Abstract
Background
Stereotactic radiotherapy (SRT) is expected to have a less detrimental effect on neurocognitive functioning and health-related quality of life (HRQoL) than whole-brain radiotherapy. To evaluate the impact of brain metastases and SRT on neurocognitive functioning and HRQoL, we performed a prospective study.
Methods
Neurocognitive functioning and HRQoL of 97 patients with brain metastases were measured before SRT and 1, 3, and 6 months after SRT. Seven cognitive domains were assessed. HRQoL was assessed with the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and BN20 questionnaires. Neurocognitive functioning and HRQoL over time were analyzed with linear mixed models and stratified for baseline Karnofsky performance status (KPS), total metastatic volume, and systemic disease.
Results
Median overall survival of patients was 7.7 months. Before SRT, neurocognitive domain and HRQoL scores were lower in patients than in healthy controls. At group level, patients worsened in physical functioning and fatigue at 6 months, while other outcome parameters of HRQoL and cognition remained stable. KPS < 90 and tumor volume >12.6 cm3 were both associated with worse information processing speed and lower HRQoL scores over 6 months time. Intracranial tumor progression was associated with worsening of executive functioning and motor function.
Conclusions
Prior to SRT, neurocognitive functioning and HRQoL are moderately impaired in patients with brain metastases. Lower baseline KPS and larger tumor volume are associated with worse functioning. Over time, SRT does not have an additional detrimental effect on neurocognitive functioning and HRQoL, suggesting that SRT may be preferred over whole-brain radiotherapy.
Keywords: brain metastases, cognition, health-related quality of life, stereotactic radiotherapy
Brain metastases are the most common type of brain cancer and develop in 9%–45% of patients with systemic cancer.1,2 Neurocognitive deficits, impaired health-related quality of life (HRQoL), and fatigue are often present in patients with brain metastases and can be caused by the metastasis itself, antitumor treatment, or supportive medication.3–6 Median survival of brain metastatic patients ranges considerably, from 2.6 to 15 months, depending on age and condition of the patient, primary tumor, and treatment.7,8 Also, due to improved treatments for primary tumors, specific patient groups survive longer.8,9 However, most patients with brain metastases cannot be cured. Therefore, preservation of neurocognitive functioning and HRQoL for maintaining daily functioning is important.
In recent years, treatment with stereotactic radiosurgery and stereotactic radiotherapy (SRT) as an alternative or addition to whole-brain radiotherapy (WBRT) has gained interest, especially in patients with 1–3 small brain metastases and relatively good prognosis.10 Because in SRT a high dose of focal irradiation is delivered to the tumor, while irradiation to healthy brain tissue is minimized, it is expected to have fewer (long-term) side effects on cognition and functional outcome than WBRT,11,12 while overall survival (OS) is comparable.13,14 Conversely, tumor control outside the initial site has been found to be worse in patients treated with SRT alone compared with SRT combined with WBRT,14 with brain tumor recurrence probably resulting in cognitive deterioration.15,16
Up to now, data on the impact of SRT on neurocognitive functioning and HRQoL have been scarce. Previous studies suggest some (transitory) decline in neurocognitive functioning and an increase in self-reported cognitive dysfunction in the months after SRT.5,16,17 HRQoL scores after radiotherapy, including SRT, may further deteriorate, stabilize, or improve.6,18–20 However, small patient samples, the use of inappropriate measurement tools, the absence of preradiation treatment assessment, and short follow-up periods hamper the interpretation of results and subsequently limit their ability to inform clinical decision making.
As preservation of neurocognitive functioning and HRQoL in patients treated with SRT is essential, we set out to investigate the impact of SRT on these outcomes prospectively in a cohort of patients with 1–3 brain metastases. In addition, we investigated the impact of several other factors that could potentially influence neurocognitive functioning and HRQoL in these patients.
Materials and Methods
Patients and Procedure
Consecutive adult patients scheduled to undergo SRT for 1–3 brain metastases, with a maximum diameter of 4 cm per metastasis, were recruited in the Medical Center Haaglanden, a tertiary hospital in The Hague, the Netherlands, between January 2009 and February 2012. Exclusion criteria were: prior treatment of brain metastases, including resection; Karnofsky performance status (KPS) <70; and insufficient command of the Dutch language. Baseline assessment was carried out in the week preceding SRT, and follow-up assessments took place 1 month (only HRQoL) and 3 and 6 months after SRT. The study protocol was approved by the medical ethics committee of the institution. All patients gave written informed consent.
Treatment and Follow-up Schedule
In all patients, the gross tumor volume was contoured on a T1-weighted contrast-enhanced MRI, the clinical target volume was equal to the gross tumor volume, and the planning target volume (PTV) was created by 3D expansion of the clinical target volume with 2 mm. Metastases with PTV <8 cm3 received 21 Gy and metastases with PTV of 8–13 cm3 received 18 Gy, both in a single fraction, whereas metastases with PTV >13 cm3 and metastases near the brainstem were given 24 Gy in 3 fractions of 8 Gy. From 1 day prior to SRT to 7 days after SRT, patients received corticosteroids (dexamethasone 16 mg/d), which were gradually discontinued afterward.
Follow-up MRI scans were made 1, 3, and 6 months after treatment. As for brain metastases no standard criteria are available for defining response to treatment21, we used the criteria of Lin et al.22 Partial response was defined as ≥50% reduction in the total volumetric sum of contrast-enhancing lesions, and intracranial progression as ≥40% increase in the total sum of enhancement of tumor volume, or the presence of any new contrast-enhancing lesions visible on MRI.22 In case of progressive disease during the study period, patients stayed in the study provided they underwent renewed SRT. Patients treated with WBRT because of progression, or with no further tumor treatment, were no longer assessed.
Neurocognitive Functioning
Neurocognitive functioning was assessed with a comprehensive test battery consisting of validated neuropsychological tests covering a wide range of neurocognitive functions found to be affected in brain tumor patients and sensitive to subtle changes over time23–25, 47–51 (Supplementary Table S1). Test scores were combined into 7 compound neurocognitive domain scores: verbal memory, visual memory, working memory, attention, information processing speed, executive functioning, and visuoconstructive abilities. Patients' performances on verbal memory, attention, information processing speed, and executive functioning were compared with healthy controls,26,27 who were individually matched to the patients with respect to age, sex, and educational level.28 For working memory, visuoconstruction, and visual memory, published norms were used,29,30 correcting for age and education. Individual test scores of patients were converted into standardized z-scores with use of means and standard deviations of controls on that test. We calculated domain summary scores at each assessment.
Health-Related Quality of Life
We assessed HRQoL with the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) and the EORTC QLQ–Brain Cancer Module (QLQ-BN20).31,32 The EORTC QLQ-C30 is a generic questionnaire, while the QLQ-BN20 assesses issues relevant for brain cancer patients. Both questionnaires showed good psychometric properties. Scores were transformed into standardized scores ranging from 0 to 100. For global health status/QoL and functional scales, a higher score means better HRQoL. For symptom scales and BN20 scales, a higher score indicates more symptoms. Based on functions previously found to be relevant for brain metastatic patients,18 we selected 7 HRQoL scales for the primary analysis, including global health status/QoL; physical functioning; role functioning; emotional functioning; self-perceived cognitive functioning; motor dysfunction; and communication deficits. The remaining HRQoL scales were analyzed on an exploratory basis. Scores on the QLQ-C30 were compared with reference data.33 Fatigue was assessed with the Fatigue Severity Scale,34 including questions on the occurrence and severity of fatigue and its impact on daily life. The total score ranges between 1 and 7, with a score of ≥4 indicating moderate to severe fatigue.35
Statistical Analysis
For each cognitive domain, we considered an individual z-score of ≥1.5 SD below the mean of healthy controls as clinical significant impairment.23 Differences in sociodemographic and clinical characteristics between patients who completed follow-up and patients who dropped out were tested using the χ2 test for categorical data and Students' t-tests or the Mann–Whitney U-test for continuous data, depending on the distribution of the tested variable. Overall survival was analyzed with Kaplan–Meier curves, and log-rank tests were used to assess differences in survival between patients with different numbers of metastases (1 vs >1), tumor volume (based on the total volume of the contrast enhancement of all metastases on MRI scan and categorized into large [>12.6 cm3], medium [4.8–12.6 cm3], and small [<4.8 cm3]), and baseline KPS. In line with previous studies on brain metastases,4,36 we compared patients with KPS < 90 with those with KPS ≥ 90. Linear mixed models were used to analyze neurocognitive functioning and HRQoL over time (unadjusted for possible confounders), as well as separately for patients categorized into high (≥90) and low (<90) baseline KPS, tumor volume (large/medium/small), and presence of active systemic disease. In linear mixed models, contrary to a complete-case analysis, data of all patients at each time point are included in the analysis by imputation of missing data. This sophisticated method of multiple imputation depends on fitting a specific covariance structure, which assesses the correlations among the different measurements at different time points.
The influence of treatment response was analyzed by comparing (i) the difference between the neurocognitive and HRQoL scores at time of progression and the patient's own baseline score with (ii) the difference scores of patients without progressive disease, using an independent samples t-test. Statistical analyses were performed with SPSS version 20.0 software. The level of significance was set at P < .05.
Results
Patient Characteristics and Survival
Baseline sociodemographic and clinical characteristics of the study population are presented in Table 1. Ninety-seven patients (mean age, 63 ± 11 y) were included prior to SRT treatment. The most frequent primary tumor site was lung (50%). In 19 patients (20%), brain metastases were the first presentation of cancer. In 5 patients, the planning MRI scan showed a fourth metastasis, which was in all cases small (<0.5 cm3). Despite this fourth metastasis, we included these patients in our study.
Table 1.
Sociodemographic and baseline clinical characteristics of the study patients
| No. of Patients | % of Patients | |
|---|---|---|
| No. of patients included | 97 | |
| Age, mean ± SD/range | 63 ± 11/33–82 | |
| Sex, male | 46 | 47 |
| Educational level,a mean ± SD | 3.1 ± 1.7 | |
| No. of brain metastases | ||
| 1 | 43 | 44 |
| 2 | 31 | 32 |
| 3 | 18 | 19 |
| 4 | 5 | 5 |
| Total tumor volume by patient, cm3 | ||
| Median (range) | 7.8 (0.12–63.9) | |
| Tertiles | < 4.8/4.8–12.6/ > 12.6 | |
| Primary cancer | ||
| Non–small cell lung | 48 | 50 |
| Renal cell carcinoma | 12 | 13 |
| Melanoma | 9 | 9 |
| Colorectal | 9 | 9 |
| Breast cancer | 8 | 8 |
| Other | 11 | 11 |
| Active systemic disease | 52 | 54 |
| Chemotherapy (<3 mo of baseline) | 13 | 13 |
| Extracranial metastases | 54 | 56 |
| Use of corticosteroids | 85 | 88 |
| Use of AEDs | 21 | 22 |
| KPS | ||
| Median (range) | 80 (60–100) | |
| KPS ≥ 90 | 35 | 36 |
aLevel 1–8.28
Median OS was 7.7 months (interquartile range: 8.1). One-year survival rate was 30%. Median OS was significantly shorter for patients with baseline KPS < 90 compared with KPS ≥ 90 (5.3 vs 11.1 mo, P = .003) (Supplementary Fig. S1) and for patients with large compared with medium or small tumor volume (4.5 vs 10.4 vs 7.7 mo, P = .042). Number of brain metastases and active systemic disease at baseline were not associated with survival (5.5 mo for 1 metastasis vs 8.2 mo for >1 metastases, P = .437; 8 mo for no active systemic disease vs 7.2 mo for systemic disease, P = .737).
During follow-up, intracranial progression was observed in 47/90 evaluable patients (52%), in 27 patients solely due to new enhancing lesions without increment in initial tumor volume. Seventeen of 47 patients (36%) had progression at more than one follow-up moment. For progression, 13 patients were treated with WBRT and 7 with renewed SRT. Reasons for no salvage therapy being initiated were poor physical condition, progressive systemic disease, and the consideration of intracranial progression to be the result of radiation (ie, pseudoprogression). Twenty-five of 90 patients (28%) had at least a radiologically partial response (≥50% decrease in total tumor volume).
Active systemic disease was present in 52/97 (54%) at baseline, in 52/84 (62%) at 1 month, in 42/70 (60%) at 3 months, and in 27/45 patients (60%) at 6 months. Of the patients with systemic disease, between 25% (13/52, at baseline) and 52% (27/52 at 1 mo; 22/42 at 3 mo; and 14/27 at 6 mo) received chemotherapy. Patients with systemic disease at baseline did not significantly differ from patients without systemic disease according to age, KPS, or tumor volume. Use of corticosteroids declined during the study period: from 91% at baseline to 56% at 1 month, 44% at 3 months, and 36% at 6 months. Because of the occurrence of one or more epileptic seizures, about 25% of patients received prophylactic anti-epileptic drugs (AEDs) on daily dosage during follow-up.
Compliance
Compliance during follow-up was at least 70% for HRQoL and 53% for neurocognitive assessments (Table 2). Reasons for noncompliance were poor neurological or physical condition, refusal because testing was considered too burdensome, and time constraints. At all measurements, no statistically significant differences between patients alive who did and did not complete assessments were found for median KPS or age.
Table 2.
Compliance with neurocognitive and HRQoL assessments
| No. of Forms/ Assessments Expecteda | No. of Forms Received/Assessments Performed |
||
|---|---|---|---|
| Neurocognition | HRQoL | ||
| Baseline | 97 | 77 (79%) | 95 (98%) |
| 1 mo | 90 | – | 75 (83%) |
| 3 mo | 73 | 39 (53%) | 51 (70%) |
| 6 mo | 49 | 29 (59%) | 41 (84%) |
aBased on alive participating patients.
Neurocognitive Functioning
Baseline
Preceding SRT, 53% of patients had an impairment in at least one neurocognitive domain. The most frequently affected domains were verbal memory (33%) and visuoconstruction (22%). Neurocognitive domain scores of patients were significantly worse compared with healthy controls for verbal memory, attention, working memory, executive functioning, and visuoconstruction (Table 3). KPS < 90 compared with KPS ≥ 90 was associated with worse executive functioning (mean z-score −0.81 vs −0.13, P = .002), information processing speed (mean z-score −0.57 vs 0.38, P = .015), and working memory (mean z-score −0.7 vs −0.27, P = .054). Patients with large volumes, compared with small or medium volumes, showed a trend toward worse verbal memory (mean z-score −0.79 vs −0.62 vs −1.5 respectively, P = .077) and information processing speed (mean z-score 0.02 vs 0.24 vs −0.82, P = .069). The number of brain metastases, presence of active systemic disease, or use of AEDs was not associated with baseline neurocognitive functioning (data not shown).
Table 3.
Neurocognitive domain scores at baseline
| Total Patient Group (n = 77) |
Patients vs Controlsa | Patients with KPS < 90 (n = 47) |
Patients with KPS ≥ 90 (n = 30) |
KPS < 90 vs KPS ≥ 90 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean Score | 95% CI | Mean Score | 95% CI | Mean Score | 95% CI | |||
| Verbal memory | −0.93 | −1.26 to −0.6 | P < .001 | −1.16 | −1.56 to −0.76 | −0.74 | −1.29 to −0.18 | P = .202 |
| Visual memory | −0.07 | −0.34 to 0.2 | P = .336 | −0.09 | −0.57 to 0.38 | −0.15 | −0.46 to 0.17 | P = .842 |
| Attention | −0.63 | −0.99 to −0.26 | P = .002 | −0.86 | −1.3 to −0.41 | −0.23 | −0.89 to 0.42 | P = .105 |
| Executive functioning | −0.52 | −0.75 to −0.29 | P < .001 | −0.81 | −1.09 to −0.53 | −0.13 | −0.43 to 0.17 | P = .002 |
| Working memory | −0.52 | −0.74 to −0.29 | P < .001 | −0.7 | −0.97 to −0.44 | −0.27 | −0.65 to 0.12 | P = .054 |
| Information processing speed | −0.18 | −0.57 to −0.2 | P = .361 | −0.57 | −1.15 to 0 | 0.38 | −0.04 to 0.8 | P = .015 |
| Visuoconstruction | −0.7 | −1.03 to −0.37 | P < .001 | −0.81 | −1.31 to −0.31 | −0.58 | −1.05 to −0.11 | P = .493 |
aMean z-scores (SD) of controls: 0 (1).
Over time
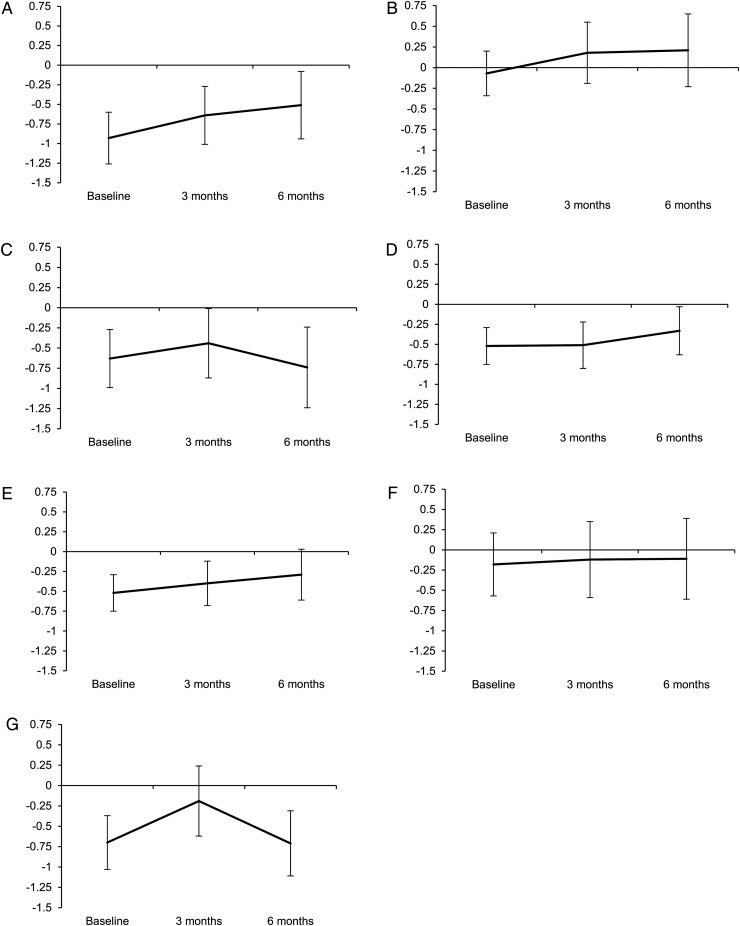
Over 6 months time, neurocognitive domain scores did not significantly change (Fig. 1). Verbal memory showed a nonsignificant trend toward improvement (mean change 0.42, P = .084). Compared with patients with baseline KPS ≥ 90, patients with KPS < 90 had worse information processing speed (mean −0.6 vs 0.5, P = .002) and executive functioning (mean −0.76 vs −0.08, P = .003) over time. Large tumor volume compared with small or medium volume was associated with worse information processing speed (mean −1.1 vs 0.2 vs 0.3, P = .02). Surprisingly, patients without systemic disease had worse information processing speed (mean −0.4 vs 0.06, P = .03) and visuoconstructive abilities (mean −0.78 vs −0.23, P = .037) over time than patients with active systemic disease, while on other domains no differences were found. Use of corticosteroids did not influence neurocognitive functioning.
Fig. 1.
Neurocognitive domain scores over time. Mean z-scores are predicted from linear mixed model analysis with their 95% CIs for (A) verbal memory, (B) visual memory, (C) attention, (D) executive functioning, (E) working memory, (F) information processing speed, and (G) visuoconstruction. Verbal memory showed a nonsignificant improvement over time (P = .084).
Progression
An association between neurocognitive functioning and intracranial changes was found only for executive functioning: patients who had radiological partial response improved, while patients with stable or progressive disease deteriorated (mean 0.43 vs −0.29, P = .032). Other neurocognitive domain scores did not differ between patients with or without progressive disease.
Health-Related Quality of Life
Baseline
Compared with the general population,33 scores on all preselected HRQoL scales were significantly lower in patients at baseline, with lowest scores in role functioning. All differences were clinically relevant. Baseline HRQoL scores were significantly lower in patients with KPS < 90 compared with KPS ≥ 90 (Table 4). Similar results were found for exploratory HRQoL items (Supplementary Table S2). Presence of active systemic disease did not result in lower HRQoL scores; indeed, physical functioning and global health status/QoL were even higher than in patients without systemic disease (mean 76 vs 61, P = .008 and 72 vs 62, P = .038, respectively). Tumor volume and number of metastases did not affect baseline HRQoL (data not shown).
Table 4.
Preselected HRQoL scores at baseline
| Total Patient Group (n = 95) |
Reference Data from General Population33 | Patients with KPS < 90 (n = 61) |
Patients with KPS ≥ 90 (n = 34) |
KPS < 90 vs KPS ≥ 90 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean Score | 95% CI | Mean Score | Mean Score | 95% CI | Mean Score | 95% CI | ||
| QLQ-C30 | ||||||||
| Global health status/QoL | 67 | 62–72 | 78 | 60 | 54–66 | 80 | 74–87 | P < .001 |
| Physical functioning | 69 | 64–75 | 90 | 58 | 52–65 | 90 | 85–95 | P < .001 |
| Emotional functioning | 73 | 69–78 | 89 | 73 | 67–79 | 74 | 66–82 | P = .785 |
| Role functioning | 58 | 51–65 | 89 | 46 | 37–54 | 80 | 73–88 | P < .001 |
| Cognitive functioning | 75 | 70–80 | 92 | 68 | 61–75 | 87 | 81–93 | P < .001 |
| BN20 | ||||||||
| Motor dysfunction | 21 | 16–26 | – | 28 | 21–35 | 8.8 | 5–13 | P < .001 |
| Communication deficits | 10 | 6.4–13 | – | 12 | 7.2–17 | 6.4 | 1.5–11 | P = .136 |
| Fatigue severity scale | 3.2 | 2.8–3.6 | – | 3.8 | 3.3–4.3 | 2.2 | 1.6–2.7 | P < .001 |
Scores >10 are rounded. Differences between total patient group and reference data are all significant (P < .05).
Over time
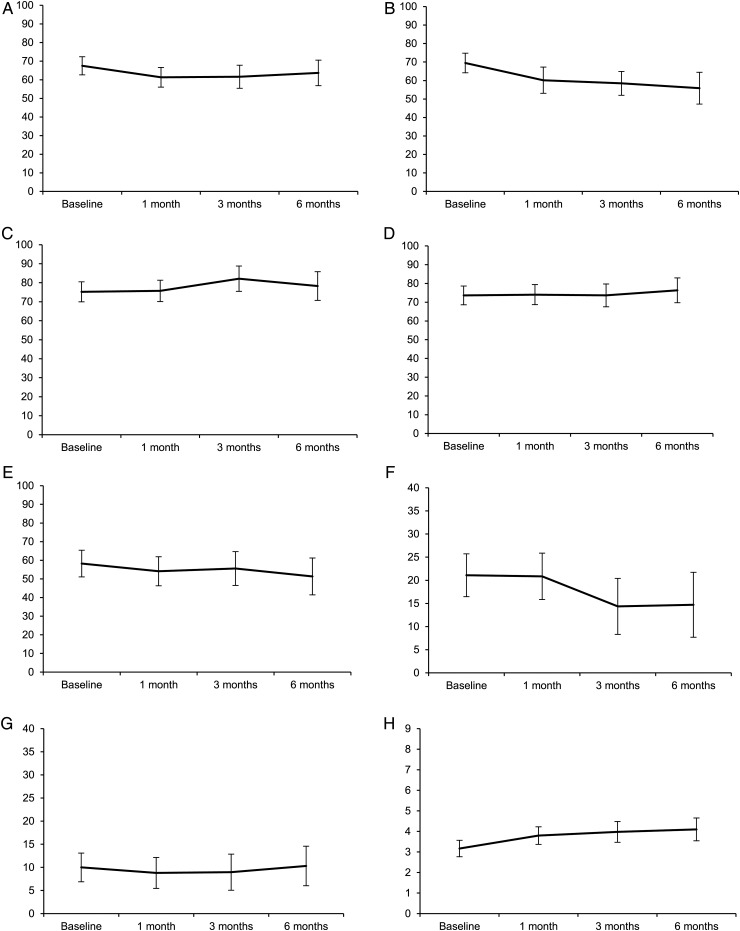
At group level, patients worsened significantly and clinically in physical functioning (mean change −14, P = .027) and fatigue (mean change 0.93, P = .009) over 6 months time, while other HRQoL scores did not change significantly (Fig. 2). Mean HRQoL scores over time were significantly lower for patients with baseline KPS < 90 compared with patients with KPS ≥ 90 for physical (54 vs 74, P < .001), self-perceived cognitive (75 vs 85, P = .029) and role functioning (46 vs 70, P < .001), motor dysfunction (21 vs 9, P = .006), and fatigue (37 vs 29, P = .015). Large tumor volume resulted in lower mean scores of physical (48 vs 66 vs 70 for large, medium, and small volumes, respectively; both P < .05) and self-perceived cognitive functioning (70 vs 84 for large and medium volumes, P = .045, 78 for small volumes) over time. Number of metastases and baseline systemic disease did not influence HRQoL scores of the preselected scales over time.
Fig. 2.
HRQoL scores over time. Data are predicted means based on linear mixed model analysis with their 95% CIs for (A) global health status/QoL, (B) physical functioning, (C) cognitive functioning, (D) emotional functioning, (E) role functioning, (F) motor dysfunction, (G) communication deficits, and (H) fatigue. Physical functioning (P = .027) and fatigue (P = .009) worsened significantly over time.
Exploratory analyses (Supplementary Table S3) showed that at group level, patients experienced significantly more nausea and appetite loss and were more bothered by hair loss over time. Patients with KPS < 90 had more fatigue, pain, visual disorders, and drowsiness than patients with KPS ≥ 90. The presence of systemic disease was associated with more nausea and appetite loss (mean scores, nausea: 10 vs 5.9, P = .043; appetite loss: 18 vs 6.8, P = .015) compared with no systemic disease. Tumor volume and number of metastases did not influence exploratory HRQoL items/scales (data not shown).
Progression
Overall, patients with intracranial progression deteriorated in preselected HRQoL scales, while patients without progressive disease showed less profound deterioration or even improvement at follow-up (Supplementary Table S4). However, a significant difference between patients with progressive disease and those with stable disease/partial response was only found for motor dysfunction (mean change 7.7 vs −10, P = .006), while role functioning showed a trend (mean change −15 vs 0.2, P = .084). For exploratory scores, no differences between patients with and without intracranial progression were found. Although the use of corticosteroids resulted in lower HRQoL at different time points, there was no significant deterioration over time.
Discussion
We found that newly diagnosed brain metastases patients suffer from moderate impairments in HRQoL and neurocognitive functioning prior to SRT. Of note, up to 6 months after SRT, overall neurocognitive and HRQoL scores remained relatively stable at group level and did not further deteriorate. To our knowledge, this is the first study in which neurocognitive functioning and HRQoL have been comprehensively and prospectively examined in a relatively large group of patients treated with SRT only. Overall survival in our study was comparable to survival after WBRT, as well as to other studies on SRT.36,37
Regarding neurocognitive functioning, we found 53% of patients to have at least some neurocognitive impairment at baseline. In earlier studies, 67%–80% of brain metastatic patients had neurocognitive impairments before any radiotherapy.5,38 These differences might be explained by the fact that other studies included patients who were scheduled to undergo WBRT38 and used different neurocognitive tests.5,38 Also, we combined test scores into domain scores, resulting in loss of information. Nevertheless, mean neurocognitive domain scores in our study were similar to scores observed in earlier studies, with memory as the most frequently impaired domain.5,39 We also observed a relatively frequent impairment in visuoconstructive abilities, which may impact spatial orientation in daily life. It may therefore be relevant to include these measures in future neurocognitive assessments.
The observation that neurocognitive functioning remained relatively stable up to 6 months after SRT is in line with a few earlier (pilot) studies in brain metastatic patients5,40–42 and with more comprehensive studies on long-term neurocognitive functioning after stereotactic irradiation for other intracranial lesions—for example, cerebral arteriovenous malformations.43 Also, patients treated with SRT alone were found to have lower risk of deteriorating in learning and memory 4 months after treatment (24%) than SRT plus WBRT patients (52%).39 Comparable results were observed in a recent trial by Brown et al,44 in which cognitive decline 3 months after treatment was more frequent in patients receiving SRT plus WBRT compared with SRT alone, despite better intracranial tumor control with the addition of WBRT. A decline in Mini-Mental State Examination (MMSE) is mainly found at recurrence.16 Even though we used instruments more sensitive to change than the MMSE,45 we did not find an association between brain tumor progression and neurocognitive decline, except for executive functioning, which deteriorated in patients with stable or progressive disease and improved in those with partial response. Perhaps our patients did not deteriorate because they often developed only small new lesions. Surprisingly, we found that information processing speed and visuoconstruction were more severely impaired over time in patients without than in patients with active systemic disease at baseline, while clinical characteristics and overall condition of patients with systemic activity did not differ from those without active systemic disease.
Baseline HRQoL outcomes in our study are comparable to the results of earlier studies evaluating radiotherapy in brain metastatic patients.18–20 This implies that patients with brain metastases are impaired already at onset in all facets of daily life, including physical and mental functioning. Some aspects of physical functioning, including nausea and fatigue, were augmented during follow-up, probably related to systemic disease or chemotherapy. Despite treatment and disease-related symptoms, most functioning scales and global health status/QoL remained stable over time, which is in line with previous results.18,42 This might be explained by the occurrence of a “response shift,” or adaptation of the patient to increased symptoms and a recalibration of priorities.9 Two other studies on HRQoL in patients with brain metastases showed either improvement or deterioration of HRQoL within 3 months after radiation compared with baseline.19,20 However, in both studies, only a minority of patients received SRT alone. The robust association between intracranial progression and HRQoL decline found in glioma patients e.g. Ref 46 might be less clear in brain metastatic patients, as we only found a significant and clinically relevant deterioration for motor dysfunction in progressive patients, while patients with stable disease or a partial response improved. Similarly, in a previous study on brain metastases, no association between intracranial recurrence and worsening of HRQoL was observed.18 Perhaps this lack of association might be related to the occurrence of pseudoprogression, or because close monitoring with MRI resulted in early detection of new small, and therefore asymptomatic, lesions.
We found that KPS and brain metastatic volume, but not number of metastases, were associated with survival, neurocognitive functioning, and HRQoL, which is in accordance with earlier findings.4,5,16,20,36 Although patients with large tumor volumes were impaired in some aspects of HRQoL and neurocognitive functioning, most scores did not differ significantly from those of patients with smaller tumors. Moreover, the median total volume of brain metastases in our study was larger than described in other studies using SRT,16,39 suggesting that also in patients with relatively large metastatic volume, SRT is adequate to achieve maintenance of HRQoL and cognition. Although HRQoL and neurocognitive functioning may be preserved in patients with low baseline performance status, their mean scores remained lower over 6 months time compared with patients with good performance status.
Our study clearly had several limitations. First, the study population was heterogeneous in that several types of primary tumors were included. As prognosis is partly based on primary tumor histology, this may have influenced survival and functional outcomes. Due to small numbers of the different tumor types, it was not feasible to assess the effect of each tumor separately. This sample is, however, representative of brain metastatic patients undergoing SRT in a tertiary hospital. Another challenge was the level of compliance during the study period, especially with the neurocognitive assessments. Noncompliance was partly due to logistic reasons, but also to poor physical or neurological condition of the patient. Therefore, our findings might have been an underestimation of truly neurocognitive impairments. We tried to overcome this problem by fitting linear mixed models, confirming that neurocognitive functioning and HRQoL were stable over time. Moreover, the level of compliance in our sample was comparable to other studies on HRQoL18–20 and neurocognitive functioning16 in radiotherapy patients. Finally, we examined effects of SRT on neurocognitive functioning and HRQoL up to 6 months, but longer follow-up periods would be informative to examine possible late effects of SRT.
To conclude, this prospective study showed that SRT is safe in terms of preservation of neurocognitive functioning and HRQoL in patients with up to 3 brain metastases. With similar OS rates as WBRT, these results suggest that SRT should be the preferred treatment. Moreover, evaluation of neurocognitive functioning and HRQoL during the disease course is essential for providing adequate information and counseling for patients with brain metastases.
Supplementary Material
Funding
This work was supported by St. Jacobusstichting, the Netherlands.
Supplementary Material
Acknowledgments
Part of this manuscript was presented at the XII European Organisation for Neuro-Oncology (EANO) meeting, October 9–12, 2014, Turin, and at the 19th annual meeting of the Society for Neuro-Oncology, November 13–16, 2014, Miami.
Conflict of interest statement. R. Wiggenraad has received a speakers fee from Brainlab; M. Taphoorn has had an ad hoc consulting role with Hoffman La Roche. The other authors report no potential conflict of interest.
References
- 1.Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, Yu C, Sloan AE, et al. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol. 2012;14(7):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaal EC, Niel CG, Vecht CJ. Therapeutic management of brain metastasis. Lancet Neurol. 2005;4(5):289–298. [DOI] [PubMed] [Google Scholar]
- 4.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–165. [DOI] [PubMed] [Google Scholar]
- 5.Chang EL, Wefel JS, Maor MH, et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery. 2007;60(2):277–283. [DOI] [PubMed] [Google Scholar]
- 6.Kondziolka D, Niranjan A, Flickinger JC, et al. Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients’ perspective regarding complications. Am J Clin Oncol. 2005;28(2):173–179. [DOI] [PubMed] [Google Scholar]
- 7.Jyothirmayi R, Saran FH, Jalali R, et al. Stereotactic radiotherapy for solitary brain metastases. Clin Oncol (R Coll Radiol). 2001;13(3):228–234. [DOI] [PubMed] [Google Scholar]
- 8.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin NU, Wefel JS, Lee EQ, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013;14(10):e407–e416. [DOI] [PubMed] [Google Scholar]
- 10.Lippitz B, Lindquist C, Paddick I, et al. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. 2014;40(1):48–59. [DOI] [PubMed] [Google Scholar]
- 11.Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118(9):2486–2493. [DOI] [PubMed] [Google Scholar]
- 12.Platta CS, Khuntia D, Mehta MP, et al. Current treatment strategies for brain metastasis and complications from therapeutic techniques: a review of current literature. Am J Clin Oncol. 2010;33(4):398–407. [DOI] [PubMed] [Google Scholar]
- 13.Mehta MP, Tsao MN, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2005;63(1):37–46. [DOI] [PubMed] [Google Scholar]
- 14.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regine WF, Scott C, Murray K, et al. Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated-hyperfractionated radiotherapy: an analysis from Radiation Therapy Oncology Group Study 91-04. Int J Radiat Oncol Biol Phys. 2001;51(3):711–717. [DOI] [PubMed] [Google Scholar]
- 16.Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68(5):1388–1395. [DOI] [PubMed] [Google Scholar]
- 17.Cole AM, Scherwath A, Ernst G, et al. Self-reported cognitive outcomes in patients with brain metastases before and after radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87(4):705–712. [DOI] [PubMed] [Google Scholar]
- 18.Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65–72. [DOI] [PubMed] [Google Scholar]
- 19.Steinmann D, Paelecke-Habermann Y, Geinitz H, et al. Prospective evaluation of quality of life effects in patients undergoing palliative radiotherapy for brain metastases. BMC Cancer. 2012;12:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caissie A, Nguyen J, Chen E, et al. Quality of life in patients with brain metastases using the EORTC QLQ-BN20+2 and QLQ-C15-PAL. Int J Radiat Oncol Biol Phys. 2012;83(4):1238–1245. [DOI] [PubMed] [Google Scholar]
- 21.Lin NU, Lee EQ, Aoyama H, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol. 2013;14(10):e396–e406. [DOI] [PubMed] [Google Scholar]
- 22.Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. [DOI] [PubMed] [Google Scholar]
- 23.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York: Oxford Univerity Press; 2004. [Google Scholar]
- 24.Meyers CA, Cantor SB. Neuropsychological assessment and treatment of patients with malignant brain tumors. In: Prigatano GP, Pliskin NH, eds. Clinical Neuropsychology and Cost Outcome Research, a Beginning. New York: Psychology Press, Inc.; 2003: 159–173. [Google Scholar]
- 25.Tucha O, Smely C, Preier M, et al. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324–333. [DOI] [PubMed] [Google Scholar]
- 26.Jolles J, Van Boxtel MP, Ponds RW, et al. [The Maastricht aging study (MAAS). The longitudinal perspective of cognitive aging]. Tijdschr Gerontol Geriatr. 1998;29(3):120–129. [PubMed] [Google Scholar]
- 27.Van der Elst W. The Neuropsychometrics of Aging: Normative Studies in the Maastricht Aging Study. Maastricht: Neuropsy publishers; 2006. [Google Scholar]
- 28.De Bie SE. [Proposal for uniformisation of questions regarding background variables and interviews]. Leiden: Leiden University Press; 1987. [Google Scholar]
- 29.Fastenau PS, Denburg NL, Hufford BJ. Adult norms for the Rey-Osterrieth Complex Figure Test and for supplemental recognition and matching trials from the Extended Complex Figure Test. Clin Neuropsychol. 1999;13(1):30–47. [DOI] [PubMed] [Google Scholar]
- 30.Kessels RP, van den Berg E, Ruis C, et al. The backward span of the Corsi Block-Tapping Task and its association with the WAIS-III Digit Span. Assessment. 2008;15(4):426–434. [DOI] [PubMed] [Google Scholar]
- 31.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 32.Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5(1):139–150. [DOI] [PubMed] [Google Scholar]
- 33.van de Poll-Franse LV, Mols F, Gundy CM, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47(5):667–675. [DOI] [PubMed] [Google Scholar]
- 34.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. [DOI] [PubMed] [Google Scholar]
- 35.Mathiowetz V, Matuska KM, Murphy ME. Efficacy of an energy conservation course for persons with multiple sclerosis. Arch Phys Med Rehabil. 2001;82(4):449–456. [DOI] [PubMed] [Google Scholar]
- 36.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 37.Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53(3):519–526. [DOI] [PubMed] [Google Scholar]
- 38.Gerstenecker A, Nabors LB, Meneses K, et al. Cognition in patients with newly diagnosed brain metastasis: profiles and implications. J Neurooncol. 2014;120(1):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 40.Onodera S, Aoyama H, Tha KK, et al. The value of 4-month neurocognitive function as an endpoint in brain metastases trials. J Neurooncol. 2014;120(2):311–319. [DOI] [PubMed] [Google Scholar]
- 41.Minniti G, Esposito V, Clarke E, et al. Stereotactic radiosurgery in elderly patients with brain metastases. J Neurooncol. 2013;111(3):319–325. [DOI] [PubMed] [Google Scholar]
- 42.Kirkpatrick JP, Wang Z, Sampson JH, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91(1):100–108. [DOI] [PubMed] [Google Scholar]
- 43.Steinvorth S, Wenz F, Wildermuth S, et al. Cognitive function in patients with cerebral arteriovenous malformations after radiosurgery: prospective long-term follow-up. Int J Radiat Oncol Biol Phys. 2002;54(5):1430–1437. [DOI] [PubMed] [Google Scholar]
- 44.Brown PD, Asher AL, Ballman KV, et al. NCCTG N0574 (Alliance): A phase III randomized trial of whole brain radiotherapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases [abstract]. J Clin Oncol. 2015;33(suppl; LBA4). [Google Scholar]
- 45.Meyers CA, Wefel JS. The use of the Mini-Mental State Examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol. 2003;21(19):3557–3558. [DOI] [PubMed] [Google Scholar]
- 46.Bosma I, Reijneveld JC, Douw L, et al. Health-related quality of life of long-term high-grade glioma survivors. Neuro Oncol. 2009;11(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmand B, Lindeboom J, Van Harskamp F. [The 15 words test A and B (a preliminary manual)]. Groningen: Afdeling Neuropyschologie, AZG; 1992. [Google Scholar]
- 48.Osterreith PA. Le test de copie d'une figure complexe [A test regarding a complex figure]. Arch Psychologie. 1944;30:206–356. [Google Scholar]
- 49.Wechsler D. Wechsler Adult Intelligence Scale III [Dutch revision. Technical manual]. Lisse: Swets Test Publishers; 2000. [Google Scholar]
- 50.Van der Elst W, Van Boxtel MP, Van Breukelen GJ, et al. The Concept Shifting Test: adult normative data. Psychol Assess. 2006;18(4):424–432. [DOI] [PubMed] [Google Scholar]
- 51.Krabbendam L, Kalff AC. The Behavioural Assessment of the Dysexecutive Syndrome: Dutch version. Lisse: Swets and Zeitlinger; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.