrier. Off-target effects directed against cells elsewhere in the body that endogenously expressed NY-ESO-1, or were induced by systemic administration of DAC, might also be minimized. While we found evidence of efficacy in our model system using the intracranial administration of T cells, only relatively small volumes can be successfully implanted by stereotactic injection, significantly limiting the number of cells that could be injected. The use of larger volumes in the brain can be injurious as well, and lead to reflux of the infusate. This fact becomes increasingly apparent when trying to deliver agents into tumor-bearing areas, where the interstitial pressure is presumably higher due to increased cell density. When extrapolated to a potential human therapy, where ∼1010 cells have been administered in successful trials,6 the volume requirement to transfer this number of cells becomes untenable for intracranial injection.
To circumvent this limitation, we first evaluated the effects of adding systemic IL-2 to enhance T cell proliferation as well as effector function after adoptive transfer. Careful studies aimed at elucidating the requirements for successful adoptive transfer immunotherapy have essentially deemed such IL-2 support a necessity for successful adoptive transfer immunotherapy.14 However, with regard to the treatment of intracranial tumors, investigators in the past have been hesitant to administer high-dose IL-2 to patients due to the theoretical concern that the ensuing inflammation could lead to malignant cerebral edema and hemorrhage. However, several large retrospective studies have revealed that the presence of brain metastases posed no additional risk for patients undergoing IL-2 therapy.15,16 The addition of IL-2 led to enhanced survival of mice treated with the DAC/adoptive transfer paradigm. However, all animals ultimately succumbed to their tumors, even when treated with intratumoral ACT plus IL-2.
As an alternative to intracranial injection, we then decided to evaluate the systemic adoptive transfer of NY-ESO-1 engineered T cells. In melanoma models, adoptive immunotherapy for established tumors in mice would require higher numbers of adoptively transferred cells (∼107) to be effective.14,17 While the maximum feasible intracranial dose is around 1–2 × 106 in mice, transfer of this number of cells is easily achieved in the higher volumes afforded by an intravenous injection of the cells into the systemic circulation. Since it is unknown what proportion of these cells might ultimately reach an intracranial tumor target, given different volumes of distribution and requirements for trafficking to a distant tumor target, a dose-to-dose comparison of systemic versus intracranial adoptive transfer becomes less meaningful. Nevertheless, it is notable that in clinical trials of ACT for the treatment of melanoma, clinical responses and regression of brain metastases have been demonstrated,10 indicating that systemically transferred cells are indeed able to traverse a tumor-affected blood–brain barrier. Additionally, clinical trials of ACT against NY-ESO in melanoma and synovial sarcoma demonstrated no toxicities attributable to on-target/off-tumor effects after the systemic infusion of adoptively transferred cells, which was a considerable theoretical concern given that NY-ESO is expressed abundantly in the testis of the adult male, and that fatal toxicities were attributed to such effects in clinical trials of adoptive transfer against other targets.6,18
In our studies, the systemic administration of a larger number of antigen-specific lymphocytes, coupled with IL-2 support, demonstrated vastly superior antitumor effects and was able to greatly prolong the survival of tumor-bearing mice to the point that animals were “cured” of their disease. Previous studies have shown that the i.v. adoptive transfer of activated antigen-specific T cells is successful in various disease paradigms where the antigen is present in the CNS.19,20 However, specific comparisons between i.v. and direct administration of T cells in the setting of a progressively growing glioma have not previously been made. We believe that the survival and proliferation of T cells is likely enhanced in the circulation, given the less hostile environment faced there. Relevant integrin homing markers may help direct these effector cells to sites of tumor and traverse the blood–brain barrier. In addition, i.v. delivery resulted in the selective enrichment of a CD8+ NY-ESO-1 T cell population, suggesting that these cells proliferated in vivo and selectively trafficked to the sites of antigen expression on glioma cells in the brain. Previous attempts to target DAC-upregulated antigens in mouse models of glioma have used only intratumoral administration and have demonstrated only short-lived antitumor effects.21 In light of the present studies, this was likely due to the limited numbers of cells infused directly into the tumor and the lack of IL-2 support of the adoptively transferred cells. We show for the first time in an orthotopic glioma model that systemic delivery of adoptively transferred T cells is more effective than local i.t. delivery, which may shift our current paradigms for immune-based treatment of this disease.
Overall, our data strongly suggest that the in vivo application of T cell–based immunotherapy can be established by systemic introduction of engineered cells, avoiding the requirement for invasive intracranial injections and their associated risks. The data presented here demonstrate that epigenetic upregulation of NY-ESO-1 by DAC, coupled with NY-ESO-1–specific T lymphocytes, has demonstrable antitumor activity against well-established glioblastomas in mouse models. While NY-ESO-1 is the first antigen we have explored, this same technique could be applied to any number of the antigens induced by DAC treatment either with adoptive transfer of engineered cells targeting multiple antigens or with polyvalent vaccines. A combination of epigenetic modulation and targeted immunotherapy provides a novel systemic treatment strategy for malignant gliomas.
Supplementary Material
Funding
This work was supported in part by NIH/NCI grants K01-CA111402 and R01-CA123396 (R.M.P.), R01-CA112358 (L.M.L.), RO1-CA125244 (C.A.K. and L.M.L.), NIH/NCATS UCLA CTSI grant no. UL1TR000124 (R.M.P., C.A.K., L.M.L.), the Eli & Edyth Broad Center of Regenerative Medicine and Stem Cell Research at UCLA (R.M.P. and L.M.L.), the STOP Cancer Foundation (R.M.P.), UCLA MSTP (J.P.A.), the Jonsson Comprehensive Cancer Center Foundation (R.G.E.), and the AANS Neurosurgery Research Education Fund (R.G.E.). Flow cytometry was performed at the UCLA Jonsson Comprehensive Cancer Center Core Facility, which is supported by NIH award P30-CA16042. Histopathology was supported by the UCLA Brain Tumor Translational Resource.
Supplementary Material
Acknowledgments
We thank Pradhan Bhat, Mitchell Flagg, and Alan J. Yaghoubian for their technical assistance.
Conflict of interest statement. None declared.
References
- 1.Mitchell DA, Sampson JH. Toward effective immunotherapy for the treatment of malignant brain tumors. Neurotherapeutics. 2009;6(3):527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vauleon E, Avril T, Collet B, Mosser J, Quillien V. Overview of cellular immunotherapy for patients with glioblastoma. Clin Dev Immunol. 2010;2010:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez GG, Varella-Garcia M, Kruse CA. Isolation of immunoresistant human glioma cell clones after selection with alloreactive cytotoxic T lymphocytes: cytogenetic and molecular cytogenetic characterization. Cancer Genet Cytogenet. 2006;165(2):121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–5525. [DOI] [PubMed] [Google Scholar]
- 5.Robbins PF, Li YF, El-Gamil M, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180(9):6116–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramis G, Martinez-Alarcon L, Quereda JJ, et al. Optimization of cytotoxicity assay by real-time, impedance-based cell analysis. Biomed Microdevices. 2013;15(6):985–995. [DOI] [PubMed] [Google Scholar]
- 8.Prins RM, Soto H, Konkankit V, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immuntherapy. Clin Cancer Res. 2011;17(6):1603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konkankit VV, Kim W, Koya RC, et al. Decitabine immunosensitizes human gliomas to NY-ESO-1 specific T lymphocyte targeting through the Fas/Fas ligand pathway. J Transl Med. 2011;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. [DOI] [PubMed] [Google Scholar]
- 14.Klebanoff CA, Gattinoni L, Palmer DC, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17(16):5343–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guirguis LM, Yang JC, White DE, et al. Safety and efficacy of high-dose interleukin-2 therapy in patients with brain metastases. J Immunother. 2002;25(1):82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell S, Dudek AZ. Single-institution outcome of high-dose interleukin-2 (HD IL-2) therapy for metastatic melanoma and analysis of favorable response in brain metastases. Anticancer Res. 2009;29(10):4189–4193. [PubMed] [Google Scholar]
- 17.Budhu S, Loike JD, Pandolfi A, et al. CD8+ T cell concentration determines their efficiency in killing cognate antigen-expressing syngeneic mammalian cells in vitro and in mouse tissues. J Exp Med. 2010;207(1):223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurschus FC. T cell mediated pathogenesis in EAE: molecular mechanisms. Biomed J. 2015;38(3):183–193. [DOI] [PubMed] [Google Scholar]
- 20.Floris S, Ruuls SR, Wierinckx A, et al. Interferon-beta directly influences monocyte infiltration into the central nervous system. J Neuroimmunol. 2002;127(1–2):69–79. [DOI] [PubMed] [Google Scholar]
- 21.Natsume A, Wakabayashi T, Tsujimura K, et al. The DNA demethylating agent 5-aza-2′-deoxycytidine activates NY-ESO-1 antigenicity in orthotopic human glioma. Int J Cancer. 2008;122(11):2542–2553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.