Abstract
Background
The efficacy of systemic antineoplastic therapy on recurrent World Health Organization (WHO) grades II and III meningiomas is unclear.
Methods
We performed a retrospective multicenter analysis of serial cranial MRI in patients with recurrent WHO II and III meningiomas treated with antineoplastic systemic therapies. Growth rates for tumor volume and diameter, as well as change rates for edema size, were calculated for all lesions.
Results
We identified a total of 34 patients (23 atypical, 11 anaplastic meningiomas) with a total of 57 meningioma lesions who had been treated at 6 European institutions. Systemic therapies included bevacizumab, cytotoxic chemotherapy, somatostatin analogues, and tyrosine kinase inhibitors. Overall, tumor growth rates decreased during systemic therapy by 51% for tumor diameter and 14% for tumor volume growth rates compared with the period before initiation of systemic therapy. The most pronounced decrease in meningioma growth rates during systemic therapy was evident in patients treated with bevacizumab, with a reduction of 80% in diameter and 59% in volume growth. Furthermore, a decrease in size of peritumoral edema after initiation of systemic therapy was exclusively observed in patients treated with bevacizumab (−107%).
Conclusions
Our data indicate that systemic therapy may inhibit growth of recurrent WHO grades II and III meningiomas to some extent. In our small cohort, bevacizumab had the most pronounced inhibitory effect on tumor growth, as well as some anti-edematous activity. Prospective studies are needed to better define the role of medical therapies in this tumor type.
Keywords: anaplastic meningioma, anti-angiogenesis, atypical meningioma, bevacizumab, chemotherapy
Meningiomas are the most frequent primary intracranial tumors in adults according to local and international brain tumor registries, with an incidence rate of about 30% and a female predominance, followed by glioblastoma and pituitary adenoma.1,2 Based on the World Health Organization (WHO) grade classification, meningiomas are histopathologically classified and graded into 3 different subgroups: benign meningiomas (WHO grade I), atypical meningiomas (WHO grade II), and anaplastic or malignant meningiomas (WHO grade III).3 The majority of these subgroups are benign, followed by atypical (up to 20% of all meningiomas) and anaplastic (about 1%–3% of all meningiomas).1
While benign meningiomas are usually curable by surgery, atypical and anaplastic meningiomas are characterized by aggressive behavior and a high recurrence rate.3 No accepted therapy standard exists for meningiomas after exhaustion of surgical and radiotherapeutic options. A variety of systemic antineoplastic therapeutic agents, including hydroxyurea, temozolomide, irinotecan, interferon-alpha, mifepristone, octreotide analogues, megestrol acetate, bevacizumab, imatinib, erlotinib, and gefitinib, have been investigated in small studies and are being used in the clinical setting.4–13 However, the validity of the available studies is limited by small sample sizes, retrospective study designs, heterogeneous patient populations, lack of control arms, and varying response criteria, thus rendering the benefit of these drugs unclear.14
In the present study, we aimed to analyze the growth rates of recurrent WHO grades II and III meningiomas before, during, and after treatment with systemic agents in order to provide novel information on the effect of drug treatment on the disease course.
Materials and Methods
Patients
Institutional review board approval for this retrospective European multicenter study was obtained from each participating institution. All participating institutions were asked to provide radiological as well as predefined epidemiological and clinical data of the patients using a prepared form (see Supplementary Material S1).
The inclusion criteria were as follows: (i) patient has histological diagnosis of WHO grade II or grade III meningioma according to 2007 WHO criteria; (ii) patient has received systemic antineoplastic therapy for tumor recurrence after previous operation and/or radiotherapy; (iii) digital datasets are available of multiple MR examinations taken before, during, and after the administration of systemic antineoplastic therapy.
Patient recruitment per institution was as follows: institution 1 (Vienna, n = 14), institution 2 (Leuven, n = 5), institution 3 (Lille, n = 4), institution 4 (Zurich, n = 4), institution 5 (Aarau, n = 4), and institution 6 (Essen, n = 3). First radiological diagnosis of meningioma was made between December 2002 and December 2013, and systemic antineoplastic therapy commenced between October 2005 and October 2013.
Image Acquisition
All patients received an MRI examination with a routine clinical imaging protocol of the brain. In total, 224 MRI examinations were included. The mean duration between 2 MRI examinations was 116 days (SD = 88 d). Each MRI examination included at least one T1-weighted sequence without and with contrast enhancement. In addition, a T2-weighted sequence was performed in 90.6% of all MRI examinations (203/224).
Image Analysis
The anonymized radiological data for participating patients from all institutions were collected in the form of digital data. The MR images were qualitatively evaluated at institution 1 on a PACS (Picture Archiving and Communication System, Centricity, GE Healthcare) workstation by an experienced neuroradiologist (J.F.) regarding their usability for further postprocessing. Image postprocessing was performed using open-source software (MRIcron).15 Maximum tumor diameter, tumor volume, and volume of peritumoral edema were measured for each of the 224 MRI examinations by the same neuroradiologist (J.F.), blinded to all clinical patient data. T1-weighted postcontrast images were selected to determine the maximum tumor diameter as well as the tumor volume. The T2-weighted images were used to depict tumor edema. Maximum tumor diameter was defined as the biggest diameter of the contrast-enhanced tumor area measured in axial, coronal, or sagittal image dimension. Tumor volume was automatically calculated on the basis of multiple manually defined regions of interests (ROIs) including the whole contrast-enhancing tumor area as well as cystic parts of the tumor in the T1-weighted postcontrast sequences. Analogous to the tumor volume, peritumoral edema was determined using multiple ROIs, including the peritumoral hyperintense signal alterations in T2-weighted images, excluding contrast-enhancing tumor areas.
Statistical Analysis
Resulting measurements from tumor volume, maximum tumor diameter, and the volume of peritumoral edema were submitted for further analysis. All lesions were subdivided into 3 different subgroups related to patients’ therapy status. The subgroup “pretherapeutic” represented all meningiomas before systemic therapy; the subgroup “therapeutic” included a pretherapeutic baseline measurement within 4 weeks before the start of systemic therapy, all measurements during systemic therapy, and the first measurement after systemic therapy if it was performed within 4 weeks after the completion of systemic therapy. All measurements after the systemic therapy are summarized in the “posttherapeutic” subgroup.
Measurements of maximum tumor diameter, maximum tumor volume, and maximum peritumoral edema volume of every lesion were used to derive gradients of decrease and increase, which are specified as average growth rates for tumor volume and diameter and average change rates for edemas throughout the manuscript. Growth and change rates were defined as the decrease or increase in tumor diameter, tumor volume, or peritumoral edema volume over a period of time (t), expressed as cm/t and cm3/t, respectively. The time period was determined by the average MR follow-up interval of the lesions. Pearson correlation coefficient was used to assess the relation between the calculated growth rates of tumor diameter and tumor volume.
A positive growth rate indicated an increase of tumor diameter, tumor volume, or edema volume, while a negative growth rate reflected a decrease of the different measurements. A growth rate of zero indicated no change in volume or diameter.
Statistical Package for the Social Sciences (SPSS) version 20.0 was used for descriptive statistics of the cohorts. Visualizations were performed in MatLab (7.14.0, Release 2012a; Mathworks). Testing for group differences between therapies was not utilized because of the small and unbalanced sample size.
Results
Patient Characteristics
We identified 34 patients with a total of 57 meningioma lesions that had been treated at 6 European institutions. Twenty-three patients (68%) had atypical meningiomas and 11 patients (32%) had anaplastic meningiomas. Systemic therapies included bevacizumab (n = 5), cytotoxic chemotherapy (n = 9), somatostatin analogues (n = 9), and tyrosine kinase inhibitors (n = 7). In 4 patients, radiological data during systemic therapy were not available, therefore these patients were included only in the “pretherapeutic” subgroup. Detailed patients’ baseline characteristics and the types of administered systemic therapies are given in Supplementary Material S2 and S3.
Thirty-two meningiomas were analyzable in the pretherapeutic time window, 37 meningiomas in the therapeutic time window, and 26 in the posttherapeutic time window. The mean time period was 140 days (SD = 124 d, range = 13–637; mean number of MR examinations = 3.65, range = 2–6) for all pretherapeutic MR examinations; 78 days (SD = 23, range = 41–156; mean number of MR examination = 3.13, range = 2–7) for systemic therapy; and 142 days (SD = 79, range = 21–334; mean number of MR examinations = 3.92, range = 2–7) for all posttherapeutic MR examinations.
Analysis of Solid Tumor Lesions
Measurements of maximum tumor diameter and tumor volume were represented as average growth rates and further subdivided into values for pretherapeutic lesions (n = 32), therapeutic lesions (n = 37), and posttherapeutic lesions (n = 26). Overall, diameter and volume growth rate values of the tumor lesions showed a high correlation in total (Pearson correlation coefficient r = 0.722; P = 1.4903×10−16), in the pretherapeutic (r = 0.794; P = 5.8995×10−8), therapeutic (r = 0.52; P = 0.001), and posttherapeutic (r = 0.892; P = 9.144×10−10) subgroups.
Table 1 and Figs 1A and B and 2A and B detail the tumor growth rates per time window and type of administered therapy. Overall, the mean tumor growth rates decreased by 51% for tumor diameter and 14% for tumor volume in the therapeutic period compared with the pretherapeutic period. Comparing the growth rates between the different therapy types, we observed the highest decrease of growth rate from the pretherapeutic to the therapeutic period in patients treated with bevacizumab (diameter: −80%, volume: −59%), followed by the subgroup of patients treated with chemotherapy (diameter: −54%, volume: +7%) and tyrosine kinase inhibitors (diameter: −40%, volume: −29%). Interestingly, in the posttherapeutic period, patients treated with bevacizumab showed the highest increase, with 200% in growth rates with regard to tumorous lesion diameter and the second highest increase in terms of tumor volume, with 50% in comparison with the therapeutic period. The lowest growth rates in the posttherapeutic period were observed with tyrosine kinase inhibitors, with regard to both tumor diameter and tumor volume.
Table 1.
Growth rates of all solid tumor lesions
| Time Window | Systemic Therapy | Analyzable Meningiomas, n | Length of Time Window, days |
Change in Diameter per Time [cm/time] |
Change in Volume per Time [cm3/time] |
|||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Pretherapeutic | All | 32 | 139.98 | 123.61 | 0.0100 | 0.0193 | 0.0488 | 0.9431 |
| Therapeutic | All | 37 | 78.00 | 22.58 | 0.0049 | 0.0080 | 0.0420 | 0.0700 |
| Bevacizumab | 9 | 75.56 | 7.50 | 0.0020 | 0.0080 | 0.0200 | 0.0430 | |
| Chemotherapy | 13 | 82.31 | 27.51 | 0.0046 | 0.0070 | 0.0523 | 0.0863 | |
| Somatostatin analogues | 4 | 73.25 | 14.84 | 0.0100 | 0.0000 | 0.0825 | 0.0860 | |
| Tyrosine kinase inhibitor | 11 | 76.64 | 27.84 | 0.0060 | 0.0120 | 0.0345 | 0.0624 | |
| Posttherapeutic | All | 26 | 141.92 | 78.52 | 0.0040 | 0.0090 | 0.0258 | 0.0569 |
| Bevacizumab | 5 | 78.40 | 4.66 | 0.0060 | 0.0134 | 0.0300 | 0.0670 | |
| Chemotherapy | 7 | 98.53 | 47.48 | 0.0040 | 0.0050 | 0.0186 | 0.0240 | |
| Somatostatin analogues | 11 | 194.98 | 87.01 | 0.0030 | 0.0090 | 0.0336 | 0.0760 | |
| Tyrosine kinase inhibitor | 3 | 154.50 | 0.00 | 0.0000 | 0.0000 | 0.0067 | 0.0060 | |
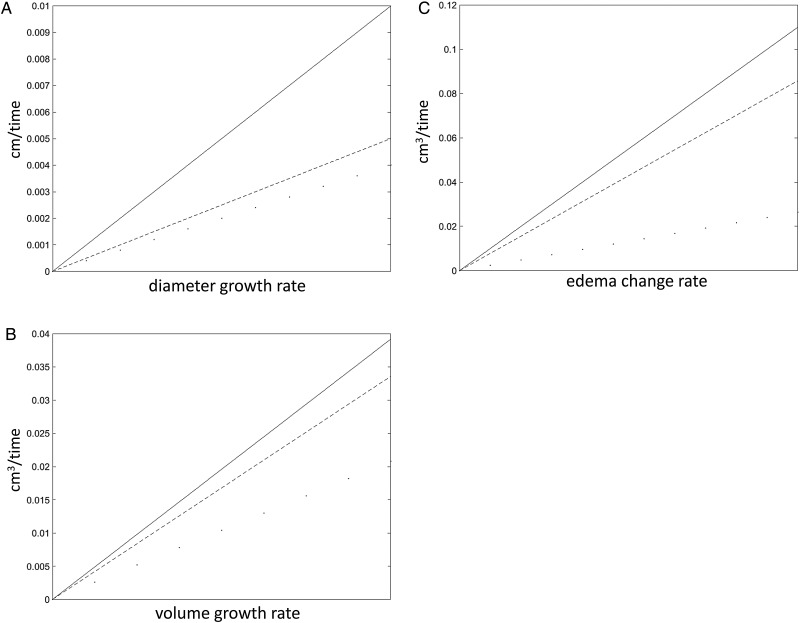
Fig. 1.
Visualization of change gradients of (A) tumor diameter, (B) tumor volume, and (C) peritumoral edema before (continuous lines), during (dashed lines), and after (dotted lines) systemic therapy. The slopes describe the direction (decrease or increase) and steepness (intensity) for the 3 groups, respectively. A very steep line therefore indicates a rapid increase. As clearly visible, growth velocity for all 3 measures (diameter growth rate, volume growth rate, edema change rate) degrades from before to after therapy.
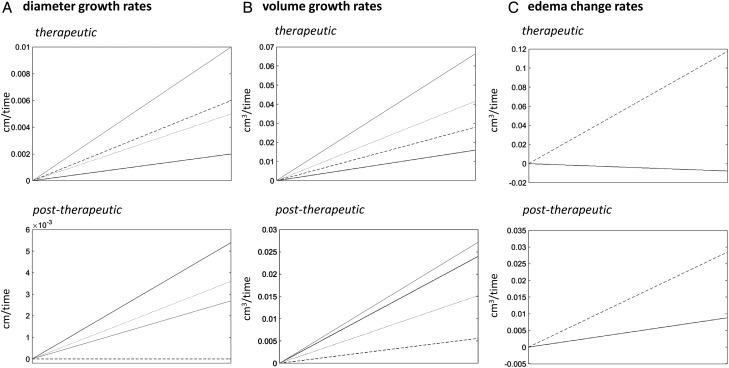
Fig. 2.
Growth rates expressed as lesion (A) diameter and (B) volume shown for different systemic therapies (black lines = bevacizumab; dotted lines = chemotherapy; grey lines = somatostatin analogues; dashed lines = tyrosine kinase inhibitor) in the therapeutic and posttherapeutic periods. Since the growth rate of lesion diameter after administration of tyrosine kinase inhibitor was zero, no dashed line is shown in this section. Column C represents the changes of peritumoral edema volumes shown for bevacizumab (black line) versus all chemotherapies, somatostatin analogues, and tyrosine kinase inhibitors pooled (dashed line) during therapy application. The slopes describe the direction (decrease or increase) and steepness (intensity) for the 3 groups, respectively. A very steep line therefore indicates a rapid increase.
Furthermore, tumor growth rates were subdivided, regarding tumor histology, into tumor diameter and volume growth rates of WHO grades II and III meningiomas per time window in Table 2. In the pretherapeutic as well as the therapeutic period, tumor growth rates (both diameter and volume) of the different histological meningioma subtypes were approximately balanced, whereas in the posttherapeutic period, tumor growth rates were considerably lower in atypical meningiomas than in anaplastic meningiomas.
Table 2.
Growth rates of solid tumor lesions and change rates of peritumoral edema by WHO grades II and III
| Time Window |
WHO Grade | Analyzable Meningiomas, n | Length of Time Window, days |
Change in Diameter per Time [cm/time] |
Change in Volume per Time [cm3/time] |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||
| Pretherapeutic | Lesion | II | 17 | 188.35 | 146.39 | 0.0100 | 0.0240 | 0.0420 | 0.0970 |
| III | 15 | 85.18 | 57.31 | 0.0100 | 0.0130 | 0.0560 | 0.0940 | ||
| Edema | II | 6 | 159.680 | 42.250 | 0.0317 | 0.0440 | |||
| III | 6 | 48.360 | 57.470 | 0.1680 | 0.3390 | ||||
| Therapeutic | Lesion | II | 24 | 81.29 | 20.84 | 0.0042 | 0.0070 | 0.0420 | 0.0670 |
| III | 13 | 71.92 | 25.19 | 0.0062 | 0.0112 | 0.0423 | 0.0780 | ||
| Edema | II | 23 | 81.610 | 21.260 | 0.0661 | 0.1580 | |||
| III | 13 | 71.920 | 25.190 | 0.1008 | 0.2840 | ||||
| Post therapeutic | Lesion | II | 20 | 155.38 | 81.27 | 0.0030 | 0.0070 | 0.0180 | 0.0350 |
| III | 6 | 97.07 | 50.99 | 0.0070 | 0.0121 | 0.0517 | 0.1025 | ||
| Edema | II | 19 | 159.770 | 81.000 | 0.0247 | 0.0413 | |||
| III | 6 | 97.070 | 50.990 | 0.0233 | 0.0367 | ||||
A further analysis using Response Evaluation Criteria In Solid Tumors during treatment with systemic therapy (therapeutic period) revealed stable disease (defined as ≤30% decrease or ≥20% increase in the maximum tumor diameter) in 25 patients and progressive disease (defined as ≥20% increase in the maximum tumor diameter) in 12 cases as the best responses during the treatment periods (see Supplementary Material S4).
Analysis of Peritumoral Edema
Changes of peritumoral edema were defined as the change rates of maximum peritumoral edema volume over a period of time and were evaluated during the pretherapeutic period in 12 meningiomas, during the therapeutic period in 36 meningiomas, and during the posttherapeutic period in 25 meningiomas. In the overall cohort, mean change rates of peritumoral edema volume were positive in all time periods and were highest in the pretherapeutic and lowest in the posttherapeutic period (Table 3, Fig. 1C). Comparing edema volume change rates between the patient populations treated with different therapies, a decrease in edema volume was evident exclusively in the therapeutic period in patients treated with bevacizumab, with a change rate of −0.007 cm3/t (SD = 0.0210) (resulting in a reduction of peritumoral edema change rate of 107% compared with the pretherapeutic period), whereas all other included therapies together showed an average increase in peritumoral edema change rate of 0.1070 cm3/t (SD = 0.2350) (resulting in an increase of peritumoral edema change rate of 7% compared with the pretherapeutic period). The remarkable effect of bevacizumab on peritumoral edema change rates during the therapy period in comparison with all other included systemic therapies is visualized in Fig. 2C.
Table 3.
Changes of peritumoral edema volume
| Time Window | Systemic Therapy | Analyzable Meningiomas, n | Length of Time Window, days |
Change in Volume per Time [cm3/time] |
||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Pretherapeutic | All | 12 | 104.02 | 75.45 | 0.1000 | 0.2410 |
| Therapeutic | All | 36 | 78.11 | 22.89 | 0.0780 | 0.0349 |
| Bevacizumab | 9 | 75.55 | 7.50 | −0.0070 | 0.0210 | |
| Chemotherapy | 12 | 83.00 | 28.62 | 0.0710 | 0.1470 | |
| Somatostatin analogues | 4 | 73.25 | 14.84 | 0.1950 | 0.2700 | |
| Tyrosine kinase inhibitor | 11 | 76.64 | 27.84 | 0.1145 | 0.3060 | |
| Posttherapeutic | All | 25 | 144.72 | 78.80 | 0.0244 | 0.0390 |
| Bevacizumab | 5 | 78.40 | 4.66 | 0.0080 | 0.0180 | |
| Chemotherapy | 6 | 102.95 | 50.41 | 0.0000 | 0.0000 | |
| Somatostatin analogues | 11 | 194.00 | 87.00 | 0.0380 | 0.0350 | |
| Tyrosine kinase inhibitor | 3 | 154.50 | 0.00 | 0.0500 | 0.0860 | |
A reduction in peritumoral edema volume change rate by 29% compared with the pretherapeutic period was detected during the application of chemotherapy, while in the case of somatostatin analogues and tyrosine kinase inhibitors, peritumoral edema showed an increase in change rates of 95% and 15%, respectively. After the termination of systemic therapy, no change of peritumoral edema volume was determined in the chemotherapy subgroup. The second lowest peritumoral edema change rate after termination of systemic therapy was determined in the bevacizumab cohort, whereas patients who were treated with somatostatin analogues or tyrosine kinase inhibitor showed the highest posttherapeutic peritumoral edema change rates.
Moreover, peritumoral edema change rates were evaluated with regard to the different histological meningioma subgroups in Table 2. Remarkably, peritumoral edema volume change rates were lower in atypical meningiomas in the pretherapeutic as well as in the therapeutic period than in anaplastic meningiomas, while there was almost no difference in the posttherapeutic period. Moreover, during systemic therapy, atypical meningiomas showed increased peritumoral edema change rates, while the peritumoral edema in anaplastic meningiomas decreased in comparison with the pretherapeutic period. After systemic therapy, peritumoral edema volumes showed the lowest change rates in atypical as well as anaplastic meningiomas in comparison with the pretherapeutic and therapeutic periods.
Discussion
The role of systemic therapies in recurrent WHO grades II and III meningiomas is ill-defined. So far, medical therapy of these tumors has been evaluated only in case reports, retrospective patient series, and small and uncontrolled prospective studies. The 6-month progression-free survival (PFS-6) rates reported among these studies show a high variability, ranging from 3% to 64.3%. A recent systematic analysis proposed a PFS-6 rate of 26% as the benchmark for historical comparisons.14 Considering this benchmark, hydroxyurea, octreotide analogues, gefitinib, and erlotinib were deemed ineffective,4,13,16 while anti-angiogenic drugs, including bevacizumab, vatalanib, and sunitinib showed potential activity, with PFS-6 rates of 37.5% to 64.3%.11,14,17–19 Interestingly, our data support a potential role of anti-angiogenic treatment in recurrent WHO grades II and III meningioma, as the most pronounced decrease of tumor growth rates was observed in patients put on bevacizumab therapy. Of note, however, we observed evidence for drug-induced tumor growth inhibition also for the other types of systemic therapies analyzed in our series, although to a considerably smaller extent than for bevacizumab. The activity of bevacizumab or other anti-angiogenic agents targeting the vascular endothelial growth factor (VEGF) pathway in WHO grades II and III meningioma seems rational from a pathobiological point of view, as prominent expression of VEGF and its receptors as well as neo-angiogenesis have repeatedly been shown in this tumor type.20,21 Ongoing prospective studies (NCT00972335, NCT01125046) will provide more data on the role of bevacizumab for aggressive meningiomas. A point of caution, however, may be deduced from our observation of increased growth rates after cessation of bevacizumab therapy that were not noted in patients treated with other drugs. “Rebound effects” characterized by more malignant behavior after termination of bevacizumab therapy have been described for vestibular schwannoma, and further studies should address this issue also in meningioma.22
Bevacizumab has shown considerable and clinically relevant anti-edematous properties in gliomas and brain metastases. Our data indicate that this effect is also relevant in meningioma patients, as we saw shrinking of peritumoral edema volumes exclusively in patients under bevacizumab treatment. This finding is well in line with reports showing an important role for VEGF in edema formation in meningioma.23–25 Thus, bevacizumab treatment may be of particular clinical benefit in meningioma patients with symptomatic peritumoral edema and may help to decrease symptomatic burden and corticosteroid need.
Our study has several limitations; despite the multicenter approach, we were able to assemble only a relatively small patient cohort in this rare tumor type. In a number of cases we were unfortunately not able to retrospectively retrieve the neuroimages (or only neuroimages of insufficient quality), because they were done at external institutions. To overcome the individual bias of heterogenic time periods between the MR follow-up examinations at each center, growth rates, which were defined as maximum change in tumor diameter or tumor volume over a period of time, were used. Another limitation is the heterogeneity of administered treatments and the retrospective mode of data analysis. Overall, it is important to note that our analyses are strictly descriptive and need to be carefully interpreted and validated by larger, optimally prospective investigations. In general, the lack of reductions in tumor sizes seen with systemic agents suggests that response rate is not an optimal endpoint for clinical trials with medical therapies in meningiomas but that progression-free survival times or the change in growth rates may be more appropriate endpoints. In any case, our study emphasizes the need for standardization of imaging protocols and clinical management algorithms in aggressive meningioma patients to overcome the wide variation in clinical practice.
Supplementary Material
Funding
None declared.
Supplementary Material
Acknowledgments
We thank the following colleagues for helpful input and support of this project: Brigitta Baumert, Frederic Dhermain, Roland Goldbrunner, Peter Hau, Thomas Hundsberger, Jaap Reijneveldt, Salvador Villa Freixa, Tatjana Seute, Jörg-Christian Tonn, Oliver Schnell, and Monia Dall’Agata.
Conflict of interest statement. None declared.
References
- 1.Wöhrer A, Waldhör T, Heinzl H, et al. The Austrian brain tumour registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol. 2009;95(3):401–411. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(suppl.):1–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumors of th Central Nervous System. 4th ed. Lyon, France: IARC Press; 2007. [Google Scholar]
- 4.Chamberlain MC. Hydroxyurea for recurrent surgery and radiation refractory high-grade meningioma. J Neurooncol. 2012;107(2):315–321. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;13(7):1210–1212. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J Neurooncol. 2006;78(3):271–276. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain MC, Glantz MJ. Interferon-alpha for recurrent World Health Organization grade 1 intracranial meningiomas. Cancer. 2008;15(8):2146–2151. [DOI] [PubMed] [Google Scholar]
- 8.Grunberg SM, Weiss MH, Russell CA, et al. Long-term administration of mifepristone (RU486): clinical tolerance during extended treatment of meningioma. Cancer Invest. 2006;24(8):727–733. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DR, Kimmel DW, Burch PA, et al. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol. 2011;13(5):530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunberg SM, Weiss MH. Lack of efficacy of megestrol acetate in the treatment of unresectable meningioma. J Neurooncol. 1990;8(1):61–65. [DOI] [PubMed] [Google Scholar]
- 11.Nayak L, Iwamoto FM, Rudnick JD, et al. Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol. 2012;109(1):187–193. [DOI] [PubMed] [Google Scholar]
- 12.Wen PY, Yung WK, Lamborn KR, et al. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01-08). Neuro Oncol. 2009;11(6):853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norden AD, Raizer JJ, Abrey LE, et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96(2):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaley T, Barani I, Chamberlain MC, et al. Historical benchmarks for medical therapy trials in surgery-and radiation-refractory meningioma: A RANO review. Neuro Oncol. 2014;16(6):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19(7):1081–1088. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain MC, Glantz MJ, Fadul CE. Recurrent meningioma: salvage therapy with long-acting somatostatin analogue. Neurology. 2007;69(10):969–973. [DOI] [PubMed] [Google Scholar]
- 17.Raizer JJ, Grimm SA, Rademaker A, et al. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J Neurooncol. 2014;117(1):93–101. [DOI] [PubMed] [Google Scholar]
- 18.Kaley TJ, Wen P, Schiff D, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17(1):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puchner MJA, Hans VH, Harati A, et al. Bevacizumab-induced regression of anaplastic meningioma. Ann Oncol. 2010;21(12):2445–2446. [DOI] [PubMed] [Google Scholar]
- 20.Baumgarten P, Brokinkel B, Zinke J, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors VEGFR1 and VEGFR2 in primary and recurrent WHO grade III meningiomas. Histol Histopathol. 2013;28(9):1157–1166. [DOI] [PubMed] [Google Scholar]
- 21.Preusser M, Hassler M, Birner P, et al. Microvascularization and expression of VEGF and its receptors in recurring meningiomas: pathobiological data in favor of anti-angiogenic therapy approaches. Clin Neuropathol. 2012;31(5):352–360. [DOI] [PubMed] [Google Scholar]
- 22.Mautner VF, Nguyen R, Knecht R, et al. Radiographic regression of vestibular schwannomas induced by bevacizumab treatment: sustain under continuous drug application and rebound after drug discontinuation. Ann Oncol. 2010;21(11):2294, 2295. [DOI] [PubMed] [Google Scholar]
- 23.Nassehi D, Sørensen LP, Dyrbye H, et al. Peritumoral brain edema in angiomatous supratentorial meningiomas: an investigation of the vascular endothelial growth factor A pathway. APMIS. 2013;121(11):1025–1036. [DOI] [PubMed] [Google Scholar]
- 24.Iwado E, Ichikawa T, Kosaka H, et al. Role of VEGF and matrix metalloproteinase-9 in peritumoral brain edema associated with supratentorial benign meningiomas. Neuropathology. 2012;32(6):638–646. [DOI] [PubMed] [Google Scholar]
- 25.Schmid S, Aboul-Enein F, Pfisterer W, et al. Vascular endothelial growth factor: the major factor for tumor neovascularization and edema formation in meningioma patients. Neurosurgery. 2010;67(6):1703–1708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.